Submitted:
25 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. PRISMA Methodology
- Inclusion of peer-reviewed research articles, conference papers, and possibly government or industry reports. Exclusion of non-peer-reviewed sources like blog posts, news articles, or opinion pieces.
- Studies specifically focusing on BM in battery recycling, its characterization, management strategies, or related environmental and hazardous aspects.
- Inclusion of research articles published in English.
- Date Range of last 15 years (since 2008).
- Ni-Cd vs. Ni-MH: These batteries are separated due to their differing electrode materials and associated environmental impacts. Ni-Cd batteries contain cadmium, a toxic heavy metal, requiring specific handling and recycling processes. In contrast, Ni-MH batteries, while also based on nickel, replace cadmium with a metal hydride, altering the composition of the resultant BM.
- Zn/Mn Mn-C vs. Zn/Mn: The division between these Zinc/Manganese battery types is based on their distinct internal chemistries. Zinc/Manganese Carbon (Mn-C) batteries typically refer to alkaline batteries with added carbon, affecting their chemical profile and recycling process. In contrast, standard Zinc/Manganese batteries, often used in household applications, present a different composition, influencing the nature of their BM.
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs |
| Li | 3,4 | 9,725 ± 0,055 | 4,39 ± 0,14 | 4,302 ± 0,03 | 3,9 | 2,6 | 3,21 | 3,9 ± 0,1 | 4,95 ± 1,35 | 2,95 ± 0,25 |
| Co | 17,9 ± 0,1 | 7,29 ± 0,02 | 8,34 ± 0,01 | 4,11 ± 0,029 | 17,5 | 5,4 | 5,3 | 31,85 ± 0,45 | 12,65 ± 4,65 | 24 ± 2 |
| Ni | 4,54 ± 0,16 | 21,395 ± 0,005 | 16,485 ± 0,085 | 12,008 ± 0,084 | 5,1 | 12,5 | 14,6 | 11,4 ± 4,1 | 11 | |
| Mn | 5,58 ± 0,08 | 7,37 ± 0,13 | 9,39 ± 0,07 | 6,076 ± 0,042 | 3 | 10,9 | 5,09 | 11,35 ± 3,75 | 0,725 ± 0,185 | |
| Al | 5,405 ± 0,05 | 7,87 ± 0,11 | 1,185 ± 0,075 | 32,815 ± 0,09 | 1,6 | 4,8 | 2,02 | 0,65 ± 0,25 | 0,1 ± 0,1 | |
| Fe | 1,615 ± 0,185 | 0,235 ± 0,145 | > 0 | 1,6 | 2,3 | 0,326 ± 0,234 | ||||
| C | 9,17 ± 1,75 | 36 | 42,1 | 39,9 | 39,6 ± 4,2 | 28 ± 15,2 | ||||
| Cd | > 0 | |||||||||
| K | > 0 | 1,25 ± 0,15 | ||||||||
| Ti | 3,91 ± 0,09 | 0,15 ± 0,03 | 0,91 | |||||||
| Si | 2,115 ± 0,085 | > 0 | 1,6 | 1,15 ± 0,15 | ||||||
| Ca | 0,105 ± 0,045 | > 0 | ||||||||
| Mg | 0,08 ± 0,04 | 0,07 ± 0,02 | ||||||||
| Cu | 3,95 ± 0,05 | 2,135 ± 0,155 | 2,11 ± 0,04 | 5,067 ± 1,403 | 3,9 | 3,1 | 1,83 | 0,5 ± 0,1 | 1,2 ± 0,4 | 6,95 ± 5,05 |
| Zn | 0,894 ± 0,045 | 0,5 ± 0,41 | ||||||||
| Pb | ||||||||||
| P | 0,55 | 0,45 ± 0,05 | 0,5 ± 0,1 | |||||||
| F | 2,5 | 2,35 ± 0,25 | 5,7 ± 0,8 | |||||||
| Na | 0,315 ± 0,015 | |||||||||
| Sn | 0,72 ± 0,28 | |||||||||
| As | ||||||||||
| Ag | ||||||||||
| Source | [7] | [7] | [7] | [5] | [17] | 17 | [18] | [19] | [19] | [20] |
| Sample | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Type | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs | Mixed LIBs |
| Li | 2,24 ± 0,02 | 6,96 | 3,4 | 3,69 | 3,9 | 1,81 | 1,65 | 4 | 3,6 | 4 ± 0,4 |
| Co | 22 ± 0,14 | 5,8 | 11,7 | 24,6 | 17,1 | 0,17 | 32,3 | 8 | 26 ± 2,6 | |
| Ni | 2,71 ± 0,04 | 0,01 | 22,2 | 11,5 | 3,9 | 2,22 | 0,14 | 7,3 | 7,3 ± 0,7 | |
| Mn | 0,75 | 0,88 | 7,3 | 8,91 | 2,9 | 4,54 | 0,1 | 7,6 | 3,2 ± 0,3 | |
| Al | 3,88 ± 0,05 | 16,4 | 5,06 | 2,1 | 1,6 | 1,9 | 3,12 | 0,9 | 0,2 | 1,4 ± 0,1 |
| Fe | 6,51 ± 0,12 | 0,2 | 0,54 | 2,5 | 10,72 | |||||
| C | 20,5 ± 0,27 | 33,9 | 33 | 49,33 | 37,1 | 35,4 | 43,2 | |||
| Cd | ||||||||||
| K | 0,12 ± 0,01 | |||||||||
| Ti | ||||||||||
| Si | 0,37 ± 0,07 | 51,1 | 1,6 | 1 | ||||||
| Ca | 1,26 | 0,13 ± 0,01 | ||||||||
| Mg | ||||||||||
| Cu | 4,69 ± 0,02 | 0,11 | 6,9 | 0,88 | 1,1 | 0,68 | 0,82 | 0,6 | 1,6 | 2,8 ± 0,3 |
| Zn | 0,11 | |||||||||
| Pb | ||||||||||
| P | 0,44 | 9,64 | 0,5 | 0,6 | ||||||
| F | 4,1 | 2,6 | 6,5 | |||||||
| Na | ||||||||||
| Sn | ||||||||||
| As | ||||||||||
| Ag | 0,32 ± 0,01 | |||||||||
| Source | [21] | [22] | [23] | [24] | [25] | [26] | [27] | [28] | [28] | [29] |
| Sample | 21 | 22 | 23 | 25 | 24 | 26 | 27 | 28 | 29 | 30 |
| Type | Mixed LIBs | Ni-Cd | Ni-Cd | Ni-Cd | Ni-MH | Zn/Mn Mn-C | Zn/Mn | Zn/Mn | Zn/Mn | Zn/Mn |
| Li | 3,87 | 0,03 ± 0,02 | 0,0515 ± 0,0105 | |||||||
| Co | 26,45 | 1,5 ± 0,1 | 2,55 ± 2,25 | 2,4 ± 0,2 | 7,1 ± 1,2 | 0,03 | ||||
| Ni | 2,74 | 37,4 ± 2,1 | 31 ± 17 | 44,5 ± 4,5 | 62,5 ± 1,5 | 0,28 | ||||
| Mn | 1,67 | 0,88 ± 0,09 | 2,45 ± 1,15 | 43,3 | 38,7 | 28,45 ± 4,35 | 54,7 ± 4,3 | 30,6 | ||
| Al | 1,64 | 0,22 ± 0,02 | 0,675 ± 0,205 | 0,46 | 1,06 | 1,33 ± 0,17 | 0,79 | 0,79 | ||
| Fe | 0,61 | 0,8 ± 0,2 | 37,08 ± 36,92 | 3,4 ± 0,5 | 4,8 ± 1,4 | 1,42 | 9,06 | 8,145 ± 0,055 | 5,76 | |
| C | 33 | 2,4 ± 0,3 | 8,2 | 24,6 | 19 | |||||
| Cd | 27,7 ± 1,6 | 26 ± 15 | 20,9 ± 2,1 | 0,128 ± 0,092 | 0,01 | |||||
| K | 0,05 | 2,4 ± 0,2 | 2,3 ± 1,8 | 2,8 ± 0,3 | 2,75 ± 0,35 | 6,7 | 0,24 | 2,735 ± 2,465 | 0,585 ± 0,085 | 0,35 |
| Ti | 6,35 ± 0,75 | 0,191 | ||||||||
| Si | 1,69 | 5 | ||||||||
| Ca | 0,03 | |||||||||
| Mg | 0,09 | 0,23 | ||||||||
| Cu | 2,72 | 0,13 | 0,15 | 0,08 | ||||||
| Zn | 0,04 | 0,955 ± 0,645 | 2,55 ± 0,45 | 26,88 | 11,1 | 11,05 ± 4,05 | 0,535 ± 0,135 | 34,8 | ||
| Pb | 0,04 | |||||||||
| P | 0,45 | |||||||||
| F | ||||||||||
| Na | 0,06 | 0,515 ± 0,135 | 0,395 ± 0,155 | 0,03 | 0,035 ± 0,005 | 0,19 | 0,09 | |||
| Sn | 2,83 ± 1,97 | 6 ± 1,7 | ||||||||
| As | 0,41 ± 0,19 | |||||||||
| Ag | ||||||||||
| Source | [30] | [31] | [20] | [32] | [20] | [33] | [34] | [35] | [35] | [36] |
2.1. Black Mass Hazardousness Classification
- Scenario One: Focused exclusively on the CLP Regulation classifications, this scenario adheres to the GHS criteria and the labeling rules agreed upon by the United Nations.
