Submitted:
30 December 2023
Posted:
30 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Viral (MHV-1) Inoculation and SPIKENET
2.3. Skin Collection and Storage
2.4. Histological staining
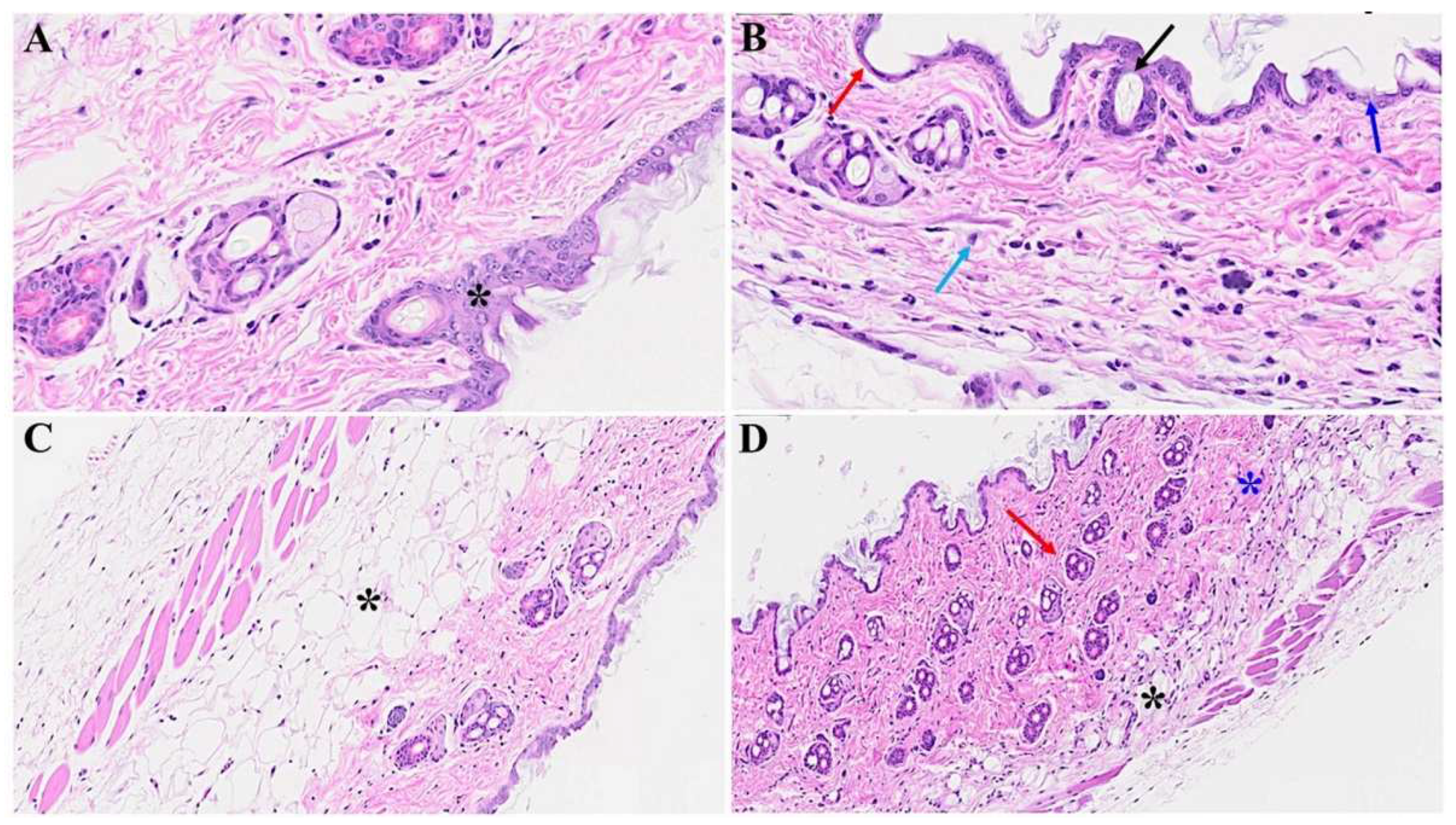
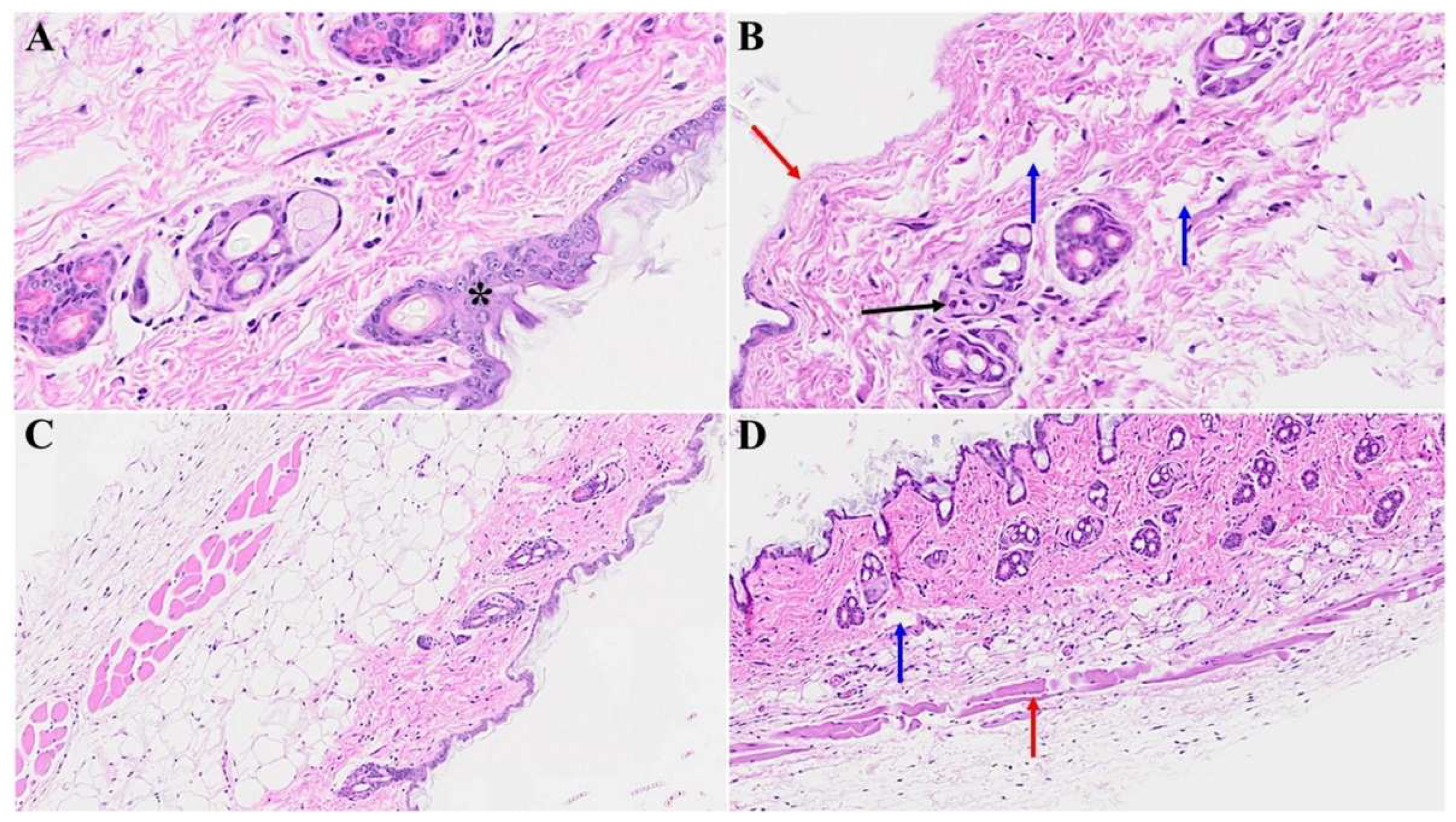
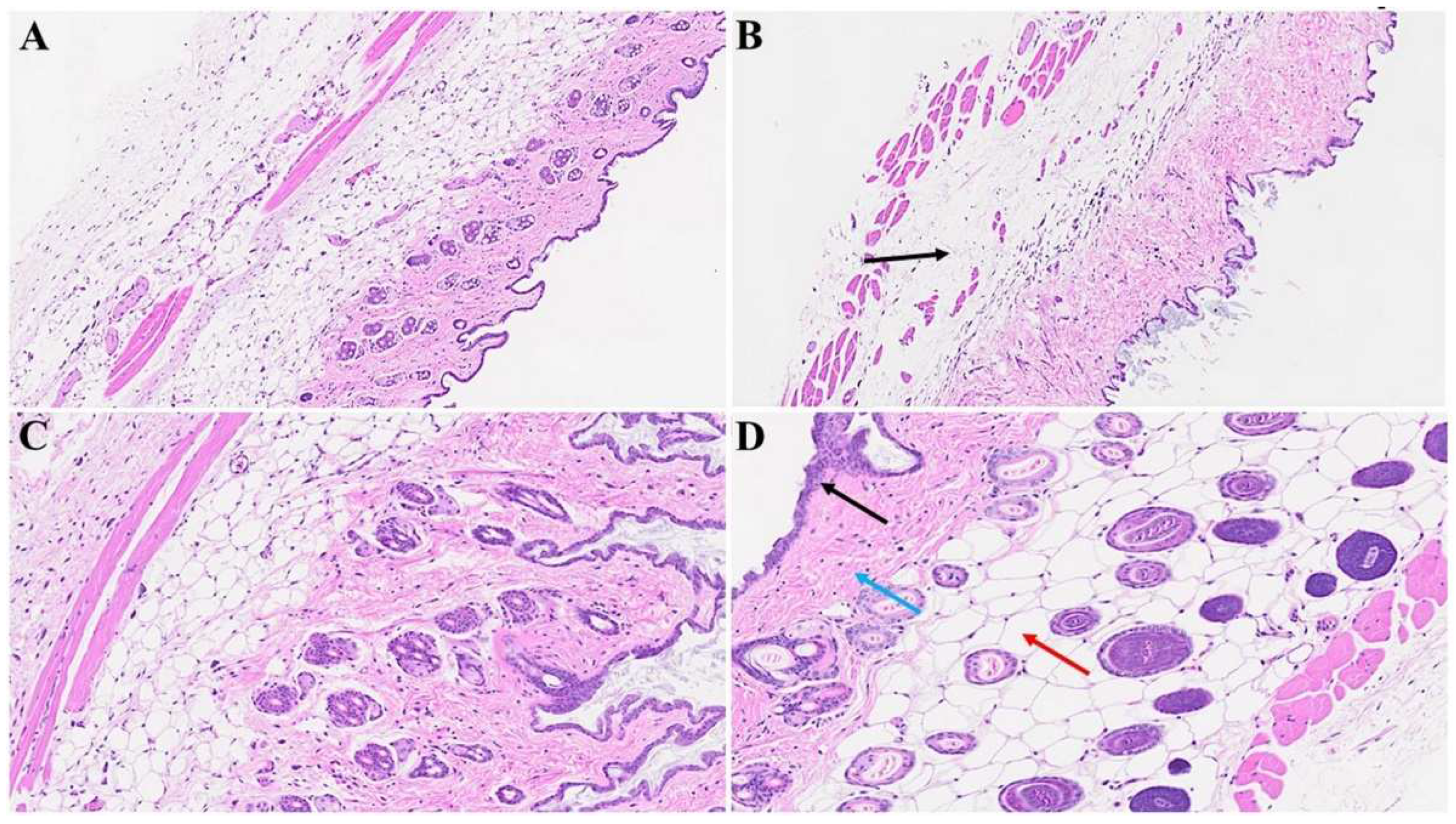
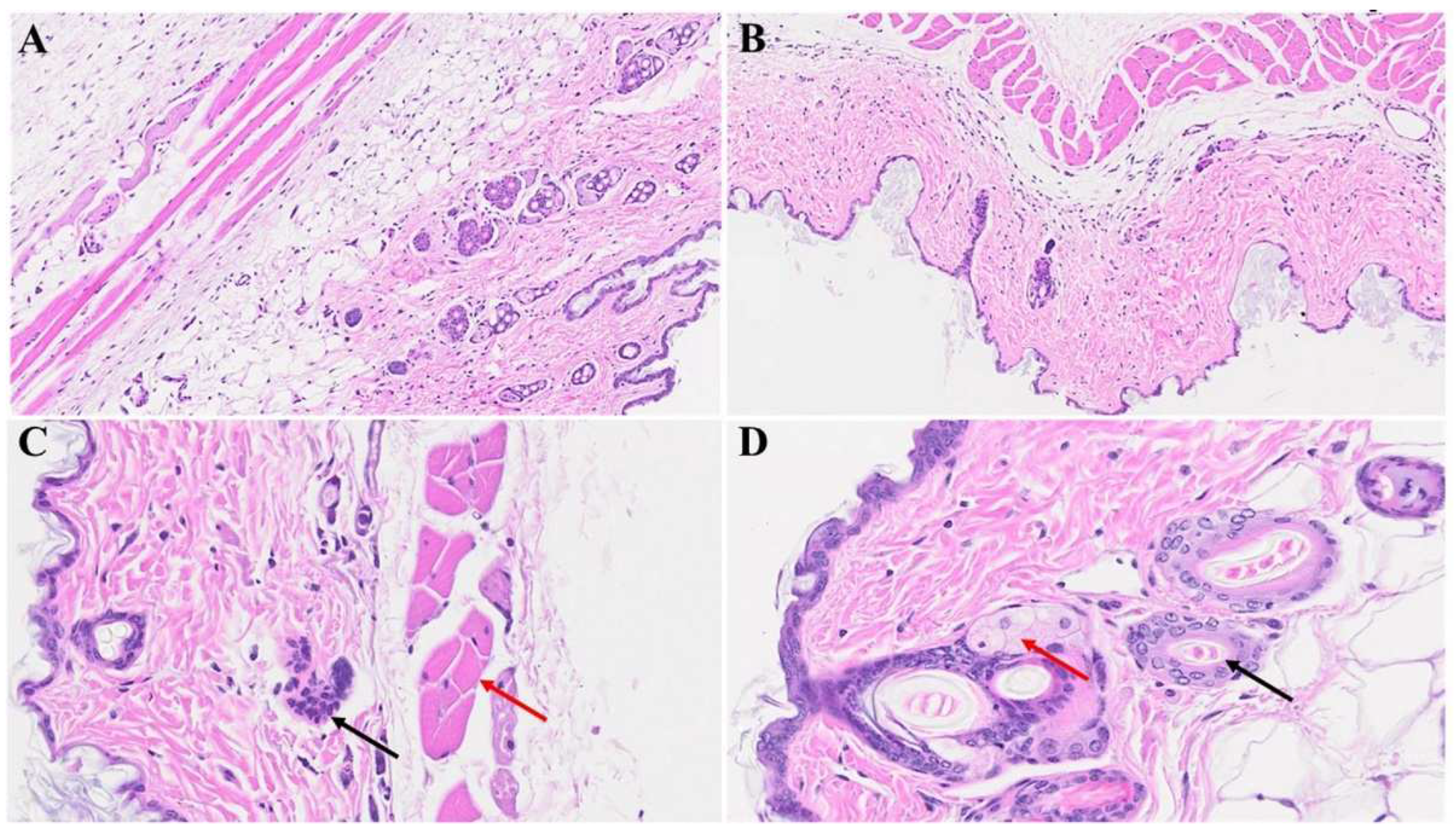
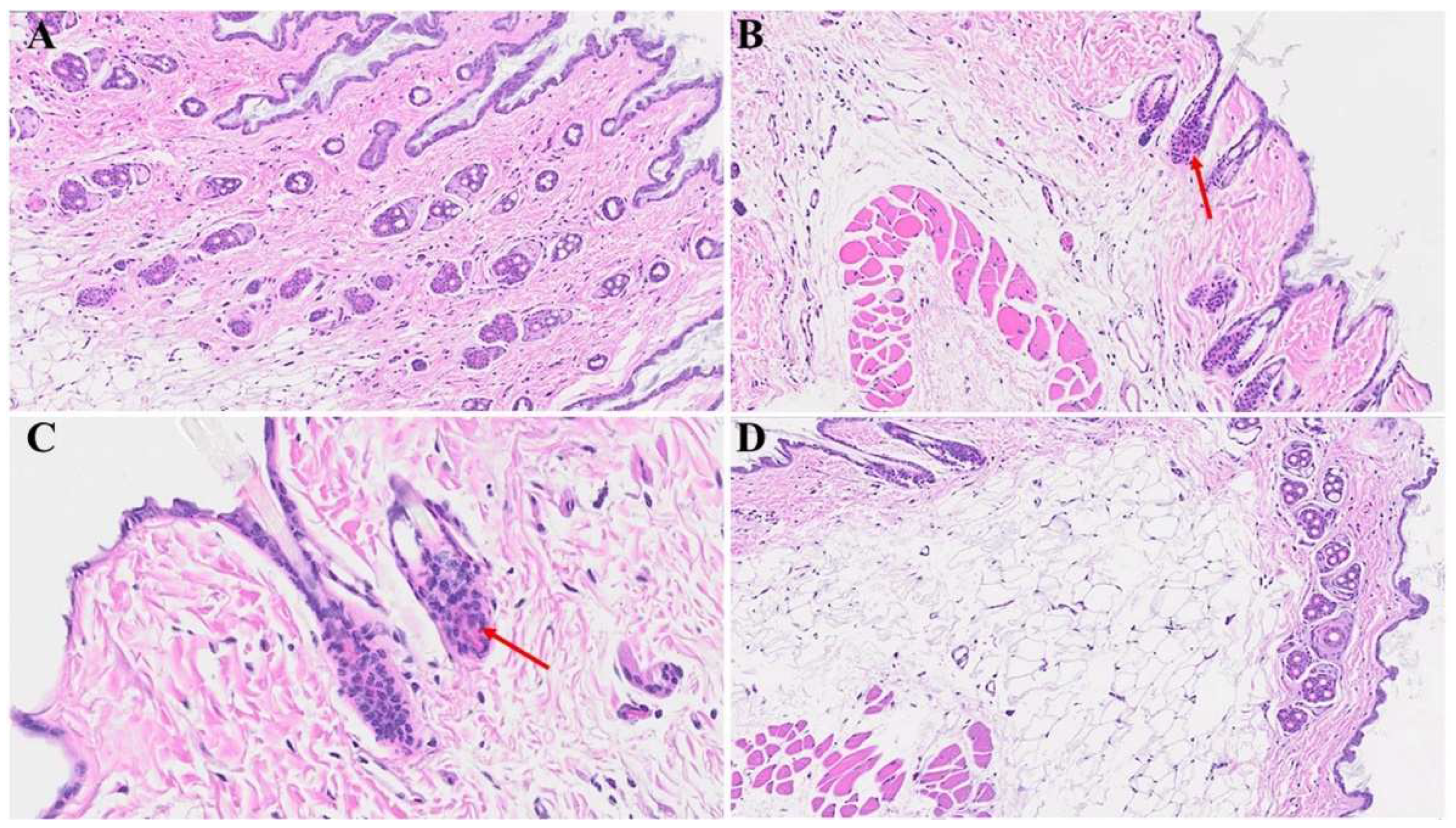
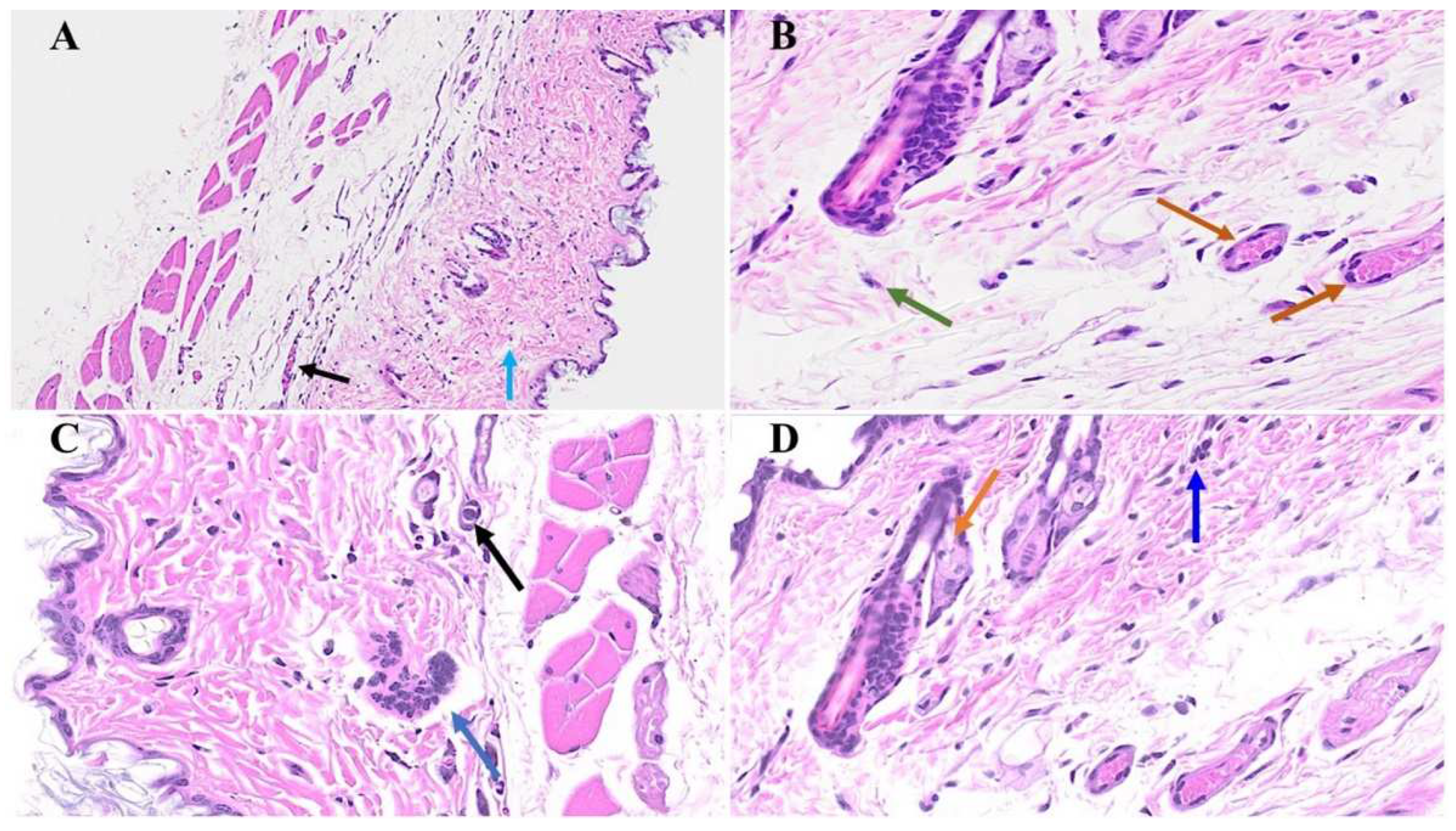
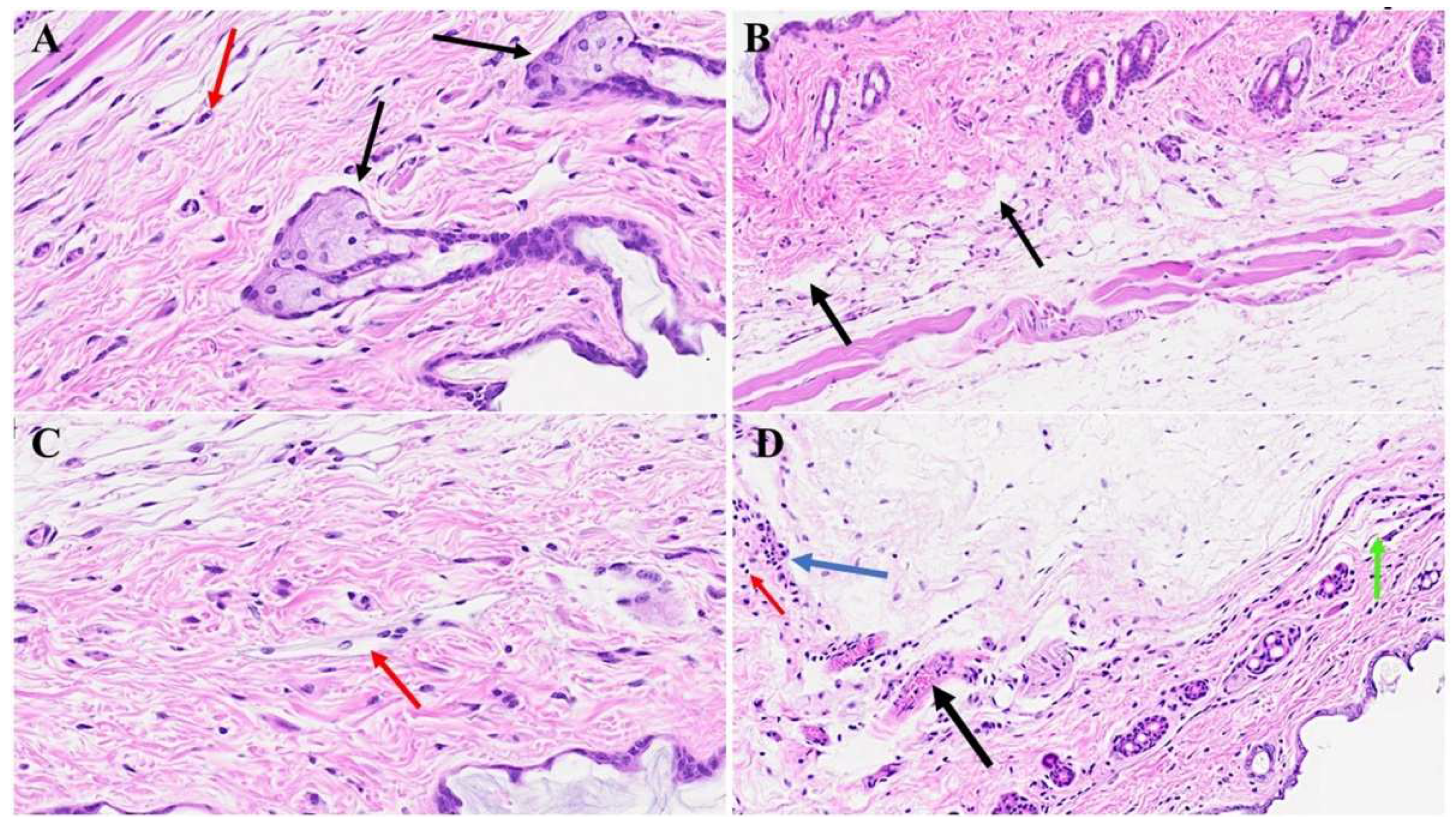
