1. Introduction
Within a demographic characterized by an increasing prevalence of older individuals, the demand for total hip arthroplasties (THAs) is experiencing a progressive escalation on a global scale,
and it is expected to augment from 1.8 million per year in 2015 to 2.8 (2.6-2.9) per year in 2050.[
1] The most important factors accounting for this tendency are the willingness of individuals to maintain a high-performance physical status and an active conduct throughout the whole lifespan and indications to perform THAs also in younger patients. [
2] THAs are primarily effectuated on the elderly, who often experience the challenges of osteoporosis and diminished bone quality. A significant complication of THAs is represented by the development of periprosthetic hip fractures (PPHFs), that can origin both intraoperatively and postoperatively. They can be located both at level of the femur and/or on the acetabulum
, with the latter being less common and more difficult to identify intraoperatively and on postoperative X-rays. [
3]
PPHFs place a significative burden on national healthcare systems worldwide. [
4,
5]
The elevation in PPHFs is due to the rising life expectancy among the population bearing prosthetic devices, which makes them more susceptible to develop bone fragility, undergo revisions or endure traumatic incidents. [
2] It is extremely important to individuate and address promptly intraoperative PPHFs, in order to avoid their propagation in postoperative settings.
PPHFs are classified based on the Vancouver classification system, developed by Duncan and Masri, which considers implant stability, fracture location and quality of the bone. Lesions are divided into type A (occurring at level of the femural trochanters), type B (further classified into B1, B2 and B3) and type C. [
6] VB1 fractures, which occur around a stable stem, account for almost one third of periprosthetic femoral fractures. [
7]
Multiple studies have been published in the past, investigating the risk factors, postoperative morbidity and management of postoperative VB1 fractures; the latter can be treated surgically either with open reduction internal fixation (ORIF) for comminuted/long oblique fractures or by employing revision arthroplasty (RA) in case of simple fractures/oblique fractures located at the tip of the stem [
2]. Conversely, fractures occurring intraoperatively around a stable stem are typically entrusted to the surgeon’s expertise, unlike other types of fractures.
Intraoperative periprosthetic femural fractures (IOPFFs), whose prevalence varies in literature from 1.7% to 5.4% [
8,
9], are more often observed in female individuals and in patients over 65 years, and they occur 14 times more often around uncemented stems (0.3% for cemented vs 5.4% for uncemented implants). [
8,
10] This could be linked to the fact that most IOPFFs occur during broaching of the femoral canal and press-fit implantation of uncemented stems [
7], when the circumferential tension at level of the proximal femur reaches the most elevated values [
11]. Most IOPFFs occur during placement of the femoral component (60%) and involve the calcar (69%). In revisions, the risk of fracture is even higher, reaching 3.6% in cemented and 21% in uncemented cases.
Notwithstanding, postoperative fracture risk is independent of age or gender, but still rises with uncemented stems. [
9,
10] The most common sites of occurrence of IOPFFs are femoral diaphisis, calcar and trochanteric region.
The most important risk factors correlated with the development of IOPFFs, other than female gender, elderhood, and cementless fixation, are the following: a diagnosis different than primary osteoarthritis, previous surgery on the same hip, and employement of direct lateral rather than posterior approach. [
12,
13] When fractures around a stable stem (B1) occur intraoperatively, the femural component is removed and cerclage wires are usually applied prior to stem reimplantation.
Effectively handling IOPFFs that occur during total hip replacement entails significant advantages for patients, enhancing both their quality of life and reoperation-free survival. However, in current literature there are no indications of a gold standard for specific treatment of VB1 fractures occurring during primary THAs.
