Submitted:
27 December 2023
Posted:
28 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
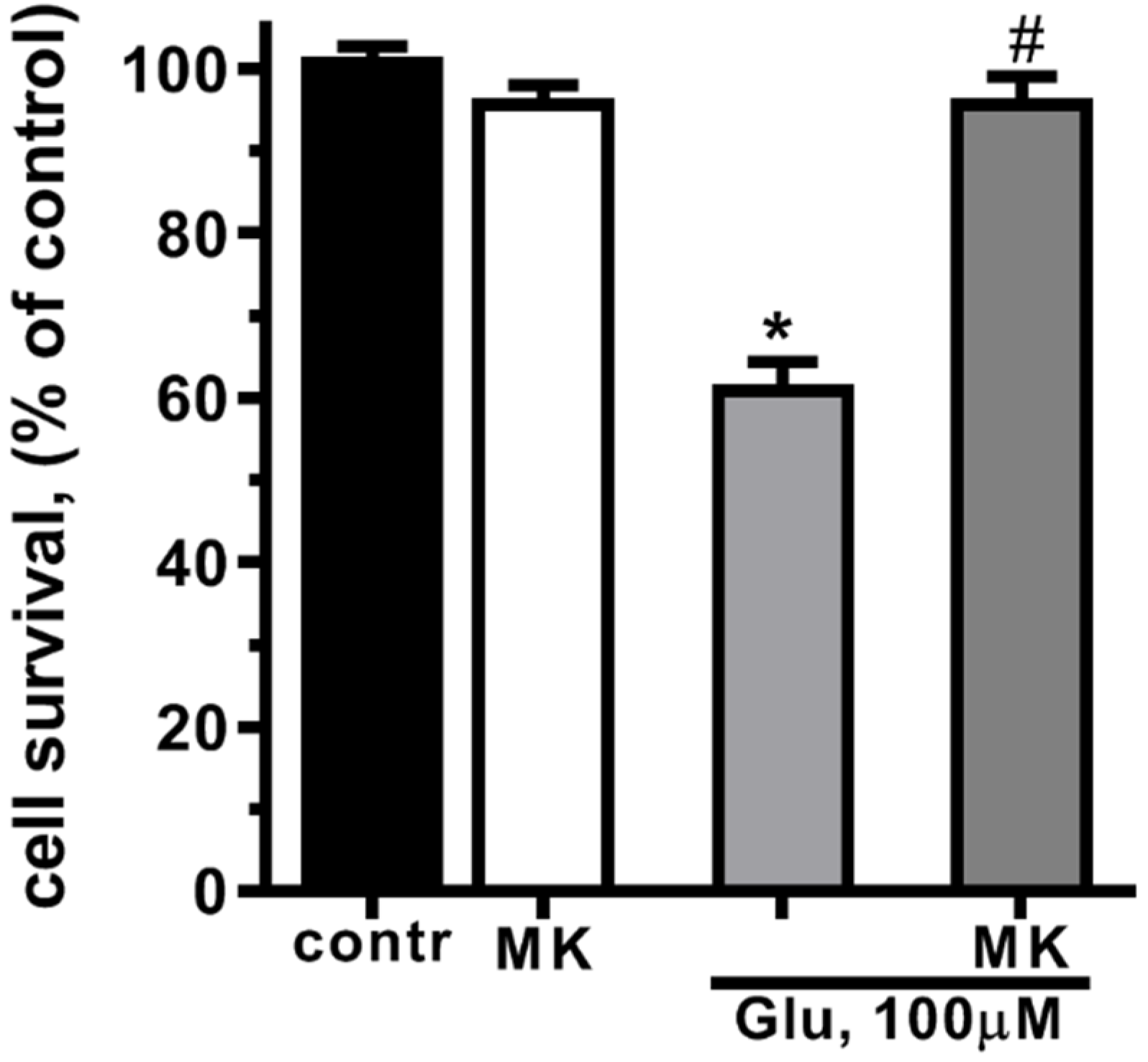
2.1. The toxic effects of glutamate and NMDA on the cultured cortical neurons
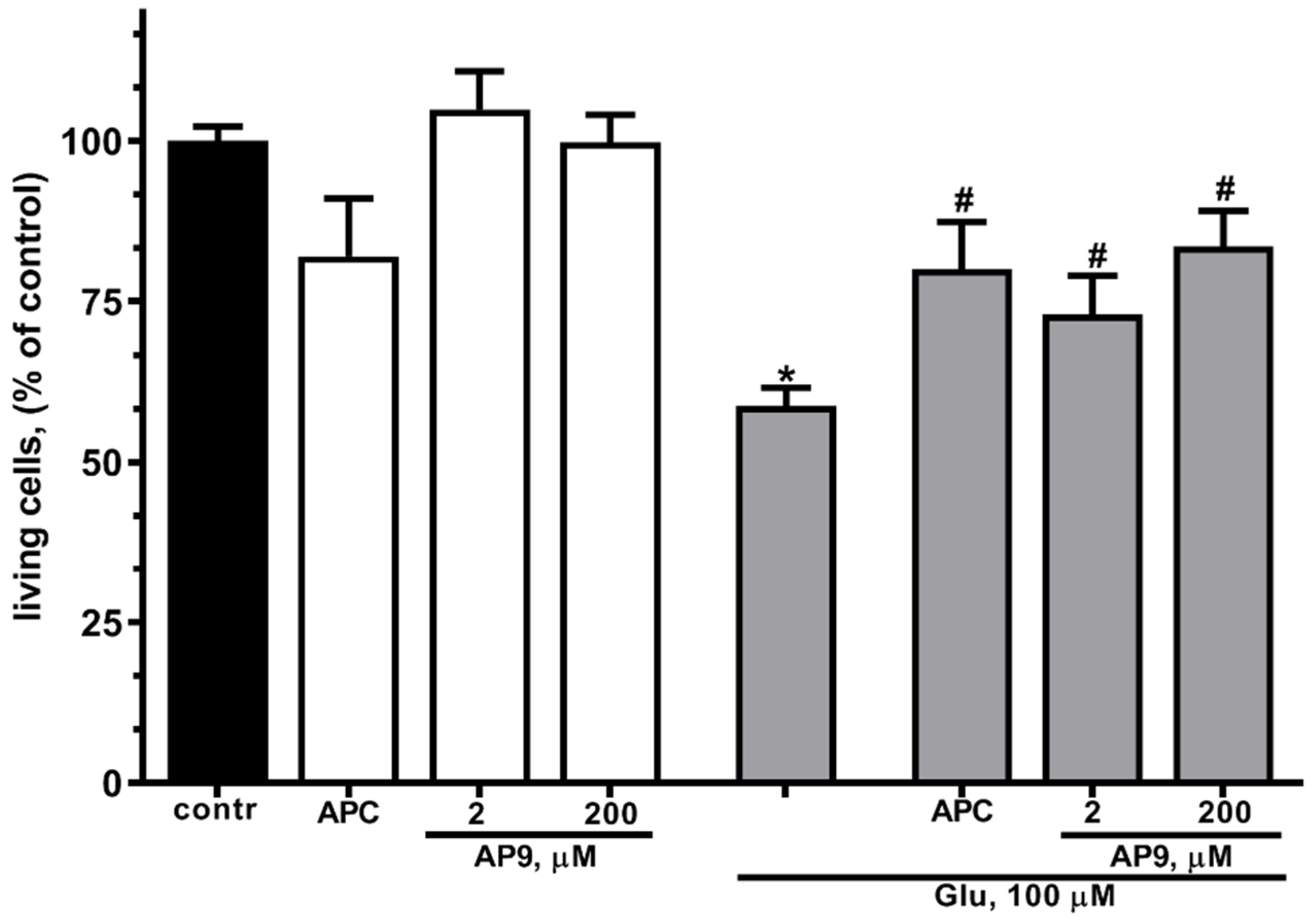
2.2. The effects of APC and AP9 on survival of cultured neurons at glutamate excitotoxicity
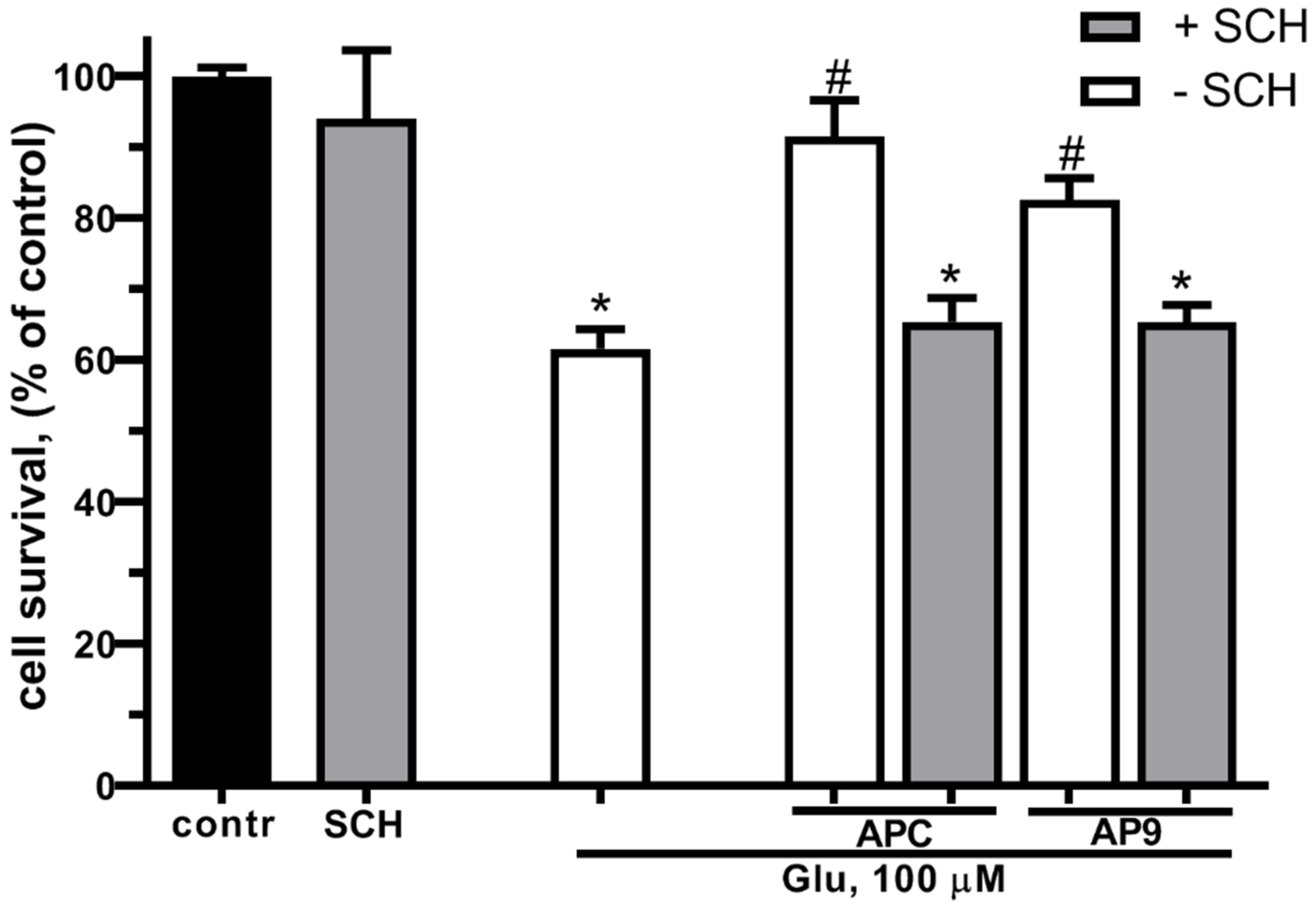
2.3. PAR1 is required for protective effects of AP9 and Glu at glutamate induced excitotoxicity
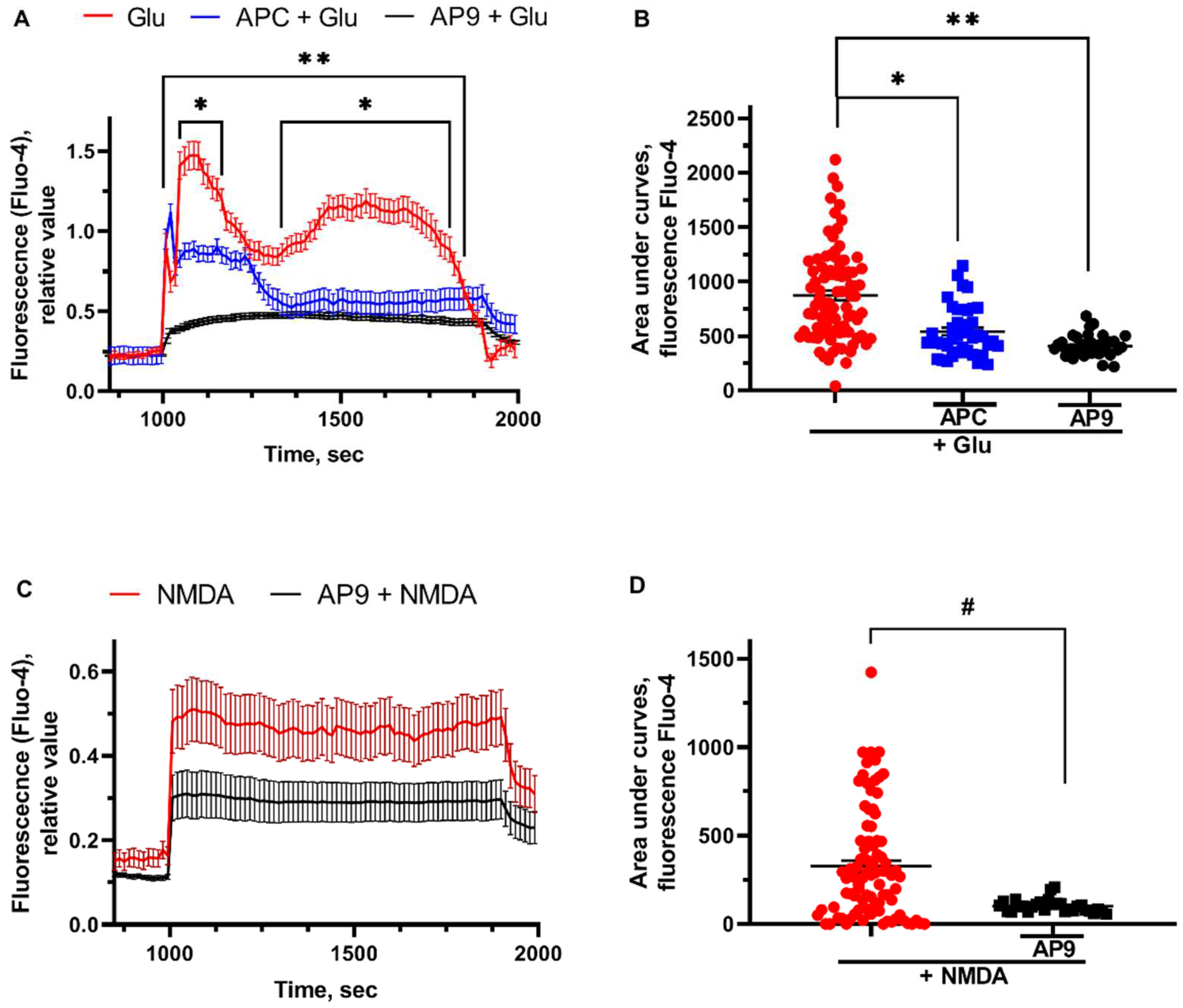
2.4. The effects of APC and AP9 on the intracellular free Ca2+ concentration ([Ca2+]i) dysregulated by Glu and NMDA
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of cell cultures
4.3. Cytotoxicity assays
4.4. Intracellular free Ca2+ ([Ca2+]i ) measurements
4.5. Data processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
References
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity From the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Kaplan-Arabaci, O.; Acari, A.; Ciftci, P.; Gozuacik, D. Glutamate Scavenging as a Neuroreparative Strategy in Ischemic Stroke. Front. Pharmacol. 2022, 13, 866738. [Google Scholar] [CrossRef]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef]
- Nian, K.; Harding, I.C.; Herman, I.M.; Ebong, E.E. Blood-Brain Barrier Damage in Ischemic Stroke and Its Regulation by Endothelial Mechanotransduction. Front. Physiol. 2020, 11, 605398. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qiao, Y.; Li, Z. New Insights into Modes of GPCR Activation. Trends in Pharmacological Sciences 2018, 39, 367–386. [Google Scholar] [CrossRef]
- Flock, T.; Hauser, A.S.; Lund, N.; Gloriam, D.E.; Balaji, S.; Babu, M.M. Selectivity Determinants of GPCR–G-Protein Binding. Nature 2017, 545, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Miyano, K.; Sudo, Y.; Yokoyama, A.; Hisaoka-Nakashima, K.; Morioka, N.; Takebayashi, M.; Nakata, Y.; Higami, Y.; Uezono, Y. History of the G Protein–Coupled Receptor (GPCR) Assays From Traditional to a State-of-the-Art Biosensor Assay. J Pharmacol Sci 2014, 126, 302–309. [Google Scholar] [CrossRef]
