1. Introduction
Background
Glioblastoma (GBM) is a poor prognosis malignant WHO grade 4 glioma whose standard therapy, after surgical resection, consists of radiotherapy (RT) and chemotherapy (CT) with temozolomide (TMZ), according to Stupp et al. However, the prognosis remains poor with a 5-year survival of 5% [
1]. Recent decades have seen a renewed interest in immunotherapy in cancer due to new drugs and effective therapies, such as immune-check points inhibitors, adoptive T-cell approaches, dendritic cell-based vaccines, or combinations of these. GBM has an immunosuppressive microenvironment due to tumor-associated factors: overexpression of inhibitory cytokines or checkpoint molecules, low level of HLA, and elevated infiltrating T-regulatory cells (T-reg) [
2]. Dendritic cells (DCs) are the most potent professional antigen-presenting cells due to their function of linkage between innate and adaptive immune responses and have become a promising way to generate a specific immune response against cancer [
3]. Regarding HGGs (High-grade gliomas), multiple phase I/II trials have been reported; however, the objective response rate was only 15.6% [
4,
5]. Conversely, two meta-analyses published in 2014, indicated improved survival (OS) and progression-free survival (PFS) with DC vaccination in HGG patients. Moreover, a recent meta-analysis confirmed the advantages of the DC vaccination in terms of OS and PFS without causing severe adverse events (AEs), despite the number of cycles, dosages, and the administration route [
6,
7]. Finally a phase III trial by Liau et al. found that OS was longer in the arm treated with the DC vaccine compared to standard therapy [
8]
Study Rationale
Since 2001, we have treated more than 80 advanced melanoma patients with a tumor lysate loaded autologous DC vaccine, obtaining a clinical benefit of 54.1%. The results show that, in patients who develop an immune response directed to the vaccination antigens (about two thirds), OS is significantly improved in comparison both to the other patients and to advanced melanoma patients treated with chemotherapy [
9]. Another study evaluated DC vaccine in combination with low-dose temozolomide aimed at reducing the number of T regulatory cells [
10] and another clinical trial is ongoing. All the studies confirm the good safety profile of the vaccine, similarly to other studies.
Our group, moreover, have published a study in which quantitative and qualitative changes in tumor-infiltrating T lymphocytes (TILs) induced by vaccination with autologous tumor lysate/homogenate loaded DCs have been investigated in a series of 16 patients with metastatic melanoma. Immunohistochemistry for CD4, CD8, Foxp3, Granzyme B (GZMB), PDL1, and HLA class I was performed in tumor biopsies collected before and after DC vaccination. The density of each marker was quantified by automated digital pathology analysis on whole slide images. Co-expression of markers defining functional phenotypes, i.e., Foxp3+ regulatory CD4+ T cells (Treg) and GZMB+ cytotoxic CD8+T cells, was assessed with sequential immunohistochemistry. A significant increase of CD8+ TILs was found in post-vaccine biopsies of patients who were not previously treated with immune-modulating cytokines or Ipilimumab. Interestingly, along with a maintained tumoral HLA class I expression, after DC vaccination we observed a significant increase of PDL1+ tumor cells, which significantly correlated with intratumoral CD8+ T cell density. This observation might explain the lack of a significant concurrent cytotoxic reactivation of CD8+ T cell, as measured by the numbers of GZMB+ T cells. Altogether our findings indicate that DC vaccination exerts an important role in intratumoral T cell activation detected in post-DC therapy lesions, that is lessened by an occurring phenomenon of adaptive immune resistance, yet the concomitant PDL1 up-regulation [
11].
On the basis of previous data below are summarized the main points of the study rationale:
After resection/radio-chemotherapy patients are in a state of minimal residual disease which is probably beneficial for immunotherapy because of the lower tumor load and depletion of immunosuppressive cells
TMZ may reduce regulatory T cell
The lymphocyte compartment recovering after chemotherapy appears to be beneficial for induction of anti-tumor responses
Dying tumor cells after radio-chemotherapy may act as danger signal and boost an effective antitumor immune response
There is an increased responsiveness to TMZ after DC vaccination
2. Materials and Methods
During the pre-screening phase, patients underwent the necessary procedures to obtain the biological material for DC vaccine preparation (leukapheresis) and are treated with standard radiochemotherapy (according to Stupp regimen).
