Submitted:
26 December 2023
Posted:
27 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental diets
2.2. Fish management before the trial
2.3. Experimental management and sampling
2.4. Calculations and statistical analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Trigueiro, N.S.D.S.; Canedo, A.; Braga, D.L.D.S.; Luchiari, A.C.; Rocha, T.L. Zebrafish as an emerging model system in the global south: Two decades of research in Brazil. Zebrafish 2020, 17, 412–425. [Google Scholar] [CrossRef]
- Canedo, A.; Saiki, P.; Santos, A.L.; da Silva Carneiro, K.; de Souza, A.M.; Qualhato, G.; da Silva Brito, R.; Mello-Andrade, F.; Rocha, T.L. Zebrafish (Danio rerio) meets bioethics: the 10Rs ethical principles in research. Cienc. Anim. Bras. 2022, 23. [Google Scholar] [CrossRef]
- Watts, S.A.; Lawrence, C.; Powell, M.L.; D’Abramo, L.R.; D’Abramo, L. The vital relationship between nutrition and health in zebrafish. Zebrafish 2016, 13 (Suppl. 1), S72–S76. [Google Scholar] [CrossRef] [PubMed]
- Brenes-Soto, A.; Tye, M.; Esmail, M.Y. The role of feed in aquatic laboratory animal nutrition and the potential impact on animal models and study reproducibility. ILAR J. 2019, 60, 197–215. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Araujo, L.; Santos, M.M.; Araújo, L.; Santos, M.M. Rearing zebrafish (Danio rerio) larvae without live food: evaluation of a commercial, a practical and a purified starter diet on larval performance. Aquac. Res. 2006, 37, 1107–1111. [Google Scholar] [CrossRef]
- Siccardi, A.J.; Garris, H.W.; Jones, W.T.; Moseley, D.B.; D’Abramo, L.R.; Watts, S.A. Growth and survival of zebrafish (Danio rerio) fed different commercial and laboratory diets. Zebrafish 2009, 6, 275–280. [Google Scholar] [CrossRef]
