Submitted:
29 December 2023
Posted:
29 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
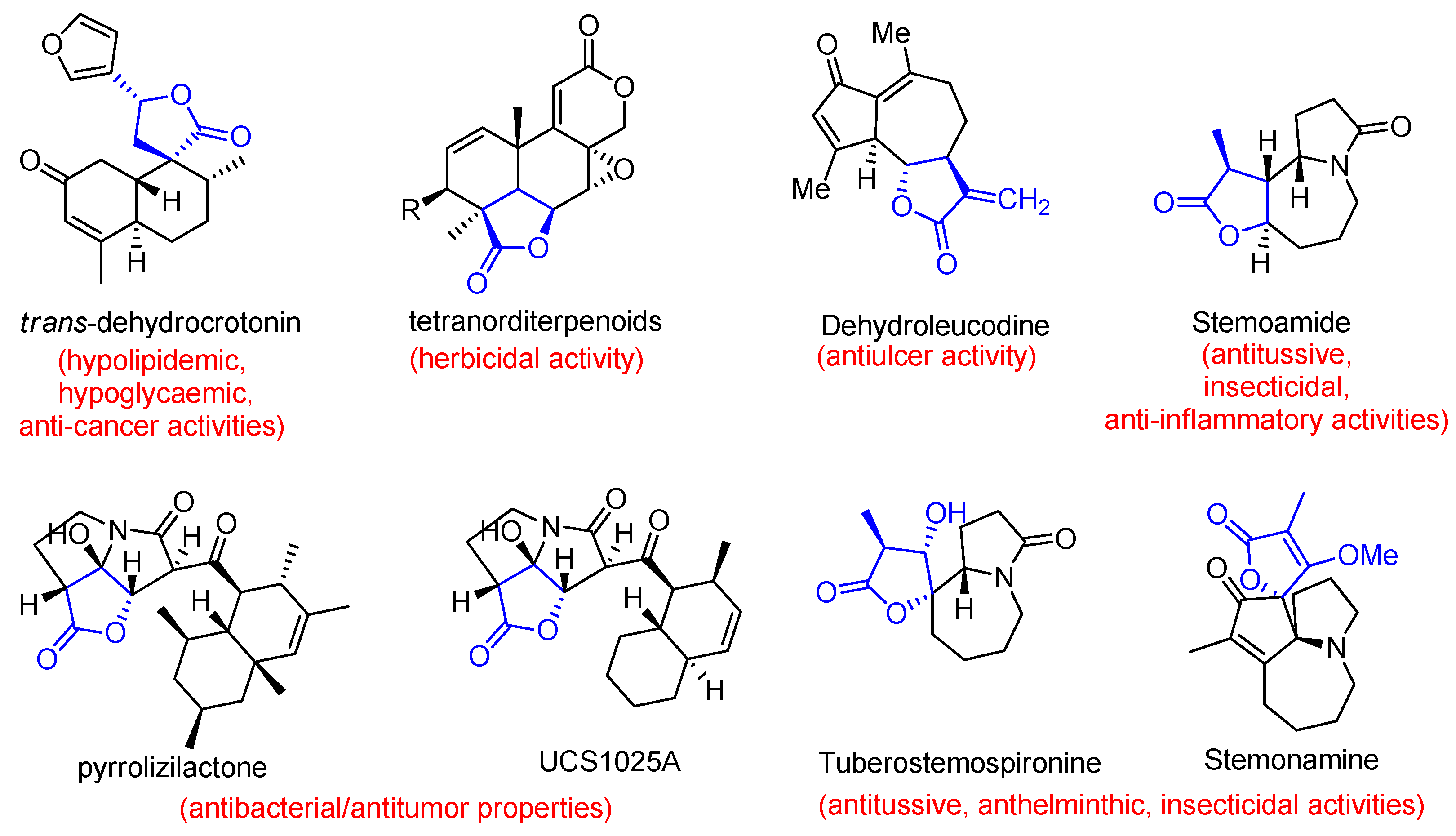
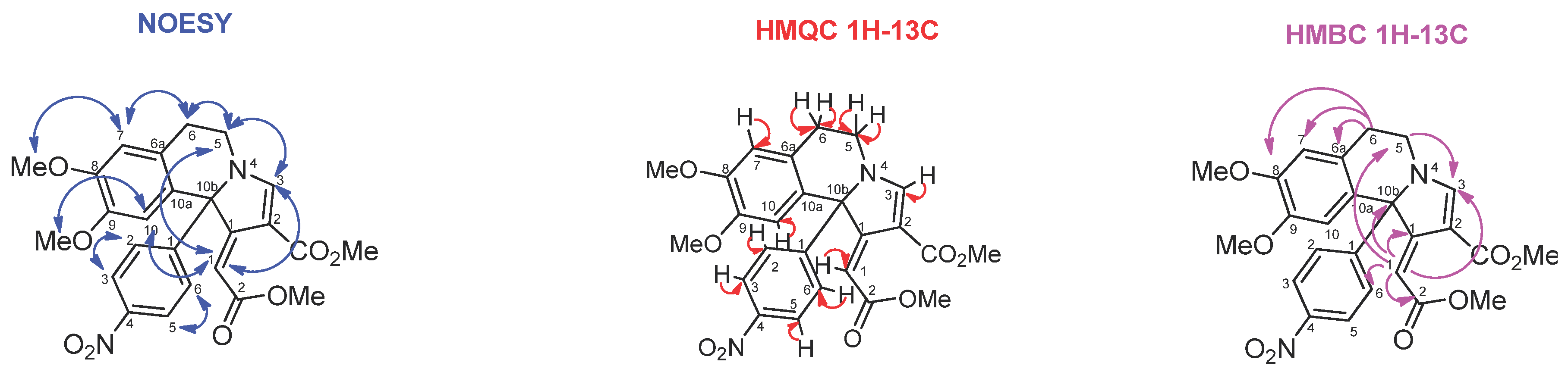
2. Results and Discussion
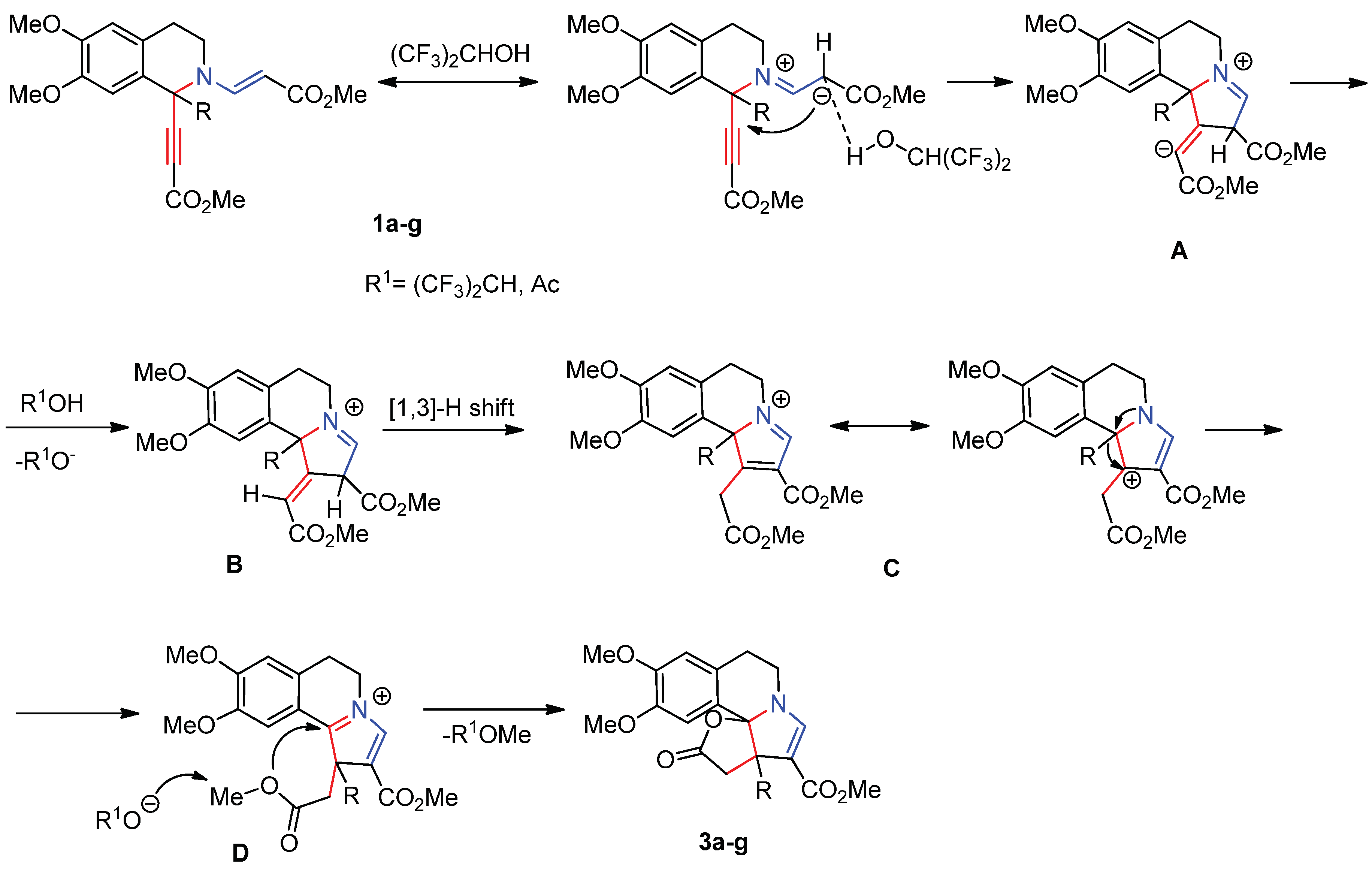
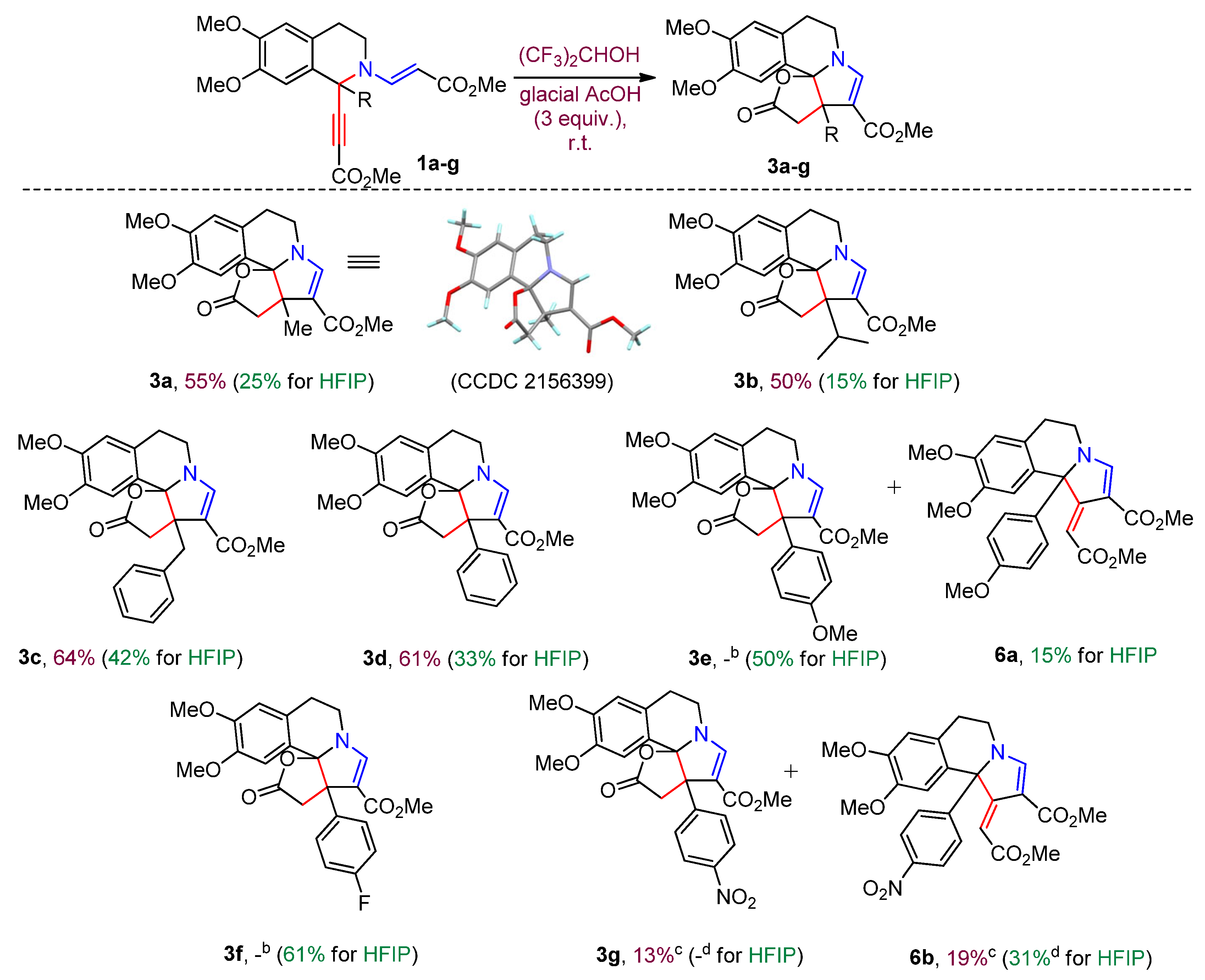
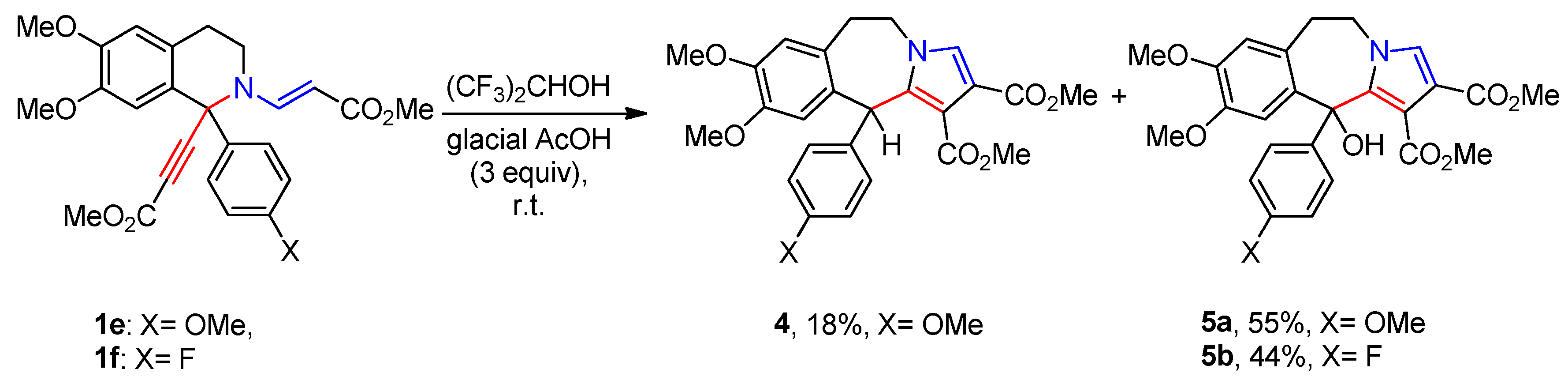
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Compound 1g
3.3. General Procedure for the Synthesis of Compounds 2a-g
3.4. General Procedure for the Synthesis of Compounds 3a-g, 4, 5a,b and 6a,b
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hitotsuyanagi, Y.; Fukaya, H.; Takeda, E.; Matsuda, S.; Saishu, Y.; Zhu, S.; Komatsu, K.; Takeya, K. Structures of stemona-amine B and stemona-lactams M–R. Tetrahedron 2013, 69, 6297–6304. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, L.; Yan, Y.; Sun, D.; Duan, Y.; Li, H.; Chen, L. Alkaloids From Stemona Tuberosa and Their Anti-Inflammatory Activity. Front. Chem. 2022, 10, 847595. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, T.; Kawatani, M.; Uramoto, M.; Okano, A.; Aono, H.; Futamura, Y.; Takahashi, S.; Osada, H. Pyrrolizilactone, a new pyrrolizidinone metabolite produced by a fungus. J. Antibiot. 