Submitted:
29 December 2023
Posted:
29 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
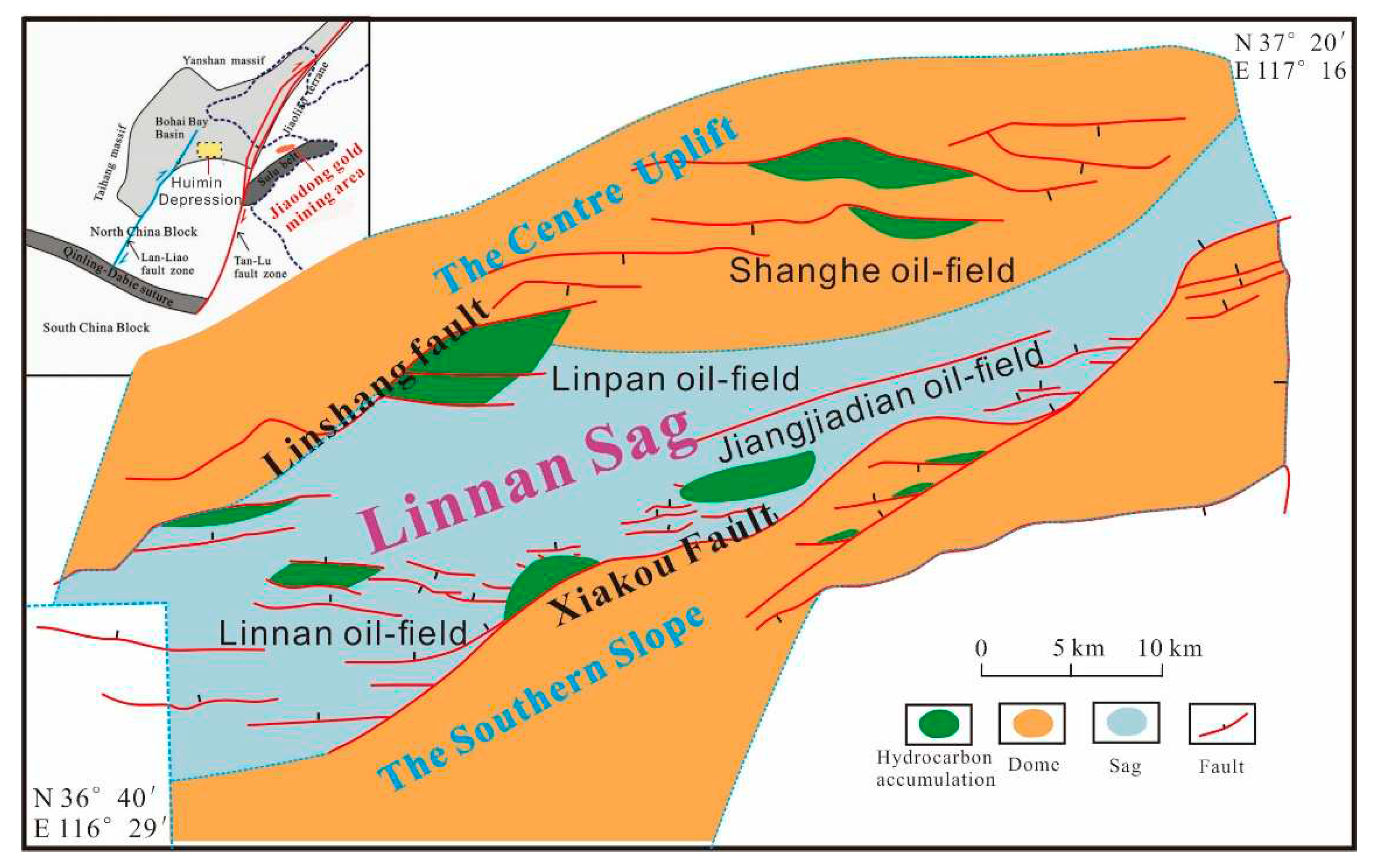
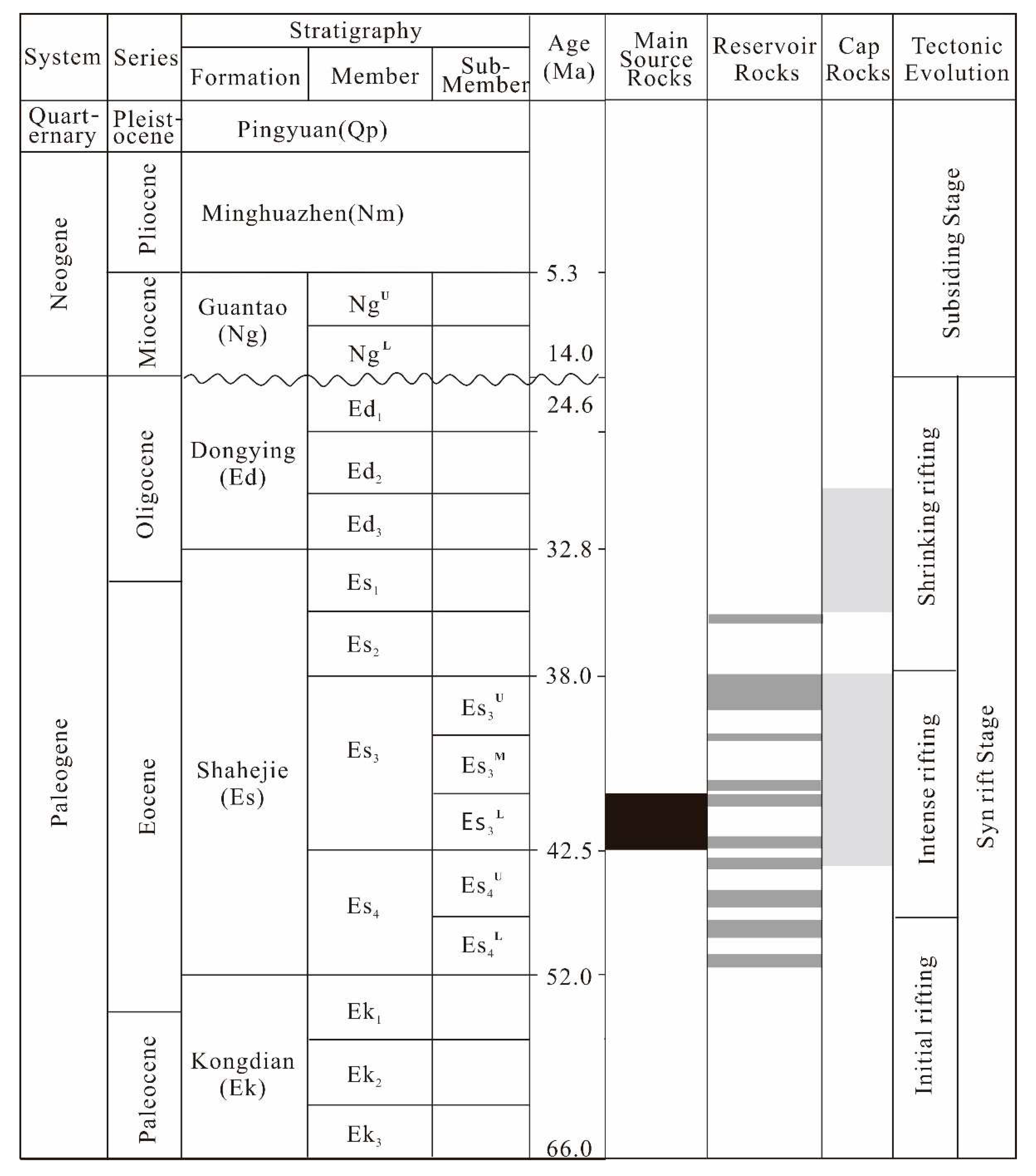
2. Geological Setting
3. Materials and Methods
3.1. Determination of Au Contents
3.2. Au-Tube Thermal Simulation
3.3. XPS Analysis
3.4. Determination of Total Acid Values of Crude Oil
4. Results
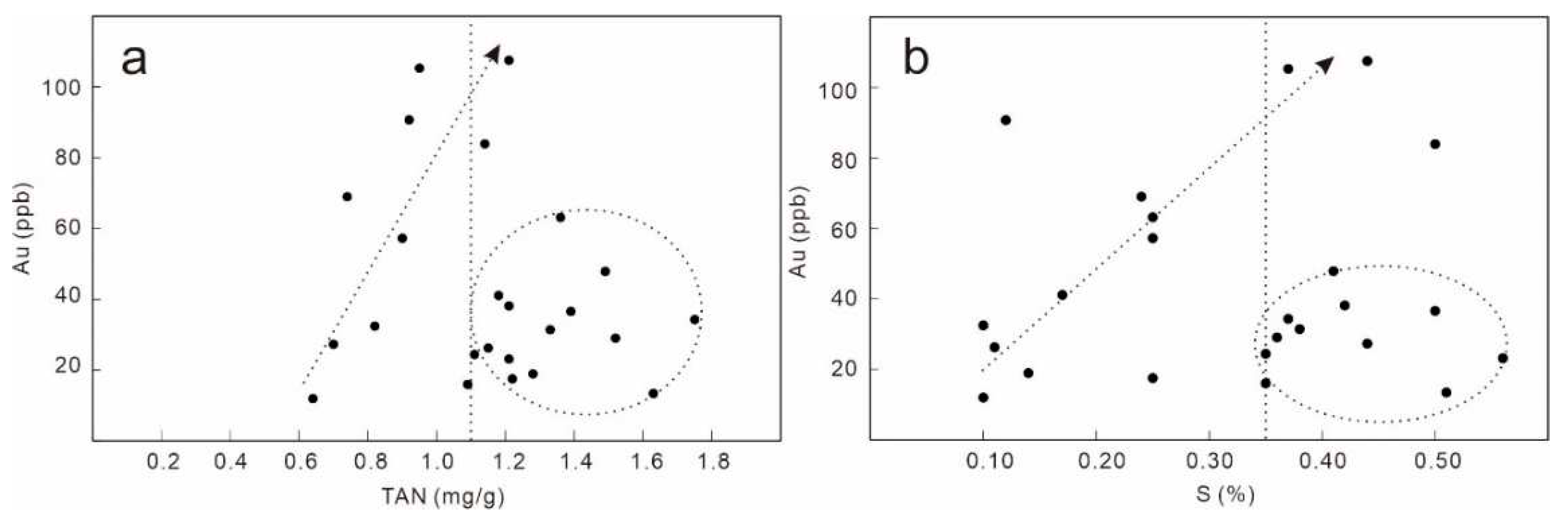
4.1. Crude oil Au, S, and Total Acid Contents
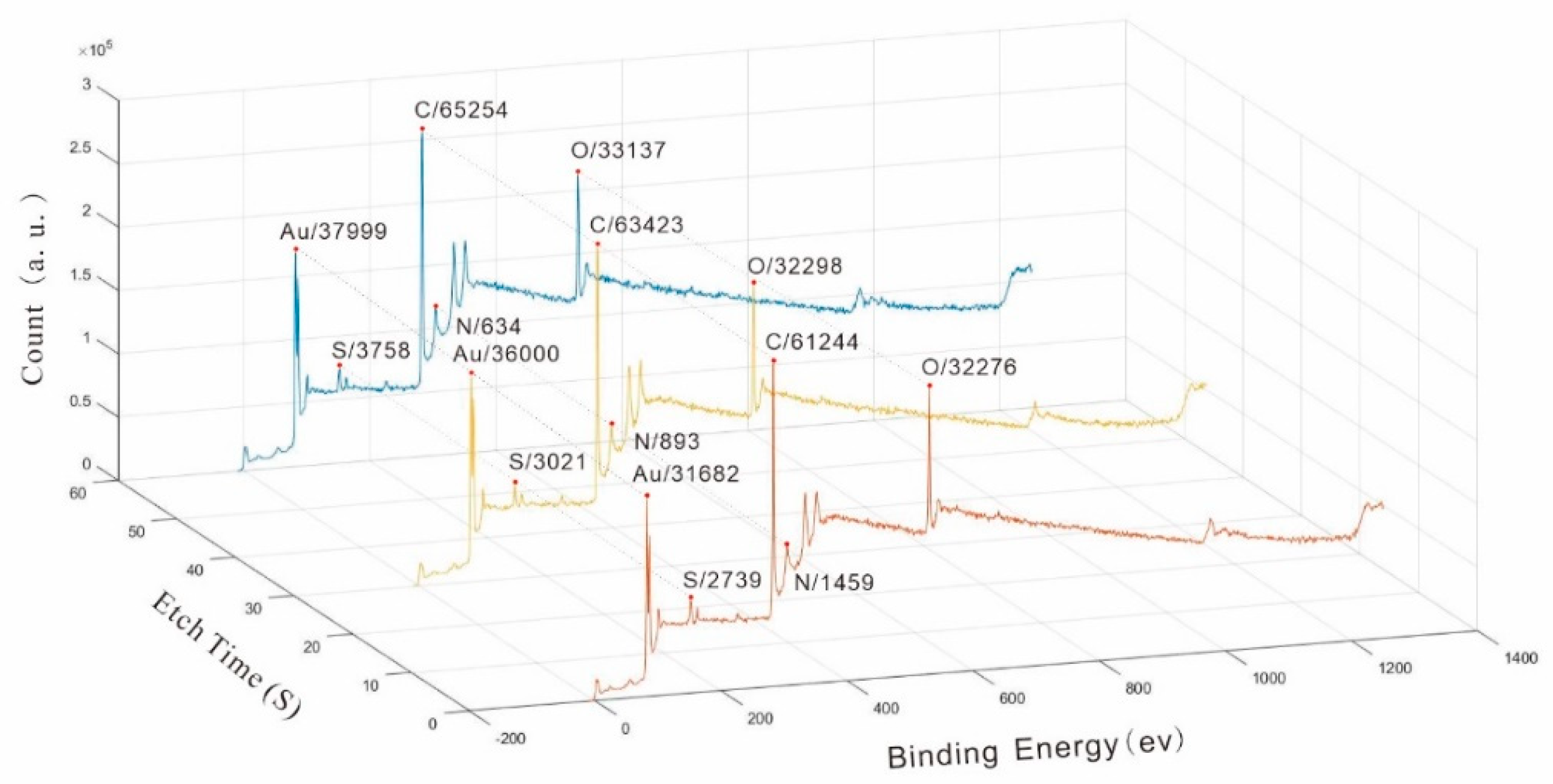
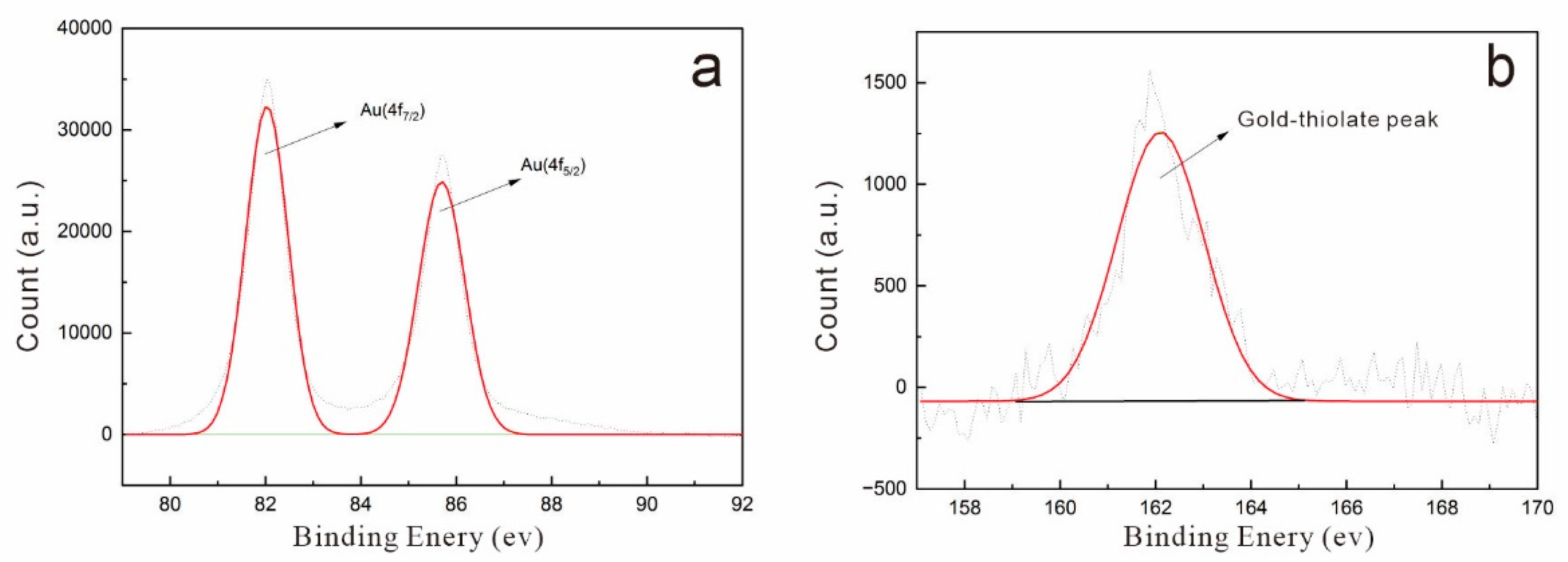
4.2. XPS Analyses
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, X.X.; Zhang, Y.M.; Li, B.H.; Xue, C.J.; Dong, S.Y.; Fu, S.H.; Cheng, W.B.; Liu, L.; Wu, C.Y. The coupling relationship between metallization and hydrocarbon accumulation in sedimentary basins. Earth Sci. Front. 2010, 17, 83–105. [Google Scholar]
- Clark, J.R.; Williams-Jones, A.E. Analogues of epithermal gold–silver deposition in geothermal well scales. Nature 1990, 346, 644–645. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Migdisov, A.A. An experimental study of the solubility of gold in crude oil: Implications for ore genesis. Geochim. Et Cosmochim. Acta 2006, 70, A703. [Google Scholar] [CrossRef]
- Emsbo, P.; Williams-Jones, A.; Koenig, A.E.; Wilson, S.A. Petroleum as an agent of metal transport: metallogenic and exploration implications. Proceding Tenth Bienn. SGA Meet. Townsv. 2009, 99–101. [Google Scholar]
- Hu, S.-Y.; Plet, C. Introduction to a special issue on organic- and microbe-metal interactions in mineral systems. Ore Geol. Rev. 2022, 146. [Google Scholar] [CrossRef]
