Submitted:
25 December 2023
Posted:
27 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fruit Sampling
2.2. Agrochemical Characteristics of Soil
2.3. RT-PCR Analysis
2.4. Extraction and Determination of Total Anthocyanins and Total Phenolics
2.5. HPLC-DAD Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. RLBV Detection
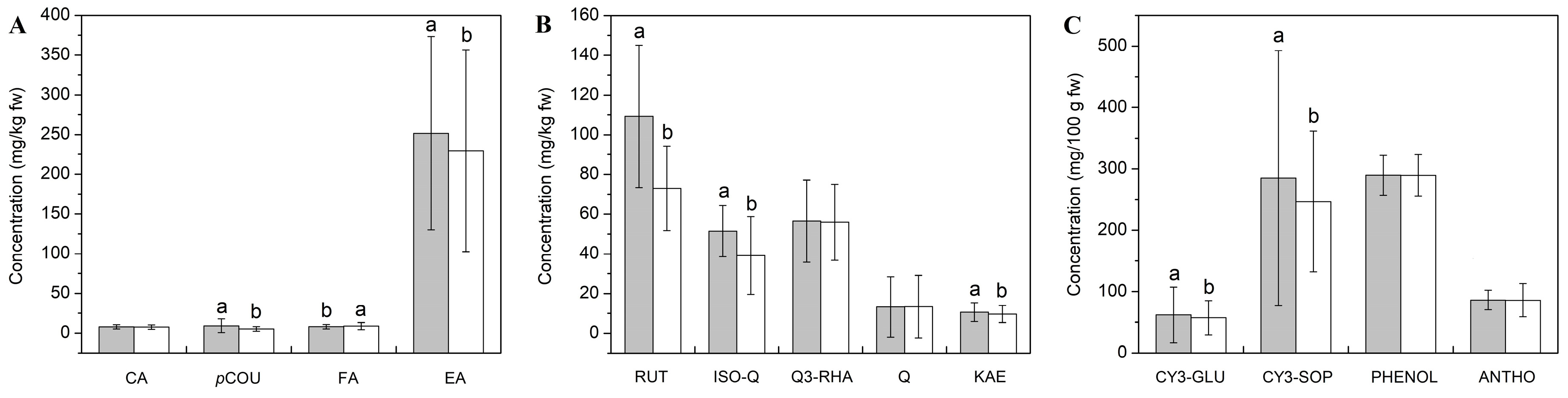
3.2. Chemical Properties of Raspberry Fruits
3.3. Influence of RLBV-infection, Harvest Year and Locality on Polyphenolic Profile of Raspberry Fruits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Chapter 29 - Chemical composition of raspberry (Rubus spp.) cultivars. In Nutritional composition of fruit cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: Cambridge, Massachusetts, USA, 2016; pp. 713–731. [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. 2018. Cri. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [CrossRef]
- Golovinskaia, O.; Wang, C.K. Review of functional and pharmacological activities of berries. Molecules 2021, 26, 3904. [CrossRef]
- FAOSTAT. 2023: Available from: https://www.fao.org/faostat/en/#data/QCL.
- Petrović, S.; Leposavić, A.; Jevremović, D. Raspberry – The management, processing and marketing. Scientific Pomological Society of Serbia: Čačak, Serbia, 2017.