2013, 66, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Salatino, M. L. F.; Negri, G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Farias, R.; Rao, V.; Viana, G.; Silveira, E.; Maciel, M.; Pino, A. Hypoglycemic Effect of Trans-Dehydrocrotonin, a Nor-Clerodane Diterpene from Croton Cajucara. Planta Med. 1997, 63, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Haun, M. Cytotoxicity of Trans-Dehydrocrotonin from Croton Cajucara on V79 Cells and Rat Hepatocytes. Planta Med. 1999, 65, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Aleman, J.; del Solar, V.; Martin-Santos, C.; Cubo, L.; Ranninger, C. N. Tandem Cyclization–Michael Reaction by Combination of Metal-and Organocatalysis. J. Org. Chem. 2011, 76, 7287–7293. [Google Scholar] [CrossRef] [PubMed]
- Herath, H. B.; Herath, W. H.; Carvalho, P.; Khan, S. I.; Tekwani, B. L.; Duke, S. O.; Tomaso-Peterson, M.; Nanayakkara, N. D. Biologically active tetranorditerpenoids from the fungus Sclerotinia homoeocarpa causal agent of dollar spot in turfgrass. J. Nat. Prod. 2009, 72, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene lactones from Artemisia genus: Biological activities and methods of analysis. J. Anal. Methods Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Greger, H. Structural classification and biological activities of Stemona alkaloids. Phytochem. Rev. 2019, 18, 463–493. [Google Scholar] [CrossRef]
- Pilli, R. A.; Rosso, G. B.; de Oliveira, M. D. C. F. The chemistry of Stemona alkaloids: An update. Nat. Prod. Rep. 2010, 27, 1908–1937. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tang, M. C.; Tang, S.; Gao, S.; Soliman, S.; Hang, L.; Xu, W.; Ye, T.; Watanabe, K.; Tang, Y. Genome mining and assembly-line biosynthesis of the UCS1025A pyrrolizidinone family of fungal alkaloids. J. Am. Chem. Soc. 2018, 140, 2067–2071. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, A.; Rodier, F.; Commeiras, L.; Parrain, J. L.; Chouraqui, G. Construction of spirolactones with concomitant formation of the fused quaternary centre–application to the synthesis of natural products. Nat. Prod. Rep. 2011, 28, 763–782. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Z.; Li, P.; Miao, T.; Wang, L. Synthesis of Spirolactones via a BF3·Et2O-Promoted Cascade Annulation of α-Keto Acids and 1,3-Enynes. Org. Lett. 2021, 23, 5698–5702. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhou, M.; Zhu, J.P.; Zhang, X. F.; Liu, Z.J.; Li, H.R.; Chen, Y.; Chen, H.