- Gu, X.X.; Zhang, Y.M.; Li, B.H.; Dong, S.Y.; Xue, C.J.; Fu, S.H. Hydrocarbon- and ore-bearing basinal fluids: a possible link between gold mineralization and hydrocarbon accumulation in the Youjiang basin, South China. Miner. Depos. 2012, 47, 663–682. [Google Scholar] [CrossRef]
- Hulen, J.B.; Collister, J.W.; Stout, B.; Curtiss, D.K.; Dahdah, N.F. The exhumed “Carlin-type” fossil oil reservoir at Yankee Basin. JOM 1998, 50, 30–34. [Google Scholar] [CrossRef]
- Collister, J.W.; Hulen, J.B. The oil-bearing, carlin-type gold deposits of Yankee Basin, Alligator Ridge District, Nevada. Econ. Geol. 1999, 94, 1029–1049. [Google Scholar]
- Wang, G.Z.; Hu, R.Z.; Su, W.C.; Zhu, L.M. Fluid and mineralization of Youjiang Basin in the Yunnan-Guizhou-Guangxi area, China. Sci. China (Ser. D: Earth Sci. ) 2002, 46, 99–109. [Google Scholar]
- Hu, Y.; Liu, W.; Wang, J.; Zhang, G.; Zhou, Z.; Han, R. Basin-scale structure control of Carlin-style gold deposits in central Southwestern Guizhou, China: Insights from seismic reflection profiles and gravity data. Ore Geol. Rev. 2017, 91, 444–462. [Google Scholar] [CrossRef]
- Hu, Y.Z.; Han, R.S.; Mao, X.X. Relationship between metal mineralization and accumulation oil and gas in the eastern Guizhou. Geol. Prospect. 2007, 43, 51–56. [Google Scholar]
- Arehart, G.B. Characteristics and origin of sediment-hosted disseminated gold deposits: A review. Ore Geol. Rev. 1996, 11, 383–403. [Google Scholar] [CrossRef]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The biomarker guide: Volume 2, Biomarkers and isotopes in petroleum systems and earth history. Cambridge University Press, 2007. [Google Scholar]