- Martin, R.R.; MacFarlane, S.; Sabanadzovic, S.; Quito, D.; Poudel, B.; Tzanetakis, I.E. Viruses and virus diseases of Rubus. Plant Dis. 2013, 97, 168–182. [CrossRef]
- Jevremović, D.; Leposavić, A.; Paunović, A.S. Genetic diversity of raspberry leaf blotch emaravirus in red raspberries from Serbia. Span. J. Agric. Res. 2019, 17: e1004. [CrossRef]
- McGavin, W.J.; Mitchell, C.; Cock, P.J.; Wright, K.M.; MacFarlane, S.A. Raspberry leaf blotch virus, a putative new member of the genus Emaravirus, encodes a novel genomic RNA. J. Gen. Virol. 2012, 93, 430–437. [CrossRef]
- Lu, Y.; McGavin, W.; Cock, P.J.A.; Schnettler, E.; Yan, F.; Chen, J.; MacFarlane, S. 2015. Newly identified RNAs of raspberry leaf blotch virus encoding a related group of proteins. J. Gen. Virol. 2015, 96, 3432–3439. [CrossRef]
- Dong, L.; Lemmetty, A.; Latvala, S.; Samuilova, O.; Valkonen, J.P.T. 2016. Occurrence and genetic diversity of Raspberry leaf blotch virus (RLBV) infecting cultivated and wild Rubus species in Finland. Ann. Appl. Biol. 2016, 168, 122–132. [CrossRef]
- Tan, J.L.; Trandem, N.; Fránová, J.; Hamborg, Z.; Blystad, D.R.; Zemek, R. Known and potential invertebrate vectors of raspberry viruses. Viruses 2022, 14, 571. [CrossRef]
- Jevremović, D.; Leposavić, A.; Miletić, N.; Vasilijević, B.; Popović, B.; Mitrović, O.; Milinković, M. Impact of raspberry leaf blotch emaravirus on red raspberry ‘Willamette’ fruits. Pestic. Fitomed. 2022, 37, 1–7. [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11‒33. [CrossRef]
- Loeppert, R.H.; Suarez, D.L. Carbonate and Gypsum. In Methods of soil analysis. Part 3. Chemical methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnson, C.T., Sumner, M.E., Eds.; American Society of Agronomy & Soil Science Society of America, Inc.: Madison, Wisconsin, USA, 1996; pp. 437–474. [CrossRef]
- Egnér, H.; Riehm, H.; Domingo, W.R. Chapter II Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. In Chemische Extraktionsmethoden zur Phosphor- und Kaliumbestimmung; Kungliga Lantbrukshögskolans Annaler; Lantbrukshögskolan: Uppsala, Sweden, 1960; Volume 26, pp. 199–215.
- Li, R.; Mock, R.; Huang, Q.; Abad, J.; Hartung, J.; Kinard, G. A reliable and inexpensive method of nucleic acid extraction for the PCR-based detection of diverse plant pathogens. J. Virol. Methods 2008, 154, 48–55. [CrossRef]
- Cervantes, L.; Martínez–Ferri E.; Soria, C.; Ariza, M.T. Bioavailability of phenolic compounds in strawberry, raspberry and blueberry: Insights for breeding programs. Food Biosci. 2020, 37, 100680. [CrossRef]
- Miletić, N., Jevremović, D., Mitić, M., Popović, B. & Petković, M. 2022. Influence of D and Rec strains of plum pox virus on phenolic profile and antioxidant capacity of fresh plum fruits of ‘Čačanska Lepotica’ cultivar. Span. J. Agric. Res. 2022, 20, e1005. [CrossRef]
- Stavang, J.A.; Freitag, S.; Foito, A.; Verrall, S.; Heide, O.M.; Stewart, D.; Sønsteby, A. Raspberry fruit quality changes during ripening and storage as assessed by colour, sensory evaluation and chemical analyses. Sci. Hortic. 2015, 195, 216–225. [CrossRef]
- Lopez–Corona, A.V.; Valencia–Espinosa, I.; González–Sánchez, F.A.; Sánchez–López, A.L.; Garcia–Amezquita, L.E.; Garcia–Varela, R. Antioxidant, anti–inflammatory and cytotoxic activity of phenolic compound family extracted from raspberries (Rubus idaeus): A general review. Antioxidants 2022, 11: 1192. [CrossRef]
- Pavlović, A.V.; Dabić, D.Č.; Momirović, N.M.; Dojčinović, B.P.; Milojković-Opsenica, D.M.; Tešić, Ž.Lj.; Natić, M.M. Chemical composition of two different extracts of berries harvested in Serbia. J. Agric. Food Chem. 2013, 61: 4188−4194. [CrossRef]
- Levaj, B.; Dragović-Uzelac, V.; Delonga, K.; Kovačević Ganić, K.; Banović, M.; Bursać Kovačević, D. Polyphenols and volatiles in fruits of two sour cherry cultivars, some berry fruits and their jams. Food Technol. Biotechnol. 2010, 48, 538–547.