- Bushi, D.; Ben Shimon, M.; Shavit Stein, E.; Chapman, J.; Maggio, N.; Tanne, D. Increased Thrombin Activity Following Reperfusion after Ischemic Stroke Alters Synaptic Transmission in the Hippocampus. Journal of Neurochemistry 2015, 135, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.H.; Fernández, J.A.; Lyden, P.D.; Zlokovic, B.V. Activated Protein C Promotes Neuroprotection: Mechanisms and Translation to the Clinic. Thrombosis Research 2016, 141, S62–S64. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, H.; Hua, Y.; Keep, R.F.; Xi, G. Role of Protease-Activated Receptor-1 in Brain Injury After Experimental Global Cerebral Ischemia. Stroke 2012, 43, 2476–2482. [Google Scholar] [CrossRef]
- Shavit-Stein, E.; Mindel, E.; Gofrit, S.G.; Chapman, J.; Maggio, N. Ischemic Stroke in PAR1 KO Mice: Decreased Brain Plasmin and Thrombin Activity along with Decreased Infarct Volume. PLoS ONE 2021, 16, e0248431. [Google Scholar] [CrossRef]
- Mosnier, L.O.; Sinha, R.K.; Burnier, L.; Bouwens, E.A.; Griffin, J.H. Biased Agonism of Protease-Activated Receptor 1 by Activated Protein C Caused by Noncanonical Cleavage at Arg46. Blood 2012, 120, 5237–5246. [Google Scholar] [CrossRef]
- Sinha, R.K.; Wang, Y.; Zhao, Z.; Xu, X.; Burnier, L.; Gupta, N.; Fernández, J.A.; Martin, G.; Kupriyanov, S.; Mosnier, L.O.; et al. PAR1 Biased Signaling Is Required for Activated Protein C in Vivo Benefits in Sepsis and Stroke. Blood 2018, 131, 1163–1171. [Google Scholar] [CrossRef]
- Künze, G.; Isermann, B. Targeting Biased Signaling by PAR1: Function and Molecular Mechanism of Parmodulins. Blood 2023, blood.2023019775. [CrossRef]