- Su, W.C. Geochemistry of the Ore-Forming Fluids of the Carlin-Type Gold Deposits in the southwestern Margin of theYangtze Block: Institute of Geochemistry. Chinese Academy of Scicnces, Guiyang, China, 2002; pp. 1–120. [Google Scholar]
- Xia, Y.; Chen, Q.N.; Zhou, W.C. The simulating experiments of gold mineralization in low temperature and open system. J. Nanjing Univ. (Nat. Sci.) 1996, 32, 634–639. [Google Scholar]
- Liu, J.Z. Ore characteristics and gold occurrence of the Shuiyindong gold deposit, guizhou. Guizhou Geol. 2003, 20, 30–34. [Google Scholar]
- Liu, J.Z.; Deng, Y.M.; Liu, C.Q.; Zhang, X.C.; Xia, Y. Metallogenic conditions and model of the superlarge Shuiyindong stratabound gold deposit in Zhenfeng County, Guizhou Province. Geol. China 2006, 33, 169–177. [Google Scholar]
- Liu, J.Z.; Xia, Y.; Zhang, X.C.; Deng, Y.M.; Su, W.C.; Tao, Y. Model of strata Karlin-type gold deposit: the Shuiyindong super-scale gold deposit. Gold Sci. Technol. 2008, 16, 1–15. [Google Scholar]
- Tu, G.Z. Gechemisty of strata-bound Deposits in China (Volume III). Science Press: Beijing, 1988. [Google Scholar]
- Fu, J.M.; Peng, P.A.; Lin, Q.; Liu, D.H.; Jia, R.F.; Shi, J.X.; Lu, J.L. Several problems in the study of organic gechemisty of strata-bound Deposits. Prog. Earth Sci. 1990, 5, 43. [Google Scholar]
- Parnell, J. Metal enrichments in solid bitumens: A review. Miner. Depos. 1988, 23, 191–199. [Google Scholar] [CrossRef]