- Kula, M.; Majdan, M.; Głód, D.; Krauze–Baranowska, M. Phenolic composition of fruits from different cultivars of red and black raspberries grown in Poland. J. Food Compos. Anal. 2016, 52, 74–82. [CrossRef]
- Cheng, G.; He, Y.N.; Yue, T.X.; Wang, J.; Zhang, Z.W. Effects of climatic conditions and soil properties on Cabernet Sauvignon berry growth and anthocyanin profiles. Molecules 2014, 19, 13683‒13703. [CrossRef]
- Li, X.X.; He, F.; Wang, J.; Li Z.; Pan, Q.H. Simple rain-shelter cultivation prolongs accumulation period of anthocyanins in wine grape berries. Molecules 2014, 19, 14843–14861. [CrossRef]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content, soil fertilization and agricultural management: a review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [CrossRef]
- Delgado, R.; Martín, P.; del Alamo, M.; González, M.R. Changes in the phenolic composition of grape berries during ripening in relation to vineyard nitrogen and potassium fertilisation rates. J. Sci. Food Agric. 2004, 84, 623–630. [CrossRef]
- Michalska, A.; Wojdyło, A.; Bogucka, B. The influence of nitrogen and potassium fertilisation on the content of polyphenolic compounds and antioxidant capacity of coloured potato. J. Food Comp. Anal. 2016, 47, 69–75. [CrossRef]
- Anjos, R.; Cosme, F.; Gonçalves, A.; Nunes, F.M.; Vilela, A.; Pinto, T. Effect of agricultural practices, conventional vs organic, on phytochemical composition of ‘Kweli’ and ‘Tulameen’ raspberries (Rubus idaeus L.). Food Chem. 2020, 328, 126833. [CrossRef]
- Piccolo, E.L.; Garcìa, L.M.; Landi, M.; Guidi, L.; Massai, R.; Remorini, D. Influences of postharvest storage and processing techniques on antioxidant and nutraceutical properties of Rubus idaeus L.: A mini–review. Horticulturae 2020, 6, 105. [CrossRef]
- Kotuła, M.; Kapusta-Duch, J.; Smoleń, S.; Doskočil, I. Phytochemical composition of the fruits and leaves of raspberries (Rubus idaeus L.) – conventional vs. organic and those wild grown. Appl. Sci. 2022, 12, 11783. [CrossRef]