After pre-screening, patients are enrolled based on subsequent main eligibility criteria:
Inclusion Criteria
Histologically confirmed glioblastoma.
The autologous surgical specimen needed for vaccine manufacturing must have been collected and sent to the Somatic Cell Therapy Lab of IRCCS IRST and must fulfill all the acceptance criteria prescribed by the GMP procedures.
Availability of sufficient leukapheretic material for the preparation of the vaccine product.
Patients must have recovered (grade 1 or less by CTCAE 5.0) from all the events related to previous treatments.
Be willing and able to provide written informed consent/assent for the trial.
Be ≥ 18 years of age on day of signing informed consent.
Have a Karnofsky performance status (KPS) ≥ 70% or a performance status of 0 or 1 on the ECOG Performance Scale.
Demonstrate adequate organ and marrow function.
Main Exclusion criteria
Patients with a diagnosis of immunodeficiency or receiving systemic steroid therapy > 20 mg prednisone equivalent or any other form of immunosuppressive therapy within 7 days prior to the first dose of trial treatment.
Patients with active autoimmune disease that has required systemic treatment in the past 2 years (i.e. with use of disease modifying agents, corticosteroids or immunosuppressive drugs). Replacement therapy (eg., thyroxine, insulin, or physiologic corticosteroid replacement therapy for adrenal or pituitary insufficiency, etc.) is not considered a form of systemic treatment.
Known history of active TB (Bacillus Tuberculosis).
Previous treatment with a cancer vaccine.
Other known malignant neoplastic diseases in the patient’s medical history with a disease-free interval of less than 5 years, except basal or squamous cell carcinoma of the skin and in situ carcinoma of the cervix uteri treated with radical surgery.
Any known history of or is positivity of any serologic marker indicative of infection by Treponema pallidum, hepatitis B virus (HBsAg, HBsAb, HBcAB), hepatitis C virus (HCVAb, HCV RNA quantitative), human immunodeficiency virus (HIV), whether actual or previous.Patients who have received a live vaccine within 30 days of planned start of study therapy.
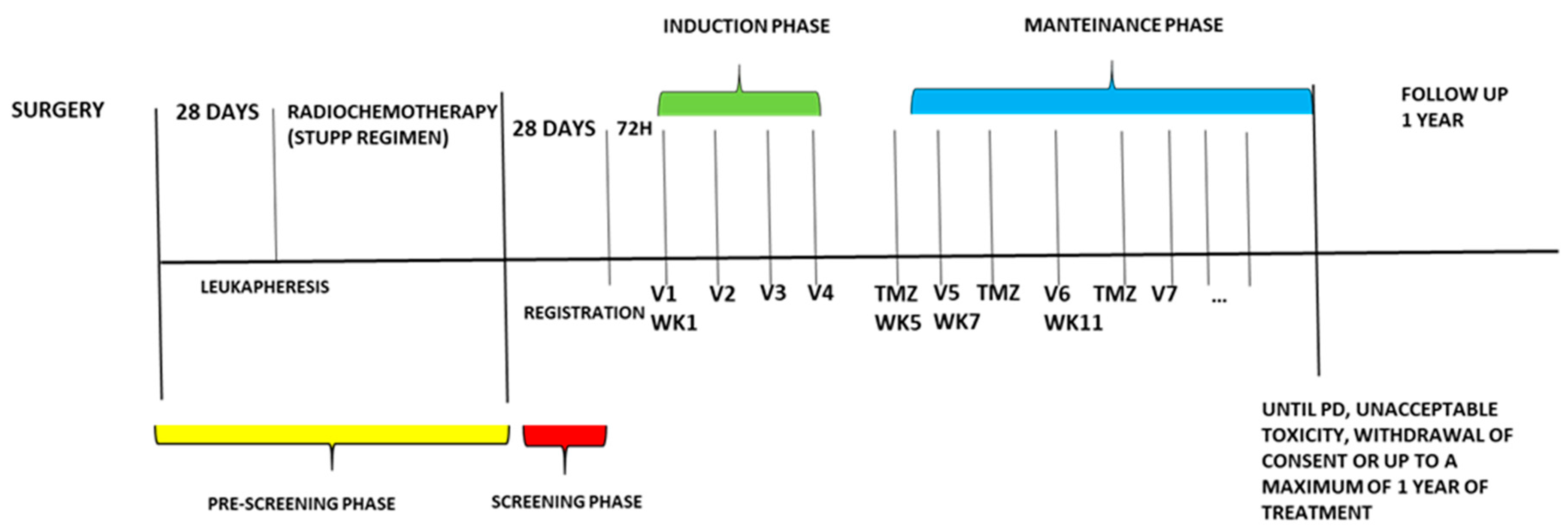
The experimental treatment consists of an induction phase with four-weekly doses of the dendritic cell vaccine (10x10
6 cells), administered intradermally (weeks one to four), followed by a maintenance phase consisting of 28-day cycles with the administration of the vaccine at the start of week seven, and temozolomide (150-200mg/m2/day) assumed orally from day one to five q28 (starting week five). The combined maintenance treatment continues until disease progression, unacceptable toxicity, withdrawal of consent by the patient, or until the maximum one-year treatment time. (
Figure 1). To evaluate the response and progression, we used the international Response Evaluation Criteria in Brain Tumors Committee (Response Assessment in Neuro-Oncology guidelines, RANO) associated with Perfusion MRI by DSC (dynamic susceptibility contrast). Our objective is to distinguish between real progression of GBM from radionecrosis and pseudo progression by evaluating the 5 ROIs of the perfusion study and correlate them with RANO imaging criteria. In particular we want to demonstrate that the presence in at least 3 ROIs of rCBV values> 2.5 identifies real disease progression if in accordance with RANO imaging criteria.
