Submitted:
27 December 2023
Posted:
29 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
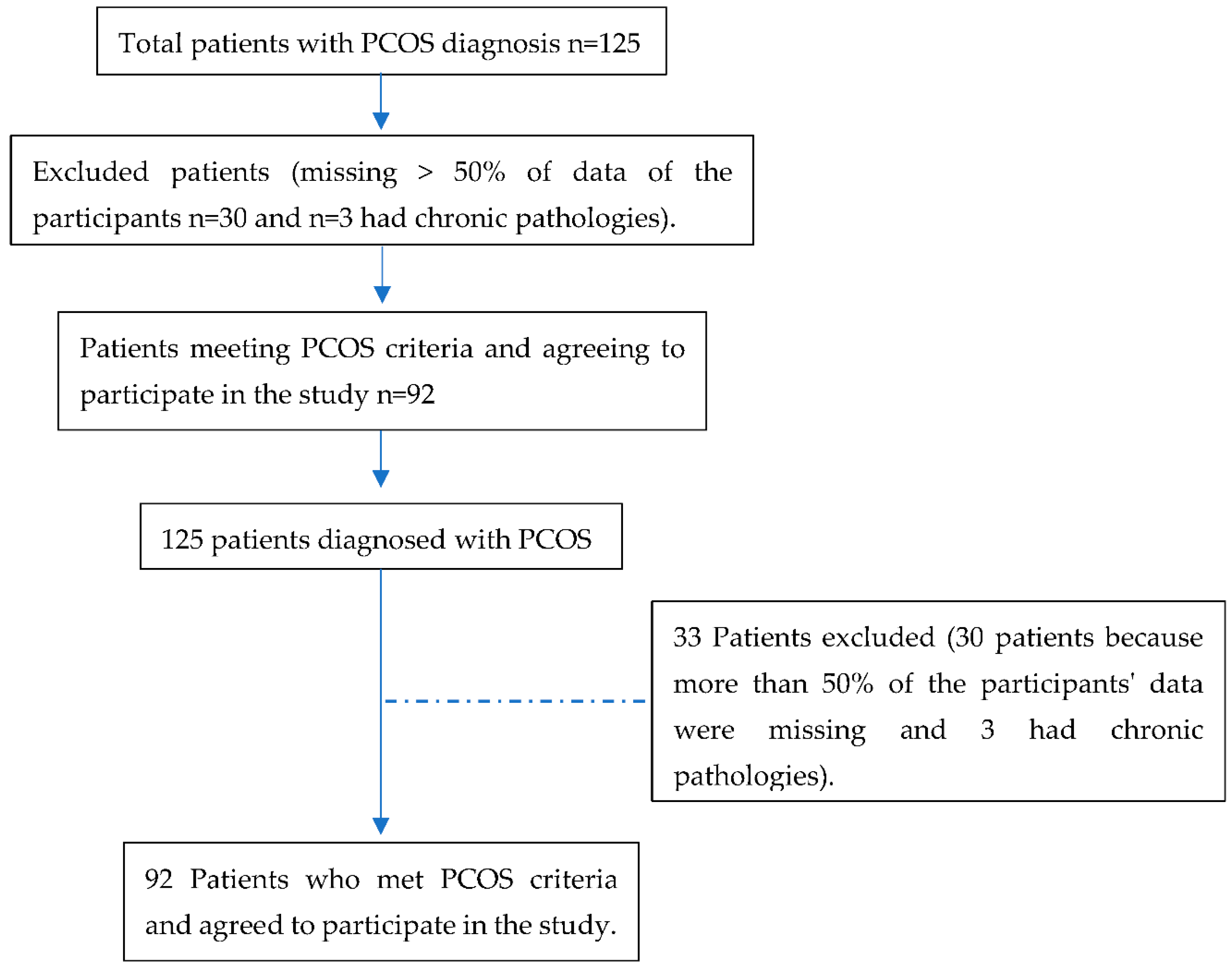
2.2. Data Collection and Participants
2.3. Inclusion and Exclusion Criteria
- 1.
- Oligo/amenorrhea (menstrual cycle disturbances between less than 21 days and more than 35 to 90 days);
- 2.
- Clinical (with the presence of hirsutism, acne, androgenic alopecia) or biochemical hyperandrogenism (with elevated levels of total testosterone, SHBG (sex hormone-binding globulin), and free androgen index;
- 3.
- Ultrasound with polycystic ovarian morphology (12 or more follicles of 2 to 9 mm, with volume >10cc).
