Submitted:
28 December 2023
Posted:
29 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
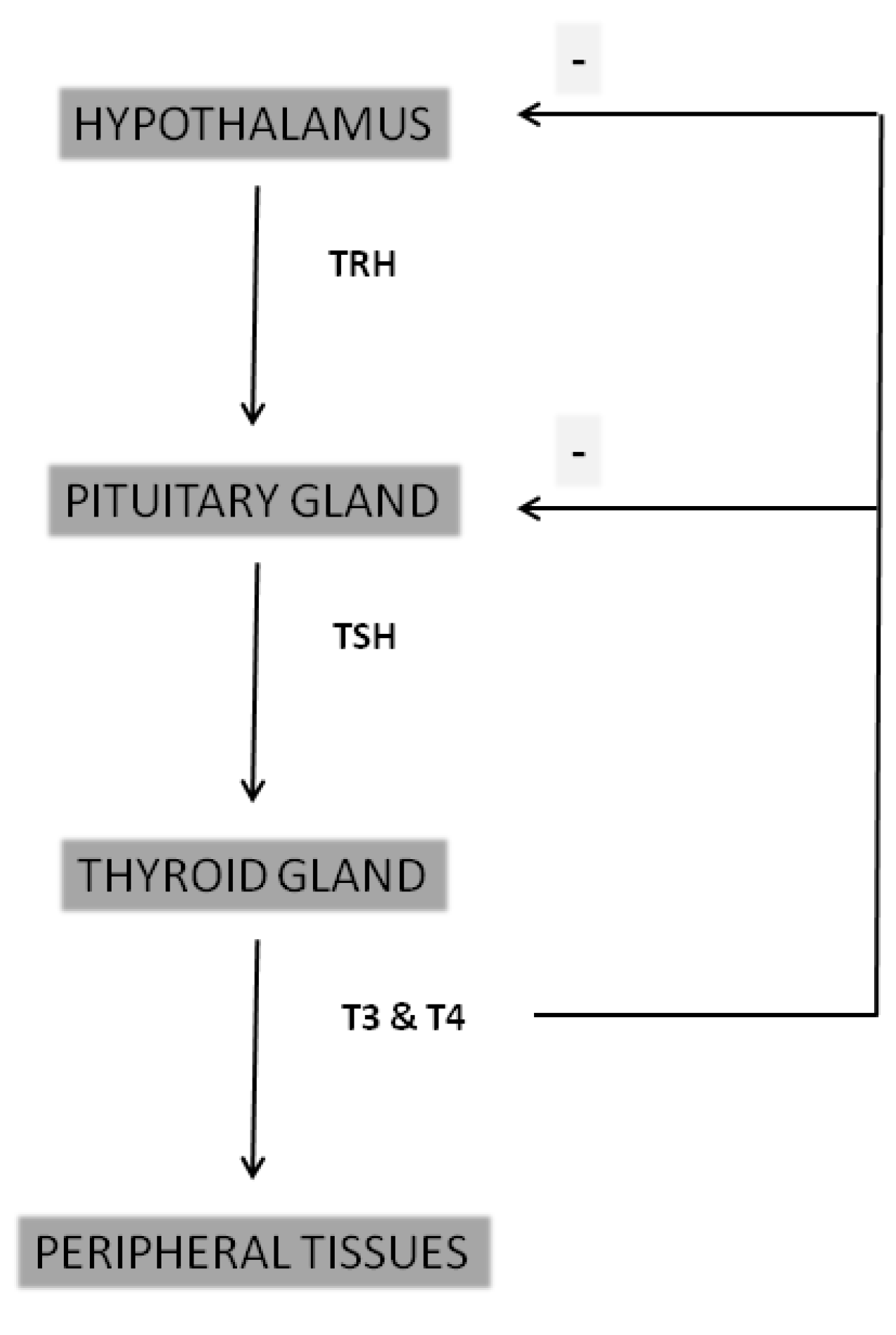
2. Thyroid Hormones Physiology
3. Methodology
4. Effects of Nutrition, Starvation/Overfeeding, and Obesity on Thyroid Function in Euthyroid Subjects
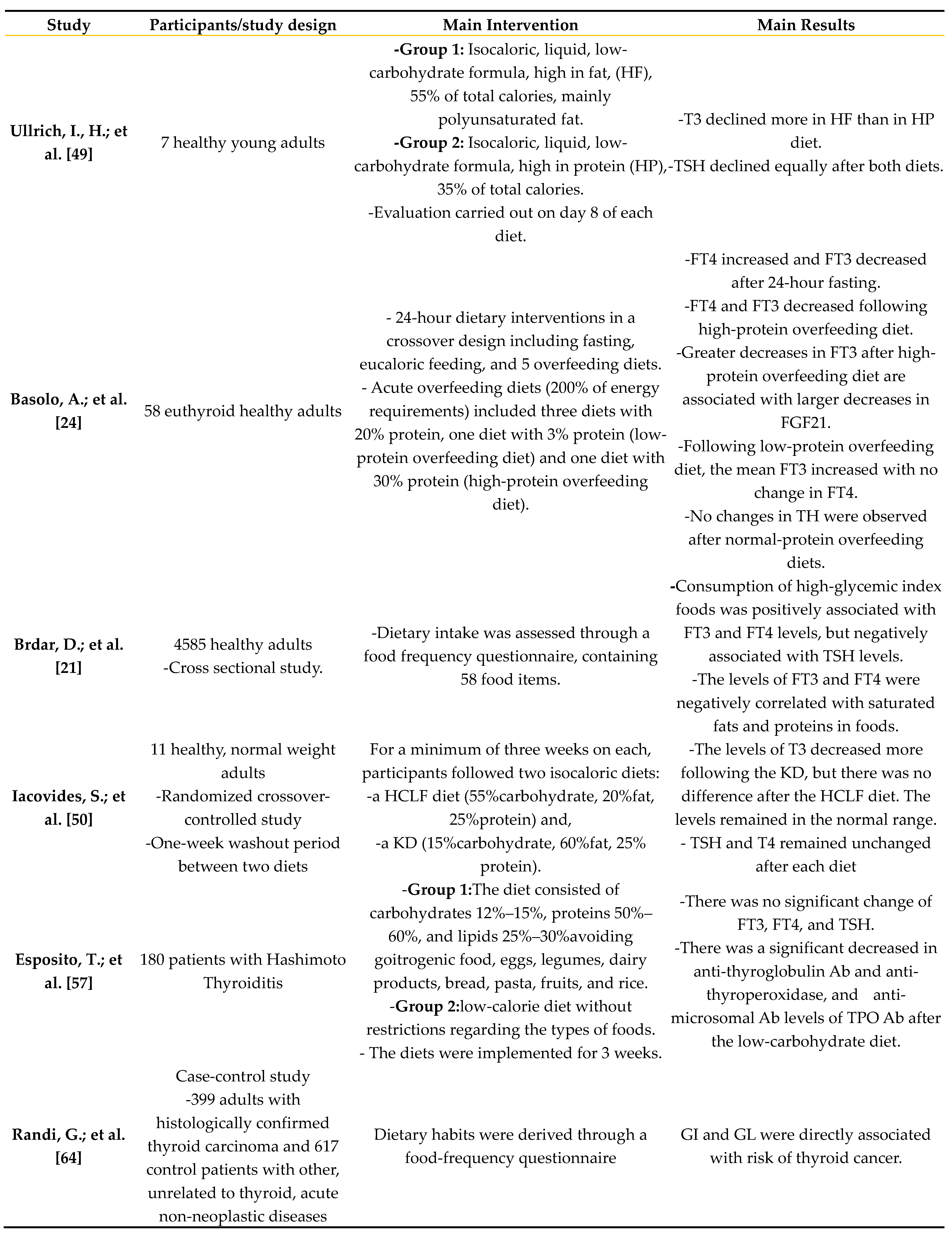
4.1. Nutrition
4.2. Starvation/overfeeding
4.3. Obesity
5. Low Glycemic Load (LGL) Diets
6. LGL Diets Lead to Subtle Changes of Thyroid Function Tests in Euthyroid Subjects
7. LGL Diets and Weight Reduction in Hypothyroidism
8. Impact of Low-Glycemic Load diets on the Risk of Thyroid Cancer
9. Perspectives and Conclusions
References
- Jing, L.; Zhang, Q. Intrathyroidal feedforward and feedback network regulating thyroid hormone synthesis and secretion. Front. Endocrinol. 2022, 13, 992883. [Google Scholar] [CrossRef] [PubMed]
- Bereketoglu, C.; Pradhan, A. Plasticizers: negative impacts on the thyroid hormone system. Environ. Sci. Pollut. Res. 2022, 29, 38912–38927. [Google Scholar] [CrossRef]
- Gereben, B.; McAninch, E.A.; Ribeiro, M.O.; Bianco, A.C. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat. Rev. Endocrinol. 2015, 11, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Brent, G. A, Mechanisms of thyroid hormone action Find the latest version: Science in medicine Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Kim, B. Thyroid Hormone as a Determinant of Energy Expenditure and the Basal Metabolic Rate. Thyroid® 2008, 18, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Chidakel, A.; Mentuccia, D.; Celi, F. Peripheral Metabolism of Thyroid Hormone and Glucose Homeostasis. Thyroid® 2005, 15, 899–903. [Google Scholar] [CrossRef]
- Pucci, E.; Chiovato, L.; Pinchera, A. Thyroid and lipid metabolism. Int. J. Obes. 2000, 24, S109–S112. [Google Scholar] [CrossRef] [PubMed]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. , Hypothalamus-Pituitary-Thyroid Axis. Compr. Physiol. 2016, 6, 1387–1428. [Google Scholar] [PubMed]
- Gauthier, B.R.; Sola-Garcia, A.; Caliz-Molina, M.A.; Lorenzo, P.I.; Cobo-Vuilleumier, N.; Capilla-Gonzalez, V.; Martin-Montalvo, A. Thyroid hormones in diabetes, cancer, and aging. Aging Cell 2020, 19, e13260. [Google Scholar] [CrossRef]
- Candido, A.C.; Azevedo, F.M.; Silva, D.L.F.; Ribeiro, S.A.V.; Franceschini, S.D.C.C. Effects of iodine supplementation on thyroid function parameter: Systematic review and meta-analysis. J. Trace Elements Med. Biol. 2023, 80, 127275. [Google Scholar] [CrossRef]