- Farias, M.; Certal, A.C. Different feeds and feeding regimens have an impact on zebrafish larval rearing and breeding performance. Int. J. Mar. Biol. Res. 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Kolb, A.; Hildebrandt, F.; Lawrence, C. Effects of diet and social housing on reproductive success in adult zebrafish, Danio rerio. Zebrafish 2018, 15, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.; Martins, S.; Farias, M.; Costa, T.; Certal, A.C. The impact of two different cold-extruded feeds and feeding regimens on zebrafish survival, growth and reproductive performance. J. Dev. Biol. 2018, 6, null. [Google Scholar] [CrossRef]
- Fowler, L.A.; Williams, M.B.; Dennis-Cornelius, L.N.; Farmer, S.C.; Barry, R.J.; Powell, M.L.; Watts, S.A. Influence of commercial and laboratory diets on growth, body composition, and reproduction in the zebrafish Danio rerio. Zebrafish 2019, 16, 508–521. [Google Scholar] [CrossRef]
- Dhanasiri, A.; Chen, X.; Dahle, D.; Siriyappagouder, P.; Fæste, C.K.; Fernandes, J.M.O. Dietary inclusion of plant ingredients induces epigenetic changes in the intestine of zebrafish. Epigenetics 2020, 15, 1035–1051. [Google Scholar] [CrossRef] [PubMed]
- Fowler, L.A.; Dennis-Cornelius, L.N.; Dawson, J.A.; Barry, R.; Davis, J.L.; Powell, M.L.; Yuan, Y.; Williams, M.B.; Makowsky, R.; D’Abramo, L.R.; et al. Both dietary ratio of n–6 to n–3 fatty acids and total dietary lipid are positively associated with adiposity and reproductive health in zebrafish. Curr. Dev. Nutr. 2020, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, S.; Luan, J.-L.; Fu, Z.-H.; Sun, M.-Z.; Zhao, X.; Feng, X.-Z. The food preservative sodium propionate induces hyperglycaemic state and neurological disorder in zebrafish. Neurotoxicol. Teratol. 2022, 93, 107123. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.A.; D’Abramo, L.R. Standardized reference diets for zebrafish: addressing nutritional control in experimental methodology. Annu. Rev. Nutr. 2021, 41, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Barry, R.J.; Powell, M.L.; Nagy, T.R.; D’Abramo, L.R.; Watts, S.A. Dietary protein source influence on body size and composition in growing zebrafish. Zebrafish 2013, 10, 439–446. [Google Scholar] [CrossRef] [PubMed]
- NRC, N.R.C. Nutrients requirements of fish and shrimp; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Cai, F.; Wang, Y.; Hu, X.; Huang, F.; Wang, F.; Liu, H. Effects of dietary lipid levels on growth performance, whole body composition and digestive enzyme activity of juvenile bighead carp (Aristichthys nobilis). Isr. J. Aquac. - Bamidgeh 2020, 72, 1–10. [Google Scholar] [CrossRef]
- Ding, L.; Chen, W.; Fu, H.; Xiao, J.; Fu, Y.; Ma, J. Estimation of the Optimum Dietary Protein to Lipid Ratio in Juvenile Pengze Crucian Carp (Carassius auratus Var. Pengze). Aquac. Nutr. 2022, 2022. [Google Scholar] [CrossRef]
- Meguro, S.; Hosoi, S.; Hasumura, T. High-fat diet impairs cognitive function of zebrafish. Sci. Rep. 2019, 9, 17063. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, D.; Jing, X.; Zhao, Y.; Hao, Q.; Zhang, Q.; Xie, M.; Yang, Y.; Ran, C.; Xu, Q.; et al. Addition of solid-state fermentation product of yeast ameliorated the effects of high-fat diet on hepatic lipid metabolism, epidermal mucus, intestine and liver health, and gut microbiota of zebrafish. Aquac. Reports 2023, 30, 101589. [Google Scholar] [CrossRef]
- Meng, Y.; Li, C.; Qin, Q.; Tong, Y.; Zhu, R.; Xu, G.; Shi, Y.; Shi, J.; Ma, R. Dietary Lipid Levels Affect the Growth Performance, Lipid Deposition, and Antioxidative Capacity of Juvenile Scaleless Carp, Gymnocypris przewalskii, on the Qinghai-Tibetan Plateau. J. World Aquac. Soc. 2018, 49, 788–797. [Google Scholar] [CrossRef]
