Introduction
Bilaterally symmetrical organisms can present different kind of asymmetries: Fluctuating Asymmetry (FA), Directional Symmetry (DA) and Antisymmetry (AS) (
Graham, Freeman, and Emlen 1993;
Graham et al. 2010). FA is defined as the deviation of the symmetry of an individual from its ideal symmetry. It is considered a negative indicator of the individual’s ability to resist random and small developmental accidents (Palmer 1996). These developmental accidents are commonly the result of genetic or environmental stress (
Auffray, Debat, and Alibert 1999;
Carter, Osborne, and Houle 2009). Inbreeding has been linked to reduced health of populations and consequently on body symmetries. DA occurs whenever there is a greater development of a character on one side than the other (
Kharlamova et al., 2010;
Lotto & Béguelin, 2014). DA have normally a genetic basis (
Leśniak 2018). Finally, AS is a bimodal asymmetry that is random with respect to side, where the lack of symmetry is distinguished by a departure from a Gaussian distribution of side differences (
Mancini, Sally, and Gurnsey 2005).
For the quantification of these asymmetries size and shape must be assessed separately
Sforza et al. 1998). Geometric morphometric approaches have been used for this purpose.
Chelonoidis carbonarius is a tortoise species from S America, distributed in open areas (
Barros et al. 2012;
Rhodin et al. 2017) from N Paraguay and Argentina through cis-Andean to Panama (
Gallego-García et al. 2015;
Cacciali et al. 2016;
Rhodin et al. 2017;
Turtle Taxonomy Working Group, 2017). They are medium-sized tortoises which can reach over 40 cm (
Barros et al. 2012), having dark-coloured, loaf-shaped carapaces with a lighter patch in the middle of each scute, although they present a great variation in morphological characteristics (
Barros et al. 2012;
Gallego-García, Cárdenas-Arévalo, and Castaño-Mora 2015). No size sex dimorphism has been described (
Barros et al. 2012), but morphological population differences are recognized in the species between areas, mainly in plastron scutes, carapace width, and head length (
Barros et al. 2012).
Chelonoidis is frequently kept as pet, and over-collection as well as habitat destruction have caused them to be close to extinction (
Gallego-García, Cárdenas-Arévalo, and Castaño-Mora 2015), appearing as “Vulnerable” in the 2011 TFTSG Draft Red List (
Rhodin et al. 2017).
In the present study we analyse and compare scutation asymmetries among sexes in the carapace of wild red-footed tortoise (Chelonoidis carbonarius) from Arauca plains using geometric morphometric techniques. The anthropogenic disturbance in the habitat brought about by the continuous human exploitation of Arauca plains contributes to the deterioration of the quality of the environment, subjecting C. carbonarius to ecological stress and thereby, promoting developmental instability in the species, but to date, not much attention has been paid to FA sexual differences in C. carbonarius. Our data pretends to compare a possible different sexual homeostasis in this species.