- Schaffner, M.; Rochau, U.; Stojkov, I.; Rushaj, V.Q.; Voelzke, H.; Marckmann, G.; Lazarus, J.H.; Oberaigner, W.; Siebert, U. Barriers Against Prevention Programs for Iodine Deficiency Disorders in Europe: A Delphi Study. Thyroid® 2021, 31, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, R.; Yuan, X.; Kobayashi, S.; Sasaki, S. Effect of excess iodine intake on thyroid diseases in different populations: A systematic review and meta-analyses including observational studies. PLOS ONE 2017, 12, e0173722. [Google Scholar] [CrossRef] [PubMed]
- Zavros, A.; Giannaki, C.D.; Aphamis, G.; Roupa, Z.; Andreou, E. The Effects of Zinc and Selenium Supplementation on Body Composition and Thyroid Function in Individuals with Overweight or Obesity: A Systematic Review. J. Diet. Suppl. 2022, 20, 643–671. [Google Scholar] [CrossRef]
- Kong, X.-Q.; Qiu, G.-Y.; Yang, Z.-B.; Tan, Z.-X.; Quan, X.-Q. Clinical efficacy of selenium supplementation in patients with Hashimoto thyroiditis: A systematic review and meta-analysis. Medicine 2023, 102, e33791. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, V.; Condorelli, R.A.; Cannarella, R.; Aversa, A.; Calogero, A.E.; La Vignera, S. Relationship between Iron Deficiency and Thyroid Function: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 4790. [Google Scholar] [CrossRef] [PubMed]
- Babiker, A.; Alawi, A.; Atawi, M.; Alwan, I. The role of micronutrients in thyroid dysfunction. Sudan. J. Paediatr. 2020, 20, 13–19. [Google Scholar] [CrossRef]
- Chartoumpekis, D.V.; Ziros, P.G.; Chen, J.-G.; Groopman, J.D.; Kensler, T.W.; Sykiotis, G.P. Broccoli sprout beverage is safe for thyroid hormonal and autoimmune status: Results of a 12-week randomized trial. Food Chem. Toxicol. 2019, 126, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.R.; He, X.; Braverman, L.E.; Narla, R.; Gupta, P.K.; Leung, A.M. , Letter to the editor. Endocr. Pract. 2017, 23, 885–886. [Google Scholar] [CrossRef]
- Larsen, D.; Singh, S.; Brito, M. , Thyroid, Diet, and Alternative Approaches. J. Clin. Endocrinol. Metab. 2022, 107, 2973–2981. [Google Scholar] [CrossRef]
- Danailova, Y.; Velikova, T.; Nikolaev, G.; Mitova, Z.; Shinkov, A.; Gagov, H.; Konakchieva, R. Nutritional Management of Thyroiditis of Hashimoto. Int. J. Mol. Sci. 2022, 23, 5144. [Google Scholar] [CrossRef]
- Brdar, D.; Gunjača, I.; Pleić, N.; Torlak, V.; Knežević, P.; Punda, A.; Polašek, O.; Hayward, C.; Zemunik, T. The effect of food groups and nutrients on thyroid hormone levels in healthy individuals. Nutrition 2021, 91-92, 111394. [Google Scholar] [CrossRef] [PubMed]