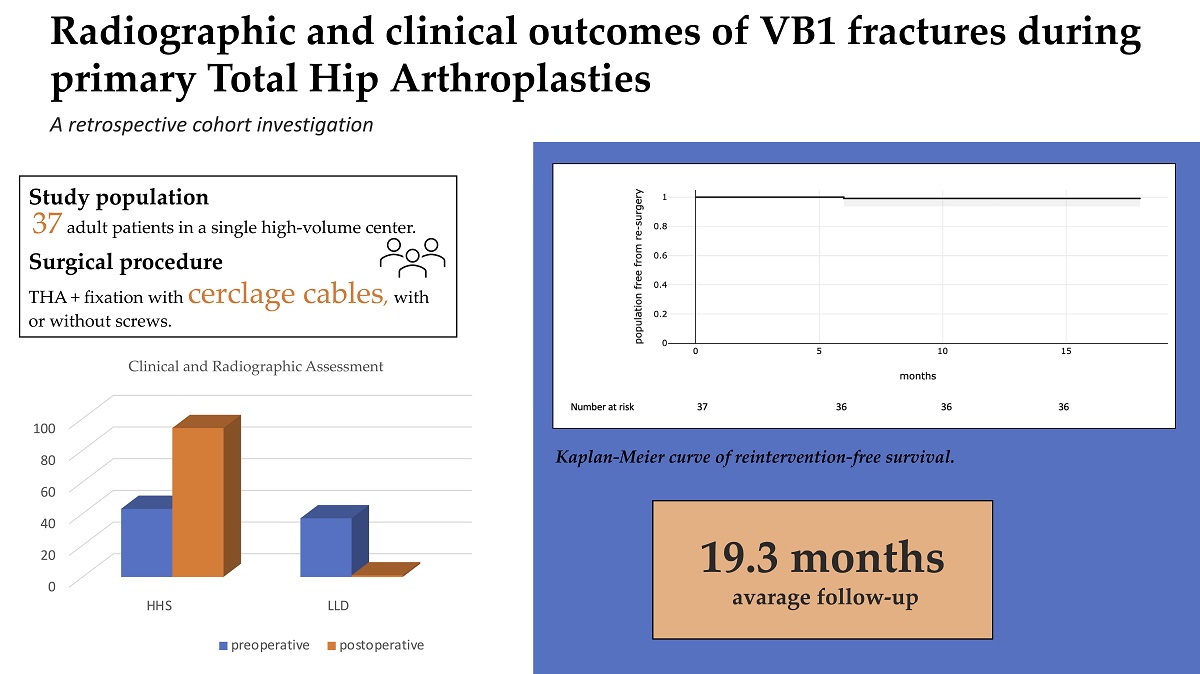
The primary outcome of this study is determining the reintervention-free survival of patients affected by IOPFFs treated with cerclage wires (with or without screws) during primary THAs. The secondary aim, instead, is to analyze clinical and radiographic assessment at the time of follow-up, based on Harris hip score (HHS), limb length discrepancy (LLD) and radiographic indicators of implant failure.
A prompt lesion identification and the employement of cerclage wires with or without screws represent an effective strategy for handling intraoperative VB1 fractures.