Methods
Area sampling
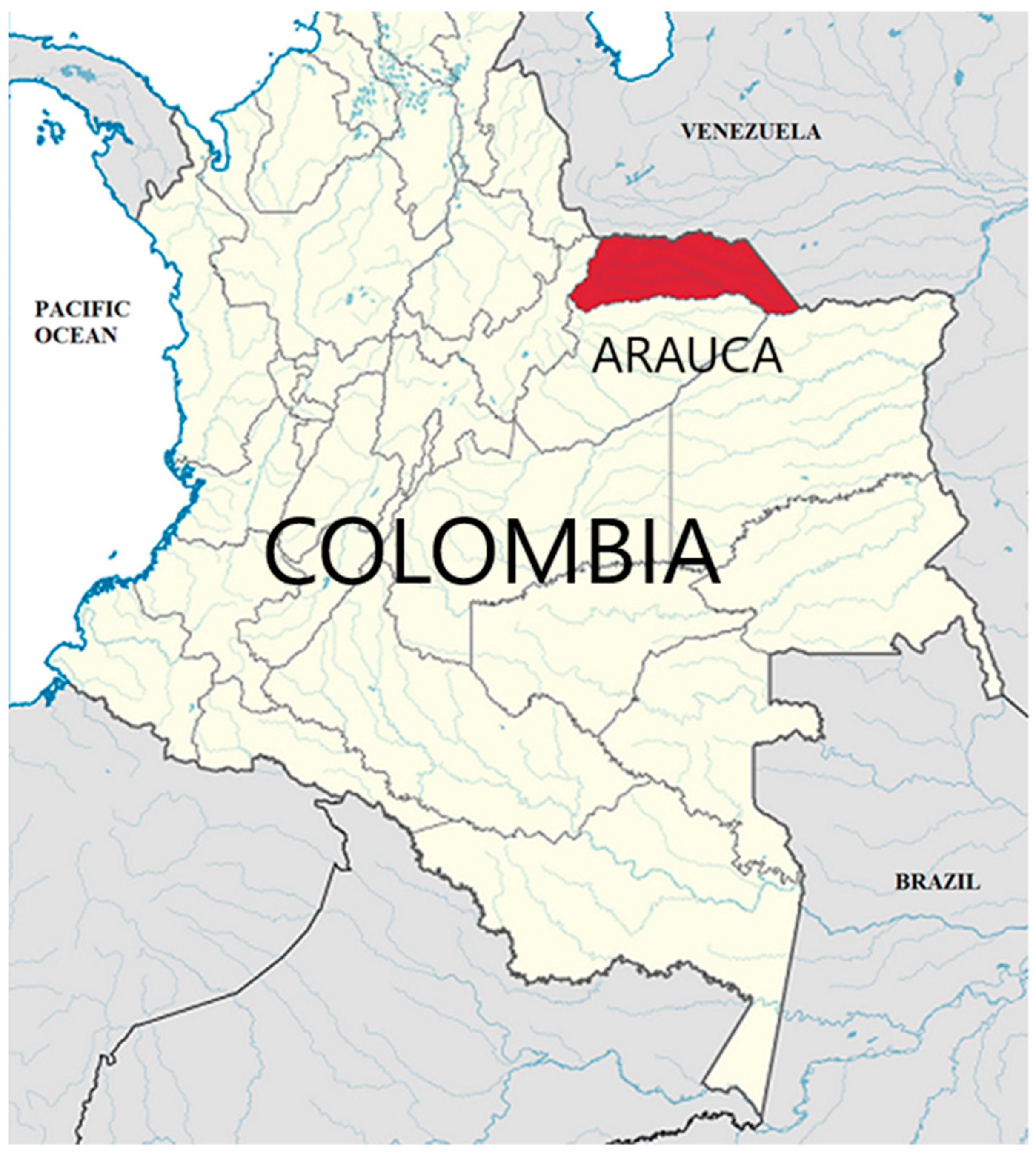
Arauca is a department of Colombia located on the Orinoco Basin (the “Llanos Orientales”) in the extreme East, bordering Venezuela, located between the Arauca and Casanare rivers, and covering a huge area of 23,818 Km
2 (
Figure 1). Seventy-five per cent of the land is flat. The temperature varies from 72°F to 97°F. This area receives low touristic impact, being waste dumped by multinational crop companies are its main threat.
Data collection
Data was obtained from 45 wild adult individuals (13 males, 34.8-53.7 cm total carapace length; and 32 females, 20.2-47.2 cm total carapace length) of Chelonoidis carbonarius, during the dry season of February-March 2018. All the field-collected specimens displayed no injuries by predators or additional scutes. Digital pictures were obtained in the field, holding the camera approximately 40-50 cm above each tortoise, with the camera focused on-centre on their central dorsal aspect. We only used the carapacial scores because the carapacial photographs are less susceptible to tilting while taking the photograph (the animal can rest flat and inside its shell). A single digital photograph of each animal was obtained. To prevent distortion and optical aberrations no zoom was used, and the tortoises were maintained without inclination, using props to reduce parallax errors. Identification of sex was based on the presence or absence of a pronounced plastron concavity. A ruler was inserted previously on each image. After their measurement and photographing, animals were released at the same site of capture.