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Who Coronavirus (COVID-19) Dashboard. World Health Organization. Available online: https://covid19.who.int/ (accessed on 20 July 2023).
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms, and recommendations. Nat. Rev. Microbiol. 2023, 3, 133–146. [CrossRef]
- Sullivan, M.K.; Lees, J.S.; Drake, T.M.; Docherty, A.B.; Oates, G.; Hardwick, H.E. Acute Kidney Injury in Patients Hospitalized With COVID-19 From the ISARIC WHO CCP-UK Study: A Prospective, Multicentre Cohort Study Michael. Nephrol. Dial. Transplant. 2021, 27708, 1–19. [CrossRef]
- Mortaz, E.; Tabarsi, P.; Jamaati, H.; Dalil Roofchayee, N.; Dezfuli, N.K.; Hashemian, S.M.; Moniri, A.; Marjani, M.; Malekmohammad, M.; Mansouri, D.; et al. Increased Serum Levels of Soluble TNF-α Receptor Is Associated with ICU Mortality in COVID-19 Patients. Front. Immunol. 2021, 12, 592727. [CrossRef]
- Schiffl, H.; Lang, S.M. Long-term interplay between COVID-19 and chronic kidney disease. Int. Urol. Nephrol. 2023, 55, 1977–1984. [CrossRef]