- Coughlin, S.R. Protease-activated Receptors in Hemostasis, Thrombosis and Vascular Biology. Journal of Thrombosis and Haemostasis 2005, 3, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.R. Thrombin Signalling and Protease-Activated Receptors. Nature 2000, 407, 258–264. [Google Scholar] [CrossRef]
- Schuepbach, R.A.; Madon, J.; Ender, M.; Galli, P.; Riewald, M. Protease-activated Receptor-1 Cleaved at R46 Mediates Cytoprotective Effects. Journal of Thrombosis and Haemostasis 2012, 10, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Mosnier, L.O.; Zlokovic, B.V.; Griffin, J.H. The Cytoprotective Protein C Pathway. Blood 2007, 109, 3161–3172. [Google Scholar] [CrossRef] [PubMed]
- Riewald, M.; Ruf, W. Protease-Activated Receptor-1 Signaling by Activated Protein C in Cytokine-Perturbed Endothelial Cells Is Distinct from Thrombin Signaling. Journal of Biological Chemistry 2005, 280, 19808–19814. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V.; Griffin, J.H. Cytoprotective Protein C Pathways and Implications for Stroke and Neurological Disorders. Trends in Neurosciences 2011, 34, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Gorbacheva, L.R.; Storozhevykh, T.P.; Pinelis, V.G.; Davydova, O.N.; Ishiwata, S.; Strukova, S.M. Activated Protein C via PAR1 Receptor Regulates Survival of Neurons under Conditions of Glutamate Excitotoxicity. Biochemistry Moscow 2008, 73, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, D.; Gelbard, H.; Cheng, T.; Insalaco, R.; Griffin, J.H.; Zlokovic, B.V. Activated Protein C Prevents Neuronal Apoptosis via Protease Activated Receptors 1 and 3. Neuron 2004, 41, 563–572. [Google Scholar] [CrossRef]
- Soh, U.J.K.; Trejo, J. Activated Protein C Promotes Protease-Activated Receptor-1 Cytoprotective Signaling through β-Arrestin and Dishevelled-2 Scaffolds. Proc. Natl. Acad. Sci. U.S.A. 2011, 108. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ubl, J.J.; Stricker, R.; Reiser, G. Thrombin (PAR-1)-Induced Proliferation in Astrocytes via MAPK Involves Multiple Signaling Pathways. American Journal of Physiology-Cell Physiology 2002, 283, C1351–C1364. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.H.; Zlokovic, B.V.; Mosnier, L.O. Activated Protein C, Protease Activated Receptor 1, and Neuroprotection. Blood 2018, 132, 159–169. [Google Scholar] [CrossRef]
- Barsi-Rhyne, B.; Manglik, A.; Von Zastrow, M. Discrete GPCR-Triggered Endocytic Modes Enable β-Arrestins to Flexibly Regulate Cell Signaling. eLife 2022, 11, e81563. [Google Scholar] [CrossRef]
- Willis Fox, O.; Preston, R.J.S. Molecular Basis of Protease-activated Receptor 1 Signaling Diversity. Journal of Thrombosis and Haemostasis 2020, 18, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.V.; Ardeshirylajimi, A.; Dinarvand, P.; Yang, L.; Rezaie, A.R. Occupancy of Human EPCR by Protein C Induces β-Arrestin-2 Biased PAR1 Signaling by Both APC and Thrombin. Blood 2016, 128, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Hazell, A. Excitotoxic Mechanisms in Stroke: An Update of Concepts and Treatment Strategies. Neurochemistry International 2007, 50, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Bano, D.; Nicotera, P. Ca2+ Signals and Neuronal Death in Brain Ischemia. Stroke 2007, 38, 674–676. [Google Scholar] [CrossRef]
- Berliocchi, L.; Bano, D.; Nicotera, P. Ca2+ Signals and Death Programmes in Neurons. Phil. Trans. R. Soc. B 2005, 360, 2255–2258. [Google Scholar] [CrossRef]
- Brittain, M.K.; Brustovetsky, T.; Sheets, P.L.; Brittain, J.M.; Khanna, R.; Cummins, T.R.; Brustovetsky, N. Delayed Calcium Dysregulation in Neurons Requires Both the NMDA Receptor and the Reverse Na+/Ca2+ Exchanger. Neurobiology of Disease 2012, 46, 109–117. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Duchen, M.R. Mechanisms Underlying the Loss of Mitochondrial Membrane Potential in Glutamate Excitotoxicity. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2008, 1777, 953–964. [Google Scholar] [CrossRef]