- Scenario Two: This intermediate scenario broadens the scope by incorporating HSCs from both CLP and REACH classifications, thus expanding the regulatory purview.
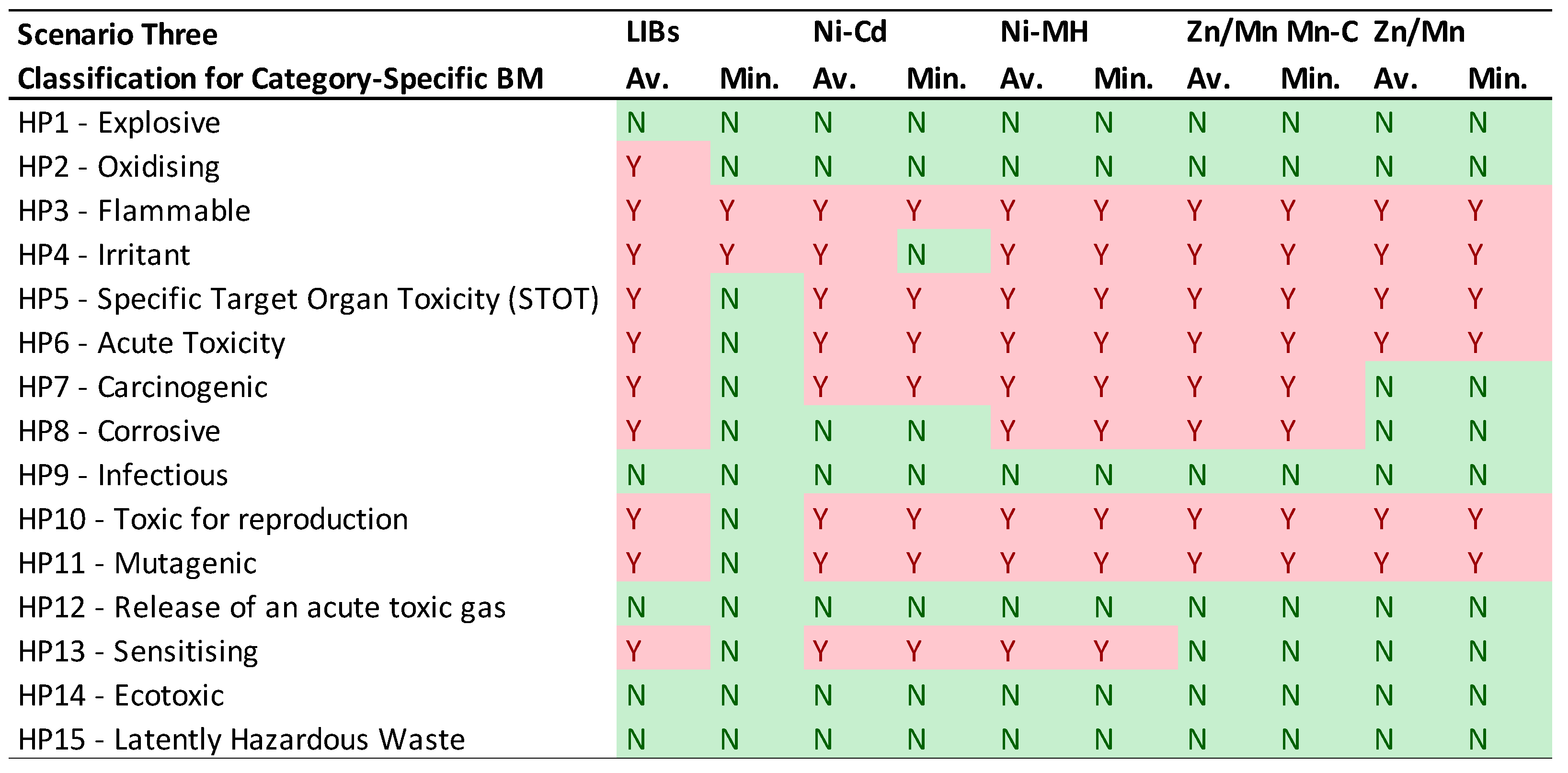
- Scenario Three: The most expansive scenario, it compiles all classifications, including those by manufacturers and importers, to reveal the full potential of HPs associated with the BM. This comprehensive view is inclusive of extra-European legislative considerations and provides the most extensive hazard potential profile.
| (%) | LIBs | Ni-Cd | Ni-MH | Zn/Mn Mn-C | Zn/Mn | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Av. | Min. | Av. | Min. | Av. | Min. | Av. | Min. | Av. | Min. | |
| Li | 3,93 | 1,65 | 0,01 | - | 0,05 | 0,04 | - | - | - | - |
| Co | 14,69 | - | 2,15 | 0,30 | 7,10 | 5,90 | 0,03 | 0,03 | - | - |
| Ni | 8,05 | - | 37,63 | 14,00 | 62,50 | 61,00 | 0,28 | 0,28 | - | - |
| Mn | 4,63 | - | 0,29 | - | 2,45 | 1,30 | 43,30 | 43,30 | 38,11 | 24,10 |
| Al | 4,51 | - | 0,07 | - | 0,68 | 0,47 | 0,46 | 0,46 | 0,99 | 0,79 |
| Fe | 1,29 | - | 13,76 | 0,16 | 4,80 | 3,40 | 1,42 | 1,42 | 5,74 | - |
| C | 22,87 | - | 0,80 | - | - | - | 8,20 | 8,20 | 10,90 | - |
| Cd | 0,00 | - | 24,87 | 11,00 | 0,13 | 0,04 | 0,01 | 0,01 | - | - |
| K | 0,07 | - | 2,50 | 0,50 | 2,75 | 2,40 | 6,70 | 6,70 | 0,98 | 0,24 |