- Kudose, S.; Batal, I.; Santoriello, D.; Xu, K.; Barasch, J.; Peleg, Y.; Canetta, P.; Ratner, L.E.; Marasa, M.; Gharavi, A.G.; Stokes MB, Markowitz GS, D’Agati VD. Kidney Biopsy Findings in Patients with COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1959–1968. [CrossRef]
- Zamoner, W.; Santos, C.A.D.S.; Magalhães, L.E.; de Oliveira, P.G.S.; Balbi, A.L.; Ponce, D. Acute Kidney Injury in COVID-19: 90 Days of the Pandemic in a Brazilian Public Hospital. Front. Med. 2021, 8, 622577. [CrossRef]
- Davis H.E.; McCorkell L.; Vogel J.M.; Topol E.J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023, 3, 133-146. [CrossRef]
- Sharma, P.; Uppal, N.N.; Wanchoo, R.; Shah, H.H.; Yang, Y.; Parikh, R.; Khanin, Y.; Madireddy, V.; Larsen, C.P.; Jhaveri, K.D.; Bijol V. COVID-19-associated kidney injury: A case series of kidney biopsy findings. J. Am. Soc. Nephrol. 2020, 31, 1948–1958. [CrossRef]
- Körner, R.W.; Majjouti, M.; Alcazar, M.A.A.; Mahabir, E. Of Mice and cMHV and Mouse Models as a Translational Approach to Understand SARS-CoV-2. Viruses 2020, 12, 880.
