Submitted:
27 December 2023
Posted:
28 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant material and experimental design
2.2. Chlorophyll a fluorescence
2.3. Determination of photosynthetic pigments
2.4. Quantification of photosynthetic performance
2.5. Growth analyzes
2.6. Leaf anatomical analysis
2.7. Ex vitro acclimatization
2.8. Statistical analyzes
3. Results
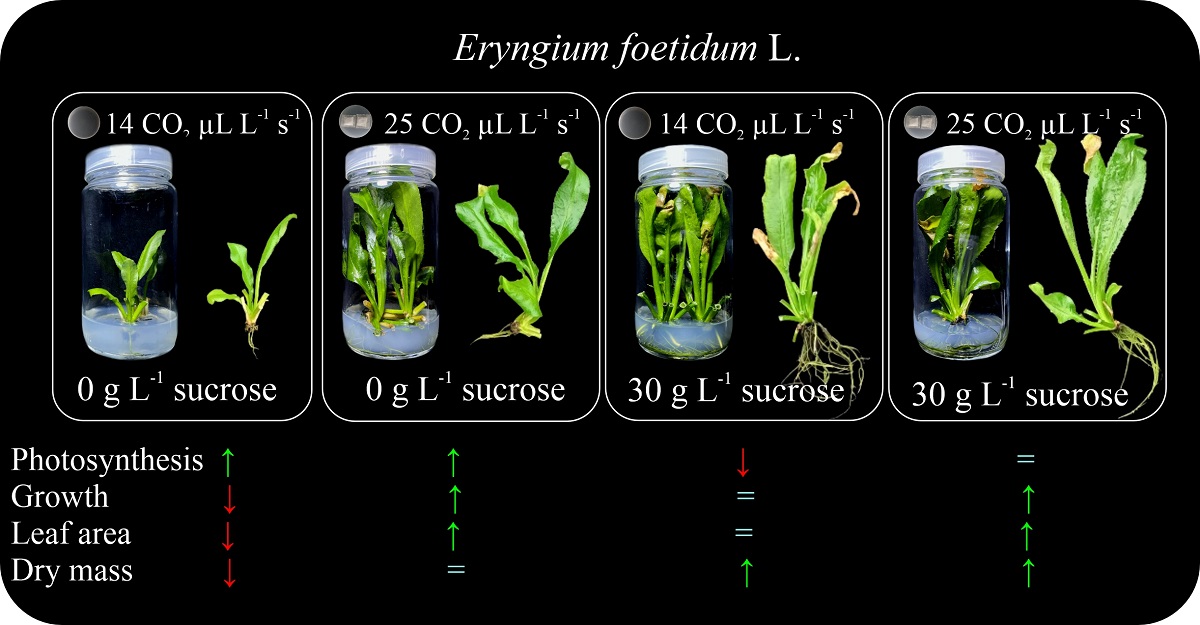
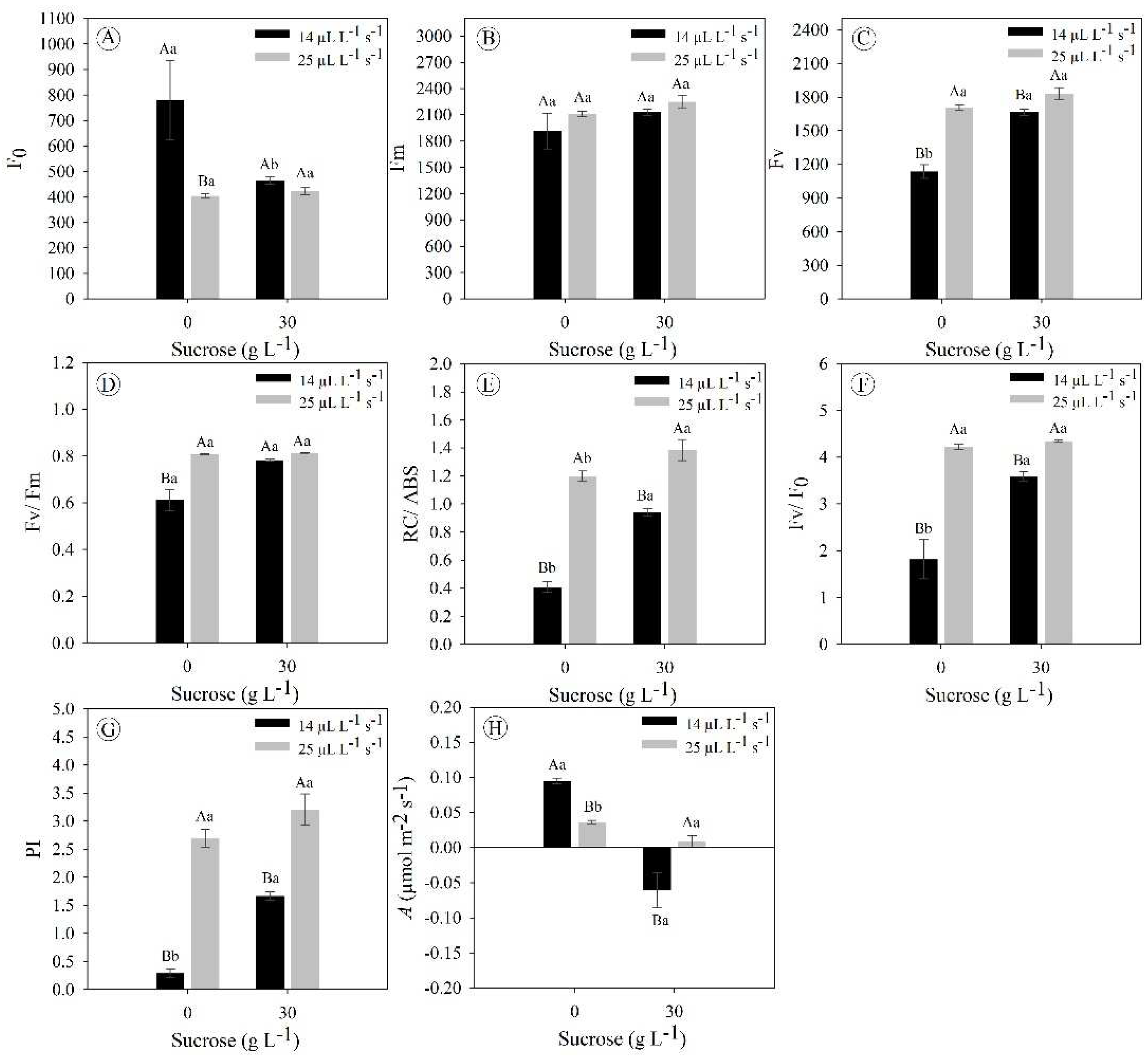
3.1. Gas exchange rates improve chlorophyll a fluorescence and photosynthetic rate in E. foetidum grown in vitro
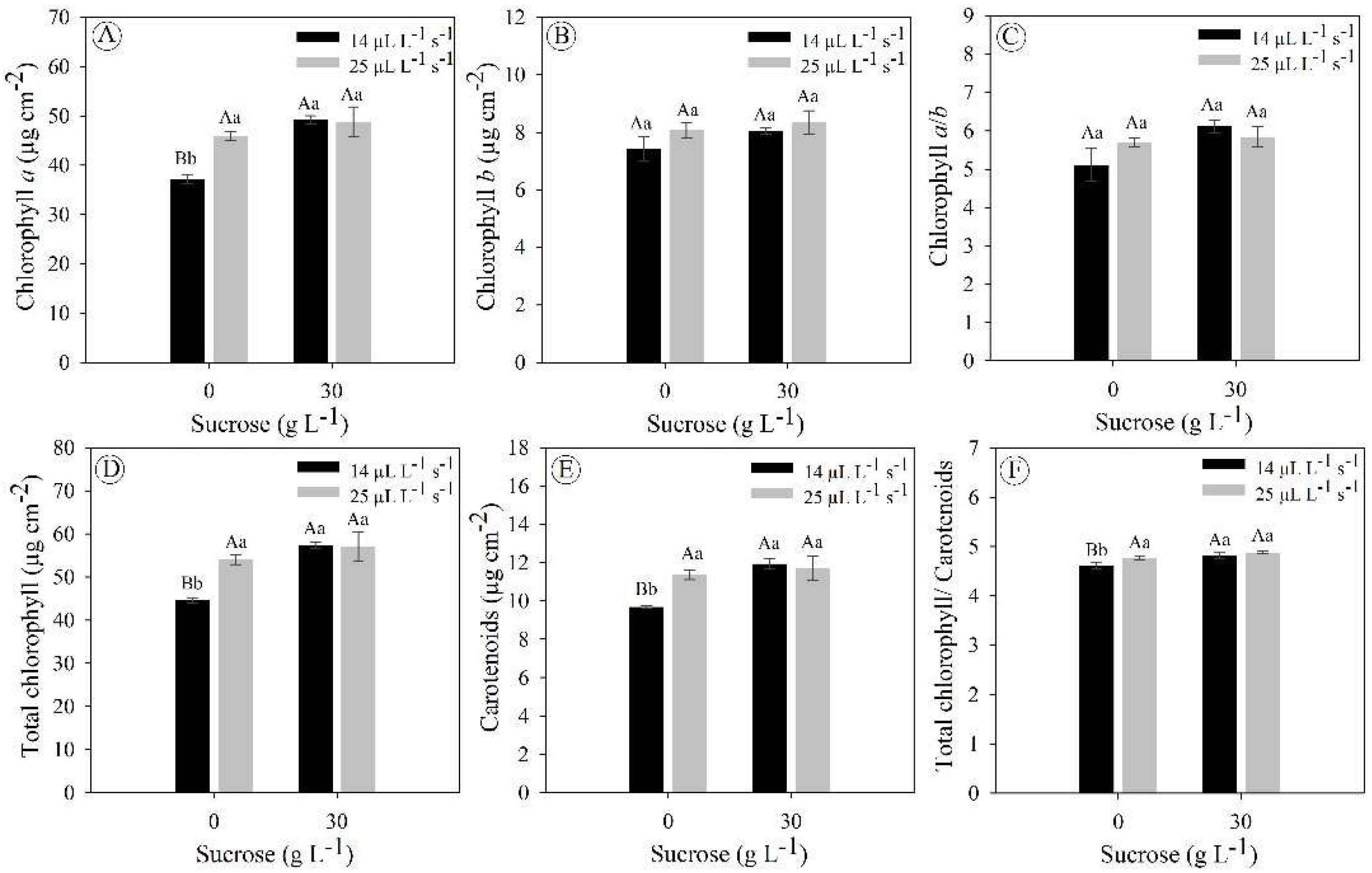
3.2. Sucrose concentrations and gas exchange rates do not modulate pigment concentration in E. foetidum grown in vitro