- Jedwab, J.; Badaut, D.; Beaunier, P. Discovery of a palladium-platinum-gold-mercury bitumen in the Boss Mine, Clark County, Nevada. Econ. Geol. 1999, 94, 1163–1172. [Google Scholar] [CrossRef]
- Baker, W.E. The role of humic acid in the transport of gold. Geochim. Et Cosmochim. Acta 1978, 42, 645–649. [Google Scholar] [CrossRef]
- Liu, J.Z.; Fu, J.M.; Lu, J.L. Experimental study on the role of organic matter in the mineralization of Sedimentary-reformed gold deposits. Sci. China (Ser. B) 1993, 9, 993–1000. [Google Scholar]
- Zhang, J.R.; Lu, J.J.; Yang, F.; Wu, J.W.; Zhu, F.H. An experimental study on gold solubility in amino acid solution and its geological signifcance. Geochimica 1996, 25, 190–195. [Google Scholar]
- Crede, L.-S.; Liu, W.; Evans, K.; Rempel, K.; Testemale, D.; Brugger, J. , Crude oils as ore fluids: An experimental in-situ XAS study of gold partitioning between brine and organic fluid from 25 to 250 °C. Geochim. Et Cosmochim. Acta 2019, 244, 352–365. [Google Scholar] [CrossRef]
- Xu, Z.H.; Zhan, Y.L.; Zhu, J.H.; Li, X. The research progress in the utilization of metal resources in crude oils. Multipurp. Util. Miner. Resour. 1998, 3, 22–26. [Google Scholar]
- Wang, D.; Wu, Z.P.; Yang, L.L.; Liu, H.; Zhang, Y. Growth and linkage of the Xiakou fault in the Linnan Sag, Jiyang Depression, Eastern China: Formation mechanism and sedimentation response. Mar. Pet. Geol. 2020, 116, 104319. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.K.; Luo, X.R.; Wang, B.; Lei, Y.H.; Cheng, M.; Luo, H.M.; Wang, C.J.; Yu, L. Modeling of overpressure generation-evolution of the Paleogene source rock and implications for the Linnan Sag, Eastern China. Front. Earth Sci. 2022, 10, 829322. [Google Scholar] [CrossRef]