| Ti | 0,24 | - | - | - | 6,35 | 5,60 | 0,19 | 0,19 | - | - |
| Si | 2,81 | - | - | - | - | - | 1,69 | 1,69 | 1,25 | - |
| Ca | 0,07 | - | - | - | - | - | - | - | - | - |
| Mg | 0,01 | - | - | - | - | - | 0,23 | 0,23 | - | - |
| Cu | 2,55 | 0,11 | 0,04 | - | 0,15 | 0,15 | 0,08 | 0,08 | - | - |
| Zn | 0,07 | - | 0,32 | - | 2,55 | 2,10 | 26,88 | 26,88 | 14,37 | 0,40 |
| Pb | - | - | - | - | - | - | 0,04 | 0,04 | - | - |
| P | 0,63 | - | - | - | - | - | - | - | - | - |
| F | 1,13 | - | - | - | - | - | - | - | - | - |
| Na | 0,02 | - | 0,17 | - | 0,40 | 0,24 | - | - | 0,09 | 0,03 |
| Sn | 0,03 | - | 0,94 | - | 6,00 | 4,30 | - | - | - | - |
| As | - | - | - | - | 0,41 | 0,22 | - | - | - | - |
| Ag | 0,02 | - | - | - | - | - | - | - | - | - |
3. Results
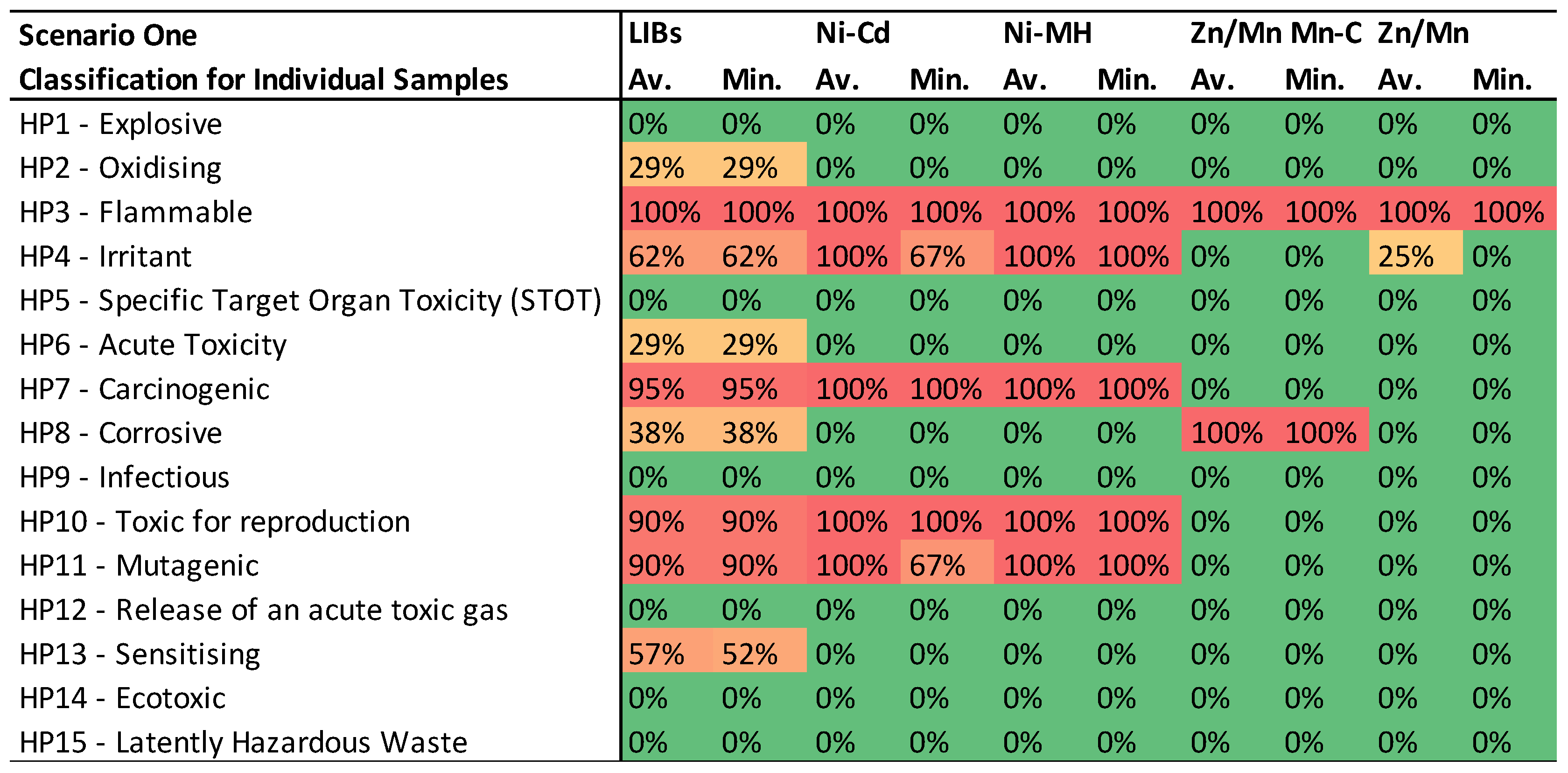
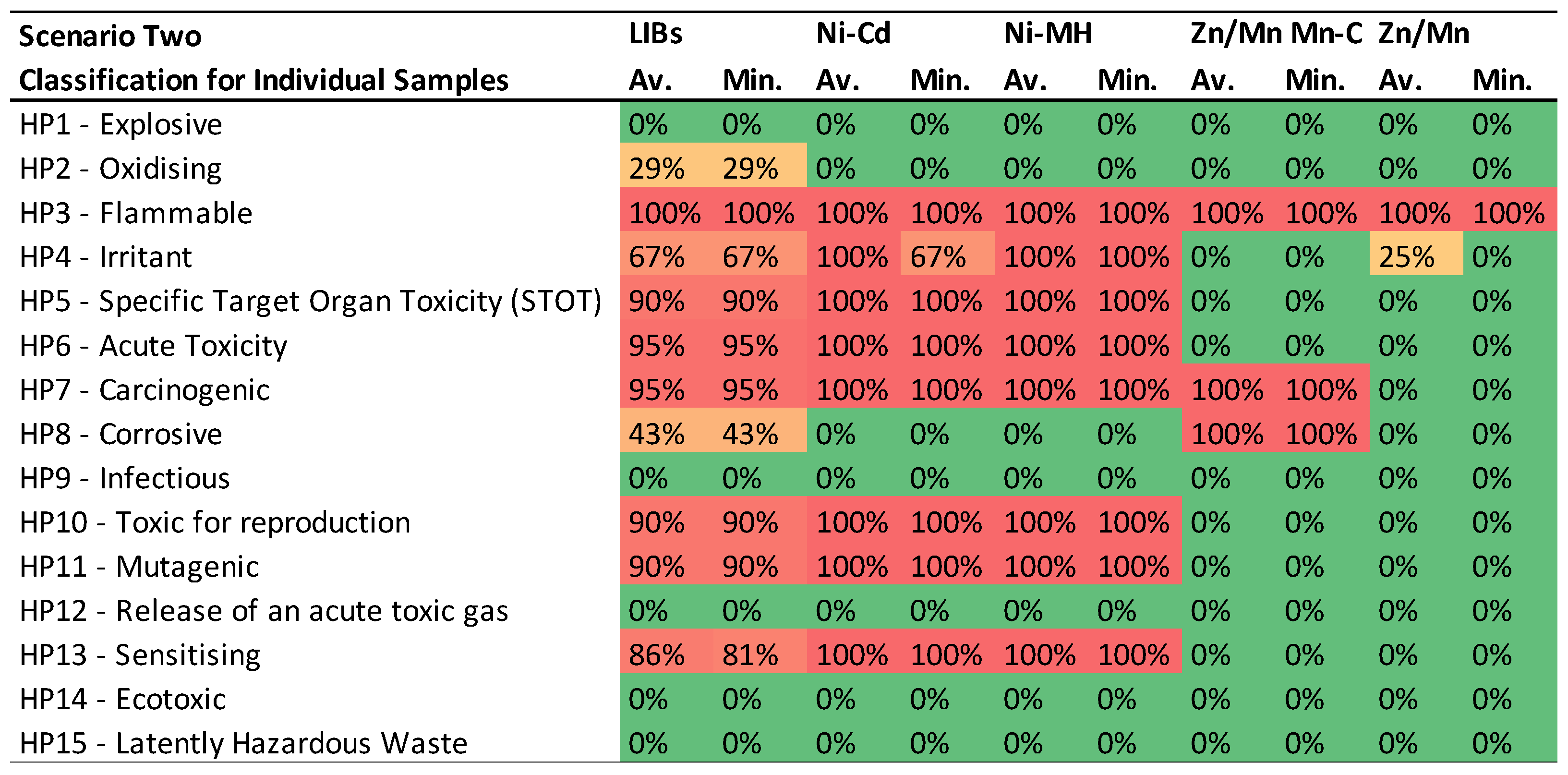
- Individual Sample Compatibility with HPs: The first table presents the compatibility of individual samples with each HP, categorized by battery type. Each value represents the percentage of samples in a battery category adhering to a specific HP, ranging from 0% (in green), indicating no sample falls under the category, to 100% (in red), denoting all samples fit the category. These percentages are displayed on a color scale to visually represent the increasing likelihood of BM from a specific battery category falling under the respective HP.