2. Patients and Methods
2.1. Protocol
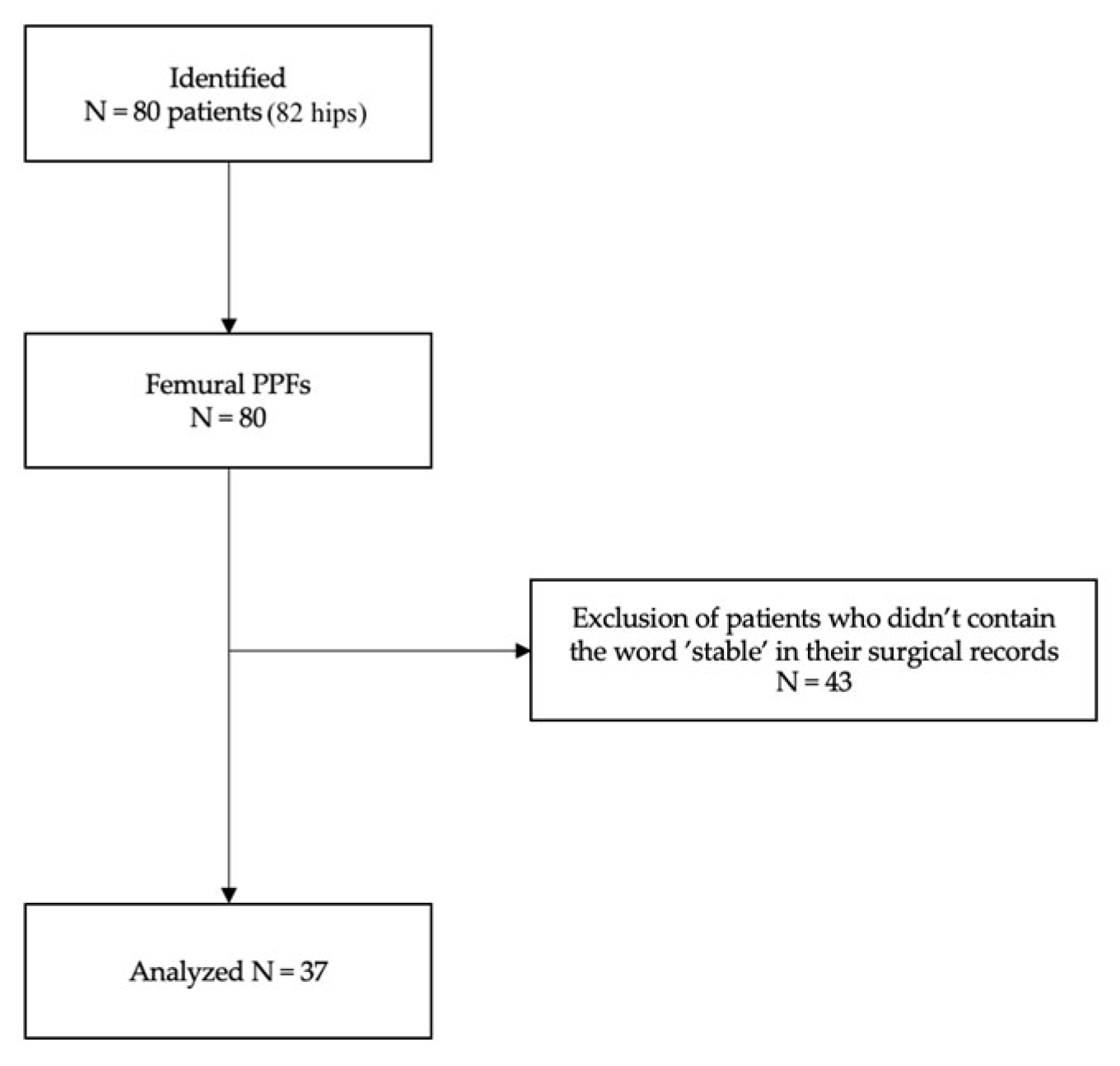
The present retrospective observational cohort study employed medical file records of patients included in a registry of orthopedic surgical procedures. It includes data from patients who developed Vancouver type B1 PPHFs during THAs from December 3rd 2020 to November 30th 2022 at the IRCCS Humanitas Research Hospital, Italy. All individual participants signed a written informed consent to perform the surgery and a written informed consent to be included in the registry of orthopedic surgical procedures within the scope of research and improvement of clinical practice. All the surgical procedures were executed by senior specialists with wide experience in joint replacement surgery. Patients were identified from hospital clinical records using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-CM) procedure codes 81.51 (total hip replacement) and diagnostic code 996.43 (periprosthetic femoral fracture). Subsequently, a second analysis was conducted, researching the word “stable” within surgical records.
Eligibility criteria included all patients aged above 18 years old who sustained an intraoperative PPHFs Vancouver type B1 during primary THAs (both cemented and uncemented) and were treated surgically with placement of cerclage wires with or without screws.
Exclusion criteria for participants included the presence of complications arising from infections and malignancies, as these conditions could potentially elevate bone fragility due to the presence of infection and osteolytic lesions. Vancouver fractures type A, B2, B3, C were excluded from this study, along with fractures that occurred postoperatively.