- Vagenakis, A.G.; Burger, A.; Portnay, G.I.; Rudolph, M.; O'Brain, J.T.; Azizi, F.; Arky, R.A.; Nicod, P.; Ingbar, S.H.; Braverman, L.E. Diversion of Peripheral Thyroxine Metabolism from Activating to Inactivating Pathways During Complete Fasting. J. Clin. Endocrinol. Metab. 1975, 41, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Heist, K.; DePaoli, A.M.; Veldhuis, J.D.; Mantzoros, C.S. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J. Clin. Investig. 2003, 111, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Basolo, A.; Begaye, B.; Hollstein, T.; Vinales, K.L.; Walter, M.; Santini, F.; Krakoff, J.; Piaggi, P. Effects of Short-Term Fasting and Different Overfeeding Diets on Thyroid Hormones in Healthy Humans. Thyroid® 2019, 29, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Bisschop, P.H.; Sauerwein, H.P.; Endert, E.; Romijn, J.A. Isocaloric carbohydrate deprivation induces protein catabolism despite a low T3-syndrome in healthy men. Clin. Endocrinol. 2001, 54, 75–80. [Google Scholar] [CrossRef]
- Fontana, L.; Klein, S.; Holloszy, J.O.; Premachandra, B.N. Effect of Long-Term Calorie Restriction with Adequate Protein and Micronutrients on Thyroid Hormones. J. Clin. Endocrinol. Metab. 2006, 91, 3232–3235. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217–155217. [Google Scholar] [CrossRef] [PubMed]
- Michalaki, M.A.; Vagenakis, A.G.; Leonardou, A.S.; Argentou, M.N.; Habeos, I.G.; Makri, M.G.; Psyrogiannis, A.I.; Kalfarentzos, F.E.; Kyriazopoulou, V.E. Thyroid Function in Humans with Morbid Obesity. Thyroid® 2006, 16, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.J.B.; Filgueiras, A.R.; Almeida, V.B.P.; de Moraes, R.C.S.; Sawaya, A.L. Changes in Thyroid and Glycemic Status and Food Intake in Children with Excess Weight Who Were Submitted for a Multi-Component School Intervention for 16 Months. Int. J. Environ. Res. Public Heal. 2020, 17, 3825. [Google Scholar] [CrossRef] [PubMed]
- Peeters, R.P. , Subclinical Hypothyroidism. N. Engl. J. Med. 2017, 376, 2556–2565. [Google Scholar] [CrossRef]
- Rotondi, M.; Leporati, P.; La Manna, A.; Pirali, B.; Mondello, T.; Fonte, R.; Magri, F.; Chiovato, L. , Raised serum TSH levels in patients with morbid obesity: Is it enough to diagnose subclinical hypothyroidism? Eur. J. Endocrinol. 2009, 160, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T. Obesity and thyroid function. Mol. Cell. Endocrinol. 2010, 316, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Magkos, F.; Brinkoetter, M.; Sienkiewicz, E.; Dardeno, T.A.; Kim, S.