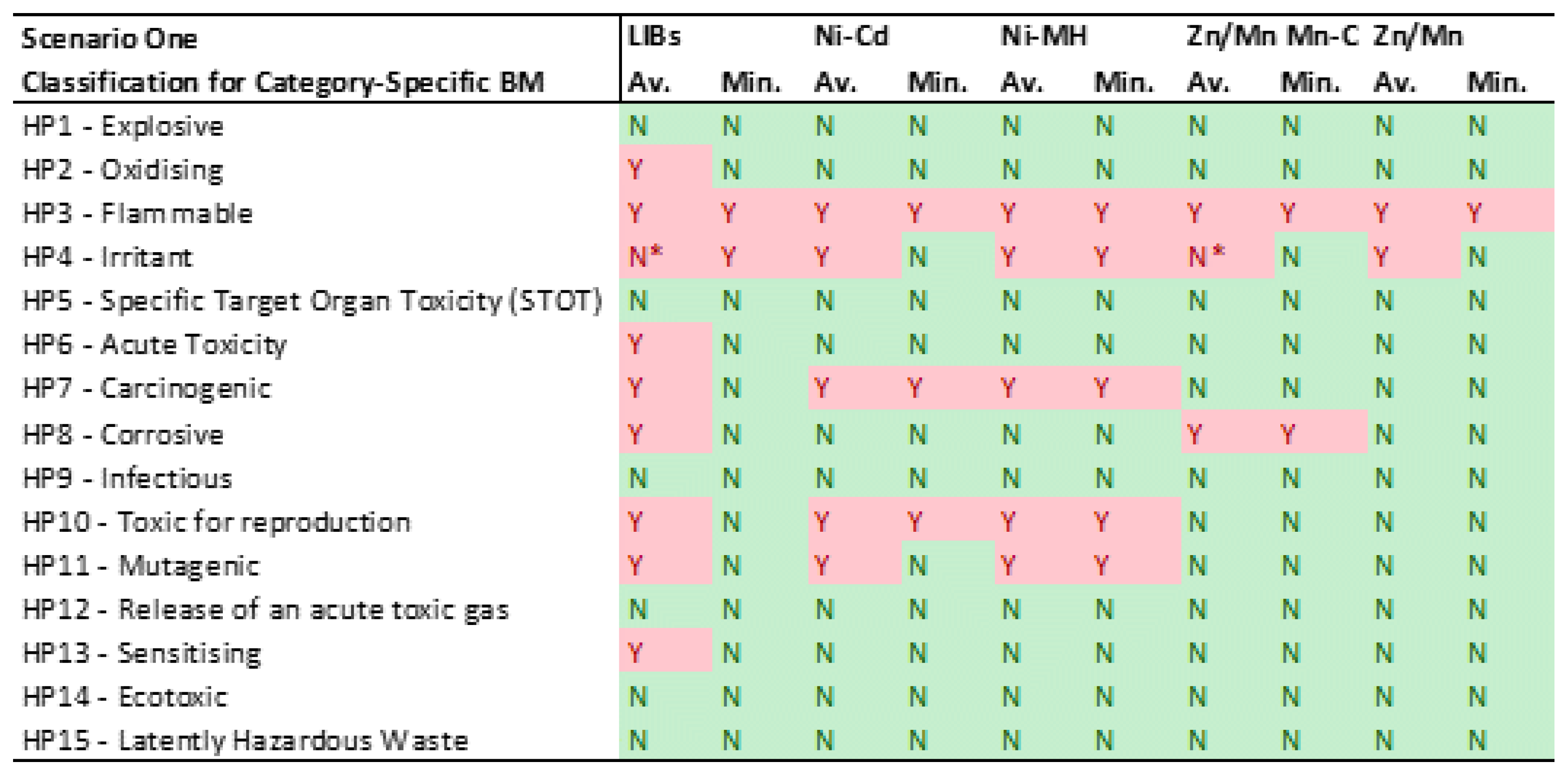
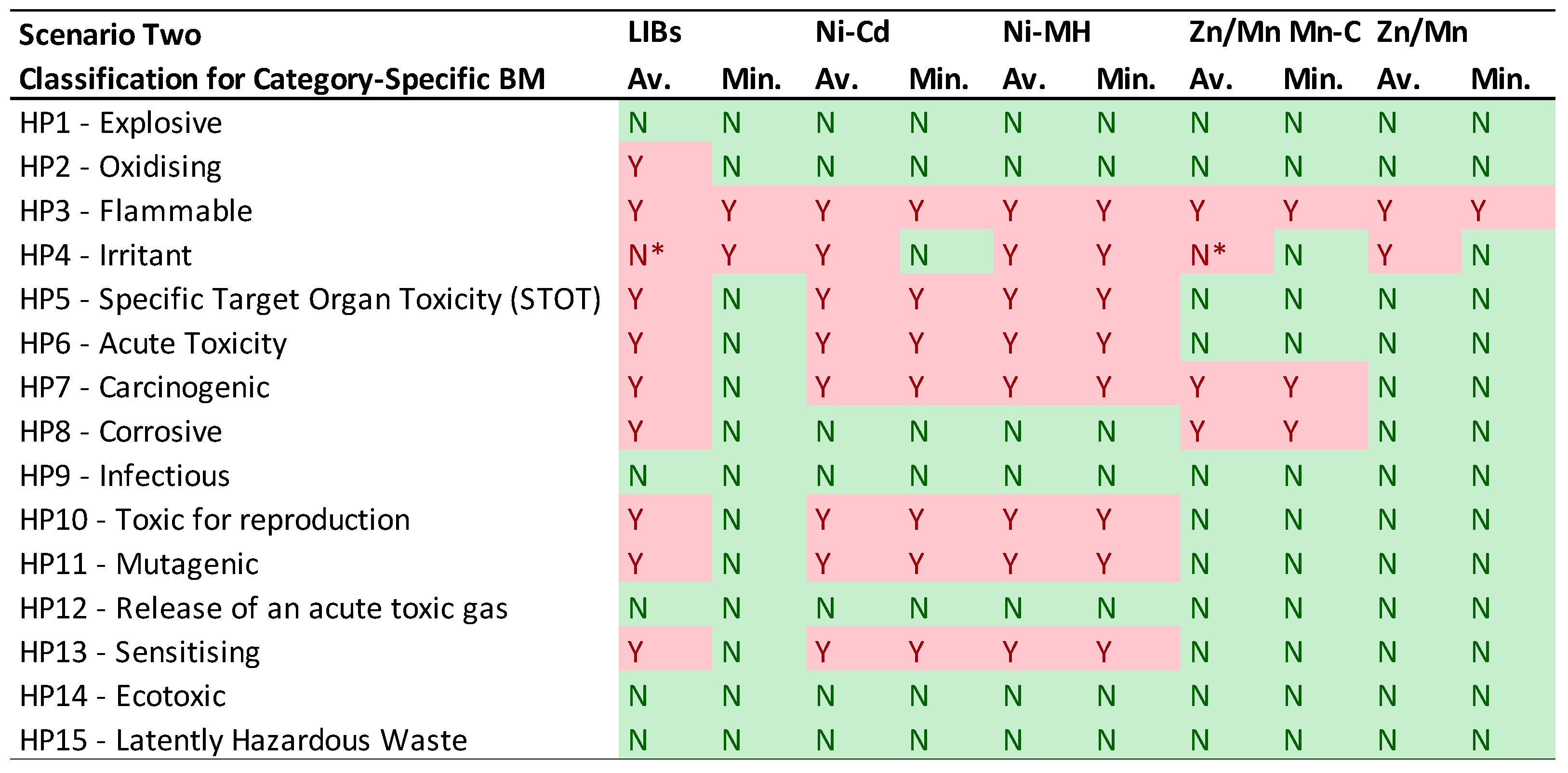
- CSBM Analysis: The second table focuses on CSBM, considering the average and minimum values as described in Table 4. It uses a simple 'Y' (Yes) in red to indicate classification under a specific HP, or 'N' (No) in green when it does not fall under that HP.
3.1. Scenario One: CLP Regulation classifications
3.2. Scenario Two: CLP Regulation and REACH classifications
3.3. Scenario Three: CLP Regulation, REACH, and Notification classifications
4. Discussion
5. Conclusions
References
- J. Fleischmann et al., “Battery 2030: Resilient, sustainable, and circular Battery demand is growing — and so is the need for,” McKinsey Co., 2023.