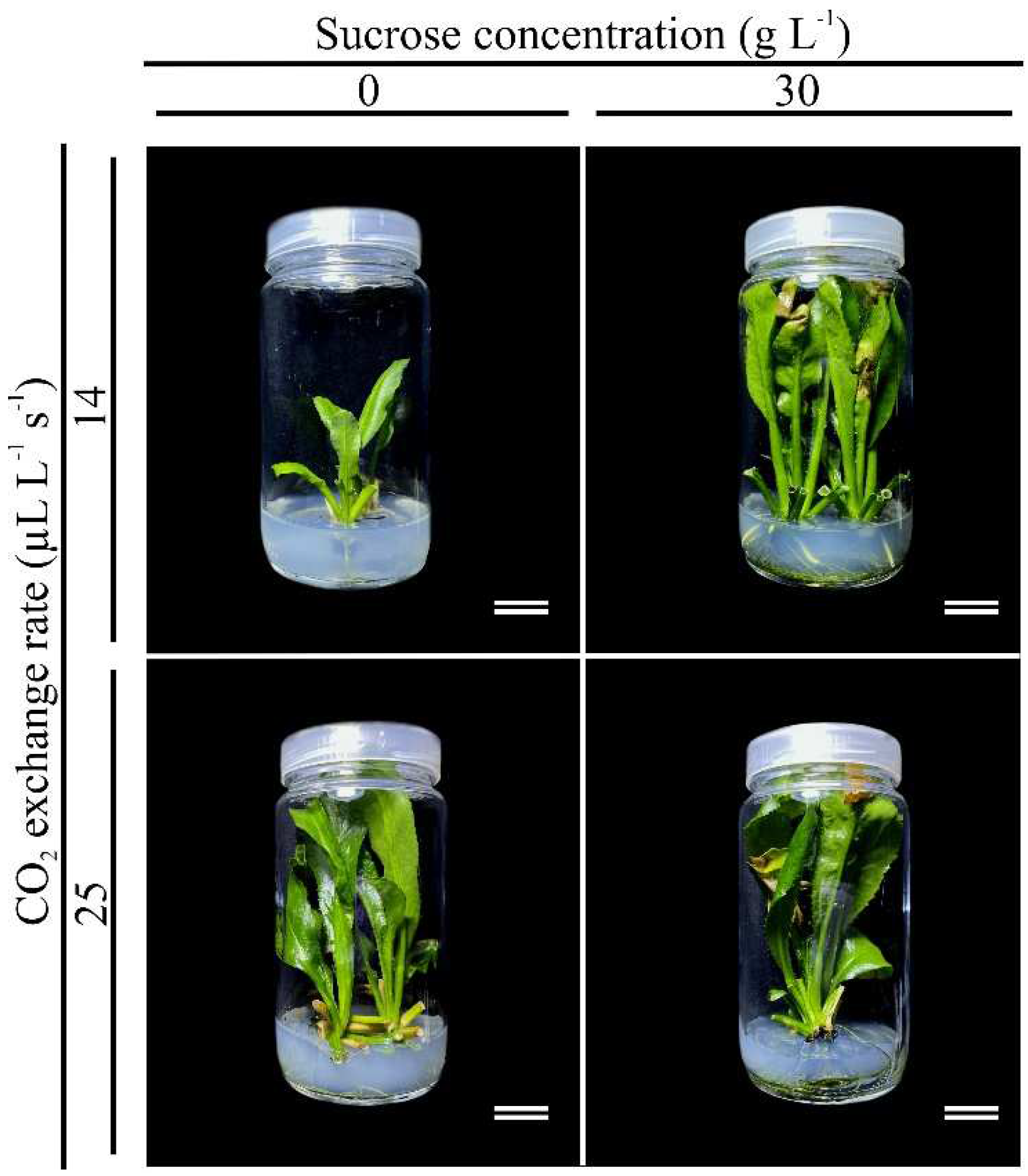
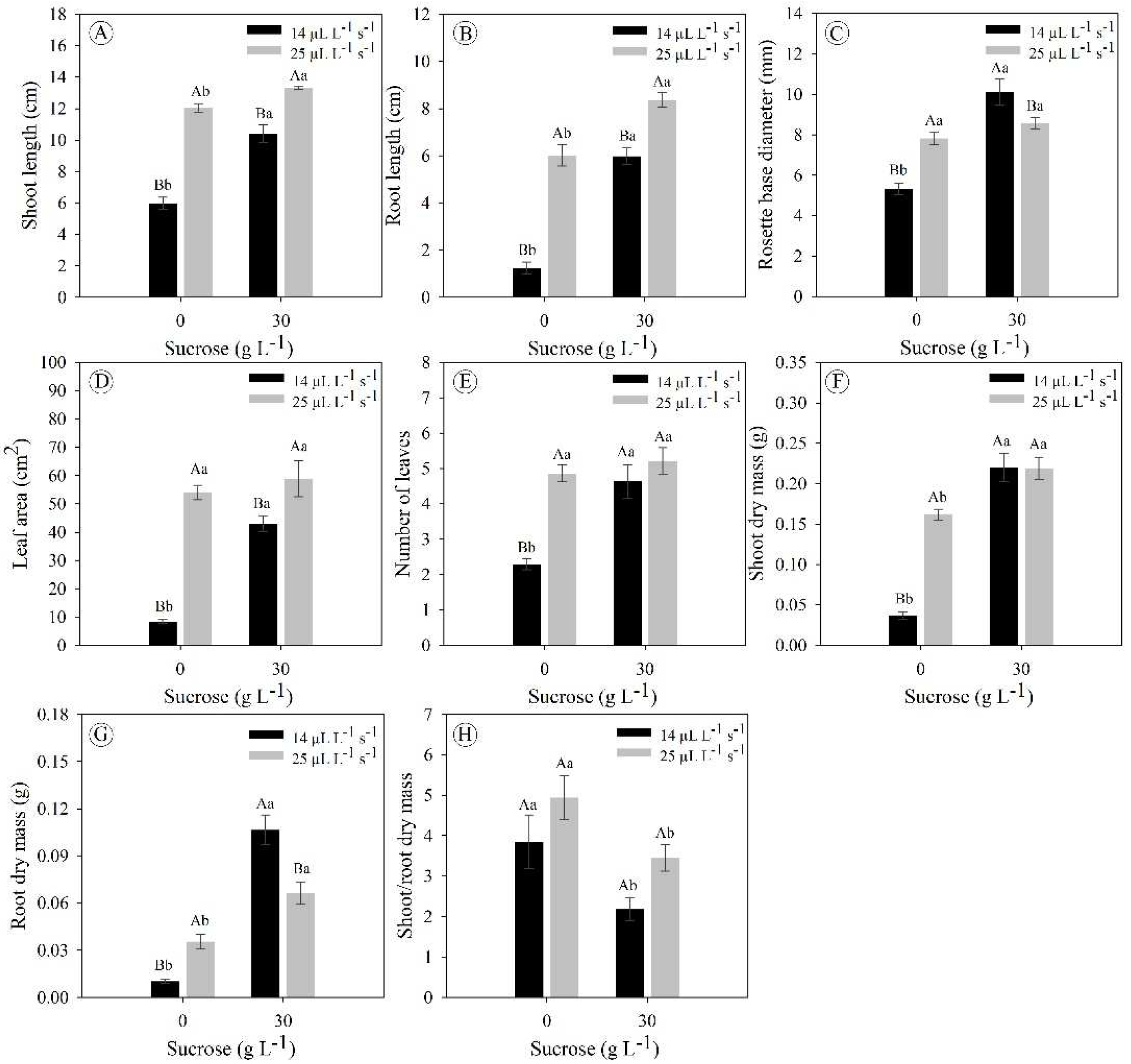
3.3. Sucrose concentrations and gas exchange rates affect the in vitro growth and development of E. foetidum
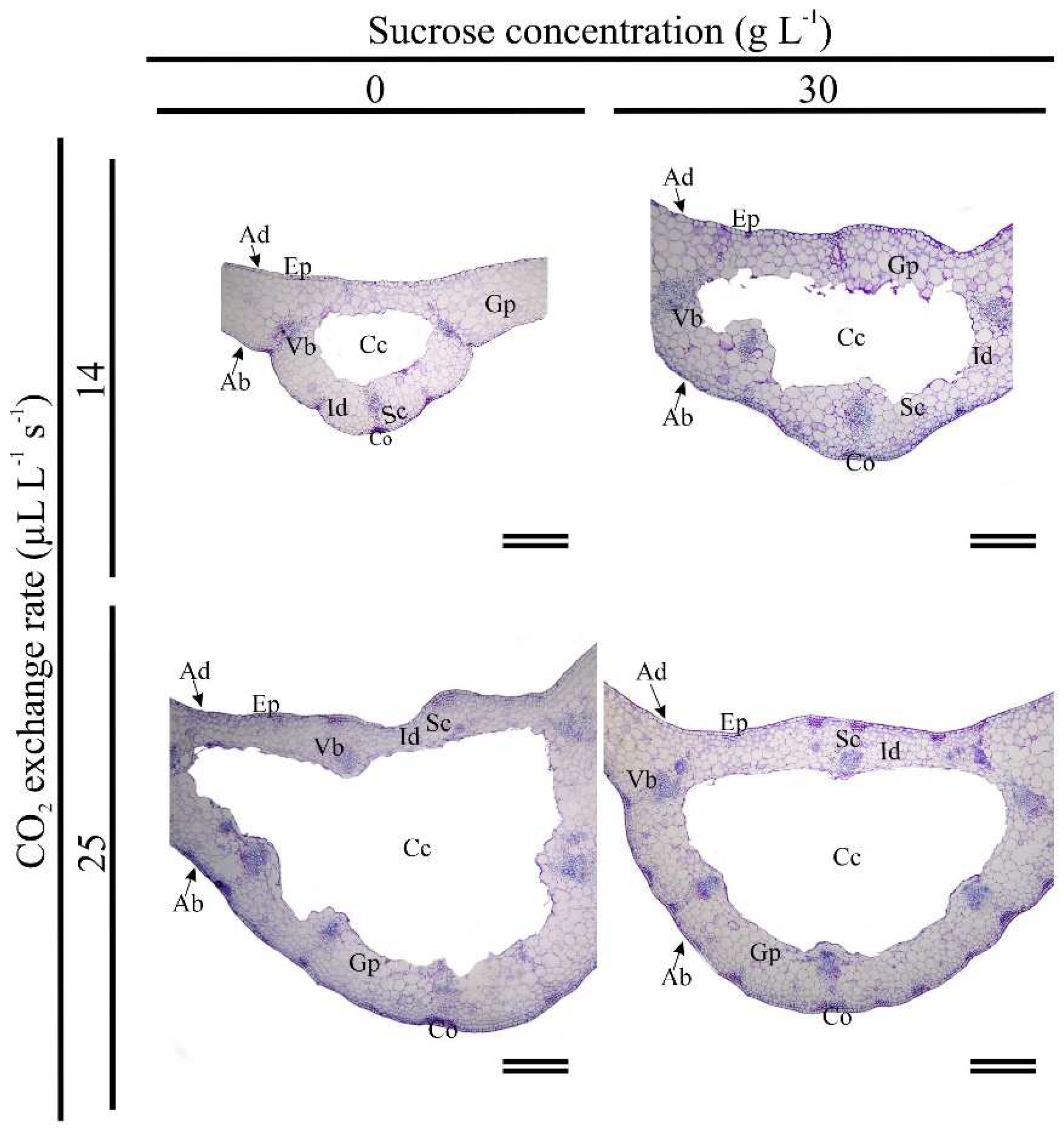
3.4. Sucrose concentrations and gas exchange rates affect leaf anatomy
3.5. Ex vitro acclimatization of E. foetidum grown under different sucrose concentrations and gas exchange rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro, T.F.D; Carneiro, W.F.; Reichel, T.; Fabem, S.L.; Machado, M.R.F; Souza, K.K.C; Resende, L.V.; Murgas, L.D.S. The toxicological effects of Eryngium foetidum extracts on zebrafish embryos and larvae depend on the type of extract, dose, and exposure time. Toxicol Res 2022, 11(5), 891–899. [CrossRef]
- Lucas, D.B.; Cardozo, A.L. Eryngium in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/FB15529 (accessed on 05 December 2023).
- POWO – Plants of the World Online. Available online: https://powo.science.kew.org/ (accessed on 30 November 2023).