- Wang, A.; Yang, W.; Shen, Y.; Han, G.; Lv, F.; Yu, Y.; Huang, J.; Zhang, J. Effects of dietary lipid levels on growth performance, whole body composition and fatty acid composition of juvenile gibel carp (Carassius auratus gibelio). Aquac. Res. 2015, 46, 2819–2828. [Google Scholar] [CrossRef]
- O’Brine, T.M.; Vrtělová, J.; Snellgrove, D.L.; Davies, S.J.; Sloman, K.A. Growth, oxygen consumption, and behavioral responses of Danio rerio to variation in dietary protein and lipid levels. Zebrafish 2015, 12, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.S.; Pandolfo, L.M.; Marshall, C.M.; Mitra, A.K.; Schech, J.M. Body condition scoring for adult zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists - AOAC. Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemists, Inc.: Arlington, Virginia, USA, 1990; Vol. 1. [Google Scholar]
- Chen, T.; Xu, M.; Tu, J.; Wang, H.; Niu, X. Relationship between omnibus and post-hoc tests : an investigation of performance of the F test in ANOVA. Shanghai Arch. Psychiatry 2018, 30, 60–64. [Google Scholar] [CrossRef]
- Jin, Y.; Tian, L.; Zeng, S.; Xie, S.; Yang, H.; Liang, G.; Liu, Y. Dietary lipid requirement on non-specific immune responses in juvenile grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2013, 34, 1202–8. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Ni, P.J.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Decreased enteritis resistance ability by dietary low or excess levels of lipids through impairing the intestinal physical and immune barriers function of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017, 67, 493–512. [Google Scholar] [CrossRef]
- Priya, K.; Pal, A.K.; Sahu, N.P.; Mukherjee, S.C. Effect of dietary lipid sources on growth, enzyme activities and immuno-hematological parameters in Catla catla fingerlings. Asian-Australasian J. Anim. Sci. 2005, 18, 1609–1616. [Google Scholar] [CrossRef]