For the purposes of this study, patients are reevaluated for response at the end of induction phase and every 2 cycles during maintenance phase. [
12]. (Protocol in supplementary documents)
DC vaccine preparation
The “autologous dendritic cell loaded with autologous tumor homogenate” is an Advanced Therapy Medicinal Product consisting of dendritic cells obtained by in vitro differentiation of peripheral blood monocytes, isolated by leukapheresis from each patient, with IL-4 and GM-CSF. Immature DC such obtained are then loaded with a homogenate of tumor tissue obtained from the same patient, matured with a cytokine cocktail containing IL1b, PGE2, IL6, and TNF-a (“maturation cocktail”). Pulsed mature dendritic cells (mDC) are collected at day 9, washed, counted, tested for quality control (vitality, purity, phenotype markers, sterility, endotoxin, mycoplasma) frozen in aliquots (at least 13×106 cells/aliquot) and stored in nitrogen vapours. The aliquots are thawed and packed in two insulin syringes for administration to the patient (10x106 total dendritic cells). The syringes are filled and closed in Class A area and report the product identity. The syringes are packed in their original packaging labeled and put in a plastic bag reporting the product and the protocol identity, according to EU-cGMP Annex XIII.
The content of each syringe is administered intradermally with 5 injections in sites close to inguinal or axillary lymph node stations that had not been site of previous surgical exeresis, preferentially alternating injection sites in consecutive vaccine administrations.
DTH test
In vivo, Immunomonitoring is measured by the delayed-type hypersensitivity (DTH) test against tumor homogenate and KLH. DTH testing is a classical method for measuring cell-mediated immune reactivity. This technique involves intradermal administration of antigen preparation and recording the degree of erythema and induration produced 24-48 hours after the injection. This response reflects antigen-specific recruitment and activation of CD4+ to release T helper-1 cytokines (IFN-γ in particular) and recruitment and activation of CD8+ effector T cells in the injection site. In our and others’ groups experience, positive response to DTH test performed with soluble antigen after vaccination with DC vaccine in patients carrying metastatic melanoma was strongly related with better clinical outcome. In addition, DTH testing does not require extensive training nor the utilization of costly equipment, and can be easily performed at the bedside. All these features make it a feasible, low-cost immunomonitoring method to evaluate immunologic efficacy in a clinical trial setting. DTH testing dose corresponds to 50 μg of autologous tumor homogenate or KLH (positive control) prepared in 0.5 ml of 0.9% sterile saline. The negative control is a dose of 0.5 ml of 0.9% sterile saline alone
Objectives and Statistical Considerations
The study’s primary objectives are Progression-free survival (PFS), intended as the proportion of patients without progression of disease at three months from leukapheresis date and safety, as a percentage of patients experiencing grade (G) 3 or higher adverse events concerning the treatment.