All the intraoperative fractures were classified based on the Vancouver classification system, which considers the site of fracture, implant stability and surrounding bone stock. Fractures are categorized into type A fractures, involving the trochanteric region, further divided into A(G) if they relate to the greater trochanter and A(L) if they concern the lesser one; type B fractures when located around the stem or immediately below it (B1: fractures occurring around a stable stem without damage of the surrounding bone stock, B2: fractures with stem loosening, and B3: fractures with stem loosening and concomitant poor bone quality); and ultimately type C fractures when they arise considerably lower than the stem position.
Total hip arthroplasties were performed with both cemented and short or conventional uncemented stems.
An analysis of medical records allowed to obtain demographic details of the patients (sex and age at surgery), affected side (left, right or bilateral), preoperative diagnosis (primary vs secondary osteoarthritis), fracture etiology, and Vancouver type, stem fixation (cemented vs uncemented), surgical strategy and stem type (straight or anatomical variant), operating time, and length of stay (LOS). 30 days readmission rate, 12 months reoperation rate and mortality rate at 30 days and 12 months were examined. Reintervention at 6 months and 1 year was recorded.
Furthermore, implant complications (non-union, loosening, dislocation, infection, heterotopic ossification) were recorded within 90 days of surgery; along with adverse events experienced by the patients, including hospital acquired pneumonia, atrial fibrillation (AF), myocardial infarction, venous thromboembolism (VTE), urinary tract infections (UTIs), surgical site infection and sciatic nerve injury.
A thorough examination of medical records was conducted during the study, encompassing clinical and surgical details, follow-up visits, and radiographic evaluations of all patients. Functional and radiographical assessments were collected both preoperatively at hospital admission and postoperatively during follow-up visits. During postoperative follow-up visits, the examiners followed a standardized protocol encompassing both clinical testing of patients’ performance status and evaluation of X-rays of the pelvis and the hips.
A quantitative assessment of functional hip recovery was made by comparing the preoperative and postoperative values of Harris Hip Score, a disease-specific questionnaire employed to measure clinical outcomes. The score combines both a questionnaire directed to patients (pain, walking support, presence of limp, walking distance, ability to walk stairs, put on shoes and sit) and a clinical evaluation of the range of movement (ROM) of the hip. [
14]
Conversely, radiographic evaluation relied on comparison of limb length discrepancy estimates before and after the surgical procedure, measured from the anteroposterior view.
Table 1.
The Vancouver classification system.
Table 1.
The Vancouver classification system.
| Classification |
Fracture location |
| A |
AG |
Greater trochanter fracture |
| AL |
Lesser trochanter fracture |
| B |
B1 |
Fracture around the prosthesis, stem well fixed
|
| B2 |
Fracture around the prosthesis, stem loose
|
| B3 |
Fracture around the prosthesis, loose stem, and poor proximal bone stock |
| C |
|
Fracture distal to tip of stem |
Surgical Technique
All the procedures were carried out by senior surgeons experienced in joint replacement surgery. All THAs were performed using a standard posterolateral approach and femur first technique with the patient in lateral decubitus.[
15] The operations were preceded by routine radiographic work-up and digital templating. Many different designs of monoblock stems were employed. In this analysis, the management of VB1 fractures occurring intraoperatively, and in most cases around uncemented stems, was investigated. All these fractures were identified during the surgical procedures, and their location and extent, along with stem stability, were assessed intraoperatively. Afterwards, the stem was removed, and fractures were treated with femur fixation by metal or iso-elastic cerclages with or without screws prior to definitive reimplantation of the femoral component, after having removed eventual bone fragments from the medullary canal. If the stem remains stable and the fracture is classified as VB1, it is viable to opt for the same stem size for replacement due to the preservation of bone stock. It is important to identify intraoperative fractures to avoid their future propagation in postoperative settings.