- Guo, X.L.; Xiong, M.; Zhou, Q.; Tian, H.; Xiao, X.M. Petroleum generation and expulsion kinetics: A sase study of the Shahejie Formation source rocks from Linnan Sag of Huimin Depression. ACTA Sedmentologica SINICA 2009, 27, 723–731. [Google Scholar]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Seward, T.M. Thio complexes of gold and the transport of gold in hydrothermal ore solutions. Geochim. Et Cosmochim. Acta 1973, 37, 379–399. [Google Scholar] [CrossRef]
- Giordano, T.H. Evaluation of Organic Ligands and Metal-Organic Complexing in Mississippi Valley-Type Ore Solutions. Econ. Geol. 1985, 80, 96–106. [Google Scholar] [CrossRef]
- Vlassopoulos, D.; Wood, S.A.; Mucci, A. , Gold speciation in natural waters : II. The importance of organic complexing- Experiments with some simple model ligands. Geochim. Et Cosmochim. Acta 1990, 54, 1575–1586. [Google Scholar] [CrossRef]
- Sanz-Robinson, J.; Williams-Jones, A.E. The solubility of Nickel (Ni) in crude oil at 150, 200 and 250°C and its application to ore genesis. Chem. Geol. 2020, 533, 119443. [Google Scholar] [CrossRef]
- Hughey, C.A.; Rodgers, R.P.; Marshall, A.G.; Walters, C.C.; Qian, K.; Mankiewicz, P. Acidic and neutral polar NSO compounds in Smackover oils of different thermal maturity revealed by electrospray high field Fourier transform ion cyclotron resonance mass spectrometry. Org. Geochem. 2004, 35, 863–880. [Google Scholar] [CrossRef]