2.4. Variables
2.5. Statistical Analysis
2.6. Ethical Aspects
3. Results
3.1. Sociodemographic Characteristics of the Participants
3.2. Clinical Features Associated with the Different PCOS Phenotypes
3.3. Metabolic Profile of the Participants
3.4. Reproductive Profile of the Participants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Charifson, M.A.; Trumble, B.C. Evolutionary Origins of Polycystic Ovary Syndrome: An Environmental Mismatch Disorder. Evol. Med. Public Heal. 2019, 2019, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Robin, G.; Peigne, M.; Dumont, A.; Plouvier, P.; Rolland, A.-L.; Catteau-Jonard, S.; Dewailly, D. Síndrome de Ovarios Poliquísticos. EMC - Ginecol. 2020, 56, 1–18. [Google Scholar] [CrossRef]
- Ortiz-Flores, A.E.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Polycystic Ovary Syndrome in Adult Women. Med. Clin. (Barc). 2019, 152, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Kintziger, K.; Li, R.; Laven, J.; Morin-Papunen, L.; Merkin, S.S.; Teede, H.; Yildiz, B.O. Recommendations for Epidemiologic and Phenotypic Research in Polycystic Ovary Syndrome: An Androgen Excess and PCOS Society Resource. Hum. Reprod. 2019, 34, 2254–2265. [Google Scholar] [CrossRef] [PubMed]
- Dietz de Loos, A.L.P.; Jiskoot, G.; Timman, R.; Beerthuizen, A.; Busschbach, J.J.V.; Laven, J.S.E. Improvements in PCOS Characteristics and Phenotype Severity during a Randomized Controlled Lifestyle Intervention. Reprod. Biomed. Online 2021, 43, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Dapas, M.; Dunaif, A. The Contribution of Rare Genetic Variants to the Pathogenesis of Polycystic Ovary Syndrome. Curr. Opin. Endocr. Metab. Res. 2020, 12, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Vanhauwaert, P.S. Síndrome de Ovario Poliquístico e Infertilidad Polycystic Ovary Syndrome and Infertility. Rev. Clínica Las Condes 2021, 32, 166–172. [Google Scholar] [CrossRef]
- Bajares, D.M.; Pizzi, R. Tratamiento Basado En El Fenotipo. 2016, 76, 93–96. [Google Scholar]
- Rodrigo Carvajal, G.; Cristian Herrera, G.; Arnaldo Porcile, J. Espectro Fenotípico Del Síndrome de Ovario Poliquístico. Rev. Chil. Obstet. Ginecol. 2010, 75, 124–132. [Google Scholar] [CrossRef]
- Goodarzi, M.O.; Carmina, E.; Azziz, R. DHEA, DHEAS and PCOS. J. Steroid Biochem. Mol. Biol. 2015, 145, 213–225. [Google Scholar] [CrossRef]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, Prevalence, and Phenotypes of Polycystic Ovary Syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Pehlivanov, B.; Orbetzova, M. Characteristics of Different Phenotypes of Polycystic Ovary Syndrome in a Bulgarian Population. Gynecol. Endocrinol. 2007, 23, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, L.B.; Ramos, R.B.; Spritzer, P.M. Metabolic Features of Women with Polycystic Ovary Syndrome in Latin America: A Systematic Review. Front. Endocrinol. (Lausanne). 2021, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dapas, M.; Lin, F.T.J.; Nadkarni, G.N.; Sisk, R.; Legro, R.S.; Urbanek, M.; Geoffrey Hayes, M.; Dunaif, A. Distinct Subtypes of Polycystic Ovary Syndrome with Novel Genetic Associations: An Unsupervised, Phenotypic Clustering Analysis. PLoS Med. 2020, 17, 1–28. [Google Scholar] [CrossRef]
- Puttabyatappa, M.; Cardoso, R.C.; Padmanabhan, V. Effect of Maternal PCOS and PCOS-like Phenotype on the Offspring’s Health. Mol. Cell. Endocrinol. 2016, 435, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.; Iturrieta, V.; Aguilera, G.; Cárcamo, G.; Galvez, L.; Valdés, P. Características Clínicas y Metabólicas de Síndrome de Ovario Poliquístico En La Ciudad de Temuco. Rev. chil. endocrinol. diabetes 2014, 7, 85–88. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Instituto Ecuatoriano de Estadísticas y Censos del Ecuador Encuesta de Estratificación Del Nivel Socioeconómico NSE 2011. 2011 2011, 1–4.