-P.; Zhao, J.; Pu, J.-X.; Yu, M.; Liu, J.-K.; Wu, B. An unexpected photoinduced cyclization to synthesize fully substituted γ-spirolactones via intramolecular hydrogen abstraction with allyl acrylates. Org. Chem. Front. 2022, 9, 2316–2321. [Google Scholar] [CrossRef]
- Nair, D.; Basu, P.; Pati, S.; Baseshankar, K.; Sankara, C. S.; Namboothiri, I. N. Synthesis of Spirolactones and Functionalized Benzofurans via Addition of 3-Sulfonylphthalides to 2-Formylaryl Triflates and Conversion to Benzofuroisocoumarins. J. Org. Chem. 2023, 88, 4519–4527. [Google Scholar] [CrossRef] [PubMed]
- Delayre, B.; Wang, Q.; Zhu, J. Natural product synthesis enabled by domino processes incorporating a 1,2-rearrangement step. ACS Cent. Sci. 2021, 7, 559–569. [Google Scholar] [CrossRef]
- Obydennik, A.Y.; Titov, A.A.; Listratova, A.V.; Borisova, T.N.; Sokolova, I.L.; Rybakov, V.B.; Van der Eycken, E.V.; Voskressensky, L.G.; Varlamov, A.V. Divergent and Nucleophile-Assisted Rearrangement in the Construction of Pyrrolo [2,1-b][3]benzazepine and Pyrido[2,1-a]isoquinoline Scaffolds. Chem.– Eur. J. 2023, e202302919. [Google Scholar] [CrossRef]