- Aminikhoei, Z.; Choi, J.; Lee, S.-M. Optimal dietary protein and lipid levels for growth of juvenile Israeli carp Cyprinus carpio. Fish. Aquat. Sci. 2015, 18, 265–271. [Google Scholar] [CrossRef]
- NRC, N.R.C. Nutrient Requirements of Laboratory Animals: Fourth Revised Edition, 4th ed.; Subcommittee on Laboratory Animal, Nutrition, Ed.; National Research Council: Washington, DC, USA, 1995. [Google Scholar]
- Takeuchi, T. Essential fatty acid requirements in carp. Arch. Anim. Nutr. 1996, 49, 23–32. [Google Scholar] [CrossRef]
- Duarte Silva, G.; Cavalcante Lucena, J.; Cavalcanti, O.; Almeida Bicudo, A. Growth and body composition of juvenile curimata pacu (Prochilodus argenteus) fed diets with different protein: lipid ratios. Lat. Am. J. Aquat. Res. 2019, 47, 114–121. [Google Scholar] [CrossRef]
- Virote, B. do C.R.; Moreira, A.M.S.; Silva Souza, J.G. da; Castro, T.F.D.; Melo, N.; Carneiro, W.F.; Drummond, C.D.; Vianna, A.R. da C.B.; Murgas, L.D.S. Obesity induction in adult zebrafish leads to negative reproduction and offspring effects. Reproduction 2020, 160, 833–842. [Google Scholar] [CrossRef]
- Williams, M.B.; Dennis-Cornelius, L.N.; Miyasaki, N.D.; Barry, R.J.; Powell, M.L.; Makowsky, R.A.; Fowler, L.A.; Watts, S.A.; Smith, D.L. Macronutrient Ratio Modification in a Semi-Purified Diet Composition: Effects on Growth and Body Composition of Juvenile Zebrafish Danio rerio. N. Am. J. Aquac. 2022, 84, 493–504. [Google Scholar] [CrossRef]
| Ingredients (%) | Dietary crude lipid levels (% of diet) | ||||
| 0.55 | 4.87 | 7.65 | 10.65 | 13.94 | |
| Casein | 42.00 | 42.00 | 42.00 | 42.00 | 42.00 |
| Corn starch | 31.43 | 26.43 | 21.43 | 16.43 | 11.43 |
| Gelatin | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 |