- INEC Censo Población y Vivienda 2010. Fasciculo Provincial Loja. Ecuador en cifras 2010, 1, 1–8. [Google Scholar]
- Carmona-Ruiz, I.O.; Saucedo-de la Llata, E.; Moraga-Sánchez, M.R.; Romeu-Sarrió, A. Síndrome de Ovario Poliquístico: ¿Ha Aumentado Su Prevalencia? Ginecol. Obstet. Mex. 2015, 83, 750–759. [Google Scholar]
- Tavares, A.; Rêgo Barros, R.C. The Prevalence of Metabolic Syndrome in the Different Phenotypes of Polycystic Ovarian Syndrome. Rev. Bras. Ginecol. e Obstet. 2019, 41, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ladrón De Guevara, A.; Fux-Otta, C.; Crisosto, N.; Szafryk De Mereshian, P.; Echiburú, B.; Iraci, G.; Perez-Bravo, F.; Sir-Petermann, T. Metabolic Profile of the Different Phenotypes of Polycystic Ovary Syndrome in Two Latin American Populations. Fertil. Steril. 2014, 101. [Google Scholar] [CrossRef]
- Daan, N.M.P.; Louwers, Y.V.; Koster, M.P.H.; Eijkemans, M.J.C.; De Rijke, Y.B.; Lentjes, E.W.G.; Fauser, B.C.J.M.; Laven, J.S.E. Cardiovascular and Metabolic Profiles amongst Different Polycystic Ovary Syndrome Phenotypes: Who Is Really at Risk? Fertil. Steril. 2014, 102, 1444–1451.e3. [Google Scholar] [CrossRef]
- Yang, R.; Li, Q.; Zhou, Z.; Qian, W.; Zhang, J.; Wu, Z.; Jin, L.; Wu, X.; Zhang, C.; Zheng, B.; et al. Changes in the Prevalence of Polycystic Ovary Syndrome in China over the Past Decade. Lancet Reg. Heal. - West. Pacific 2022, 25, 1–10. [Google Scholar] [CrossRef]
- Singh, P.; Barbieri, J.S.; James, W.D.; Thiboutot, D. Letter to the Editor from Singh et Al: “Female Adult Acne and Androgen Excess: A Report from the Multidisciplinary Androgen Excess and PCOS Committee. ” J. Endocr. Soc. 2022, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.Y.; Knochenhauer, E.S.; Bartolucci, A.A.; Azziz, R. Phenotypic Spectrum of Polycystic Ovary Syndrome: Clinical and Biochemical Characterization of the Three Major Clinical Subgroups. Fertil. Steril. 2005, 83, 1717–1723. [Google Scholar] [CrossRef]
- Gabrielli, L.; Aquino, E.M.L. Polycystic Ovary Syndrome in Salvador, Brazil: A Prevalence Study in Primary Healthcare. Reprod. Biol. Endocrinol. 2012, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Goldenberg, N. Characteristics of Obesity in Polycystic Ovary Syndrome: Etiology, Treatment, and Genetics. Metabolism. 2019, 92, 108–120. [Google Scholar] [CrossRef]
- Lim, S.S.; Kakoly, N.S.; Tan, J.W.J.; Fitzgerald, G.; Bahri Khomami, M.; Joham, A.E.; Cooray, S.D.; Misso, M.L.; Norman, R.J.; Harrison, C.L.; et al. Metabolic Syndrome in Polycystic Ovary Syndrome: A Systematic Review, Meta-Analysis and Meta-Regression. Obes. Rev. 2019, 20, 339–352. [Google Scholar] [CrossRef]
- Jamil, A.S.; Alalaf, S.K.; Al-Tawil, N.G.; Al-Shawaf, T. A Case-Control Observational Study of Insulin Resistance and Metabolic Syndrome among the Four Phenotypes of Polycystic Ovary Syndrome Based on Rotterdam Criteria Female Fertility. Reprod. Health 2015, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Tay, C.T.; Laven, J.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; M. Redman, L.; A. Boyle, J.; et al. Recommendations from the 2023 International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil. Steril. 2023, 120, 767–793. [Google Scholar] [CrossRef] [PubMed]