- Q. Dai, J. C. Kelly, L. Gaines, and M. Wang, “Life cycle analysis of lithium-ion batteries for automotive applications,” Batteries, vol. 5, no. 2, 2019. [CrossRef]
- A. Bhuyan, A. Tripathy, R. K. Padhy, and A. Gautam, “Evaluating the lithium-ion battery recycling industry in an emerging economy: A multi-stakeholder and multi-criteria decision-making approach,” J. Clean. Prod., vol. 331, 2022, [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85120832737&doi=10.1016%2Fj.jclepro.2021.130007&partnerID=40&md5=5e2fa19dcb54dd5c9b970ba340125a31.
- Motus E, Strategy&, and Politecnico Milano, “Il riciclo delle batterie dei veicoli elettrici @ 2050: scenari evolutivi e tecnologie abilitanti,” 2023.
- L. Donnelly et al., “The Recycling of End-of-Life Lithium-Ion Batteries and the Phase Characterisation of Black Mass,” Recycling, vol. 8, no. 4, 2023. [CrossRef]
- AquaMetals, “What Exactly is Lithium Battery ‘Black Mass’?” [Online]. Available: https://aquametals.com/recyclopedia/what-exactly-is-black-mass/.
- M. Dadé, T. Wallmach, and O. Laugier, “Detailed Microparticle Analyses Providing Process Relevant Chemical and Microtextural Insights into the Black Mass,” Minerals, vol. 12, no. 2, 2022. [CrossRef]
- European Commission, COM (2023) 160 final. https://eur-lex.europa.eu/resource.html?uri=cellar:903d35cc-c4a2-11ed-a05c-01aa75ed71a1.0001.02/DOC_1&format=PDF, 2023. [CrossRef]
- European Commission, Regulation (EC) No 1272/2008. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1272, 2008. [Online]. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1272.
- European Commission, Regulation (EC) No 1907/2006. https://eur-lex.europa.eu/eli/reg/2006/1907/2023-08-06, 2006. [Online]. Available: https://eur-lex.europa.eu/eli/reg/2006/1907/2023-08-06.
- S. Frédéric, “EU urged to restrict export of black mass from used electric vehicles,” Euractive.
- European Commission, Regulation (EU) No 2023/1542. 2023. [Online]. Available: https://eur-lex.europa.eu/eli/reg/2023/1542/oj.
- European Parliament, P9_TA (2023)0325. https://www.europarl.europa.eu/doceo/document/TA-9-2023-0325_EN.pdf. [Online]. Available: https://www.europarl.europa.eu/doceo/document/TA-9-2023-0325_EN.pdf.
- European Commission, Regulation (EU) No 1357/2014. [Online]. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32014R1357.
- W. Mengist, T. Soromessa, and G. Legese, “Method for conducting systematic literature review and meta-analysis for environmental science research,” MethodsX, vol. 7, p. 100777, 2020. [CrossRef]
- M. Pellegrini and F. Marsili, “Evaluating software tools to conduct systematic reviews: a feature analysis and user survey,” Form@re - Open J. per la Form. rete, vol. 21, no. 2, pp. 124–140, 2021. [CrossRef]
- E. Mousa et al., “Characterization and Thermal Treatment of the Black Mass from Spent Lithium-Ion Batteries,” Sustain., vol. 15, no. 1, 2023. [CrossRef]
- C. Stallmeister and B. Friedrich, “Influence of Flow-Gas Composition on Reaction Products of Thermally Treated NMC Battery Black Mass,” Metals (Basel)., vol. 13, no. 5, 2023. [CrossRef]
- S. Babanejad et al., “High-Temperature Behavior of Spent Li-Ion Battery Black Mass in Inert Atmosphere,” J. Sustain. Metall., vol. 8, no. 1, pp. 566–581, 2022. [CrossRef]
- I. Vassura, L. Morselli, E. Bernardi, and F. Passarini, “Chemical characterisation of spent rechargeable batteries,” Waste Manag., vol. 29, no. 8, pp. 2332–2335, 2009. [CrossRef]
- M. Sommerfeld et al., “A combined pyro-and hydrometallurgical approach to recycle pyrolyzed lithium-ion battery black mass part 1: Production of lithium concentrates in an electric arc furnace,” Metals (Basel)., vol. 10, no. 8, pp. 1–27, 2020. [CrossRef]
- J. Klimko et al., “A combined pyro-and hydrometallurgical approach to recycle pyrolyzed lithium-ion battery black mass part 2: Lithium recovery from li enriched slag— thermodynamic study, kinetic study, and dry digestion,” Metals (Basel)., vol. 10, no. 11, pp. 1–31, 2020. [CrossRef]
- E. Gerold, R. Lerchbammer, and H. Antrekowitsch, “Evaluation of the Influence Exerted by Increased Silicon Contents on the Leaching Behavior of NMC-Based Black Mass,” Metals (Basel)., vol. 13, no. 4, 2023. [CrossRef]
- L. Schwich, T. Schubert, and B. Friedrich, “Early-stage recovery of lithium from tailored thermal conditioned black mass part i: Mobilizing lithium via supercritical co2-carbonation,” Metals (Basel)., vol. 11, no. 2, pp. 1–30, 2021. [CrossRef]
- D. Amalia, P. Singh, W. Zhang, and A. N. Nikoloski, “The Effect of a Molasses Reductant on Acetic Acid Leaching of Black Mass from Mechanically Treated Spent Lithium-Ion Cylindrical Batteries,” Sustain., vol. 15, no. 17, 2023. [CrossRef]
- B. Makuza et al., “Synergetic carbothermic reduction and selective hydrochlorination of spent Li-ion batteries black mass towards enhanced metal recovery,” J. Clean. Prod., vol. 386, no. December 2022, p. 135831, 2023. [CrossRef]
- T. Zhao et al., “Direct selective leaching of lithium from industrial-grade black mass of waste lithium-ion batteries containing LiFePO4 cathodes,” Waste Manag., vol. 171, no. January, pp. 134–142, 2023. [CrossRef]
- S. Babanejad, H. Ahmed, C. Andersson, and E. P. Heikkinen, “Mechanical Activation-Assisted Recovery of Valuable Metals from Black Mass in the Form of Fe/Cu Alloys,” J. Sustain. Metall., vol. 9, no. 2, pp. 522–536, 2023. [CrossRef]
- A. Łukomska, A. Wiśniewska, Z. Dąbrowski, D. Kolasa, S. Luchcińska, and U. Domańska, “Separation of cobalt, lithium and nickel from the ‘black mass’ of waste Li-ion batteries by ionic liquids, DESs and organophosphorous-based acids extraction,” J. Mol. Liq., vol. 343, 2021. [CrossRef]
- J. Biswas, S. Ulmala, X. Wan, J. Partinen, M. Lundström, and A. Jokilaakso, “Selective Sulfation Roasting for Cobalt and Lithium Extraction from Industrial LCO-Rich Spent Black Mass,” Metals (Basel)., vol. 13, no. 2, 2023. [CrossRef]
- C. Hazotte, N. Leclerc, S. Diliberto, E. Meux, and F. Lapicque, “End-of-life nickel-cadmium accumulators: Characterization of electrode materials and industrial Black Mass,” Environ. Technol. (United Kingdom), vol. 36, no. 6, pp. 796–805, 2015. [CrossRef]
- A. Łukomska et al., “New method for recovery of nickel and cadmium from the ”black mass” of spent Ni-Cd batteries by solvent extraction,” J. Mol. Liq., vol. 357, pp. 1–9, 2022. [CrossRef]