- Castner, D.G.; Hinds, K.; Grainger, D.W. X-ray Photoelectron Spectroscopy Sulfur 2p Study of Organic Thiol and Disulfide Binding Interactions with Gold Surfaces. Langmuir 1996, 12, 5083–5086. [Google Scholar] [CrossRef]
- Sanz-Robinson, J.; Sugiyama, I.; Williams-Jones, A.E. The solubility of palladium (Pd) in crude oil at 150, 200 and 250°C and its application to ore genesis. Chem. Geol. 2020, 531, 119320. [Google Scholar] [CrossRef]
- Lewan, M.D. Factors controlling the proportionality of vanadium to nickel in crude oils. Geochim. Et Cosmochim. Acta 1984, 48, 2231–2238. [Google Scholar] [CrossRef]
- Giordano, T.H. Metal Transport in Ore Fluids by Organic Ligand Complexation. In Organic Acids in Geological Processes; Pittman, E.D., Lewan, M.D., Eds. Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1994; pp. 319–354. [Google Scholar]
- Speight, J.G. Handbook of Petroleum Analysis; John Wiley & Sons Inc: Hoboken, NJ, USA, 2001. [Google Scholar]
- Park, L.K.E.; Liu, J.; Yiacoumi, S.; Borole, A.P.; Tsouris, C. Contribution of acidic components to the total acid number (TAN) of bio-oil. Fuel 2017, 200, 171–181. [Google Scholar] [CrossRef]
- Kawamurat, K.; Tannenbaum, E.L.I.; Huizinga, B.J.; Kaplan, I.R. Long-chain carboxylic acids in pyrolysates of Green River kerogen. Adv. Org. Geochem. 1986, 10, 1059–1065. [Google Scholar] [CrossRef]
- Shock, E.L. Organic acid metastability in sedimentary basins. Geology 1988, 16, 886–890. [Google Scholar] [CrossRef]
- Head, I.M.; Jones, D.M.; Larter, S.R. Biological activity in the deep subsurface and the origin of heavy oil. Nature 2003, 426, 344–352. [Google Scholar] [CrossRef]