Geometric morphometrics
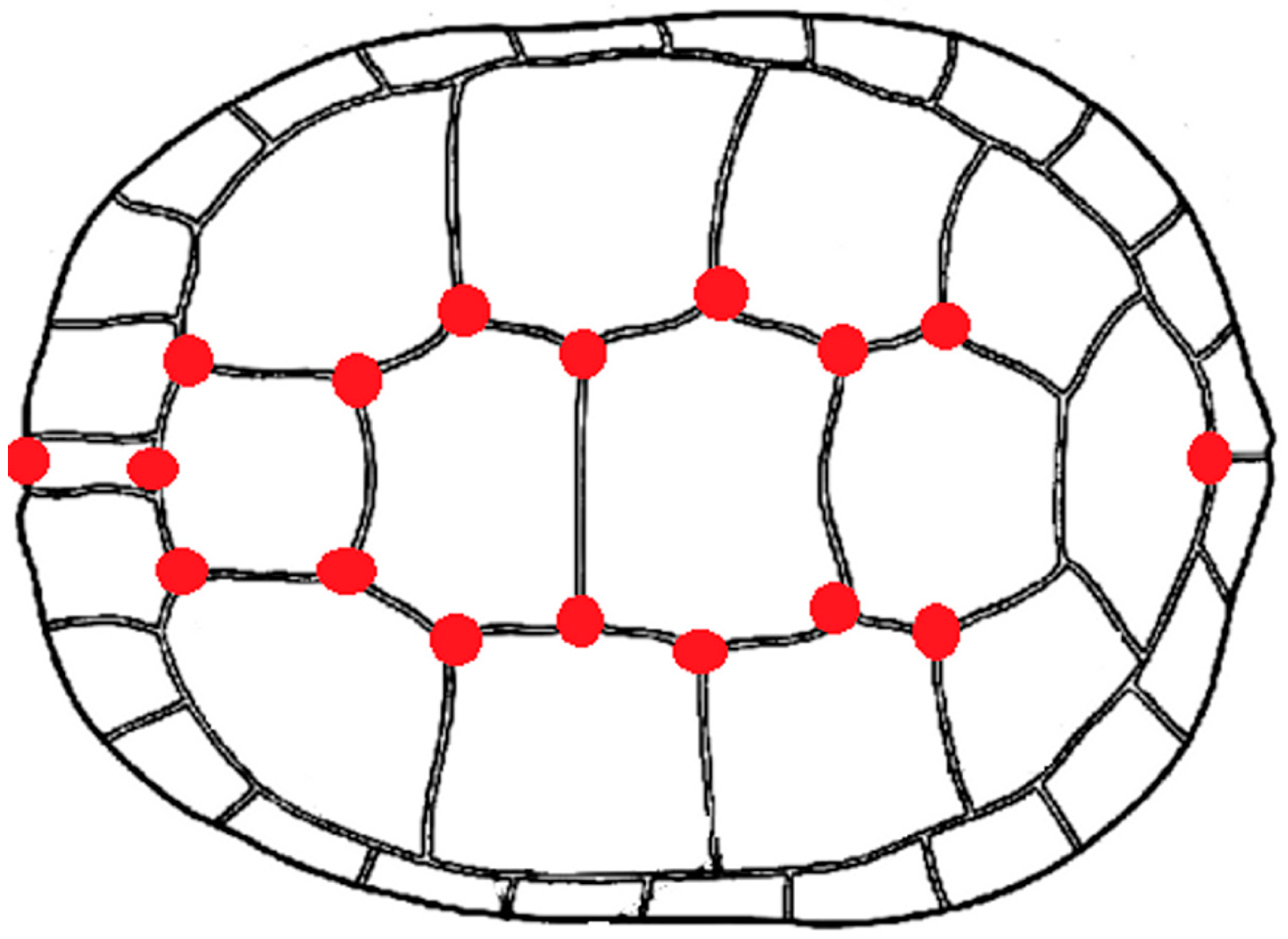
A landmark configuration based on dorsal scute sutures of the carapace, of 7 symmetric pairs with 3 landmarks along the axial plane was tested (
Figure 2). Three types of landmarks can be recognized. Our landmarks were of type I, e.g., localized in the intersection of sutures and so easy to identify repetitively. The captured images were transformed to TpsUtil v. 1.40 software (
Rohlf 2015a) and landmarks recorded using TpsDig v. 2.26 software (
Rohlf 2010). Scale was eliminated by setting the centroid size (the square root of the sum of squared distance between each landmark and the carapace centroid) the same in all specimens (
Bookstein 1991). Because measurement error may potentially either hide or bias real differences between sides (
Palmer 1994), the first author repeated the measurements for all traits in all samples twice to lessen inconsistencies or error in plotting landmark points. The set of original configurations and mirrored copies (including the replicated measurements) were then superimposed (scaled, translated, and rotated) following the Procrustes method of generalized least squares superimposition.
Shape spaces are curved, non-Euclidean spaces. To apply the usual methods of statistics, it is necessary to project shape space onto linear Euclidean space so the distribution of points in the tangent space can be used as a good approximation of their distribution in shape space (
Klingenberg and McIntyre 1998). As the process of extracting tangent landmark coordinates from curved objects can introduce an error due to non-coplanarity (
Klingenberg and McIntyre 1998;
Webster and Sheets 2010) we tested whether the observed shape variation was sufficiently small. This analysis was done with TpsSmall v. 1.33 software (
Rohlf 2015b).
The asymmetrical component was quantified as a difference between the original and the ideally symmetrical configuration based on the displacement of unpaired 3 landmarks along the mid-sagittal axis and averaged right and left 7 paired landmarks moving in any direction. For the quantification of asymmetry, a displacement of unpaired landmarks is allowed in the direction perpendicular to the mid-sagittal axis while paired landmarks move freely. The analysis done were specifically for object symmetry –with the symmetry axis passing through the structure-.
Statistical analyses
A multivariate regression of the asymmetric component against centroid size to detect allometry was also done for each sex. There was no change of asymmetry according to size, shape asymmetric variables and centroid size being found to be largely uncorrelated in the sample (p=0.0661; 10,000 randomization rounds).