-Y.; Hamnvik, O.-P.R.; Koniaris, A. Leptin in human physiology and pathophysiology. Am. J. Physiol. Metab. 2011, 301, E567–E584. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Sui, H.; Wang, Z.; Zhang, T.; Zheng, J.; Yan, H.; Li, Q.; Mo, Z.; Liu, L. Thyroid hormone sensitivity and diabetes onset: a longitudinal cross-lagged cohort. Front. Endocrinol. 2023, 14, 1267612. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hu, Y.; Zhao, H.; Zhou, G.; Wang, G.; Ma, C.; Xu, Y. , Exploring the association between triglyceride-glucose index and thyroid function. Eur. J. Med. Res. 2023, 28, 508. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Hu, F.B.; Tappy, L.; Brand-Miller, J. Dietary carbohydrates: role of quality and quantity in chronic disease. BMJ 2018, 361, k2340. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: a systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef]
- Vlachos, D.; Malisova, S.; Lindberg, F.A.; Karaniki, G. Glycemic Index (GI) or Glycemic Load (GL) and Dietary Interventions for Optimizing Postprandial Hyperglycemia in Patients with T2 Diabetes: A Review. Nutrients 2020, 12, 1561. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Lee, D.; Ahmed, A.; Cheung, A.; A Khan, T.; Blanco, S.; Mejia; Mirrahimi, A. ; A Jenkins, D.J.; Livesey, G.; et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: systematic review and meta-analysis of randomised controlled trials. BMJ 2021, 374, n1651. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Tobias, D.K.; Malik, V.S.; Pan, A.; Hruby, A.; E Manson, J.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am. J. Clin. Nutr. 2014, 100, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Mirrahimi, A.; de Souza, R.J.; Chiavaroli, L.; Sievenpiper, J.L.; Beyene, J.; Hanley, A.J.; Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A. Associations of Glycemic Index and Load With Coronary Heart Disease Events: A Systematic Review and Meta-Analysis of Prospective Cohorts. J. Am. Heart Assoc. 2012, 1, e000752. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Soltani, S.; Jenkins, D.; Sievenpiper, J.; Shab-Bidar, S. Dietary glycemic index, glycemic load, and chronic disease: an umbrella review of meta-analyses of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2020, 62, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.F.; Passos, E.P.; Moulin, C.C. Short-term effects of a hypocaloric diet with low glycemic index and low glycemic load on body adiposity, metabolic variables, ghrelin, leptin, and pregnancy