- Panidis, D.; Tziomalos, K.; Misichronis, G.; Papadakis, E.; Betsas, G.; Katsikis, I.; Macut, D. Insulin Resistance and Endocrine Characteristics of the Different Phenotypes of Polycystic Ovary Syndrome: A Prospective Study. Hum. Reprod. 2012, 27, 541–549. [Google Scholar] [CrossRef] [PubMed]
| Biodemographic variables | Totaln (%) |
Phenotypes A y B n(%) |
Phenotypes C y D n(%) |
|---|---|---|---|
| Sample | 92(100) | 69(75.3) | 23(24.7) |
| Age (years ± SD) | 22±3.4 | 22.6±3.8 | 23.6±5.4 |
| Ethnic origin | |||
| Mestiza | 90(97.8) | 67(98.5) | 23(95.8) |
| Origin | |||
| Urban | 88(95.7) | 67(98.5) | 21(87.5) |
| Marital status | |||
| Single | 87(94.6) | 64(94.1) | 23(95.8) |
| Educational level | |||
| Higher | 88(95.7) | 65(95.6) | 23(95.8) |
| Socioeconomic level | |||
| Upper middle | 52(75.4) | 40(76.9) | 12(70.6) |
| Clinical variables | Total (%) | Phenotypes A y B n (%) |
Phenotypes C y D n (%) |
OR | IC 95% |
|---|---|---|---|---|---|
| Oligomenorrhea* | 78(85.7) | 66(97.1) | 12(52.2) | 30.3 | 5.9; 153.9 |
| Hirsutism | 61(78.2) | 45(78.9) | 16(76.2) | 1.2 | 0.4; 3.8 |
| Acne | 74(87.1) | 56(88.9) | 18(81.8) | 1.8 | 0.4; 6.8 |
| Alopecía | 22(25.3) | 14(21.2) | 8(38.1) | 0.4 | 0.2; 1.3 |
| Acanthosis nigricans | 40(48.2) | 31(50.0) | 9(42.9) | 1.3 | 0.5; 3.6 |
| Polycystic ovaries ultrasound volume ≥ 10 cc | 65(86.7) | 44(83.0) | 21(95.5) | 0.2 | 0.1; 1.9 |
| Metabolic variables | Total (%) | Phenotypes A y B n (%) |
Phenotypes C y D n (%) |
OR | IC 95% |
|---|---|---|---|---|---|
| BMI ≥ 25.0 | 29(34.1) | 24(38.1) | 5(22.7) | 2.1 | 0.7; 6.4 |
| Waist to height/ratio ≥0.49 | 45( 60.0) | 36(63.2) | 9(50.0) | 1.7 | 0.6; 4.9 |
| Waist hip/radio ≥ 0.86 | 30(40.5) | 22(40.0) | 8(42.1) | 0.9 | 0.3; 2.6 |
| Total Colesterol ≥ 200 mg/dl | 25(34.2) | 19(33.9) | 6(35.3) | 0.9 | 0.3; 2.9 |
| Triglycerides ≥ 150 mg/dl * | 21(29.2) | 20(36.4) | 1(5.9) | 9.1 | 1.1; 74.2 |
| LDL≥ 160.1mg/dl | 21( 30.0) | 16(29.6) | 5(31.3) | 0.9 | 0.3; 3.1 |
| ALT≥33 U/l | 17(24.3) | 14(25.9) | 3(18.8) | 1.4 | 0.3; 5.6 |
| AST≥ 32 U/l | 11(15.7) | 8(14.8) | 3(18.8) | 0.8 | 0.2; 3.3 |
| Direct Bilirrubin ≥ 0.30 mg/dl | 6(9.7) | 4(8.2) | 2(15.4) | 0.5 | 0.1; 3.0 |
| Uric Acid≥ 5.8 mg/dl | 8(10.3) | 6(10.9) | 2(8.7) | 1.3 | 0.2; 6.7 |
| HOMA-IR ≥ 2.8 | 49(59.0) | 37(59.7) | 12(57.1) | 1.1 | 0.4; 3.0 |
| Insulin≥ 25.1 uUI/ml | 13(15.5) | 12(19.0) | 1(4.8) | 4.7 | 0.6-38.6 |
| Blood pressure>120/80 mm/Hg | 42(50.0) | 32(51.6) | 10(45.5) | 1.3 | 0.5-3.4 |
| Vitamin D | 31(43.7) | 25(43.9) | 6(42.9) | 1.1 | 0.3-3.4 |
| Reproductive variables | Total (%) |
Phenotypes A y B (%) |
Phenotypes C y D (%) |
OR | IC 95% |
|---|---|---|---|---|---|
| DHEAS≥ 430 ug/dl | 6(7.7) | 5(8.3) | 1(5.6) | 1.5 | 0.2; 14.2 |
| Total Testosterona ≥ 0.482 ng/ml | 45(48.9) | 37(54.4) | 8(33.3) | 2.4 | 0.9; 6.3 |
| 17-OH Progesterone ≥ 1.4 ng/ml | 37(51.4) | 32(57.1) | 5(31.3) | 2.9 | 0.9; 9.6 |
| Androstenedione≥ 3.9 ng/ml | 22(29.3) | 17(29.3) | 5(29.4) | 1.0 | 0.3; 3.2 |
| LH ( Folicular phase) ≥11.7 mUI/ml | 30(40.0) | 25(44.6) | 5(26.3) | 2.3 | 0.7; 7.1 |
| SHBG≥114.1 nmol/l | 14(16.3) | 11(17.2) | 3(13.6) | 0.8 | 0.2; 3.0 |
| Free androgen index(FAI) | 11(13.9) | 10(16.7) | 1(5.3) | 3.6 | 0.4; 30.1 |
| AMH> 2.5 (ng/mL) | 51(91.1) | 41(93.2) | 10(83.3) | 3.4 | 0.2; 65.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