- Usenik, V.; Kastelec, D.; Stampar, F.; Marn, M.V. Effect of plum pox virus on chemical composition and fruit quality of plum. J. Agric. Food Chem. 2015, 63, 51–60. [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of phenolic compounds in plant–defensive mechanisms. In Plant phenolics in sustainable agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020; pp. 517–532. [CrossRef]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. 17 – Role of phenols and polyphenolics in plant defense response to biotic and abiotic stresses. In Biocontrol agents and secondary metabolites – Applications and immunization for plant growth and protection; Jogaiah, S., Ed.; Woodhead Publishing: Sawston, Cambridge, UK, 2021; pp. 419–441. [CrossRef]
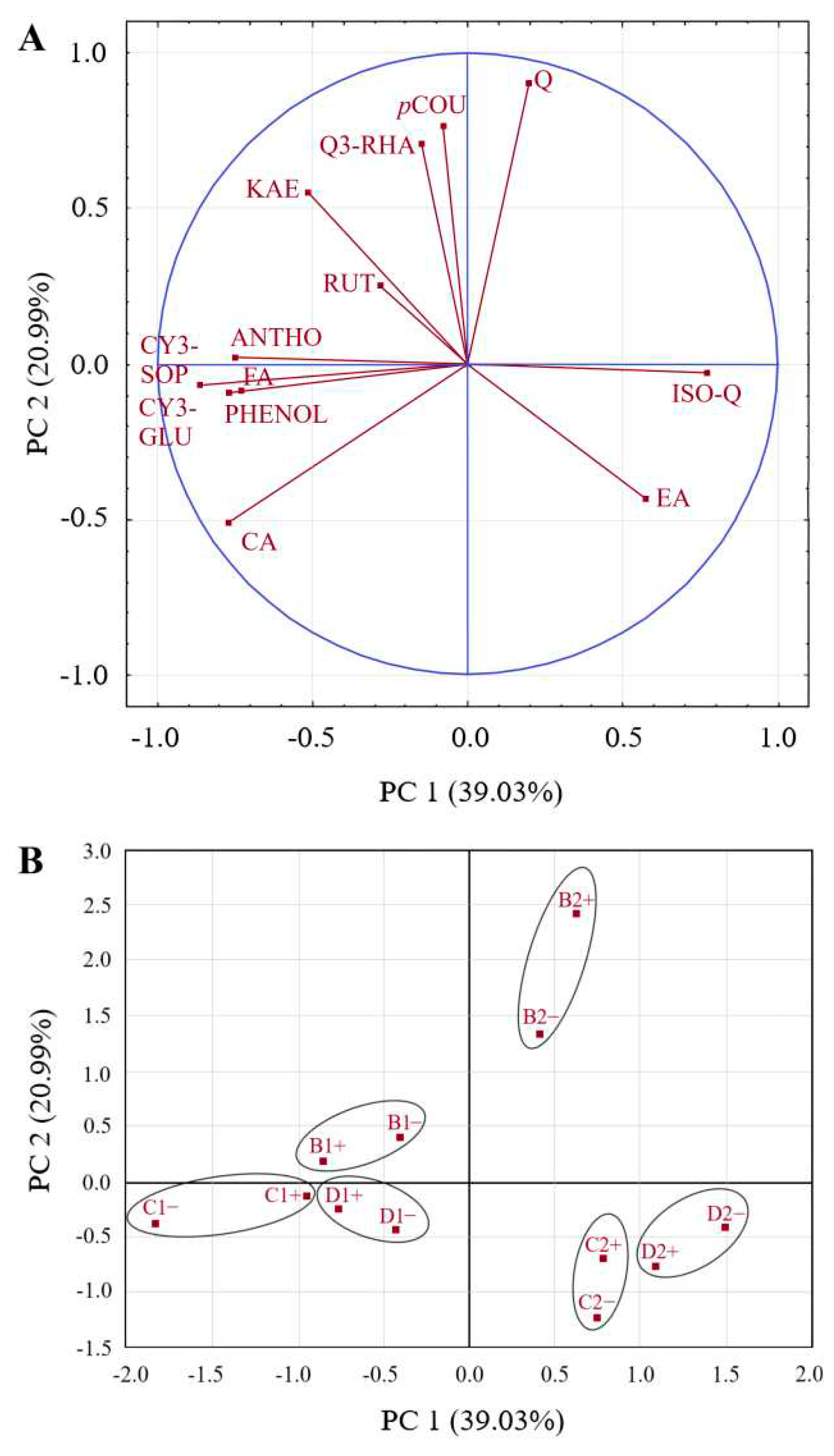
| Location | Harvest year | |||
|---|---|---|---|---|
| 2019 | 2020 | |||
| RLBV + | RLBV ‒ | RLBV + | RLBV ‒ | |
| Bedina Varoš | B1+ | B1‒ | B2+ | B2‒ |
| Devići | D1+ | D1‒ | D2+ | D2‒ |
| Cerova | C1+ | C1‒ | C2+ | C2‒ |
| Location | Average air temperature (°C) |
Average precipitation (mm) |
|||
|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | ||
| April | Bedina Varoš | 11.7 | 10.5 | 166.8 | 70.0 |
| Devići | 7.7 | 6.9 | 57.9 | 42.1 | |
| Cerova | 11.7 | 10.1 | 101.9 | 22.0 | |
| May | Bedina Varoš | 13.1 | 14.1 | 110.2 | 197.2 |
| Devići | 9.6 | 11.3 | 84.4 | 67.9 | |
| Cerova | 13.2 | 14.4 | 176.3 | 99.0 | |
| June | Bedina Varoš | 20.4 | 17.7 | 328.4 | 231.0 |
| Devići | 17.2 | 14.3 | 126.8 | 112.9 | |
| Cerova | 20.7 | 18.3 | 110.5 | 137.3 | |
| July | Bedina Varoš | 20.1 | 19.4 | 107.2 | 17.4 |
| Devići | 16.8 | 16.5 | 89.1 | 74.4 | |
| Cerova | 20.3 | 20.0 | 80.8 | 84.8 | |
| August | Bedina Varoš | 21.3 | 20.6 | 71.2 | 46.6 |
| Devići | 17.8 | 16.9 | 9.3 | 265.0 | |
| Cerova | 21.2 | 20.5 | 68.0 | 154.4 | |
| September | Bedina Varoš | 16.5 | 17.2 | 2.4 | 49.6 |
| Devići | 13.2 | 13.6 | 54.7 | 52.8 | |
| Cerova | 15.9 | 16.6 | 22.6 | 9.9 | |
| Bedina Varoš | 12.4 | 11.8 | 5.4 | 152.6 | |
| October | Devići | 8.6 | 8.4 | 24.8 | 80.6 |
| Cerova | 11.3 | 11.1 | 32.5 | 64.4 | |
| Compounds/ class of compounds |
Bedina Varoš | Devići | Cerova | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||||||||||||