The secondary objectives are to evaluate the immune response in vivo by analyzing the prognostic role of a positive DTH skin test after treatment and, additionally, to measure the clinical outcome in terms of OS and the efficacy of the treatment to enhance the number of circulating immune effectors specific for tumor antigens. Additionally, the study aims to evaluate the persistence of anti-tumor immune response, the degree of plasma levels and proangiogenic factors of inflammatory cytokines, as well as assess the prognostic and predictive role of tumor antigen expression in tumor tissue, and analyze the prognostic and predictive role of immune cells in the peripheral blood and the tumor microenvironment.
Simon's two-stage design (Simon, 1989) was used for the sample size calculation. The null hypothesis that 70% of patients did not experience disease progression three months after leukapheresis was tested against a one-sided alternative. At stage one of the study, we enrolled nine patients. Three months from the start of leukapheresis, at least six patients showed no signs of disease progression. For the second stage of the trial, we will enroll 19 new patients for a total of 28. If more than 22 patients out of 28 show no evidence of disease progression at three months, the null hypothesis will be rejected. This design yields a 10% false positive rate and an 80% true positive rate for an 87% true proportion.
The calculation determines the percentage of patients who did not experience progression within three months. Furthermore, non-progressive patients are tracked from the date of leukapheresis to the date of first progression, the date of death from any cause, or the date of the last restaging. The OS is measured from the date of leukapheresis until the date of death from any cause or the last date the patient was known to be alive.
PFS and OS are calculated with the Kaplan-Meier method, and the analysis is carried out on the eligible population. The proportion of patients experiencing vaccine-related G≥ 3 AEs during the treatment will be inferred using the two-sided Clopper-Pearson, or a more appropriate one, with a 95% confidence interval. Descriptive statistics are used to assess the extent of the secondary endpoints.
The preplanned interim analysis for the safety and efficacy of the first step has been concluded.
3. Results
In December 2022, nine patients were evaluated for safety and efficacy, allowing the pre-planned analysis to be performed. All the patients (five males and four females) with a median age of 58 years (range: 58-69) underwent radical surgery and leukapheresis before starting radiochemotherapy. One week after completing radiochemotherapy (RTC), they began the vaccine induction phase. The most represented tumor sites were frontal lobe and temporal lobe, five patients had MGMT methylation >5% (
Table 1) and 7 patients were IDH1 (IHC) wild type. Five patients had a positive DTH test against KLH (2 G1, 2 G2, and 1 G3) Of nine enrolled patients, all were evaluable for analysis of AE (all patients had at least 30 days of observation after first vaccine administration). The median vaccine cycles was 6 (range: 3-12).
Table 2 summarizes the targeted AEs reported by AE type and maximum grade: the maximum grade consolidates the reports of a given type of AE for a patient over time by taking the maximum across time. All of the G3-4 toxicities observed were related to temozolomide and in line with the drug’s expected ones Two patients progressed within three months after leukapheresis. (
Table 2). At the cut-off date, all patients were alive except one and were being treated with temozolomide. However, the patients that progressed, continued temozolomide out of the study and none had to start a second line of treatment. At the start of the induction phase, the median amount of dexamethasone consumed by patients was 2 milligrams, which gradually reduced during the four weeks of treatment (compatible with the patient’s symptoms).
Table 2.
Treatment related Adverse Events.
Table 2.
Treatment related Adverse Events.