- De Albuquerque, N.; Baig, E.; Ma, X.; Zhang, J.; He, W.; Rowe, A.; Habal, M.; Liu, M.; Shalev, I.; Downey, G.P.; Gorczynski R.; Butany J.; Leibowitz J.; Weiss S.R.; McGilvray I.D.; Phillips M.J.; Fish E.N.; Levy G.A. Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. J. Virol. 2006, 80, 10382–10394. [CrossRef]
- Gong, H.H.; Worley, M.J.; Carver, K.A.; Goldstein, D.R.; Deng, J.C. Neutrophils drive pulmonary vascular leakage in MHV-1 infection of susceptible A/J mice. Front. Immunol. 2023, 13, 1089064. [CrossRef]
- Cox, G.; Gonzalez, A.J.; Ijezie, E.C.; Rodriguez, A.; Miller, C.R.; Van Leuven, J.T.; Miura, T.A. Priming With Rhinovirus Protects Mice Against a Lethal Pulmonary Coronavirus Infection. Front. Immunol. 2022, 13, 886611. [CrossRef]
- Gain, C.; Song, S.; Angtuaco, T.; Satta, S.; Kelesidis, T. The role of oxidative stress in the pathogenesis of infections with coronaviruses. Front. Microbiol. 2023, 13, 1111930. [CrossRef]
- Paidas, M.J.; Cosio, D.S.; Ali, S.; Kenyon, N.S.; Jayakumar, A.R. Long-Term Sequelae of COVID-19 in Experimental Mice. Mol. Neurobiol. 2022, 59, 5970–5986. [CrossRef]
- Paidas, M.J.; Sampath, N.; Schindler, E.A.; Cosio, D.S.; Ndubizu, C.O.; Shamaladevi, N.; Kwal, J.; Rodriguez, S.; Ahmad, A.; Kenyon, N.S.; Jayakumar A.R. Mechanism of Multi-Organ Injury in Experimental COVID-19 and Its Inhibition by a Small Molecule Peptide. Front. Pharmacol. 2022, 30, 864798. [CrossRef]
- Caldera-Crespo, L.A.; Paidas, M.J.; Roy, S.; Schulman, C.I.; Kenyon, N.S.; Daunert, S.; Jayakumar, A.R. Experimental Models of COVID-19. Front. Cell Infect. Microbiol. 2022, 11, 792584. [CrossRef]
- Paidas, M.J.; Mohamed, A.B.; Norenberg, M.D.; Saad, A.; Barry, A.F.; Colon, C.; Kenyon, N.S.; Jayakumar, A.R. Multi-Organ Histopathological Changes in a Mouse Hepatitis Virus Model of COVID-19. Viruses 2021, 13, 1703. [CrossRef]
- Gottlieb, M.; Long, B. Dermatologic manifestations and complications of COVID-19. The American journal of emergency medicine. 2020, 9, 1715-1721. [CrossRef]
- Gao, J. C.; Huang, A.; Desai, A.; Safai, B.; Marmon, S. “COVID toes”: A true viral phenomenon or a diagnosis without a leg to stand on?. JAAD international. 2022, 9, 1-6. [CrossRef]
- Muntean, I. A.; Pintea, I.; Bocsan, I. C.; Dobrican, C. T.; Deleanu, D. COVID-19 disease leading to chronic spontaneous urticaria exacerbation: a Romanian retrospective study. In Healthcare. 2021, 9, 1144. [CrossRef]
- Sameni, F.; Hajikhani, B.; Yaslianifard, S.; Goudarzi, M.; Owlia, P.; Nasiri, M. J.; Dadashi, M. COVID-19 and skin manifestations: an overview of case reports/case series and meta-analysis of prevalence studies. Frontiers in Medicine. 2020, 7, 573188. [CrossRef]
- Becker, R.C. COVID-19-associated vasculitis and vasculopathy. Journal of thrombosis and thrombolysis. 2020, 50, 499-511. [CrossRef]
- Iba, T.; Connors, J. M.; Levy, J. H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflammation Research. 2020, 69, 1181-1189. [CrossRef]
- Sahara, T.; Yokota, K. Livedo reticularis associated with COVID-19. Internal Medicine. 2022, 3, 441-441. [CrossRef]
- Jaimes, J. A.; Millet, J. K.; Whittaker, G. R. Proteolytic cleavage of the SARS-CoV-2 spike protein and the role of the novel S1/S2 site. IScience.2020, 6, 22-50. [CrossRef]
- Rajan, M.B.; Kumar, M.P.; Bhardwaj, A. The trend of cutaneous lesions during COVID-19 pandemic: lessons from a meta-analysis and systematic review. International Journal of Dermatology. 2020, 11, 1358-1370. [CrossRef]
- Tan, S.W.; Tam, Y.C.; Oh, C.C. Skin manifestations of COVID-19: a worldwide review. JAAD international. 2020, 2, 119-133. [CrossRef]
- Ramamoorthy, R.; Hussain H.; Ravelo, N.; Sriramajayam, K.; Di Gregorio, D.M.; Paulrasu K.; Chen, P.; Young, K.; Masciarella, A.D.; Jayakumar, A.R.; Paidas, M.J. Kidney Damage in Long COVID: Studies in Experimental Mice. Biology (Basel). 2023 30,1070. [CrossRef]
- Bancroft, J. D.; Gamble, M. Theory and Practice of Histological Techniques (6th ed.). Churchill Livingstone, Elsevier, China, 2008.