Postoperative Rehabilitation
The rehabilitation process after THAs complicated by intraoperative VB1 fractures remained consistent with standard postoperative management for uncomplicated hip replacement surgeries. The recovery regimen began in the immediate postoperative period and was tailored to the specific needs and physical status of each patient. Early mobilization exercises, including soft activities for range of motion (ROM) recovery and ambulation with assistive devices, were initiated, to prevent joint stiffness and muscle atrophy development. As the healing progressed, the physiotherapeutic program evolved to include strengthening exercises, balance training, and activities aimed at restoring normal gait. Partial weightbearing was suggested for the first 3 to 4 weeks postoperatively. The primary goal of postoperative rehabilitation was to improve patients’ functional abilities, reduce pain, and promote a safe return to daily activities. Close collaboration between the surgical team, physical therapists, and patients has been essential to ensure a successful rehabilitation process and achieve the best possible outcomes in terms of mobility and quality of life.
2.2. Statistical Analysis
Data analysis was performed with STATA 17, considering a p-value under 0.05 as significant.
Discrete variables were described as numbers and percentages, whereas for continuous variables the mean value and standard deviation were obtained, with range if necessary. Adherence of continuous variables to Gaussian distribution was checked with Shapiro Wilks test. Change of continuous variables between pre-surgery and last follow up was explored with Wilcoxon test, due to non-Gaussian data distribution.
Re-intervention free survival time was calculated from surgery date to re-intervention date, or last contact date. Re-intervention-free survival was plotted according to the Kaplan-Meier method. The estimate of reintervention-free survival for any reason was calculated at six months and one year and presented as percentage and 95% confidence interval (CI).
3. Results
3.1. Selection of Study Population
A total of 37 periprosthetic femoral component fractures were identified and enrolled in the present study, following an exclusion process. The details about flow chart of the patients’ selection and inclusion process are shown in
Figure 1. Demographic results are reported in
Table 1.
3.2. Characteristics of Study Population
Most of the primary THAs were uncemented (35, 95.59%) and only 2 (5.41%) employed cemented stems. All the stems employed were monoblock.
Mean LOS was 5.81±1.56 days (range 4-9). The detailed analysis of the operative management is presented in
Table 2.
The Kaplan-Meier estimated that overall freedom from reintervention was 100% at 30 and 90 days, and would stabilise at 99% (95%CI) at 6 months. There was only one case of reintervention among the population observed, concerning a monoblock uncemented stem (Wagner Conus, Zimmer Biomet, Warsaw, Indiana, US).
Figure 2 shows Kaplan-Meier curve of reintervention-free survival.
Two implant-related complications were observed in the present cohort (5.41% of the population), both occurring during the first 30 days following the THA: one case of stem loosening and dislocation in a female over 65 (2.70%), and one case of non-union of the PPHF (2.70%). The first patient underwent revision arthroplasty (RA) within the first 6 months following operation, whereas the second patient did not experience significant impairment in hip functionality and did not require revision surgery up to present time.
Only one patient (2.70%) sustained medical complications within 30 days postoperatively, experiencing a sudden rise in c-reactive protein (CRP) during the 5th postoperative day, provoked by a local infection managed with antibiotic regimen administration.
One patient (2.70%) experienced an accidental fall at two months from the intervention, with no noteworthy impact on the clinical course. No patients died during the follow-up period. Patients received an average follow-up of 19.30 months (range 12-35).
Table 3 lists the post-operative mechanical and medical complications.
The mean HHS and LLD were subject to significative postoperative improval, as reported in
Table 4.
4. Discussion
The main finding of this retrospective study is the effectiveness of treating VB1 PPHFs that occur in primary THAs with cerclage wires with or without screws; this strategy entails a good reintervention-free survival rate at 1 year of follow-up and improvement of radiographic outcomes and functional assessment.