| Dicalcium phosphate | 6.20 | 6.20 | 6.20 | 6.20 | 6.20 |
| Microfine cellulose | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Mineral and vitamin supplement1 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Carboxymethylcellulose | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Dextrin | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Betaine | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Soy lecithin | 0.00 | 1.00 | 2.00 | 3.00 | 4.00 |
| Fish oil | 0.00 | 2.00 | 4.00 | 6.00 | 8.00 |
| Soybean oil | 0.00 | 2.00 | 4.00 | 6.00 | 8.00 |
| BHT | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Analyzed chemical composition | |||||
| Moisture (%) | 93.6 | 94.3 | 95.9 | 96.3 | 97.0 |
| Crude protein (%) | 42.3 | 42.1 | 41.4 | 40.5 | 39.1 |
| Crude lipid (%) | 0.55 | 4.87 | 7.65 | 10.65 | 13.94 |
| Ash (%) | 10.25 | 10.88 | 10.97 | 11.23 | 10.61 |
| Gross energy (kcal/kg) | 4030 | 4223 | 4612 | 4869 | 4858 |
| Ingredients (%) | Dietary crude lipid levels (% of diet) | p-value | ||||
| 0.55 | 4.87 | 7.65 | 10.65 | 13.94 | ||
| Initial weight (mg/fish) | 236.67±21.26 a | 232.08±23.40 a | 232.92±23.76 a | 232.08±1.91 a | 225.83±10.63 a | 0.9656 |
| Final weight (mg/fish) | 310.33±41.22 b | 468.75±40.10 a | 470.42±21.84 a | 445.00±26.34 a | 414.58±27.68 a | 0.0006 |
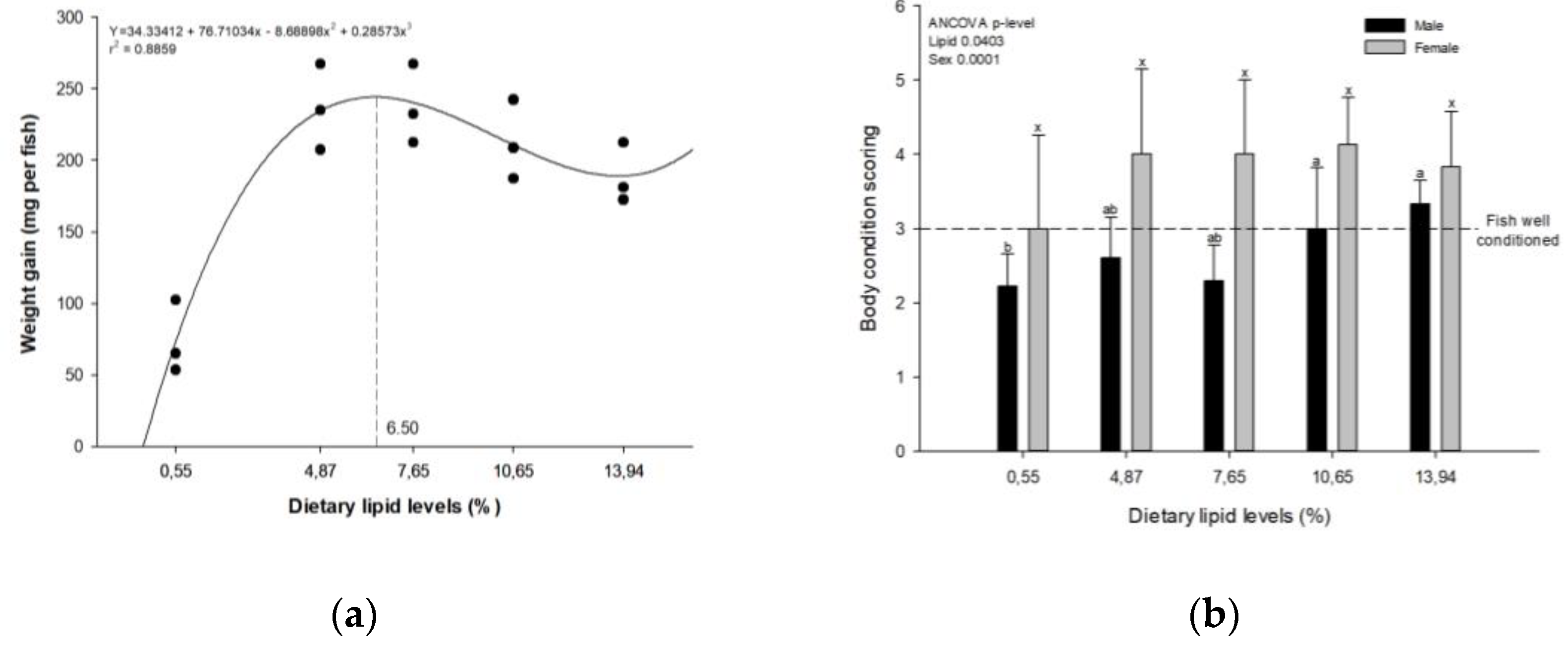
| Weight gain (mg/fish) | 73.67±25.62 b | 236.67±30.03 a | 237.50±27.84 a | 212.92±27.74 a | 188.75±21.03 a | 0.0001 |
| Specific growth rate (% live weight/day) | 0.67±0.18 b | 1.76±0.19 a | 1.76±0.26 a | 1.63±0.16 a | 1.52±0.11 a | 0.0001 |
| Fork length (mm) | 28.26±2.07 c | 30.54± 3.69 ab | 30.78±1.75 ab | 32.06±1.92 a | 29.84±1.71 bc | 0.0396 |
| Body condition scoring | 2.53±0.92 b | 3.42±1.16 a | 3.00±1.13 ab | 3.75±0.87 a | 3.42±0.79 a | 0.0225 |