- Surin, A.M.; Gorbacheva, L.R.; Savinkova, I.G.; Sharipov, R.R.; Khodorov, B.I.; Pinelis, V.G. Study on ATP Concentration Changes in Cytosol of Individual Cultured Neurons during Glutamate-Induced Deregulation of Calcium Homeostasis. Biochemistry Moscow 2014, 79, 146–157. [Google Scholar] [CrossRef]
- Lipton, S.A. Paradigm Shift in Neuroprotection by NMDA Receptor Blockade: Memantine and Beyond. Nat Rev Drug Discov 2006, 5, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Chinopoulos, C.; Adam-Vizi, V. Calcium, Mitochondria and Oxidative Stress in Neuronal Pathology: Novel Aspects of an Enduring Theme. The FEBS Journal 2006, 273, 433–450. [Google Scholar] [CrossRef]
- Li, V.; Wang, Y. Molecular Mechanisms of NMDA Receptor-Mediated Excitotoxicity: Implications for Neuroprotective Therapeutics for Stroke. Neural Regen Res 2016, 11, 1752. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Fernandes, J.; Burgeiro, A.; Thomas, G.M.; Huganir, R.L.; Duarte, C.B.; Carvalho, A.L.; Santos, A.E. Excitotoxicity through Ca2+-Permeable AMPA Receptors Requires Ca2+-Dependent JNK Activation. Neurobiology of Disease 2010, 40, 645–655. [Google Scholar] [CrossRef]
- Hollenberg, M.D. Physiology and Pathophysiology of Proteinase-Activated Receptors (PARs): Proteinases as Hormone-Like Signal Messengers: PARs and More. J Pharmacol Sci 2005, 97, 8–13. [Google Scholar] [CrossRef]
- Suo, Z.; Citron, B.A.; Festoff, B.W. Thrombin: A Potential Proinflammatory Mediator in Neurotrauma and Neurodegenerative Disorders. Current Drug Targets - Inflammation & Allergy 2004, 3, 105–114. [Google Scholar] [CrossRef]
- Ruf, W. Is APC Activation of Endothelial Cell PAR1 Important in Severe Sepsis? : Yes. Journal of Thrombosis and Haemostasis 2005, 3, 1912–1914. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Reiser, G.; Keep, R.F. The Role of Thrombin and Thrombin Receptors in Ischemic, Hemorrhagic and Traumatic Brain Injury: Deleterious or Protective? Journal of Neurochemistry 2003, 84, 3–9. [Google Scholar] [CrossRef]