| AE |
N° of patients (%) |
| G1 |
G2 |
G3 |
G4 |
| Asthenia |
1 |
0 |
0 |
0 |
| Fatigue |
1 |
0 |
0 |
0 |
| Local reaction at vaccine |
3 |
0 |
0 |
0 |
| Nausea# |
1 |
1 |
0 |
0 |
| Neutropenia# |
1 |
0 |
0 |
1 |
| Pain, specify |
1 |
0 |
0 |
0 |
| Pruritus, spec if gen |
3 |
1 |
0 |
0 |
| Redness in site of injection |
1 |
0 |
0 |
0 |
| Skin, specify |
1 |
0 |
0 |
0 |
| Thrombocytopenia# |
0 |
0 |
1 |
0 |
| Constipation |
1 |
0 |
0 |
0 |
| Hypokalemia |
0 |
1 |
0 |
0 |
As of June 2023, the median follow-up after surgery was 13.8 months (range: 13.3-24.7 months) (
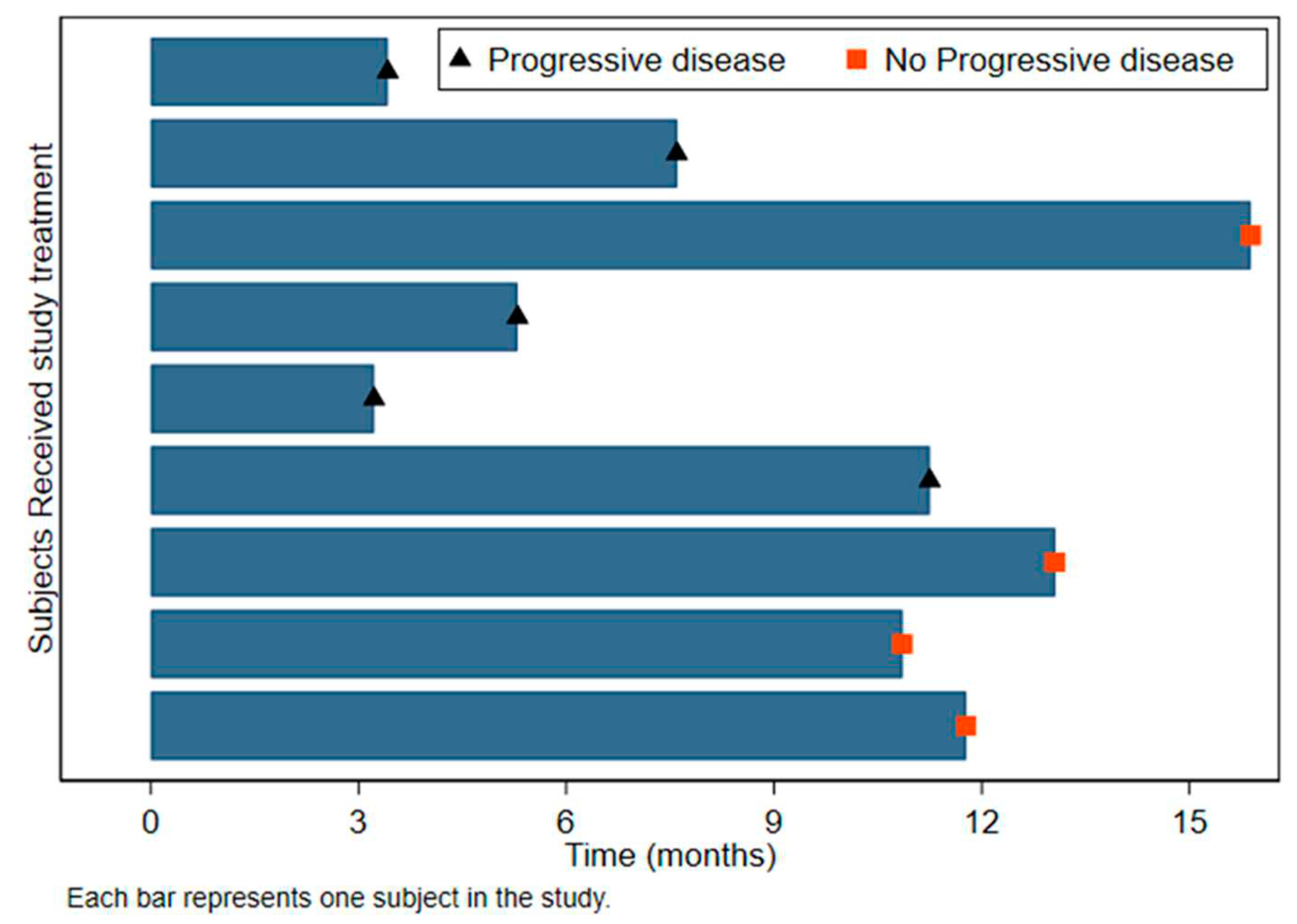
Table 3). The median PFS from leukapheresis was 11.3 months (95%CI: 3.2-Not estimable) and 12.2 months from surgery (95%CI: 4.7-Not estimable)(
Figure 2) The median OS from leukapheresis was 23.1 months and 24.7 from surgery (95%:13.0-NE) (
Table 4).