- L. A. Romo, A. López-Fernández, I. García-Díaz, P. Fernández, A. Urbieta, and F. A. López, “From spent alkaline batteries to ZnxMn3−xO4 by a hydrometallurgical route: synthesis and characterization,” RSC Adv., vol. 8, no. 58, pp. 33496–33505, 2018. [CrossRef]
- S. C. Kim, M. K. Kim, S. C. Jung, H. Y. Jung, H. Kim, and Y. K. Park, “Effect of palladium on the black mass-based catalyst prepared from spent Zn/Mn alkaline batteries for catalytic combustion of volatile organic compounds,” Chemosphere, vol. 276, p. 130209, 2021. [CrossRef]
- B. S. Kim, S. C. Jung, H. Y. Jung, M. A. Khan, B. H. Jeon, and S. C. Kim, “The use of black mass in spent primary battery as an oxidative catalyst for removal of volatile organic compounds,” J. Ind. Eng. Chem., vol. 114, pp. 323–330, 2022. [CrossRef]
- Y. K. Park, W. G. Shim, S. C. Jung, H. Y. Jung, and S. C. Kim, “Catalytic removal of volatile organic compounds using black mass from spent batteries,” Korean J. Chem. Eng., vol. 39, no. 1, pp. 161–166, 2022. [CrossRef]
- D. S. Premathilake, A. B. Botelho Junior, J. A. S. Tenório, D. C. R. Espinosa, and M. Vaccari, “Designing of a Decentralized Pretreatment Line for EOL-LIBs Based on Recent Literature of LIB Recycling for Black Mass,” Metals (Basel)., vol. 13, no. 2, 2023. [CrossRef]
- C. Wilke, D. M. Werner, A. Kaas, and U. A. Peuker, “Influence of the Crusher Settings and a Thermal Pre-Treatment on the Properties of the Fine Fraction (Black Mass) from Mechanical Lithium-Ion Battery Recycling,” Batteries, vol. 9, no. 10, 2023. [CrossRef]
- T. Punt, S. M. Bradshaw, P. van Wyk, and G. Akdogan, “The Efficiency of Black Mass Preparation by Discharge and Alkaline Leaching for LIB Recycling,” Minerals, vol. 12, no. 6, 2022. [CrossRef]
- E. Guyot, C. Boulanger, and J. M. Lecuire, “Leaching optimization of battery black mass for lithium recovery by Electrochemical Junction Transfer (ETJ) technology,” Chem. Eng. Trans., vol. 41, no. Special Issue, pp. 67–72, 2014. [CrossRef]
- A. Porvali, T. Mäkelä, and J. Bachér, “Observations on the Leaching of Milled Black Mass with Additives,” J. Sustain. Metall., vol. 9, no. 2, pp. 816–825, 2023. [CrossRef]
- R. Golmohammadzadeh et al., “Removal of polyvinylidene fluoride binder and other organics for enhancing the leaching efficiency of lithium and cobalt from black mass,” J. Environ. Manage., vol. 343, no. May, p. 118205, 2023. [CrossRef]
- N. Vieceli, P. Benjamasutin, R. Promphan, P. Hellström, M. Paulsson, and M. Petranikova, “Recycling of Lithium-Ion Batteries: Effect of Hydrogen Peroxide and a Dosing Method on the Leaching of LCO, NMC Oxides, and Industrial Black Mass,” ACS Sustain. Chem. Eng., vol. 11, no. 26, pp. 9662–9673, 2023. [CrossRef]
- A. Łukomska et al., “Recovery of zinc and manganese from ‘black mass’ of waste Zn-MnO2 alkaline batteries by solvent extraction technique with ionic liquids, DESs and organophosphorous-based acids,” J. Mol. Liq., vol. 338, 2021. [CrossRef]
- C. Hazotte, E. Meux, N. Leclerc, and F. Lapicque, “Electroassisted leaching of black mass solids from Ni-Cd batteries for metal recovery: Investigation of transport and transfer phenomena coupled to reactions,” Chem. Eng. Process. Process Intensif., vol. 96, pp. 83–93, 2015. [CrossRef]
- V. Gupta, X. Yu, H. Gao, C. Brooks, W. Li, and Z. Chen, “Scalable Direct Recycling of Cathode Black Mass from Spent Lithium-Ion Batteries,” Adv. Energy Mater., vol. 13, no. 6, pp. 1–8, 2023. [CrossRef]

| Element | EC/List no. | CAS no. | CLP Regulation HSCs | REACH HSCs | Notifications HSCs |
|---|---|---|---|---|---|
| Li | 231 102 5 | 7439 93 2 | H314 (Skin Corr. 1B); H260 (Water-react. 1) | H314 (Skin Corr. 1B); H260 (Water-react. 1) | H228; H260; H301; H314; H318; H371; H413 |
| Co | 231 158 0 | 7440 48 4 | H317 (Skin Sens. 1); H334 (Resp. Sens. 1) H341 (Muta. 2); H413 (Aquatic Chronic 4) | H302 (Acute Tox. 4); H317 (Skin Sens. 1); H319 (Eye Irrit. 2); H330 (Acute Tox. 1); H334 (Resp. Sens. 1B); H341 (Muta. 2); H360 (Repr. 1B); H373 (STOT RE 2); H400 (Aquatic Acute 1); H410 (Aquatic Chronic 1); H411 (Aquatic Chronic 2); H412 (Aquatic Chronic 3); H413 (Aquatic Chronic 4) | H228; H250; H260; H302; H315; H317; H319; H330; H332; H334; H341; H350; H351; H360; H361; H372; H373; H400; H410; H411; H412; H413 |
| Ni | 231 111 4 | 7440 02 0 | H317 (Skin Sens. 1); H334 (Resp Sens. 1); H341 (Muta. 2); H350 (Carc. 1B); H350i (Carc. 1A); H351 (Carc. 2); H360F (Repr. 1B); H372 (STOT RE 1); H411 (Aquatic Chronic 2); H412 (Aquatic Chronic 3) | H228; H250; H317; H334; H341; H350; H350i; H351; H360; H370; H372; H400; H411; H412 | |
| Mn | 231 105 1 | 7439 96 5 | H411 (Aquatic Chronic 2); H412 (Aquatic Chronic 3) | H228; H260; H302; H312; H315; H319; H332; H335; H340; H341; H360; H361; H370; H372; H373; H411; H412 | |
| Al | 231 072 3 | 7429 90 5 | H228 (Flam. Sol. 1); H261 (Water-react. 2) | H228; H250; H261; H302; H311; H315; H317; H331; H332; H372; H373; H400; H413 | |
| Fe | 231 096 4 | 7439 89 6 | H228 (Flam. Sol. 1); H251 (Self-heat. 1) | H228; H251; H261; H302; H315; H319; H335; H370; H371; H372; H373; H400; H410 | |
| C | 231 153 3 | 7440 44 0 | H226; H228; H251; H252; H300; H302; H315; H319; H335; H373; H412 | ||
| Cd | 231 152 8 | 7440 43 9 | H250 (Pyr. Sol 1); H330 (Acute Tox. 1); H341 (Muta. 2); H350 (Carc. 1B); H361 (Repr. 2); H372 (STOT RE 1); H400 (Aquatic Acute 1); H410 (Aquatic Chronic 1) | H250; H301; H330; H335; H341; H350; H361; H372; H400; H410 | |
| K | 231 119 8 | 7440 09 7 | H260 (Water-react. 1); H314 (Skin Corr. 1B) | H260 (Water-react. 1); H314 (Skin Corr. 1B); H318 (Eye Dam. 1) | H260; H314; H318; H412 |
| Ti | 231 142 3 | 7440 32 6 | H228 (Flam. Sol. 1) | H228; H250; H251; H252; H260; H315; H319; H335 | |
| Si | 231 130 8 | 7440 21 3 | H228; H315; H319; H335; H373 | ||
| Ca | 231-179-5 | 7440 70 2 | H261 (Water-react. 2) | H261 (Water-react. 2) | H250; H261; H314; H315; H318; H319; H371 |
| Mg | 231 104 6 | 7439 95 4 | H228 (Flam. Sol. 1); H250 (Pyr. Sol 1); H252 (Self-heat. 1 and 2); H260 (Water-react. 1); H261 (Water-react. 2) | H228; H250; H251; H252; H260; H261; H315; H318; H335; H413 | |
| Cu | 231 159 6 | 7440 50 8 | H411 (Aquatic Chronic 2) | H400 (Aquatic Acute 1); H411 (Aquatic Chronic 2) | H228; H302; H315; H317; H319; H331; H332; H335; H361; H370; H371; H372; H373; H400; H410; H411; H412; H413 |