- Rohatgi, T.; Sedehizade, F.; Reymann, K.G.; Reiser, G. Protease-Activated Receptors in Neuronal Development, Neurodegeneration, and Neuroprotection: Thrombin as Signaling Molecule in the Brain. Neuroscientist 2004, 10, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, F.; Vergnolle, N.; McArthur, J.C.; Silva, C.; Vodjgani, M.; Andrade-Gordon, P.; Hollenberg, M.D.; Power, C. Proteinase-Activated Receptor-2 Induction by Neuroinflammation Prevents Neuronal Death during HIV Infection1. The Journal of Immunology 2005, 174, 7320–7329. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.E.; Lyuboslavsky, P.; Traynelis, S.F.; McKeon, R.J. PAR-1 Deficiency Protects against Neuronal Damage and Neurologic Deficits after Unilateral Cerebral Hypoxia/Ischemia. J Cereb Blood Flow Metab 2004, 24, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.-Q.; Lim, C.S.; Hwang, J.K.; Ha, I.; Han, J.-S. Anti-Oxidant and Anti-Inflammatory Activities of Macelignan in Murine Hippocampal Cell Line and Primary Culture of Rat Microglial Cells. Biochemical and Biophysical Research Communications 2005, 331, 1264–1269. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Budd, S.L. Mitochondria and Neuronal Survival. Physiological Reviews 2000, 80, 315–360. [Google Scholar] [CrossRef]
- Khodorov, B. Glutamate-Induced Deregulation of Calcium Homeostasis and Mitochondrial Dysfunction in Mammalian Central Neurones. Progress in Biophysics and Molecular Biology 2004, 86, 279–351. [Google Scholar] [CrossRef]
- Gupta, K.; Hardingham, G.E.; Chandran, S. NMDA Receptor-Dependent Glutamate Excitotoxicity in Human Embryonic Stem Cell-Derived Neurons. Neuroscience Letters 2013, 543, 95–100. [Google Scholar] [CrossRef]