rate in overweight and obese infertile women: a randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzopoulou, I.; Theodoridis, X.; Kotzakioulafi, E.; Evripidou, K.; Chourdakis, M. The Effectiveness of a Low Glycemic Index/Load Diet on Cardiometabolic, Glucometabolic, and Anthropometric Indices in Children with Overweight or Obesity: A Systematic Review and Meta-Analysis. Children 2023, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Chekima, K.; Yan, S.W.; Lee, S.W.H.; Wong, T.Z.; Noor, M.I.; Ooi, Y.B.; Metzendorf, M.-I.; Lai, N.M. Low glycaemic index or low glycaemic load diets for people with overweight or obesity. . 2023, 6, CD005105. [Google Scholar] [PubMed]
- Zafar, M.I.; E Mills, K.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.-L. Low-glycemic index diets as an intervention for diabetes: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, I.H.; Peters, P.J.; Albrink, M.J. Effect of low-carbohydrate diets high in either fat or protein on thyroid function, plasma insulin, glucose, and triglycerides in healthy young adults. J. Am. Coll. Nutr. 1985, 4, 451–459. [Google Scholar] [CrossRef]
- Iacovides, S.; Maloney, S.K.; Bhana, S.; Angamia, Z.; Meiring, R.M. Could the ketogenic diet induce a shift in thyroid function and support a metabolic advantage in healthy participants? A pilot randomized-controlled-crossover trial. PLOS ONE 2022, 17, e0269440. [Google Scholar] [CrossRef]
- Warner, A.; Mittag, J. Thyroid hormone and the central control of homeostasis. J. Mol. Endocrinol. 2012, 49, R29–R35. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B. Thyroid and Obesity: An Intriguing Relationship. J. Clin. Endocrinol. Metab. 2010, 95, 3614–3617. [Google Scholar] [CrossRef] [PubMed]
- Cerit, E.T.; Akturk, M.; Altinova, A.E.; Tavil, Y.; Ozkan, C.; Yayla, C.; Altay, M.; Demirtas, C.; Cakir, N. Evaluation of body composition changes, epicardial adipose tissue, and serum omentin-1 levels in overt hypothyroidism. Endocrine 2014, 49, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Song, R.-H.; Wang, B.; Yao, Q.-M.; Li, Q.; Jia, X.; Zhang, J.-A. The Impact of Obesity on Thyroid Autoimmunity and Dysfunction: A Systematic Review and Meta-Analysis. Front. Immunol. 2019, 10, 2349. [Google Scholar] [CrossRef] [PubMed]
- Vettor, R.; Mingrone, G.; Manco, M.; Granzotto, M.; Milan, G.; Scarda, A.; Lombardi, A.; Greco, A.; Federspil, G. Reduced expression of uncoupling proteins-2 and -3 in adipose tissue in post-obese patients submitted to biliopancreatic diversion. Eur. J. Endocrinol. 2003, 148, 543–550. [Google Scholar] [CrossRef]