Table 3.
Median Follow-up.
Table 3.
Median Follow-up.
| |
N° patients |
Median fw (min-max) |
| From surgery |
9 |
13.8 (13.3-24.7) |
| From leukapheresis |
9 |
12.9 (12.2-23.2) |
Figure 2.
Swimmer plot detailing progression survival (PFS).
Figure 2.
Swimmer plot detailing progression survival (PFS).
Table 4.
Clinical Outcomes.
Table 4.
Clinical Outcomes.
| |
|
N° patients |
N° events |
Median Value (95%CI) |
| Progression-free survival |
|
|
|
|
| |
From surgery |
9 |
5 |
12.2 (4.7-NE) |
| |
From leukapheresis |
9 |
5 |
11.3 (3.2-NE) |
| |
|
|
|
|
| Overall survival |
|
|
|
|
| |
From surgery |
9 |
2 |
24.7 (13.0-NE) |
| |
From leukapheresis |
9 |
2 |
23.1 (11.9-NE) |
4. Discussion
GBM has a poor prognosis with a median survival of approximately 12-14 months and a less than 5% five-year survival, even when the patients received the Stupp regimen treatment post-surgery. In January 2023, Liau et al. published a phase III trial regarding GBM patients. They compared OS between the Stupp regimen and treatment with the DC vaccine. Three hundred thirty-one patients were enrolled. The trial outcome showed evidence of a statistically significant longer OS for patients receiving the combination therapy. In addition to their primary endpoint of OS, they conducted an exploratory analysis on biomarkers and immunogenicity that may correlate with OS and responses to DC vaccine [
13]. Similar results were reported by the Lepski G. et al. study, which investigated the difference between a combination treatment and an allogeneic DC vaccine after a standard radiochemotherapy regimen. In recurrent patients, the vaccinations lead to an OS of 27.6 months [
14]. These recent trials and our first-step results emphasize the potential impact of immunotherapy, particularly DC cell-based approaches concerning patients with a poor prognosis. Our previous data (on melanoma patients) demonstrated that temozolomide could selectively reduce the circulating Foxp3+ T lymphocytes, and the vaccine may favor objective responses to subsequent therapies [
10,
15]. Based on the data, we propose adding the vaccine to standard therapies, believing it can increase the synergistic effect. A positive impact on patient outcomes is suggested because only one patient had to proceed to second-line therapy. The remaining PD patients continued the temodal therapy as a clinical practice due to its clinical benefit and the patient’s only slight disease progression. The advantage of using autologous antigens and a tumor homogenate instead of allogeneic antigens could be to target the antigen's repertoire while addressing the extreme heterogeneity of glioblastoma. Our vaccine's favorable toxicity profile and action mechanism make it an ideal candidate for combination therapies.
5. Conclusions
When used with standard radiochemotherapy treatments, the autologous dendritic cell vaccines are safe and have met the intended objectives, allowing enrollment to continue.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Vaccine Investigator Brochure, Vaccine IMPD, Study Protocol.
Author Contributions
LR: LG conceived the idea for the study; LR, LG, NRi, FDR, MG, VF, NRa, SC, EA, PC, AG, AP, and GP were responsible for patients' treatment and follow-up under GCP guidelines; MDA and IB were responsible for data collection: FF performed the statistical analysis; JB and MT were responsible for translational research and immunomonitoring; MP, AMG, and EP were responsible for Dendritic cell GMP manufacturing, GM and LT performed the surgery and provided the surgical specimens under GMP rules; DB performed histological analysis. All the authors read and approved the manuscript for submission.
Funding
The work was partially supported by a contribution from Ricerca Corrente by the Italian Ministry of Health within research line two.
Ethical approval
The study was reviewed and approved by IRCCS IRST and the Area Vasta Romagna Ethics Committee (17/02/2022– Amendment 1.0 Eudract Number 2020-003755-15). The study was conducted following the 1964 Helsinki Declaration and its later amendments and following the Good Clinical Practice (GCP) guidelines.