- Simões, A.P.; Silva, C.G.; Marques, J.M.; Pochmann, D.; Porciúncula, L.O.; Ferreira, S.; Oses, J.P.; Beleza, R.O.; Real, J.I.; Köfalvi, A.; et al. Glutamate-Induced and NMDA Receptor-Mediated Neurodegeneration Entails P2Y1 Receptor Activation. Cell Death Dis 2018, 9, 297. [Google Scholar] [CrossRef]
- Savinkova, I.G.; Gorbacheva, L.R.; Bespalova, Z.D.; Pinelis, V.G.; Strukova, S.M. Peptides Analogous to Tethered Ligands Liberated by Activated Protein C Exert Neuroprotective Effects in Glutamate-Induced Excitotoxicity. Biochem. Moscow Suppl. Ser. A 2014, 8, 116–120. [Google Scholar] [CrossRef]
- Gorbacheva, L.; Pinelis, V.; Ishiwata, S.; Strukova, S.; Reiser, G. Activated Protein C Prevents Glutamate- and Thrombin-Induced Activation of Nuclear Factor-κB in Cultured Hippocampal Neurons. Neuroscience 2010, 165, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.H.; Zlokovic, B.V.; Mosnier, L.O. Activated Protein C: Biased for Translation. Blood 2015, 125, 2898–2907. [Google Scholar] [CrossRef] [PubMed]
- Pendurthi, U.R.; Rao, L.V.M. Endothelial Cell Protein C Receptor-Dependent Signaling. Current Opinion in Hematology 2018, 25, 219–226. [Google Scholar] [CrossRef]
- Randall, R.; Thayer, S. Glutamate-Induced Calcium Transient Triggers Delayed Calcium Overload and Neurotoxicity in Rat Hippocampal Neurons. J. Neurosci. 1992, 12, 1882–1895. [Google Scholar] [CrossRef] [PubMed]
- Canto, I. ; J. K. Soh, U.; Trejo, J. Allosteric Modulation of Protease-Activated Receptor Signaling. MRMC 2012, 12, 804–811. [Google Scholar] [CrossRef]
- Bae, J.-S.; Yang, L.; Rezaie, A.R. Lipid Raft Localization Regulates the Cleavage Specificity of Protease Activated Receptor 1 in Endothelial Cells. Journal of Thrombosis and Haemostasis 2008, 6, 954–961. [Google Scholar] [CrossRef]
- Blandini, F.; Porter, R.H.P.; Greenamyre, J.T. Glutamate and Parkinson’s Disease. Molecular Neurobiology 1996, 12, 73–94. [Google Scholar] [CrossRef]