- Mikulska, A.A.; Karaźniewicz-Łada, M.; Filipowicz, D.; Ruchała, M.; Główka, F.K. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management—An Overview. Int. J. Mol. Sci. 2022, 23, 6580. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.; Esposito, T.; Lobaccaro, J.-M.; Esposito, M.G.; Monda, V.; Messina, A.; Paolisso, G.; Varriale, B.; Monda, M. Effects of low-carbohydrate diet therapy in overweight subject with autoimmune thyroiditis: possible synergism with ChREBP. Drug Des. Dev. Ther. 2016, ume 10, 2939–2946. [Google Scholar] [CrossRef]
- Fagin, J.A.; Wells, S.A., Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef]
- Park, S.; Oh, C.-M.; Cho, H.; Lee, J.Y.; Jung, K.-W.; Jun, J.K.; Won, Y.-J.; Kong, H.-J.; Choi, K.S.; Lee, Y.J.; et al. Association between screening and the thyroid cancer “epidemic” in South Korea: evidence from a nationwide study. BMJ 2016, 355, i5745. [Google Scholar] [CrossRef]
- Powers, A.E.; Marcadis, A.R.; Lee, M.; Morris, L.G.T.; Marti, J.L. Changes in Trends in Thyroid Cancer Incidence in the United States, 1992 to 2016. JAMA 2019, 322, 2440–2441. [Google Scholar] [CrossRef]
- Veiga, L.H.S.; Holmberg, E.; Anderson, H.; Pottern, L.; Sadetzki, S.; Adams, M.J.; Sakata, R.; Schneider, A.B.; Inskip, P.; Bhatti, P.; et al. Thyroid Cancer after Childhood Exposure to External Radiation: An Updated Pooled Analysis of 12 Studies. Radiat. Res. 2016, 185, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Pfeiffer, R.M.; Sosa, J.A.; Shiels, M.S. Impact of Overweight and Obesity on US Papillary Thyroid Cancer Incidence Trends (1995-2015). JNCI J. Natl. Cancer Inst. 2020, 112, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Bandrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA: A Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Randi, G.; Ferraroni, M.; Talamini, R.; Garavello, W.; Deandrea, S.; Decarli, A.; Franceschi, S.; La Vecchia, C. Glycemic index, glycemic load and thyroid cancer risk. Ann. Oncol. 2007, 19, 380–383. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Rinaldi, S.; Tsilidis, K.K.; Weiderpass, E.; Boutron-Ruault, M.-C.; Rostgaard-Hansen, A.L.; Tjønneland, A.; Clavel-Chapelon, F.; Mesrine, S.; Katzke, V.A.; et al. Energy and macronutrient intake and risk of differentiated thyroid carcinoma in the European Prospective Investigation into Cancer and Nutrition study. Int. J. Cancer 2015, 138, 65–73. [Google Scholar] [CrossRef]
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