- Singh, S.; Singh, D.R.; Banu, S.; Salim, K.M. Determination of bioactives and antioxidant activity in Eryngium foetidum L.: a traditional culinary and medicinal herb. Proc Natl Acad Sci, India, Sect B Biol Sci 2013, 83, 453–460. [CrossRef]
- Anju, T.; Rai, N.K.S.; Uthirchamkavu, I.; Sreedharan, S.; Ndhlala, A.R.; Singh, P.; Kumar, A. Analysis of nutritional and antioxidant potential of three traditional leafy vegetables for food security and human wellbeing. S Afr J Bot 2022, 145, 99–110. [CrossRef]
- Leitão, D.d.S.T.C.; Barbosa-Carvalho, A.P.P.; de Siqueira, F.C.; Sousa, R.P.e.; Lopes, A.S.; Chisté, R.C. Extracts of Eryngium foetidum Leaves from the Amazonia Were Efficient Scavengers of ROS and RNS. Antioxidants 2023, 12, 1112. [CrossRef]
- Rodrigues, T.L.M.; Silva, M.E.P.; Gurgel, E.S.C.; Oliveira, M.S.; Lucas, F.C.A. Eryngium foetidum L. (Apiaceae): a literature review of traditional uses, chemical composition, and pharmacological activities. Evid Based Complement Alternat Med 2022, 14, 2896895. [CrossRef]
- Paw, M.; Gogoi, R.; Sarma, N.; Saikia, S.; Chanda, S.K.; Lekhak, H.; Lal, M. Anti-microbial, anti-oxidant, anti-diabetic study of leaf essential oil of Eryngium foetidum L. along with the chemical profiling collected from North East India. J Essent Oil-Bear 2022, 26, 814–829. [CrossRef]
- Zhang, X.; Chen, J.; Zhou, S.; Zhao, H. Ethanol extract of Eryngium foetidum leaves induces mitochondrial associated apoptosis via ROS generation in human gastric cancer cells. Nutr Cancer 2022, 74, 2996–3006. [CrossRef]
- Rodrigues, T.L.M. Desempenho fisiológico e perfil químico do óleo essencial. Orientadora: Eloisa Helena de Aguiar Andrade. 2019. 60 f. Dissertação (Mestrado em Ciências Biológicas/ Botânica Tropical) – Universidade Federal Rural da Amazônia/ Museu Paraense Emílio Goeldi, Belém, 2019. Available online: http://repositorio.ufra.edu.br/jspui/handle/123456789/812 (accessed on 10 December 2023).
- Devi, P.B.; Deb, P.; Singh, H.B. Promotion, utilization, and commercial cultivation of local spices with special reference to eryngo (Eryngium foetidum L.) as a measure for livelihood improvement towards achieving the goal of sustainable development in the indo-burma biodiversity hotspot: a case study from manipur, North-East India. In: Sharma, S.; Kuniyal, J.C.; Chand, P.; Singh, P. (eds) Climate change adaptation, risk management and sustainable practices in the Himalaya. Cham: Springer International Publishing, United States, 2023; pp. 253–266. [CrossRef]
- Rajamohan, S. Harbouring the potential of medicinal and aromatic plants of India: novel biotechnological approach and extraction technologies. In: Máthé, Á.; Khan, I.A. (eds) Medicinal and aromatic plants of India Vol. 1. Medicinal and aromatic plants of the world, vol 8. Cham: Springer International Publishing, United States, 2022; pp. 323–339. [CrossRef]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res 2009, 3(13), 1222–1239. [CrossRef]
- Fazili, M.A.; Bashir, I.; Ahmad, M.; Yaqoob, U.; Geelani, S.N. In vitro strategies for the enhancement of secondary metabolite production in plants: a review. Bull Natl Res Cent 2022, 46, 35. [CrossRef]
- Nhut, D.T. General information: some aspects of plant tissue culture. In: Nhut, D.T., Tung, H.T., YEUNG, E.CT. (eds) Plant Tissue Culture: new techniques and application in horticultural species of tropical region. Singapore: Springer Singapore, Singapore, 2022; pp. 1–23. [CrossRef]
- Kozai, T., Kubota, C. Concepts, definitions, ventilation methods, advantages and disadvantages. In: Kozai, T., Afreen, F., Zobayed, S. (eds) Photoautotrophic (sugar–free medium) Micropropagation as a New Micropropagation and Transplant Production System. Springer: Dordrecht, Dordrecht, 2005, pp. 19–30. [CrossRef]
- Pires, H.P.; Felipe, S.H.S.; Pinheiro, M.V.M.; Paula Alves, A.; Alves, G.L.; Catunda, F.E.A.; Figueiredo, F.A.M.M.A.; Reis, F.O.; Ferraz, T.M.; Corrêa, T.R. Natural ventilation and sucrose concentrations in the in vitro culture system affect the acclimatization of "Perola" pineapple plants under different substrates. Aust J Crop Sci 2023, 17(1), 90–98. [CrossRef]
- Saldanha, C.W.; Otoni, C.G.; Azevedo, J.L.F.; Dias, L.L.C.; Rêgo, M.M.; Otoni, W.C. A low-cost alternative membrane system that promotes growth in nodal cultures of Brazilian ginseng [Pfaffia glomerata (Spreng.) Pedersen]. Plant Cell Tiss Organ Cult 2012, 110, 413–422. [CrossRef]
- Fortini, E.A.; Batista, D.S.; Mamedes-Rodrigues, T.C.; Felipe, S.H.S.; Correia, L.N.F.; Chagas, K.; Silva, P.O.; Rocha, D.I.; Otoni, W.C. Gas exchange rates and sucrose concentrations affect plant growth and production of flavonoids in Vernonia condensata grown in vitro. Plant Cell Tiss Organ Cult 2021, 144, 593–605. [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 1962, 15, 473–497. [CrossRef]
- Batista, D.S.; Castro, K.M.; Ribeiro, D.M.; Caixeta, E.T.; Oliveira Santos, M.; Viccini, L.F.; Otoni, W. C. Ethylene responses and ACC oxidase gene expression in Lippia alba (Verbenaceae) chemotypes with varying ploidy levels. In Vitro Cell Dev Biol -Plant 2017, 53, 278–284. [CrossRef]
- Bolhar-Nordenkampf, H.R.; Long, S.P.; Baker, N.R.; Oquist, G.; Schreiber, U.L.E.G.; Lechner, E.G. Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: a review of current instrumentation. Funct Ecol 1989, 3(4), 497-514. [CrossRef]
- Santos, R.P.; Ferreira Da Cruz, A.C.; Iarema, L.; Kuki, K.N.; Campos Otoni, W. Protocolo para extração de pigmentos foliares em porta-enxertos de videira micropropagados. Rev Ceres 2008, 55, 356–364.