The management of intraoperative VB1 fractures represents a significative challenge for surgeons, since their propagation in the postoperative setting could determine implant failure and reintervention. The objective of promptly treating intraoperative periprosthetic fractures is to ensure bone healing, avoid their postoperative propagation, and ultimately enable full functional recovery of the hip, thereby averting further surgical interventions. Despite their growing incidence, there is currently no established gold standard for treating VB1 IOPFFs, and surgeons often rely on their expertise in managing these cases. Previous biomechanical studies have investigated the effectiveness of treating VB1 IOPFFs with extramedullary cerclage constructs, proving their effectiveness both in axial load and torsional load testing, especially when using metallic wires with positive locking system. [
16] Another case-control study has evidenced favorable outcomes for cerclage cabling in fractures of the calcar occurring during cementless THAs (focusing on clinical outcomes, amount of stem subsidence, and implant survivorship).[
17] Furthermore, additional research has underscored the importance of precise risk assessment during preoperative planning and emphasized the significance of identifying intraoperatively the surgical steps with higher fracture risk (canal preparation and component insertion). [
18]
The incidence of periprosthetic fractures is expected to increase, due to the raising diffusion of THAs and aging of population. A study conducted in this field reports an incidence of femural PPFs of 1.70% (564 events on 32.644 surgical procedures). [
8]
The overall incidence of IOPFFs in the surgical unit where this study was conducted was around 1.42% (37 events on 2606 procedures, performed on 2350 patients).
None of the patients included in the study population have encountered mortality within one year post-surgery, aligning with the existing mortality rates observed following primary THA (0.30%).[
19] Furthermore, in our center, the awareness of possible major systemic complications after invasive procedures leads to comprehensive perioperative management, which could lead to better outcomes.
Stem mobilization rate within one year for our cohort was (2.70%, n=1). The event was most likely due to a flawed classification of an IOPFF with stem instability as VB1, along with poor adherence of the patient to postoperative recommendations.
IOPFFs occurrence entailed a slight increase in the average LOS with comparison to that reported for primary THAs in previous literature (5.81 vs 4.00 days). [
20] Regardless, it is important to note that the calculation of LOS varies depending on the time of patients' admission (whether it's the night before the intervention or on the same day). In the centre where the present study was conducted, protocol requires admitting patients the day before the scheduled operation. Therefore, if it was calculated starting from the date of intervention, the LOS of each patient would be diminished of one day, narrowing the deviation from the values documented in existing literature.
The incidence of IOPFFs is increasing. IOPFFs are 14 times more likely to occur within uncemented stems in females older than 65 years old, mainly during femoral broaching or stem insertion.[
8] This aligns with our findings, since the majority of intraoperative Vancouver B1 fractures occurred around uncemented rather than cemented stems (95.59% vs 5.41%). To mitigate fracture risk, cemented implants should be the primary treatment choice in elderly patients of female sex, as suggested by the Spotorno scoring system, that classifies patients according to gender, age, Singh’s Index and morphous-cortical index.
This study has several limitations. First, it is a retrospective observational study; thereby, as with any database, the quality of data and missing data may introduce errors. Additionally, as a cohort study, the absence of a control group doesn’t allow for comparison between cerclage cabling (with or without wires) and other therapeutic strategies. The inclusion of patients solely coming from a single centre may also introduce selection bias into the analyzed data. Lastly, one limitation is represented by the reduced length of patients’ follow-up, which anyhow was sufficient for determining the endpoints of this study.
The main strength of this study is that, to the best of our knowledge, no gold standard exists for treating intraoperative VB1 IOPFFs in primary THAs. This is the first study focusing exclusively on the effectiveness of treating intraoperative VB1 fractures occurring during THAs with cerclage cabling. Moreover, this study relies on arthroplasties performed in a single high-volume center; thereby, the centralized patient selection enhances the homogeneity of perioperative treatment of the study population, providing a more consistent and specific analysis.
5. Conclusions
Cerclage wires prove to be a reliable and secure surgical method for addressing intraoperative Vancouver B1 fractures in total hip arthroplasties. This technique is linked to favorable outcomes, including optimal fracture healing, complete functional recovery, and a high reintervention-free survival rate at the one year. Radiographic and clinical evaluations of patients undergoing this surgical approach reveal a favorable postoperative clinical course.