| RLBV + | RLBV ‒ | RLBV + | RLBV ‒ | RLBV + | RLBV ‒ | RLBV + | RLBV ‒ | RLBV + | RLBV ‒ | RLBV + | RLBV ‒ | ||||||
| CA | 9.2 ± 1.6 | 5.8 ± 1.0 | 3.8 ± 0.3 | 4.6 ± 0.6 | 8.6 ± 0.7 | 10.2 ± 0.7 | 5.9 ± 0.7 | 5.2 ± 0.8 | 11.9 ± 0.7 | 11.9 ± 0.2 | 7.7 ± 0.1 | 7.9 ± 0.3 | |||||
| bc | de | e | e | bc | ab | de | e | A | a | cd | cd | ||||||
| pCOU | 4.1 ± 1.1 | 1.7 ± 0.5 | 26.3 ± 1.5 | 7.9 ± 0.2 | 6.4 ± 0.4 | 6.9 ± 0.3 | 4.1 ± 0.2 | 4.3 ± 0.2 | 12.6 ± 1.8 | 8.6 ± 1.5 | 1.8 ± 0.3 | 2.3 ± 0.3 | |||||
| ef | f | a | c | cde | cd | ef | def | b | c | f | f | ||||||
| FA | 10.7 ± 1.6 | 6.4 ± 0.7 | 8.3 ± 0.9 | 4.9 ± 1.0 | 12.1 ± 0.6 | 11.1 ± 0.8 | 6.0 ± 0.3 | 6.1 ± 0.3 | 5.6 ± 0.6 | 17.5 ± 0.6 | 5.8 ± 0.8 | 6.6 ± 0.6 | |||||
| b | cd | c | d | b | b | d | cd | d | a | d | cd | ||||||
| EA | 194.2 ± 10.0 | 118.6 ± 6.3 | 212.8 ± 8.0 | 162.3 ± 7.3 | 130.1 ± 4.0 | 106.3 ± 5.9 | 248.3 ± 10.5 | 358.2 ± 8.4 | 222.3 ± 15.5 | 198.3 ± 6.5 | 502.4 ± 10.9 | 434.0 ± 6.6 | |||||
| f | h | ef | g | h | h | d | c | E | ef | a | b | ||||||
| RUT | 145.9 ± 5.6 | 92.9 ± 6.1 | 142.7 ± 6.4 | 33.4 ± 1.9 | 71.4 ± 3.2 | 69.5 ± 3.9 | 54.6 ± 3.8 | 67.7 ± 5.7 | 124.2 ± 7.8 | 91.5 ± 6.3 | 116.5 ± 5.3 | 83.1 ± 5.9 | |||||
| a | c | a | f | d | de | e | de | b | c | b | cd | ||||||
| ISO-Q | 50.7 ± 3.0 | 35.6 ± 2.5 | 57.5 ± 2.3 | 32.4 ± 1.9 | 37.1 ± 1.5 | 29.6 ± 1.5 | 54.3 ± 2.6 | 78.0 ± 2.1 | 36.6 ± 0.9 | 17.1 ± 1.0 | 72.6 ± 2.0 | 42.5 ± 1.7 | |||||
| c | e | b | ef | de | f | bc | a | e | g | a | d | ||||||
| Q3-RHA | 63.2 ± 0.9 | 64.9 ± 1.0 | 82.8 ± 2.1 | 79.8 ± 0.8 | 39.7 ± 1.4 | 35.3 ± 2.7 | 22.6 ± 1.3 | 57.2 ± 1.1 | 58.4 ± 1.5 | 70.2 ± 1.6 | 72.2 ± 1.3 | 27.9 ± 1.5 | |||||
| c | c | a | a | e | e | g | d | d | b | b | f | ||||||
| Q | 6.6 ± 0 .5 | 11.6 ± 1.3 | 46.0 ± 1.7 | 47.3 ± 1.3 | 10.3 ± 0.7 | 5.2 ± 0.5 | 5.5 ± 0.4 | 5.3 ± 0.4 | 6.2 ± 0.4 | 5.8 ± 0.5 | 5.0 ± 0.3 | 5.3 ± 0.2 | |||||
| c | b | a | a | b | c | c | c | c | c | c | c | ||||||
| KAE | 18.1 ± 1.7 | 16.0 ± 0.5 | 12.5 ± 0.6 | 11.9 ± 1.1 | 12.6 ± 0.7 | 12.2 ± 0.6 | 6.9 ± 0.4 | 5.9 ± 0.6 | 10.1 ± 0.8 | 8.3 ± 0.4 | 3.6 ± 0.4 | 3.7 ± 0.2 | |||||