| Zn | 231 175 3 | 7440 66 6 | H410 (Aquatic Chronic 1) | H228; H250; H251; H252; H260; H261; H302; H311; H315; H319; H331; H332; H335; H400; H410; H413 | |
| Pb | 231 100 4 | 7439 92 1 | H350 (Carc. 1A); H360 (Repr. 1A); H362 (Lact.); H372 (STOT RE 1); H400 (Aquatic Acute 1); H410 (Aquatic Chronic 1); H411 (Aquatic Chronic 2) | H302; H311; H315; H318; H332; H341; H350; H351; H360; H362; H371; H372; H373; H400; H410; H411; H413 | |
| P | 231 768 7 | 7723 14 0 | H228 (Flam. Sol. 1); H250 (Pyr. Sol 1); H300 (Acute Tox. 1 and 2); H314 (Skin Corr. 1B); H330 (Acute Tox. 2); H400 (Aquatic Acute 1); H412 (Aquatic Chronic 3) | H228; H250; H251; H300; H312; H314; H318; H330; H370; H373; H400; H412 | |
| F | 231 954 8 | 7782 41 4 | H270 (Ox. Gas 1); H314 (Skin Corr. 1B); H330 (Acute Tox. 2) | H270 (Ox. Gas 1); H280 (Press. Gas Comp.); H314 (Skin Corr. 1B); H318 (Eye Dam. 1); H330 (Acute Tox. 1 and 2) | H270; H280; H310; H314; H318; H330 |
| Na | 231 132 9 | 7440 23 5 | H260 (Water-react. 1); H314 (Skin Corr. 1B) | H260 (Water-react. 1); H314 (Skin Corr. 1B) | H260; H314; H318; H370; H412 |
| Sn | 231 141 8 | 7440 31 5 | H228; H302; H311; H315; H319; H331; H332; H334; H335; H372; H400; H413 | ||
| As | 231 148 6 | 7440 38 2 | H301 (Acute Tox. 3); H331 (Acute Tox. 3); H400 (Aquatic Acute 1); H410 (Aquatic Chronic 1) | H301 (Acute Tox. 3); H315 (Skin Irrit. 2); H318 (Eye Dam. 1); H331 (Acute Tox. 3); H350 (Carc. 1A); H360D (Repr. 1A); H372 (STOT RE 1); H400 (Aquatic Acute 1); H410 (Aquatic Chronic 1) | H228; H251; H300; H301; H315; H318; H331; H341; H350; H360; H361; H371; H372; H373; H400; H410; H411 |
| Ag | 231 131 3 | 7440 22 4 | H360D (Repr. 1A); H400 (Aquatic Acute 1); H410 (Aquatic Chronic 1) | H312; H315; H317; H319; H332; H335; H351; H360; H370; H372; H400; H410; H411 |
| Hazardous Property | HSCs | Element | Condition |
|---|---|---|---|
| HP2 - Oxidizing | H270 (Ox. Gas 1) | F | Presence |
| HP3 - Flammable | H226 (Flam. Liq. 3) | C | Presence |
| H228 (Flam. Sol. 1-2) | Li, Co, Ni, Mn, Al, Fe, C, Ti, Si, Mg, Cu, Zn, P, Sn, As | Presence | |
| H250 (Pyr. Sol. 1) | Co, Ni, Al, Cd, Ti, Ca, Mg, Zn, P | Presence | |
| H251 (Self-heat.1) | Fe, C, Ti, Mg, Zn, P, As | Presence | |
| H252 (Self-heat. 2) | C, Ti, Mg, Zn | Presence | |
| H260 (Water-react. 1) | Li, Co, Mn, K, Ti, Mg, Zn, Na | Presence | |
| H261 (Water-react. 2-3) | Al, Fe, Ca, Mg, Zn | Presence | |
| HP4 - Irritant | H314 (Skin corr. 1A-1B) | Li, K, Ca, P, F, Na | ≥1%; <5% - Sum |
| H315 (Skin irrit. 2) | Co, Mn, Al, Fe, C, Ti, Si, Ca, Mg, Cu, Zn, Sn, As, Ag | ≥20% - Sum | |
| H318 (Eye dam. 1) | Li, K, Ca, Mg, P, F, Na, As | ≥10% - Sum | |
| H319 (Eye irrit. 2) | Co, Mn, Fe, C, Ti, Si, Ca, Cu, Zn, Sn, Ag | ≥20% - Sum | |
| HP5 - Specific Target Organ Toxicity (STOT) | H370 (STOT SE 1) | Ni, Mn, Fe, Cu, P, Na, Ag | ≥1% - Ind. |
| H371 (STOT SE 2) | Li, Fe, Ca, Cu, As | ≥10% - Ind. | |
| H335 (STOT SE 3) | Mn, Fe, C, Cd, Ti, Si, Mg, Cu, Zn, Sn, Ag | ≥20% - Ind. | |
| H372 (STOT RE 1) | Co, Ni, Mn, Al, Fe, Cd, Cu, Sn, As, Ag, Pb | ≥1% - Ind. | |
| H373 (STOT RE 2) | Co, Mn, Al, Fe, C, Si, Cu, P, As, | ≥10% - Ind. | |
| HP6 - Acute Toxicity | H300 (Acute Tox.2 (Oral)) | C, P, As | ≥25% - Sum |
| H301 (Acute Tox.3 (Oral)) | Li, Cd, As | ≥5% - Sum | |
| H302 (Acute Tox.2 (Oral)) | Co, Mn, Al, Fe, C, Cu, Zn, Sn | ≥25% - Sum | |
| H310 (Acute Tox.1 (Dermal)) | F | ≥0,25% - Sum | |
| H311 (Acute Tox.3 (Dermal)) | Al, Zn, Sn | ≥15% - Sum | |
| H312 (Acute Tox.4 (Dermal)) | Mn, P, Ag | ≥55% - Sum | |
| H330 (Acute Tox.1 (Inhal.)) | F | ≥0,1% - Sum | |
| H330 (Acute Tox.2 (Inhal.)) | Co, Cd, P, F | ≥0,5% - Sum | |
| H331 (Acute Tox.3) (Inhal.)) | Al, Cu, Zn, Sn, As | ≥3,5% - Sum | |
| H332 (Acute Tox.4 (Inhal.)) | Co, Mn, Al, Cu, Zn, Sn, Ag | ≥22,5% - Sum | |
| HP7 - Carcinogenic | H350 (Carc. 1A-1B) | Co, Ni, Cd, As | ≥0,1% - Ind. |
| H351 (Carc. 2) | Co, Ni, Ag | ≥1% - Ind. | |
| HP8 - Corrosive | H314 (Skin corr. 1A-1B) | Li, K, Ca, P, F, Na | ≥5% - Sum |
| HP10 - Toxic for reproduction | H360 (Repr. 1A-1B) | Co, Ni, Mn, Pb, As, Ag | ≥0,3% - Ind. |
| H361 (Repr. 2) | Co, Mn, Cd, Cu, As | ≥3% - Ind. | |
| HP11 - Mutagenic | H340 (Muta. 1A) | Mn | ≥0,1% - Ind. |
| H341 (Muta. 2) | Co, Ni, Mn, Cd, As | ≥1% - Ind. | |
| HP13 - Sensitizing | H317 (Skin Sens. 1) | Co, Ni, Al, Cu, Ag | ≥10% - Ind. |
| H334 (Resp. Sens. 1) | Co, Ni, Sn | ≥10% - Ind. |

| CSBM | Baseline Scenario | Comprehensive Scenario |
|---|---|---|
| LIBs | HP3 - Flammable; HP4 - Irritant; | HP2 - Oxidizing; HP3 - Flammable; HP4 - Irritant; HP5 - Specific Target Organ Toxicity (STOT); HP6 - Acute Toxicity; HP7 - Carcinogenic; HP8 - Corrosive; HP10 - Toxic for reproduction; HP11 - Mutagenic; HP13 - Sensitizing |
| Ni-Cd | HP3 - Flammable; HP7 - Carcinogenic; HP10 - Toxic for reproduction; | HP3 - Flammable; HP4 - Irritant; HP5 - Specific Target Organ Toxicity (STOT); HP6 - Acute Toxicity; HP7 - Carcinogenic; HP10 - Toxic for reproduction; HP11 - Mutagenic; HP13 - Sensitizing |
| Ni-MH | HP3 - Flammable; HP4 - Irritant; HP7 - Carcinogenic; HP10 - Toxic for reproduction; HP11 - Mutagenic | HP3 - Flammable; HP4 - Irritant; HP5 - Specific Target Organ Toxicity (STOT); HP6 - Acute Toxicity; HP7 - Carcinogenic; HP8 - Corrosive; HP10 - Toxic for reproduction; HP11 - Mutagenic; HP13 - Sensitizing |
| Zn/Mn Mn-C | HP3 - Flammable; HP8 - Corrosive | HP3 - Flammable; HP4 - Irritant; HP5 - Specific Target Organ Toxicity (STOT); HP6 - Acute Toxicity; HP7 - Carcinogenic; HP8 - Corrosive; HP10 - Toxic for reproduction; HP11 - Mutagenic |
| Zn/Mn | HP3 - Flammable | HP3 - Flammable; HP4 - Irritant; HP5 - Specific Target Organ Toxicity (STOT); HP6 - Acute Toxicity; HP10 - Toxic for reproduction; HP11 - Mutagenic |
| Generic BM | HP3 - Flammable | HP3 - Flammable; HP4 - Irritant; HP5 - Specific Target Organ Toxicity (STOT); HP6 - Acute Toxicity; HP10 - Toxic for reproduction; HP11 - Mutagenic |
| Technology | Description | BM Type | Recovered Elements | TRL | Limitations | Sources |
|---|---|---|---|---|---|---|
| Electrochemical junction transfer (ETJ) | A cation recovery process using intercalation into a host matrix, with a focus on lithium recovery from spent Li-ion batteries leachate. | Spent Li-ion batteries leachate. | Lithium (close to 100% faradic yield after thermal treatment and pH adjustment). | TRL4-5 | Presence of organic compounds and leachate acidity can block transfer; requires thermal treatment and pH adjustment. | [40] |