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 1994, 144(3), 307-313. [CrossRef]
- Castro, K.M.; Batista, D.S.; Fortini, E.A.; Silva, T.D.; Felipe, S.H.S.; Fernandes, A.M.; Sousa, R.M.J.; Nascimento, L.S.Q., Campos, V.R.; Grazul, R.M.; Viccini, L.F.; Otoni, W.C. Photoperiod modulates growth, morphoanatomy, and linalool content in Lippia alba L. (Verbenaceae) cultured in vitro. Plant Cell Tiss Organ Cult 2019, 139, 139–153. [CrossRef]
- Silva, T.D.; Batista, D.S.; Fortini, E.A.; Castro, K.M.; Felipe, S.H.S.; Fernandes, A.M.; Sousa, R.M.J.; Chagas, K.; Silva, J.V.S.; Correia, L.N.F.; Farias, L.M.; Leite, J.P.V.; Rocha, D.I.; Otoni, W.C. Blue and red light affects morphogenesis and 20-hydroxyecdisone content of in vitro Pfaffia glomerata accessions. J Photochem Photobiol B, Biol 2020, 203, 111761. [CrossRef]
- Johansen, D.A. Plant microtechnique. Mac Graw-Hill Book Co., Inc., New York, 1940; 523 pp.
- O’Brien, T.P.; McCully, M.E. The study of plant structure: principles and selected methods; Termarcarphi Pty. Ltd.: Melbourne, Australia, 1981.
- Cruz, C.D. Genes: a software package for analysis in experimental statistics and quantitative genetics. Acta Sci Agron 2013, 35, 271-276. [CrossRef]
- Ferreira, P.R.B.; Cruz, A.C.F.; Batista, D.S.; Nery, L.A.; Andrade, I.G.; Rocha, D. I.; Felipe, S.H.S.; Koehler, A.D.; Nunes-Nesi, A. CO2 enrichment and supporting material impact the primary metabolism and 20-hydroxyecdysone levels in Brazilian ginseng grown under photoautotrophy. Plant Cell Tiss Organ Cult 2019, 139, 77–89. [CrossRef]
- Louback, E.; Batista, D.S.; Pereira, T.A.R.; Mamedes-Rodrigues, T.C.; Silva, T.D.; Felipe, S.H.S.; Rocha, D.I.; Steinmacher, D.A.; Otoni, W.C. CO2 enrichment leads to altered cell wall composition in plants of Pfaffia glomerata (Spreng.) Pedersen (Amaranthaceae). Plant Cell Tiss Organ Cult 2021, 145, 603–613. [CrossRef]
- Luis, S.J.; Jabín, B.J. CO2–enriched air in a temporary immersion system induces photomixotrophism during in vitro multiplication in vanilla. Plant Cell Tiss Organ Cult 2023, 155, 29–39. [CrossRef]
- Wolf, S.; Kalman-Rotem, N.; Yakir, D.; Zrv, M. Autotrophic and heterotrophic carbon assimilation of in vitro grown potato (Solanum tuberosum L) plants. J Plant Physiol 1998, 153(5–6), 574–580. [CrossRef]
- Shin, KS.; Park, SY.; Paek, KY. Sugar metabolism, photosynthesis, and growth of in vitro plantlets of Doritaenopsis under controlled microenvironmental conditions. In Vitro Cell Dev Biol -Plant 2013, 49, 445–454. [CrossRef]
- Alves, J.P.; Pinheiro, M.V.M.; Corrêa, T.R.; Alves, G.L.; Marinho, T.R.S.; Batista, D.S.; Figueiredo, F.A.M.M.A.; Reis, F.O.; Ferraz, T.M.; Campostrini, E. Morphophysiology of Ananas comosus during in vitro photomixotrophic growth and ex vitro acclimatization. In Vitro Cell Dev Biol -Plant 2023, 59, 106–120. [CrossRef]
- Vinh, B.V.T.; Tung, H.T.; Mai, N.T.N.; Khai, H.D.; Luan, V.Q.; Phong, T.H.; Phong, T.N.P.; Nhut, D.T. Enhanced efficient micropropagation and reduced abnormal phenomena in Phyllanthus amarus plantlets cultured on medium containing silver nanoparticles. S Afr J Bot 2023, 163, 217–225. [CrossRef]
- Askari, N.; Aliniaeifard, S.; Visser, R.G.F. Low CO2 Levels are detrimental for in vitro plantlets through disturbance of photosynthetic functionality and accumulation of reactive oxygen species. Horticulturae 2022, 8, 44. [CrossRef]