- Beal, M.F. Oxidative Damage in Neurodegenerative Diseases. Neuroscientist 1997, 3, 21–27. [Google Scholar] [CrossRef]
- Krieger, C.; Duchen, M.R. Mitochondria, Ca2+ and Neurodegenerative Disease. European Journal of Pharmacology 2002, 447, 177–188. [Google Scholar] [CrossRef]
- Burnier, L.; Mosnier, L.O. Novel Mechanisms for Activated Protein C Cytoprotective Activities Involving Noncanonical Activation of Protease-Activated Receptor 3. Blood 2013, 122, 807–816. [Google Scholar] [CrossRef]
- Galkov, M.; Kiseleva, E.; Gulyaev, M.; Sidorova, M.; Gorbacheva, L. New PAR1 Agonist Peptide Demonstrates Protective Action in a Mouse Model of Photothrombosis-Induced Brain Ischemia. Front. Neurosci. 2020, 14, 335. [Google Scholar] [CrossRef]
- Konradi, C.; Heckers, S. Molecular Aspects of Glutamate Dysregulation: Implications for Schizophrenia and Its Treatment. Pharmacology & Therapeutics 2003, 97, 153–179. [Google Scholar] [CrossRef]
- Gingrich, M.B.; Junge, C.E.; Lyuboslavsky, P.; Traynelis, S.F. Potentiation of NMDA Receptor Function by the Serine Protease Thrombin. J. Neurosci. 2000, 20, 4582–4595. [Google Scholar] [CrossRef] [PubMed]
- National Research Council; Division on Earth and Life Studies; Institute for Laboratory Animal Research; Committee for the Update of the Guide for the Care and Use of Laboratory Animals Guide for the Care and Use of Laboratory Animals: Eighth Edition; National Academies Press, 2010; ISBN 0-309-18663-3.
- Krasil’nikova, I.; Surin, A.; Sorokina, E.; Fisenko, A.; Boyarkin, D.; Balyasin, M.; Demchenko, A.; Pomytkin, I.; Pinelis, V. Insulin Protects Cortical Neurons Against Glutamate Excitotoxicity. Front. Neurosci. 2019, 13, 1027. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. Journal of Immunological Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid Colorimetric Assay for Cell Growth and Survival. Journal of Immunological Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