- Wenger, L.M.; Davis, C.L.; Isaksen, G.H. Multiple Controls on Petroleum Biodegradation and Impact on Oil Quality. SPE Reserv. Eval. Eng. 2002, 5, 375–383. [Google Scholar] [CrossRef]
| Oilfield | Well | Fm. | Depth (m) | Density (g/cm3) | Viscosity (mpa.s) | S (%) | TAN (mg/g) | Au (ppb) |
|---|---|---|---|---|---|---|---|---|
| Linpan | L2-x201 | G2 | 1414.4-1423.6 | 0.945 | 377.75 | 0.51 | 1.63 | 13.4 |
| Linpan | L23-11 | G3 | 1642.2-1645.0 | 0.923 | 90.90 | 0.37 | 0.95 | 105.3 |
| Linpan | L55 | S3 | 2626.6-2992.8 | 0.866 | 11.07 | 0.12 | 0.92 | 90.7 |
| Linpan | L7-x28 | S2 | 1799.2-1823.6 | 0.909 | 27.46 | 0.10 | 0.64 | 12.0 |
| Linpan | L75-17 | S3 | 1859.2-1909.0 | 0.918 | 138.00 | 0.35 | 1.11 | 24.4 |
| Linpan | L75-x30 | S4 | 2474.0-2512.6 | 0.895 | 18.00 | 0.35 | 1.09 | 16.0 |
| Linpan | L76-3 | S4 | 2069.0-2094.0 | 0.893 | 41.00 | 0.10 | 0.82 | 32.4 |
| Linpan | P14-6 | S4 | 1588.6-1594.4 | 0.986 | 5951.00 | 0.50 | 1.39 | 36.6 |
| Linpan | P18-x3 | S3 | 1597.1-1630.3 | 0.935 | 119.00 | 0.42 | 1.21 | 38.1 |
| Linpan | P20-6 | S1 | 1556.0-1599.4 | 0.936 | 352.30 | 0.25 | 1.36 | 63.1 |
| Linpan | P2-504 | S3 | 1576.0-1606.4 | 0.965 | 921.00 | 0.56 | 1.21 | 23.2 |
| Linpan | P40-12 | G | 1337.6-1354.0 | 0.960 | 384.00 | 0.41 | 1.49 | 47.9 |
| Linpan | P40-17 | G | 1345.4-1350.4 | 0.951 | 324.00 | 0.38 | 1.33 | 31.4 |
| Shanghe | S23-70 | S2 | 1710.0-1734.2 | 0.897 | 48.94 | 0.25 | 0.9 | 57.2 |
| Shanghe | S25-11 | S4 | 2044.2-2126.8 | 0.899 | 48.84 | 0.14 | 1.28 | 18.9 |