Author Contributions
Conceptualization, G.G., and M.L.; methodology, M.L.; software, K.C.; validation, M.L.; formal analysis, C.R., K.C.; investigation, C.R., V.D.M.; resources, C.R., V.D.M.; data curation, K.C., M.L., G.G.; writing - original draft preparation, C.R.; writing - review and editing, V.D.M., M.L., C.R.; visualization, V.D.M., M.L., C.R.; supervision, V.D.M., K.C. and M.L.; project administration, G.G. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. The study protocol for the development of this registry was approved by Ethics Committee of IRCCS Humanitas Research Hospital (protocol code 618/17).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study: all individual participants signed a written informed consent before the surgical procedure and a written informed consent to be included in the registry of orthopaedic surgical procedures.
Data Availability Statement
The data supporting reported results can be found in a repository (Zenodo).
Acknowledgments
The Livio Sciutto Foundation ([9]) for Medical Research is gratefully acknowledged. This is a non-profit social organization that recorded in its database the demographic and surgical data of the patients included in the study, with the previous consent of the patients and respecting the current law on the privacy. Finally, all the patients who participated in the study.
Conflicts of Interest
G.G. declares royalties and licenses from Zimmer Biomet, Innomed, and Adler Ortho; Financial support for attending symposia and educational programs from Zimmer Biomet. M.L. declares Research grant as principal investigator (2022YME9N3) from the Italian Ministry of University and Research; Research grant as co-principal investigator (GR-2019-12371158) from the Italian Ministry of Health; Research grant as principal investigator (GR-2018-12367275) from the Italian Ministry of Health; Research grants as principal investigator for postmarket study for medical devices from Zimmer Biomet; Financial support for attending symposia and educational programs from Zimmer Biomet; Scientific Director of Fondazione Livio-Sciutto. C.R., V.D.M. and K.C. declare no conflict of interest.
References
- Pabinger, C.; Lothaller, H.; Portner, N.; Geissler, A. Projections of Hip Arthroplasty in OECD Countries up to 2050. Hip Int 2018, 28, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Patsiogiannis, N.; Kanakaris, N.K.; Giannoudis, P.V. Periprosthetic Hip Fractures: An Update into Their Management and Clinical Outcomes. EFORT Open Rev 2021, 6, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Gunther, T.; Farkashazi, M.; Mihalik, G.; Nyoger, Z.; Kovacs, T. Functional Outcome after Lower Limb Periprosthetic Fractures. Injury 2021, 52, S44–S47. [Google Scholar] [CrossRef]
- González-Martín, D.; Pais-Brito, J.L.; González-Casamayor, S.; Guerra-Ferraz, A.; González-Pérez, J.M.; Jiménez-Sosa, A.; Herrera-Pérez, M. Economic Impact of Periprosthetic Hip Fractures. Revista Española de Cirugía Ortopédica y Traumatología 2022, 66, 477–484. [Google Scholar] [CrossRef]
- Bottle, A.; Griffiths, R.; White, S.; Wynn-Jones, H.; Aylin, P.; Moppett, I.; Chowdhury, E.; Wilson, H.; Davies, B.M. Periprosthetic Fractures: The next Fragility Fracture Epidemic? A National Observational Study. BMJ Open 2020, 10, e042371. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.P.; Masri, B.A. Fractures of the Femur after Hip Replacement. Instr Course Lect 1995, 44, 293–304. [Google Scholar] [PubMed]