- Silva, L.M.; Carvalho, V.S.; Generoso, A.L.; Miranda, D.P.; Costa Júnior, O.D.; Simioni, P.F.; Santana, D.B.; Cunha, M.; Oliveira, J.G.; Viana, A.P. Micropropagation of interspecific hybrids of Vitis spp. in microenvironments with different gas exchanges. Sci Hortic 2022, 305, 111413. [CrossRef]
- Carrari-Santos, R.; Vettorazzi, R.G.; Pinto, V.B.; Pinto, V.B.; Sena, E.O.A.; Oliveira, J.G.; Campostrini, E.; Silveira, V.; Santa-Catarina, C. Microporous membrane and culture medium affect in vitro seedling development of Dalbergia nigra (Vell.) Ex Benth. (Fabaceae) by modulation of the protein profile and accumulation of ethylene and CO2. Plant Cell Tiss Organ Cult 2023, 153, 559–576. [CrossRef]
- Iarema, L.; Cruz, A.C.F.; Saldanha, C.W.; Dias, L.L.C.; Vieira, R.F.; Oliveira, E.J.; Otoni, W.C. Photoautotrophic propagation of Brazilian ginseng [Pfaffia glomerata (Spreng.) Pedersen]. Plant Cell Tiss Organ Cult 2012, 110, 227–238. [CrossRef]
- Wang, K.L.C.; Li, H.; Ecker, J.R. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14, Issue suppl_1, S131–S151. [CrossRef]
- Kubota, C. Concepts and background of photoautotrophic micropropagation. In: Morohoshi N, Komamine A (eds) Molecular breeding of woody plants. Elsevier, Amsterdam, 2001; pp. 325–334. [CrossRef]
- Cournac, L.; Dimon, B.; Carrier, P.; Lohou, A.; Chagvardieff, P. Growth and photosynthetic characteristics of Solanum tuberosum plantlets cultivated in vitro in different conditions of aeration, sucrose supply, and CO2 enrichment. Plant Physiol 1991, 97 (1), 112–117. [CrossRef]
- Desjardins, Y.; Hdider, C.; Riek, J. Carbon nutrition in vitro — regulation and manipulation of carbon assimilation in micropropagated systems. In: Aitken-Christie, J., Kozai, T., Smith, M.A.L. (eds) Automation and environmental control in plant tissue culture. Springer: Dordrecht, 1995; pp. 441–471. [CrossRef]
- Mayak, S.; Tirosh, T.; Ilan, A.; Duvdevani, A.; Khayat, E. Growth and development of pineapple (Ananas comosus L.) plantlets cultured in vitro at enriched and ambient CO2 environments. In International Symposium on Biotechnology of Tropical and Subtropical Species Part 2 1998, 461 (pp. 225-230). [CrossRef]
- Marques, I.; Fernandes, I.; Paulo, O.S.; Lidon, F.C.; DaMatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. A transcriptomic approach to understanding the combined impacts of supra-optimal temperatures and CO2 revealed different responses in the polyploid Coffea arabica and its diploid progenitor C. canephora. Int J Mol Sci 2021, 22, 3125. [CrossRef]
- Martins, J.P.R.; Almeida-Rodrigues, L.C.; Santos, E.R.; Gontijo, A.B.P.L.; Falqueto, A.R. Impacts of photoautotrophic, photomixotrophic, and heterotrophic conditions on the anatomy and photosystem II of in vitro-propagated Aechmea blanchetiana (Baker) L.B. Sm. (Bromeliaceae). In Vitro Cell.Dev.Biol.-Plant 2020, 56, 350–361. [CrossRef]
- Rocha, T.T.; Araújo, D.X.; Silva, A.M.; Oliveira, J.P.V.; Carvalho, A.A.; Gavilanes, M.L.; Bertolucci, S.K.V.; Alves, E.A.; Pinto, J.E.B.P. Morphoanatomy and changes in antioxidant defense associated with the natural ventilation system of micropropagated Lippia dulcis plantlets. Plant Cell Tiss Organ Cult 2022, 151, 467–481. [CrossRef]
- Soares, J.S.; Ramos, J.C.M.; Sorgato, J.C.; Ribeiro, L.M.; Reis, L.C. Brassavola tuberculata Hook.: in vitro growth and ex vitro establishment as a function of the micropropagation system and sucrose. Braz J Biol 2023, 83, e270892. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