Acknowledgments
This work was partially supported thanks to the contribution from Ricerca Corrente by the Italian Ministry of Health within the research line “Terapie innovative, trials di phase I-III e di strategia terapeutica basati su modelli preclinici, meccanismi onco-immunologici, nanovettori.”.
Conflicts of Interest
The authors declare no competing interests.
References
- Stupp, R.; Mason,W.P.; van den Bent, M.J.;Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [CrossRef]
- Kurz SC1, Wen PY2. Quo Vadis-Do Immunotherapies Have a Role in Glioblastoma? Curr Treat Options Neurol. 2018 Apr 18;20(5):14. [CrossRef]
- Rodríguez Pérez Á, Campillo-Davo D, Van Tendeloo VFI, Benítez-Ribas D. Cellular immunotherapy: a clinical state-of-the-art of a new paradigm for cancer treatment. Clin Transl Oncol. 2020 Apr 7. [CrossRef]
- Polyzoidis S, Tuazon J, Brazil L, Beaney R, Al-Sarraj ST, Doey L, Logan J, Hurwitz V, Jarosz J, Bhangoo R, Gullan R, Mijovic A, Richardson M, Farzaneh F, Ashkan K. Active dendritic cell immunotherapy for glioblastoma: Current status and challenges. Br J Neurosurg. 2015 Apr;29(2):197-205. [CrossRef]
- Eagles ME1, Nassiri F2,3, Badhiwala JH et al.Dendritic cell vaccines for high-grade gliomas.Ther Clin Risk Manag. 2018 Jul 26;14:1299-1313.
- Cao JX, Zhang XY, Liu JL, Li D, Li JL, et al. Clinical efficacy of tumor antigen-pulsed DC treatment for high-grade glioma patients: evidence from a metaanalysis. PLoS One. 2014;9:e107173. [CrossRef]
- Wang X, Zhao HY, Zhang FC, et al.Dendritic cell-based vaccine for the treatment of malignant glioma: a systematic review.Cancer Invest. 2014 Nov;32(9):451-7. [CrossRef]
- Liau LM, Ashkan K, Tran DD, Campian JL et al First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018 May 29;16(1):142. [CrossRef]
- de Rosa F, Ridolfi L, Fiammenghi L,et al. Dendritic cell vaccination for metastatic melanoma: a 14-year monoinstitutional experience. Melanoma Res. 2017 Aug;27(4):351-357. [CrossRef]
- Ridolfi L, Petrini M, Granato AM, et al .Low-dose temozolomide before dendritic-cell vaccination reduces (specifically) CD4+CD25++Foxp3+ regulatory T-cells in advanced melanoma patients. J Transl Med. 2013 May 31;11:135. [CrossRef]
- Bulgarelli J, Tazzari M, Granato et al. Dendritic Cell Vaccination in Metastatic Melanoma Turns "Non-T Cell Inflamed" Into "T-Cell Inflamed" Tumors. Front Immunol. 2019 Oct 9;10:2353. [CrossRef]
- Benjamin M. Ellingson, Patrick Y. Wen, Timothy F. Cloughesy; “Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials” Neurotherapeutics (2017) 14:307–320. [CrossRef]
- Liau LM, Ashkan K, Brem S, Campian JL, et all. Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination With Extension of Survival Among Patients With Newly Diagnosed and Recurrent Glioblastoma: A Phase 3 Prospective Externally Controlled Cohort Trial. JAMA Oncol. 2023 Jan 1;9(1):112-121. [CrossRef]
- Lepski G, Bergami-Santos PC, Pinho MP, Chauca-Torres NE, et all. Adjuvant Vaccination with Allogenic Dendritic Cells Significantly Prolongs Overall Survival in High-Grade Gliomas: Results of a Phase II Trial. Cancers (Basel). 2023 Feb 15;15(4):1239. [CrossRef]
- Ridolfi L, Petrini M, Fiammenghi L, Granato AM, Ancarani V, Pancisi E, Scarpi E, Guidoboni M, Migliori G, Sanna S, Tauceri F, Verdecchia GM, Riccobon A, Valmorri L, Ridolfi R. Unexpected high response rate to traditional therapy after dendritic cell-based vaccine in advanced melanoma: update of clinical outcome and subgroup analysis. Clin Dev Immunol. 2010: 504979. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).