| CY3-GLU | 132.8 ± 7.6 | 69.9 ± 6.8 | 15.1 ± 1.7 | 56.5 ± 3.6 | 62.4 ± 4.5 | 51.4 ± 4.4 | 20.5 ± 1.1 | 22.3 ± 0.8 | 105.8 ± 5.5 | 107.6 ± 3.1 | 35.1 ± 1.3 | 36.3 ± 1.3 | |||||
| a | c | f | d | cd | d | f | f | b | b | e | e | ||||||
| CY3-SOP | 594.4 ± 6.2 | 304.0 ± 6.3 | 95.2 ± 4.4 | 186.1 ± 5.3 | 304.7 ± 6.0 | 238.3 ± 6.9 | 87.3 ± 2.4 | 123.7 ± 4.8 | 503.0 ± 8.6 | 460.8 ± 5.0 | 124.0 ± 4.5 | 167.3 ± 5.4 | |||||
| a | d | i | f | d | e | i | h | b | c | h | g | ||||||
| PHENOL | 259.8 ± 6.0 | 303.5 ± 0.4 | 267.6 ± 3.2 | 286.9 ± 7.5 | 337.9 ± 15.2 | 296.9 ± 5.5 | 255.1 ± 8.0 | 233.6 ± 3.0 | 315.7 ± 6.9 | 340.9 ± 8.1 | 300.2 ± 1.4 | 273.8 ± 3.6 | |||||
| e | bc | de | cd | a | bc | e | f | b | a | bc | de | ||||||
| ANTHO | 68.9 ± 15.1 | 102.6 ± 7.2 | 87.2 ± 3.7 | 72.3 ± 1.1 | 103.3 ± 4.5 | 78.8 ± 16.3 | 65.4 ± 0.6 | 54.9 ± 0.4 | 98.3 ± 7.1 | 134.1 ± 6.4 | 94.4 ± 0.7 | 73.5 ± 1.2 | |||||
| ef | b | bcde | def | b | cde | ef | f | bc | a | bcd | def | ||||||
| Compounds/ class of compounds |
A (viral status) |
B (harvest year) |
C (locality) |
[A×B] | [A×C] | [B×C] | [A×B×C] |
|---|---|---|---|---|---|---|---|
| CA | ns | *** | *** | ns | * | ns | *** |
| pCOU | *** | ** | *** | *** | *** | *** | *** |
| FA | * | *** | *** | *** | *** | *** | *** |
| EA | *** | *** | *** | *** | *** | *** | *** |
| RUT | *** | *** | *** | *** | *** | *** | *** |
| ISO-Q | *** | *** | *** | * | *** | *** | *** |
| Q3-RHA | ns | ** | *** | *** | *** | *** | *** |
| Q | ns | *** | *** | ns | *** | *** | *** |
| KAE | ** | *** | *** | ns | ns | ns | ns |
| CY3-GLU | ** | *** | *** | *** | ** | *** | *** |
| CY3-SOP | *** | *** | *** | *** | *** | *** | *** |
| PHENOL | ns | *** | *** | *** | *** | *** | *** |
| ANTHO | ns | *** | *** | *** | *** | *** | *** |
| Locality of orchard |
pH (in 1M KCl) |
CaCO3 (%) |
Humic substances (%) |
Total nitrogen (%) |
Accessible phosphorus (mg P2O5/100 g) | Accessible potassium (mg K2O/100 g) |
|---|---|---|---|---|---|---|
| Bedina Varoš | 4.08 | 0.5 | 6.04 | 0.30 | 40.45 | 61.4 |
| Devići | 4.15 | 0.5 | 6.07 | 0.30 | 1.46 | 26.2 |
| Cerova | 5.02 | 0.0 | 3.33 | 0.17 | 7.28 | 16.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