| Daily feed intake (% live weight/day) | 5.64±1.84 a | 4.20±0.09 ab | 3.70±0.30 b | 3.71±0.26 b | 4.49±0.15 ab | 0.0963 |
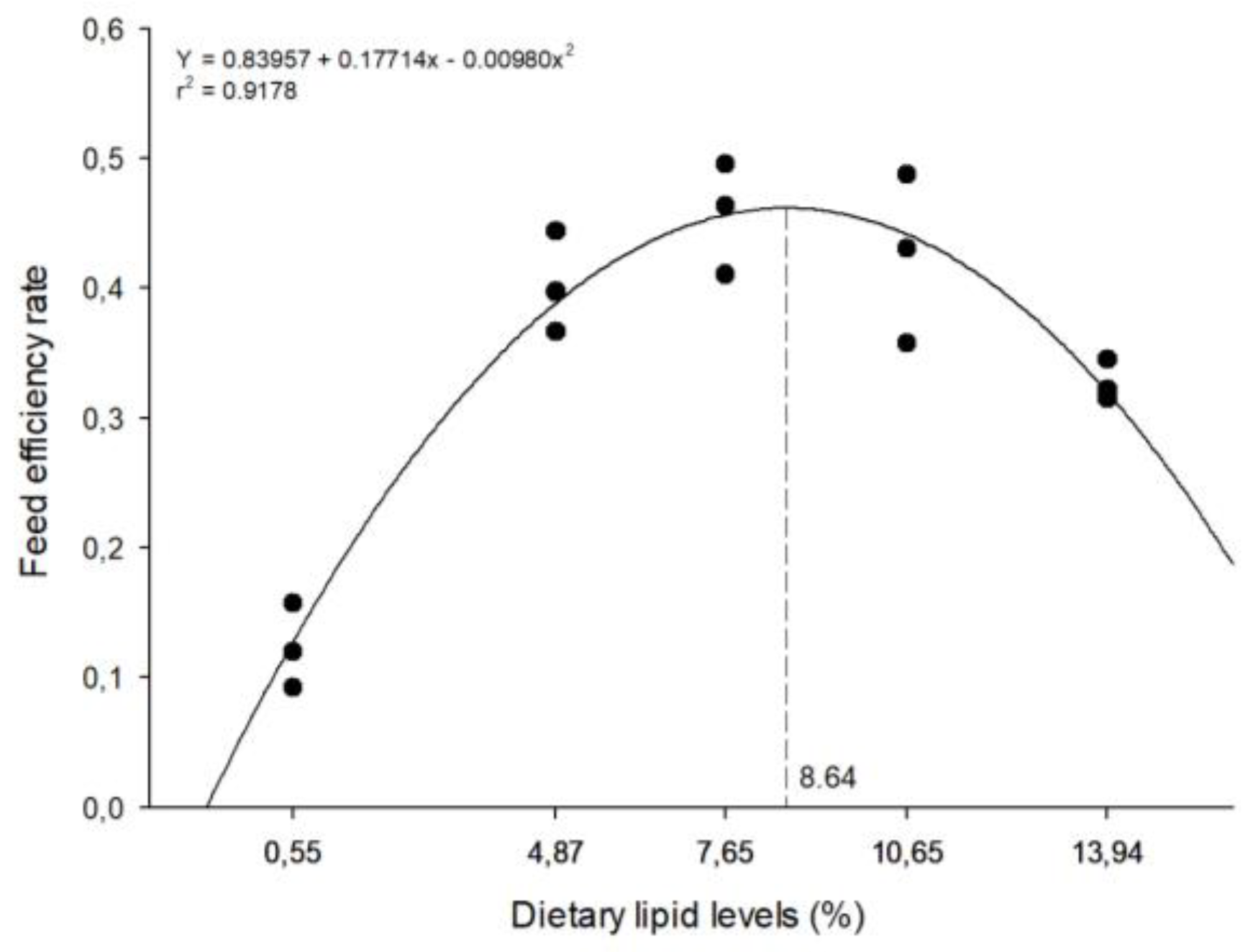
| Feed efficiency ratio (g:g) | 0.12±0.04 c | 0.40±0.04 a | 0.46±0.05 a | 0.43±0.07 a | 0.33±0.01 b | 0.0001 |
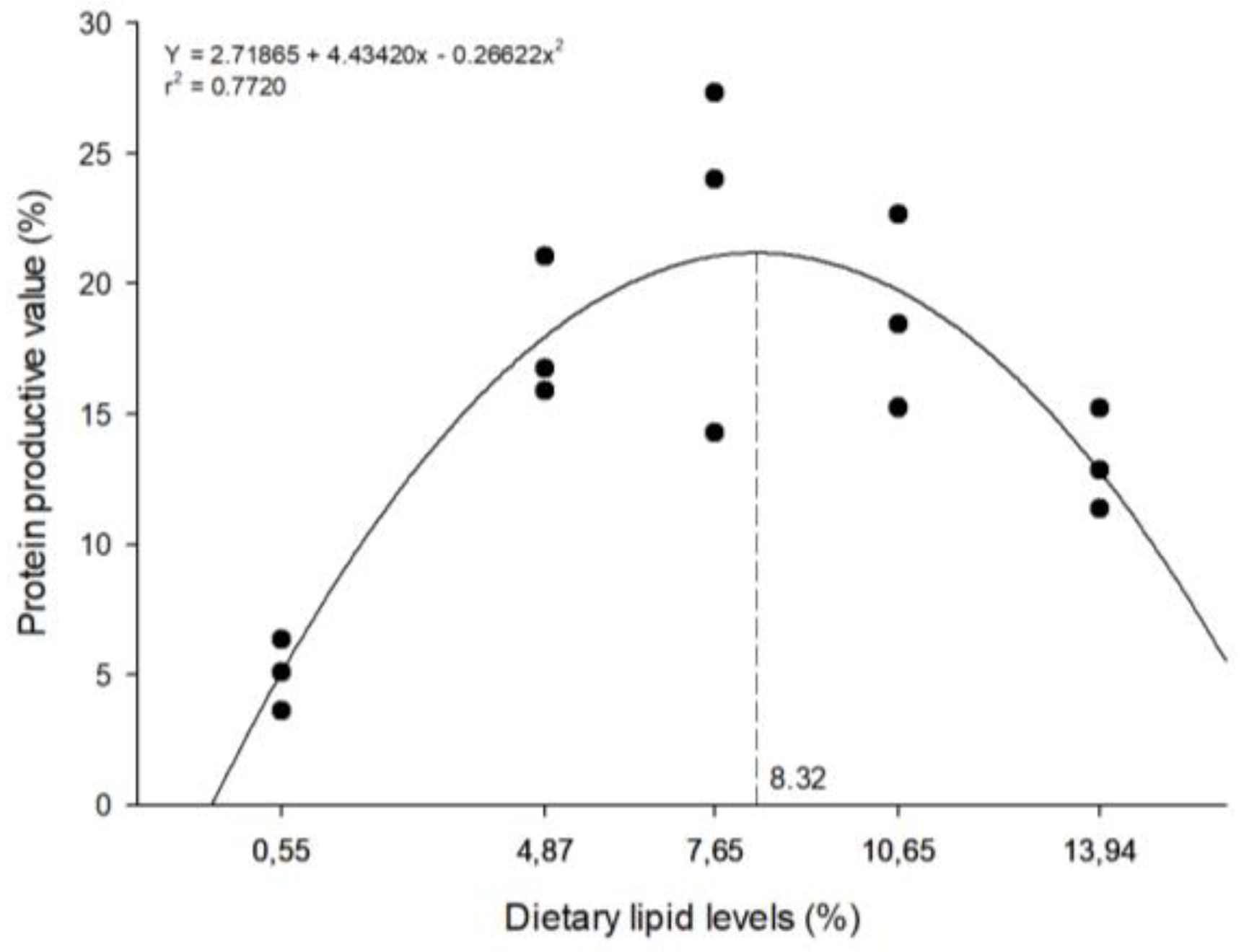
| Protein productive value (%) | 5.01±1.35 c | 17.89±2.76 ab | 21.88±6.79 a | 18.78±3.71 ab | 13.13±1.95 b | 0.0025 |
| Survival rate (%) | 70.83±26.02 b | 100.00±0.00 a | 100.00±0.00 a | 100.00±0.00 a | 100.00±0.00 a | 0.0404 |
| Ingredients (%) | Dietary crude lipid levels (% of diet) | p-value | ||||
| 0.55 | 4.87 | 7.65 | 10.65 | 13.94 | ||
| Moisture (%) | 73.74±1.23 | 73.75± 0.57 | 72.91± 2.68 | 72.84± 0.32 | 73.49± 1.04 | 0.8782 |
| Crude protein (%) | 17.03±0.06 | 17.01± 0.50 | 17.41± 1.97 | 16.99± 0.36 | 16.67± 0.67 | 0.9211 |
| Crude lipid (%) | 0.94±0.41 b | 1.46± 0.25 ab | 1.62± 0.48 a | 1.62± 0.13 a | 1.40± 0.33 ab | <.0001 |
| Ingredients (%) | Dietary crude lipid levels (% of diet) | p-value | ||||
| 0.55 | 4.87 | 7.65 | 10.65 | 13.94 | ||
| Intestinal villi height (µm) | 204.56±20.12 | 199.34±30.06 | 199.12±28.66 | 258.94± 74.99 | 207.89±11.21 | 0.3586 |
| Intestinal villi width (µm) | 77.08±14.48 | 83.25±8.07 | 71.39± 11.01 | 79.53± 8.32 | 74.89± 17.63 | 0.8097 |
| Muscularis mucosa thickness (µm) | 26.60±3.49 | 24.45±8.34 | 22.96±3.81 | 22.17± 8.36 | 25.47± 6.97 | 0.9174 |
| External perimeter (×103 µm) | 2.52± 0.37 | 3.45±0.74 | 2.78± 0.52 | 2.55±0.51 | 2.49± 0.29 | 0.1904 |
| Absorptive perimeter (×103 µm) | 4.22±1.95 | 1.95±0.95 | 1.93±1.23 | 3.18±0.05 | 3.63±2.63 | 0.5971 |
| Adipocyte average diameter | 30.82±4.54 c | 48.22±10.18 ab | 42.97±4.24 bc | 61.85±11.61 a | 50.46±7.07 ab | 0.0107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