- Kiernan, J.A. Histological and Histochemical Methods: Theory and Practice (4th ed.). Cold Spring Harbor Laboratory Press, 2008.
- Strahan, A.G.; Lubov, J.E.; Prasad, S.; Fox, L.P.; McMahon, D.E.; Singh, R.; Rosenbach, M.; Desai, S.R.; Lim, H.W.; Thiers, B.H.; Hruza, G.J.; French, L.E.; Freeman, E.E. The impact of the American Academy of Dermatology/International League of Dermatological Societies COVID-19 Registry during the pandemic: 2500 cases across 72 countries. J Am Acad Dermatol. 2023, 89, e225-e227. [CrossRef]
- Jiang, L.; Tang, K.; Irfan, O.; Li, X.; Zhang, E.; Bhutta, Z. Epidemiology, clinical features, and outcomes of multisystem inflammatory syndrome in children (MIS-C) and adolescents—a live systematic review and meta-analysis. Current pediatrics reports. 2022, 2, 19-30. [CrossRef]
- Rafferty, M.S.; Burrows, H.; Joseph, J.P.; Leveille, J.; Nihtianova, S.; Amirian, E.S. Multisystem inflammatory syndrome in children (MIS-C) and the coronavirus pandemic: Current knowledge and implications for public health. J Infect Public Health. 2021, 4, 484-494. [CrossRef]
- Roe, K. A. viral infection explanation for Kawasaki disease in general and for COVID-19 virus-related Kawasaki disease symptoms. Inflammopharmacology. 2020, 28, 1219-1222. [CrossRef]
- Horesh, E.J.; Chéret, J.; Paus, R. Growth Hormone and the Human Hair Follicle. Int J Mol Sci. 2021, 8,13205. [CrossRef]
- Trüeb, R.M. Further Clinical Evidence for the Effect of IGF-1 on Hair Growth and Alopecia. Skin Appendage Disord. 2018, 4, 90-95. [CrossRef]
- Sawaya, M.E. Steroid chemistry and hormone controls during the hair follicle cycle. Ann N Y Acad Sci. 1991, 26, 642:376-83; discussion 383-4. [CrossRef]
- Hamada, K.; Thornton, M.J.; Laing, I.; Messenger, A.G.; Randall, V.A. The metabolism of testosterone by dermal papilla cells cultured from human pubic and axillary hair follicles concurs with hair growth in 5 alpha-reductase deficiency. J Invest Dermatol. 1996, 106,1017-22. [CrossRef]
- Babadjouni, A.; Reddy, M.; Zhang, R.; Raffi, J.; Phong, C.; Mesinkovska, N. Melatonin and the Human Hair Follicle. J Drugs Dermatol. 2023, 22, 260-264. [CrossRef]
- Heidelbaugh, J.J. Endocrinology Update: Hirsutism. FP Essent. 2016, 451, 17-24.
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal Effects on Hair Follicles. Int J Mol Sci. 2020, 28, 5342. [CrossRef]
- Holub, B.S.; Kloepper, J.E.; Tóth, B.I.; Bíro, T.; Kofler, B.; Paus, R. The neuropeptide galanin is a novel inhibitor of human hair growth. Br J Dermatol. 2012, 167, 10-6. [CrossRef]
- Zhu, H.L.; Gao, Y.H.; Yang, J.Q.; Li, J.B.; Gao, J. Serenoa repens extracts promote hair regeneration and repair of hair loss mouse models by activating TGF-β and mitochondrial signaling pathway. Eur Rev Med Pharmacol Sci. 2018, 22, 4000-4008.
- Randall, V.A.; Thornton, M.J.; Messenger, A.G.; Hibberts, N.A.; Loudon, A.S.; Brinklow, B.R. Hormones and hair growth: variations in androgen receptor content of dermal papilla cells cultured from human and red deer (Cervus elaphus) hair follicles. J Invest Dermatol. 1993, 101, 114S-120S. [CrossRef]
- Lei, V.; Petty, A.J.; Atwater, A.R.; Wolfe, S.A.; MacLeod, A.S. Skin Viral Infections: Host Antiviral Innate Immunity and Viral Immune Evasion. Front Immunol. 2020, 11, 593901. [CrossRef]
- De La Cruz, N.C.; Möckel, M.; Wirtz, L.; Knebel-Mörsdorf, D. Ex vivo Human Skin Infection with Herpes Simplex Virus 1. Bio Protoc. 2022, 12, e4411. [CrossRef]
- Knöpfel, N.; Noguera-Morel, L.; Latour, I.; Torrelo, A. Viral exanthems in children: A great imitator. Clin Dermatol. 2019, 37, 213-226. [CrossRef]
- Hussain, H.; Paidas, M.J.; Fadel, A.; Ramamoorthy, R.; Garcia, E.; Saadoon, Z.F.; Casmartino, E.; Mendez, L.; Williams, E.A.; Ruiz-Cordero, R.; Jayakumar, A.R. Prior viral infection determines the mode and severity of monkeypox virus. Int J Infect Dis. 2023, 131, 95-99. [CrossRef]
- Cunningham, A.L.; Carbone, F.; Geijtenbeek, T.B. Langerhans cells and viral immunity. Eur J Immunol. 2008, 38, 2377-85. [CrossRef]
- Hasannejad, H.; Takahashi, R.; Kimishima, M.; Hayakawa, K.; Shiohara, T. Selective impairment of Toll-like receptor 2-mediated proinflammatory cytokine production by monocytes from patients with atopic dermatitis. J Allergy Clin Immunol. 2007, 120, 69-75. [CrossRef]
- Kawamura, T.; Ogawa, Y.; Aoki, R.; Shimada, S. Innate and intrinsic antiviral immunity in skin. J Dermatol Sci. 2014, 75, 159-66. [CrossRef]
- Magro, C.; Crowson, A.N.; Franks, L.; Schaffer, P.R.; Whelan, P.; Nuovo, G. The histologic and molecular correlates of COVID-19 vaccine-induced changes in the skin. Clin Dermatol. 2021, 39, 966-984.