- Motiwala, H.F.; Armaly, A.M.; Cacioppo, J.G.; Coombs, T.C.; Koehn, K.R.; Norwood IV, V.M.; Aube, J. HFIP in organic synthesis. Chem. Rev. 2022, 122, 12544–12747. [Google Scholar] [CrossRef]
- Listratova, A. V.; Titov, A. A.; Obydennik, A. Y.; Varlamov, A. V. N-propargyl aza-Claisen rearrangement in the synthesis of heterocycles. Tetrahedron 2022, 121, 132914. [Google Scholar] [CrossRef]
- Lee, J. Y.; Lee, Y. S.; Chung, B. Y.; Park, H. Asymmetric synthesis of both enantiomers of novel tetracyclic heterocycle, furo [3′,2′:2,3]pyrrolo[2,1-a] isoquinoline derivative via a diastereoselective N-acyliminium ion cyclization. Tetrahedron 1997, 53, 2449–2458. [Google Scholar] [CrossRef]
- Titov, A.A.; Kobzev, M.S.; Borisova, T.N.; Listratova, A.V.; Evenko, T.V.; Varlamov, A.V.; Voskressensky, L.G. Facile Methods for the Synthesis of 8-Ylidene-1,2,3,8-tetrahydrobenzazecines. Eur. J. Org. Chem. 2020, 2020, 3041–3049. [Google Scholar] [CrossRef]
- Titov, A.A.; Purgatorio, R.; Obydennik, A.Y.; Listratova, A.V.; Borisova, T.N.; De Candia, M.; Catto, M.; Altomare, C.D.; Varlamov, A.V.; Voskressensky, L.G. Synthesis of Isomeric 3-Benzazecines Decorated with Endocyclic Allene Moiety and Exocyclic Conjugated Double Bond and Evaluation of Their Anticholinesterase Activity. Molecules 2022, 27, 6276. [Google Scholar] [CrossRef] [PubMed]