| Shanghe | S25-40 | S4 | 1969.6-2267.8 | 0.897 | 58.23 | 0.44 | 1.21 | 107.5 |
| Shanghe | S42-2 | S2 | 1655.2-1675.4 | 0.932 | 140.30 | 0.44 | 0.7 | 27.3 |
| Shanghe | S70-x5 | S2 | 2348.5-2374.0 | 0.863 | 11.26 | 0.25 | 1.22 | 17.5 |
| Shanghe | S73-13 | S2 | 2275.8-2334.2 | 0.892 | 40.10 | 0.37 | 1.75 | 34.3 |
| Shanghe | S8-29 | S2 | 1854.2-1859.4 | 0.887 | 21.49 | 0.36 | 1.52 | 29.0 |
| Shanghe | S8-300 | S2 | 1776.0-1833.0 | 0.900 | 27.90 | 0.24 | 0.74 | 69.0 |
| Shanghe | S8-33 | S1 | 1653.0-1675.0 | 0.946 | 322.10 | 0.50 | 1.14 | 83.9 |
| Shanghe | S84-4 | S1 | 1899.0-1903.6 | 0.872 | 24.27 | 0.25 | 1.31 | - |
| Shanghe | S847 | S1 | 1884.6-1890.4 | 0.883 | 29.89 | 0.17 | 1.18 | 41.1 |
| Shanghe | S8-52 | S2 | 1846.0-1861.0 | 0.897 | 37.84 | 0.11 | 1.15 | 26.2 |
| Elements | Spectral Line | Etch Time (s) | Peak Area | Peak Center (ev) | Elements | Spectral Line | Etch Time (s) | Peak Area | Peak Center (ev) |
|---|---|---|---|---|---|---|---|---|---|
| Au | 4f5/2 | 0 | 31682 | 82 | N | 1s | 0 | 1459 | 398 |
| Au | 4f5/2 | 30 | 36000 | 82 | N | 1s | 30 | 893 | 398 |
| Au | 4f5/2 | 60 | 38000 | 82 | N | 1s | 60 | 634 | 398 |
| Au | 4f7/2 | 0 | 26368 | 86 | O | 1s | 0 | 32276 | 530 |
| Au | 4f7/2 | 30 | 30378 | 86 | O | 1s | 30 | 32299 | 530 |
| Au | 4f7/2 | 60 | 32330 | 86 | O | 1s | 60 | 33137 | 530 |
| C | 1s | 0 | 61244 | 283 | S | 4p3/2 | 0 | 2739 | 162 |
| C | 2s | 30 | 63423 | 283 | S | 4p3/2 | 30 | 3021 | 162 |
| C | 3s | 60 | 65254 | 283 | S | 4p3/2 | 60 | 3758 | 162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