- Lindahl, H.; Malchau, H.; Herberts, P.; Garellick, G. Periprosthetic Femoral Fractures: Classification and Demographics of 1049 Periprosthetic Femoral Fractures from the Swedish National Hip Arthroplasty Register. The Journal of Arthroplasty 2005, 20, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Abdel, M.P.; Watts, C.D.; Houdek, M.T.; Lewallen, D.G.; Berry, D.J. Epidemiology of Periprosthetic Fracture of the Femur in 32 644 Primary Total Hip Arthroplasties: A 40-Year Experience. The Bone & Joint Journal 2016, 98-B, 461–467. [Google Scholar] [CrossRef]
- Ricioli, W.; Queiroz, M.C.; Guimarães, R.P.; Honda, E.K.; Polesello, G.; Fucs, P.M. de M.B. Prevalence and Risk Factors for Intra-Operative Periprosthetic Fractures in One Thousand Eight Hundred and Seventy Two Patients Undergoing Total Hip Arthroplasty: A Cross-Sectional Study. International Orthopaedics (SICOT) 2015, 39, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J. EPIDEMIOLOGY: Hip and Knee. Orthopedic Clinics of North America 1999, 30, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.J.; Nagao, M.; Chu, Y.H.; Carbone, J.J.; Lennox, D.W.; Chao, E.Y. Medial Cortex Strain Distribution during Noncemented Total Hip Arthroplasty. Clin Orthop Relat Res 2000, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.N.; Matharu, G.S.; Redmond, A.; Judge, A.; West, R.M.; Pandit, H.G. Risk Factors for Intraoperative Periprosthetic Femoral Fractures During Primary Total Hip Arthroplasty. An Analysis From the National Joint Registry for England and Wales and the Isle of Man. The Journal of Arthroplasty 2019, 34, 3065–3073.e1. [Google Scholar] [CrossRef] [PubMed]
- BRÜGGEMANN, H.; DALEN, I.; BACHE-MATHIESEN, L.K.; FENSTAD, A.M.; HALLAN, G.; FOSSE, L. Incidence and Risk Factors of Intraoperative Periprosthetic Femoral Fractures during Primary Total Hip Arthroplasty: 218,423 Cases Reported to the Norwegian Arthroplasty Register between 1987 and 2020. Acta Orthop 2022, 93, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Brimer, M.A. Chapter 22 - Total Hip Arthroplasty. In Geriatric Rehabilitation Manual (Second Edition); Kauffman, T.L., Barr, J.O., Moran, M., Eds.; Churchill Livingstone: Edinburgh, 2007; pp. 141–145. ISBN 978-0-443-10233-2. [Google Scholar]
- Loppini, M.; Longo, U.G.; Caldarella, E.; Rocca, A.D.; Denaro, V.; Grappiolo, G. Femur First Surgical Technique: A Smart Non-Computer-Based Procedure to Achieve the Combined Anteversion in Primary Total Hip Arthroplasty. BMC Musculoskeletal Disorders 2017, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- Frisch, N.B.; Charters, M.A.; Sikora-Klak, J.; Banglmaier, R.F.; Oravec, D.J.; Silverton, C.D. Intraoperative Periprosthetic Femur Fracture: A Biomechanical Analysis of Cerclage Fixation. The Journal of Arthroplasty 2015, 30, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-W.; Lim, S.-J.; Ye, D.-H.; Park, Y.-S. Outcomes of Cerclage Cabling for Intraoperative Calcar Cracks in Cementless Total Hip Arthroplasty Using Broach-Only, Tapered Wedge Stems. J Arthroplasty 2020, 35, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.; Pike, J.; Garbuz, D.; Duncan, C.P.; Masri, B.A. Intraoperative Periprosthetic Fractures During Total Hip Arthroplasty: Evaluation and Management. JBJS 2008, 90, 2000. [Google Scholar] [CrossRef] [PubMed]
- Berstock, J.R.; Beswick, A.D.; Lenguerrand, E.; Whitehouse, M.R.; Blom, A.W. Mortality after Total Hip Replacement Surgery. Bone Joint Res 2014, 3, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Papalia, R.; Zampogna, B.; Torre, G.; Papalia, G.F.; Vorini, F.; Bravi, M.; Albo, E.; De Vincentis, A.; Denaro, V. Preoperative and Perioperative Predictors of Length of Hospital Stay after Primary Total Hip Arthroplasty—Our Experience on 743 Cases. J Clin Med 2021, 10, 5053. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).