- Dantignana, V.; Milan, M.; Cussó, O.; Company, A.; Bietti, M.; Costas, M. Chemoselective Aliphatic C–H Bond Oxidation Enabled by Polarity Reversal. ACS Cent. Sci. 2017, 3, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Call, A.; Cianfanelli, M.; Besalú-Sala, P.; Olivo, G.; Palone, A.; Vicens, L.; Ribas, X.; Luis, J. M.; Bietti, M.; Costas, M. Carboxylic Acid Directed γ-Lactonization of Unactivated Primary C–H Bonds Catalyzed by Mn Complexes: Application to Stereoselective Natural Product Diversification. J. Am. Chem. Soc. 2022, 144, 19542–19558. [Google Scholar] [CrossRef] [PubMed]
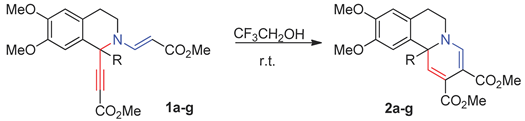
| Entry | R | Product | Yield, % |
| 1 | Me | 2a | 95 a |
| 2 | i-Pr | 2b | 55 |
| 3 | Bn | 2c | 56 |
| 4 | Ph | 2d | 71 |
| 5 | 4-OMe-C6H4- | 2e | 79 |
| 6 | 4-F-C6H4- | 2f | 80 |
| 7 | 4-NO2-C6H4- | 2g | 68 |
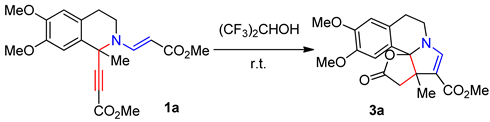
| Entry | glacial AcOH (equiv.) | Yield 3a, % | Yield 2a, % |
| 1 | - | 25 | 71 |
| 2 | 0.5 | 43 | 43 |
| 3 | 3.0 | 55 | - a |
| 4 | 5.0 | 56 | - a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