- Thangavel, H.; Dhanyalayam, D.; Lizardo, K.; Oswal, N.; Dolgov, E.; Perlin, D.S.; Nagajyothi, J.F. Susceptibility of Fat Tissue to SARS-CoV-2 Infection in Female hACE2 Mouse Model. Int J Mol Sci. 2023, 24, 1314. [CrossRef]
- Hofman, P.; Copin, M.C.; Tauziede-Espariat, A.; Adle-Biassette, H.; Fortarezza, F.; Passeron, T.; Salmon, I.; Calabrese, F. Les lésions histologiques associées à l’infection par le SARS-CoV-2 [Histopathological features due to the SARS-CoV-2]. Ann Pathol. 2021, 41, 9-22. [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006, 168, 1861-8. [CrossRef]
- Schmidtchen, A.; Carlstedt, I.; Malmström, A.; Fransson, L.A. Inventory of human skin fibroblast proteoglycans. Identification of multiple heparan and chondroitin/dermatan sulphate proteoglycans. Biochem J. 1990, 265, 289-300. [CrossRef]
- Lee, D.H.; Oh, J.H.; Chung, J.H. Glycosaminoglycan and proteoglycan in skin aging. J Dermatol Sci. 2016, 83, 174-81. [CrossRef]
- Metze, D.; Bhardwaj, R.; Amann, U.; Eades-Perner, A.M.; Neumaier, M.; Wagener, C.; Jantscheff, P.; Grunert, F.; Luger, T.A. Glycoproteins of the carcinoembryonic antigen (CEA) family are expressed in sweat and sebaceous glands of human fetal and adult skin. J Invest Dermatol. 1996, 106, 64-9. [CrossRef]
- Makihara, H.; Hidaka, M.; Sakai, Y.; Horie, Y.; Mitsui, H.; Ohashi, K.; Goshima, Y.; Akase, T. Reduction and fragmentation of elastic fibers in the skin of obese mice is associated with altered mRNA expression levels of fibrillin-1 and neprilysin. Connect Tissue Res. 2017, 58, 479-486. [CrossRef]
- Li, H.; Zhao, Y.; Zhou, L.; Hu, J. Cutaneous, skin histopathological manifestations and relationship to COVID-19 infection patients. Dermatol Ther. 2020, 33, e14157. [CrossRef]
- Huang, J.; Heng, S.; Zhang, W.; Liu, Y.; Xia, T.; Ji, C.; Zhang, L.J. Dermal extracellular matrix molecules in skin development, homeostasis, wound regeneration and diseases. Semin Cell Dev Biol. 2022, 128, 137-144. [CrossRef]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022, 29,1161-1180. [CrossRef]
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The Extracellular Matrix in Skin Inflammation and Infection. Front Cell Dev Biol. 2021, 6, 682414. [CrossRef]
- Daraban Bocaneti, F.; Altamura, G.; Corteggio, A.; Tanase, O.I.; Dascalu, M.A.; Pasca, S.A.; Hritcu, O.; Mares, M.; Borzacchiello, G. Expression of matrix metalloproteinases (MMPs)-2/-7/-9/-14 and tissue inhibitors of MMPs (TIMPs)-1/-2 in bovine cutaneous fibropapillomas associated with BPV-2 infection. Front Vet Sci. 2022, 28, 1063580. [CrossRef]
- Alpantaki, K.; Zafiropoulos, A.; Tseliou, M.; Vasarmidi, E.; Sourvinos, G. Herpes simplex virus type-1 infection affects the expression of extracellular matrix components in human nucleus pulposus cells. Virus Res. 2019, 259, 0-17. [CrossRef]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J Biol Chem. 2022, 298, 101530. [CrossRef]
- Chen, J.; Chen, J.K.; Nagai, K.; Plieth, D.; Tan, M.; Lee, T.C.; Threadgill, D.W.; Neilson, E.G.; Harris, R.C. EGFR signaling promotes TGFβ-dependent renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 215–224. [CrossRef]
- Sisto, M.; Ribatti, D.; Lisi, S. Organ Fibrosis and Autoimmunity; The Role of Inflammation in TGFβ-Dependent EMT. Biomolecules 2021, 11, 310. [CrossRef]
- Bao, M.; Feng, Q.; Zou, L.; Huang, J.; Zhu, C.; Xia, W. Endoplasmic reticulum stress promotes endometrial fibrosis through the TGF-β/SMAD pathway. Reproduction 2023, 165, 171–182. [CrossRef]
- Guo, Y.; Tian, G.; Chen, X.; Hou, Y.; Zhang, X.; Xue, X.; Zhao, L.; Wu, Y. GL-V9 ameliorates liver fibrosis by inhibiting TGF-β/smad pathway. Exp. Cell Res. 2023, 425, 113521. [CrossRef]
- Zhang, T.; He, X.; Caldwell, L.; Goru, S.K.; Ulloa Severino, L.; Tolosa, M.F.; Misra, P.S.; McEvoy, C.M.; Christova, T.; Liu, Y.; Atin C.; Zhang J.; Hu C.; Vukosa N.; Chen X.; Krizova A.; Kirpalani A.; Gregorieff A.; Ni R.; Chan, K.; Gill, M.K.; Attisano, L.; Wrana, J.L.; Yuen, D.A. NUAK1 promotes organ fibrosis via YAP and TGF-β/SMAD signaling. Sci. Transl. Med. 2022, 14, eaaz4028. [CrossRef]
- Laloglu, E.; Alay, H. Role of transforming growth factor-beta 1 and connective tissue growth factor levels in coronavirus disease-2019-related lung Injury: a prospective, observational, cohort study. Rev Soc Bras Med Trop. 2022, 55, e06152021. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