| Mechanochemical treatment and acid leaching | Utilizing reductive reagents in milling for crystal structure modification and subsequent leaching with acid. | From e-bike lithium-ion batteries. | Lithium (maximum extraction of 29.9% in water leaching), nickel extraction improved from 78% to 92% with CoS in acid leaching. | TRL4-5 | No significant improvements in crystal structure post-milling; pervasive fluoride presence. | [41] |
| Combined pyro- and hydrometallurgical process | Electric arc furnace smelting to generate lithium-enriched slag and mixed metal alloys. | From spent LIBs treated pyrometallurgically. | Lithium (82.4% yield in slag), cobalt (81.6%), nickel (93.3%), copper (90.7%). | TRL3-5 | High-temperature processing complexity. | [21] |
| Hydrometallurgical recovery from lithium slag | Direct leaching of lithium slag in H2SO4 and dry digestion method to reduce silicon gel formation. | Pyrolyzed lithium-ion battery BM. | Lithium (close to 100% efficiency after 30 mins at 20°C), Al and Si (variable efficiency) | TRL3-5 | Silicon gel formation complicating filtration and recovery | [22] |
| Early-stage lithium recovery (ESLR) using thermal treatment and supercritical CO2 leaching | Lithium recovery shifted to the start of the chemo-metallurgical process, with CO2 supercritical state enabling selective lithium leaching. | From NCM-based electric vehicle cells, thermally treated. | Lithium (up to 79% yield with supercritical CO2 treatment). | TRL3-5 | Complexity in managing lithium yield influencing factors, such as pyrolysis temperature and autoclave carbonation setup. | [24] |
| Acid leaching using molasses as reductant | Utilizes acetic acid and molasses for metal leaching from BM, reducing higher oxidation states of metals. | From spent cylindrical lithium-ion batteries. | Co, Li, Ni, Mn (96% to 99% recovery efficiency). | TRL5-6 | Balancing molasses concentration and timing; sediment formation management. | [25] |
| Carbothermic reduction and hydrochlorination | Involves reducing metallic oxides to metals, converting Li and Mn into soluble chlorides, and Co and Ni into magnetic alloys. The Al remains in the form of Al2O3 and does not react. | From spent lithium-ion batteries | Li (97.28%), Mn (98.13%), Ni and Co in magnetic fraction (93.03%, 91.37%), Al in non-magnetic fraction (95.28%). | TRL5-6 | Managing material streams, specific separation requirements, precise process condition control. | [26] |
| Direct selective leaching of lithium. | Selective lithium leaching from BM using formic acid and hydrogen peroxide, allowing for targeted extraction while leaving other metals. | Industrial BM from Li-ion batteries with LiFePO4 cathodes. | Lithium >97%; other metals (Fe, Cu, Al, Ni, Co, Mn) <1% leaching. | TRL5-6 | Need for operational condition optimization. | [27] |
| Use of organic solvents (DMF, DMAc, DMSO) and alkaline solutions (NaOH, KOH). | Organic Solvents used are DMF, DMAc, DMSO; Alkaline Solutions are: NaOH and KOH. | Mixed chemistries from Li-ion batteries. | Lithium efficiency improved from 47.2% to 78.7% with DMSO; Cobalt efficiency improved from 28.5% to 61.3% with DMSO; Lithium extraction up to 90.1% with NaOH; Cobalt extraction up to 74.4% with NaOH. | TRL5-6 | High temperatures (>150 °C) for organic solvents; concentrated alkaline solutions pose corrosion and handling risks. | [42] |
| Leaching of industrial BM. | Leaching with a solid-liquid ratio of 1:10 or 1:20 g/mL. | From spent Li-ion batteries. | Co from LCO ~32%; Li from LCO ~63%; Li, Mn, Ni 100%; Co 100% after 60 min; Al 68% after 60 min; Cu 98% after 5 min then decreases to 60%. | TRL5-6 | Operational conditions optimization needed. | [43] |
| Solvent extraction for Zn and Mn recovery. | Solvent extraction using ionic liquids, organophosphorus-based acids, and Deep Eutectic Solvents (DESs). | From spent Zn-MnO2 alkaline batteries. | 100% for Zn (II) with (Cyanex 272 + diethyl phosphite); 100% for Mn (II) using DES. | TRL5-6 | Further studies needed for process optimization and extraction efficiency improvement. | [44] |
| Solid–liquid-liquid extraction | Extraction of heavy metals from Ni-Cd battery BM using Deep Eutectic Solvents (DESs) and other extractants in toluene or naphtha. | From spent Ni-Cd batteries. | Ni (II): 30 wt.%; Cd (II): 100 wt.% | TRL 4-6 | Lower extraction efficiency for Ni compared to Cd; complexity in the extraction process | [32] |
| Electroassisted leaching | Involves electroassisted leaching for metal recovery from BM, followed by electrochemical deposition. | From dismantled Ni–Cd batteries. | Cd: High recovery rate; Ni and Co: Slower dissolution, specific rates not detailed. | TRL 4-6 | Proton generation control at the anode, slower dissolution of Ni and Co compared to Cd, high energy consumption, complex process design and operation | [45] |
| Extraction with Ionic liquids (ILs), deep eutectic solvents (DESs), organophosphorous-based acids | Low-temperature method for extracting cobalt, nickel, lithium, and other metals from spent LIB BM without pre-leaching. | From spent lithium-ion batteries. | Co (II): 90-100 wt.% using DESs; Li(I): Up to 100 wt.; Ni (II): Up to 52 wt.% | TRL 4-6 | Complex extraction process, variability in recovery rates based on methods and conditions | [29] |
| selective sulfation roasting | Extracting cobalt and lithium from LCO-rich BM using selective sulfation roasting, followed by water leaching. | From industrial LCO-rich spent batteries. | Co: Up to 61.21%; Li: Up to 99.51%; Ni: Up to 33.00%; Mn: Up to 68.36%; Cu: Up to 24.53% | TRL 4-6 | Dependency on roasting conditions and carbon presence, complexity in process design and operation | [30] |
| Scalable direct recycling of cathode BM from spent LIBs | Integrates pretreatment and relithiation of cathode BM from EoL LIBs. | From EV batteries, specifically NCM cathode materials. | 100% electrochemical performance recovery, 91% yield rate, regenerated cathode material exhibits 82% ICE with 176 mAh/g discharge capacity, 94% capacity retention after 200 cycles. | TRL 6-7. | Precision control required for hydrothermal and annealing conditions, efficient removal of impurities | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