To examine the amount of asymmetric variation we used a Procrustes two-way mixed model ANOVA (
Klingenberg, McIntyre, and Zaklan 1998;
Klingenberg and Monteiro 2005) for each sex and for shape and shape separately. The “individuals” effect is the individual variation of size/shape. The main effect is “sides” which indicates the variation between sides. The “individual*side interaction” is the mixed effect, the failure of the individuals to be the same between sides (left and right). Lastly, measurement error, estimated from the total variation of the entire landmark configuration was included. The “individuals” effect is the variation among individual samples and can be interpreted as symmetry variation. The effect of “individual*side interaction” is the measure of FA and the effect of “side” is the measure of DA (
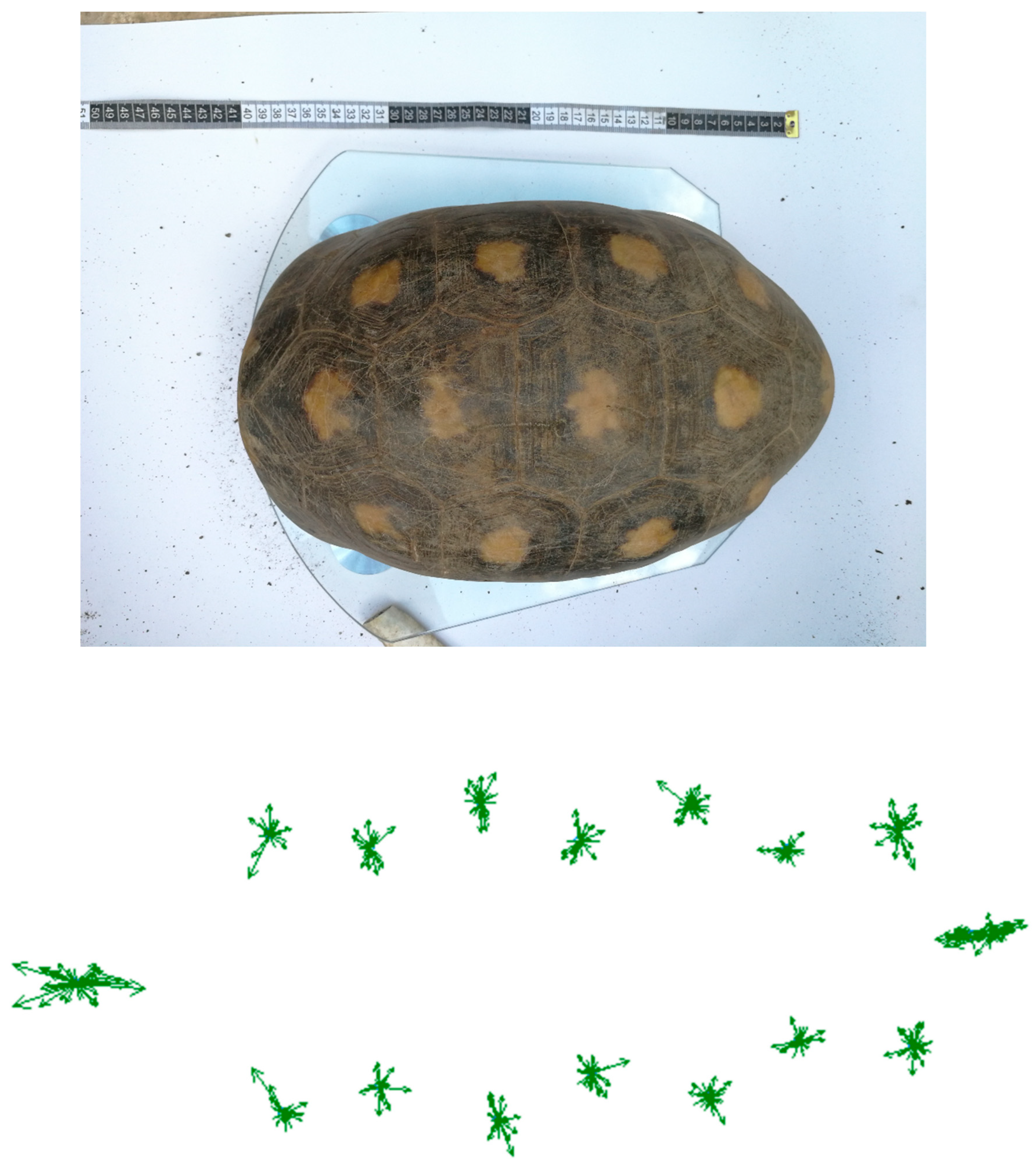
Klingenberg 2002). To check the data for AS, we visually examined scatter plots of vectors of left-right differences for each landmark after superimposition by the Procrustes algorithm. This vector plot depicts displacement of all landmarks between the target form to those on the reference form. Analytical procedures that incorporate geometric morphometrics in testing FA are available from numerous seminal publications (
Klingenberg, McIntyre, and Zaklan 1998;
Klingenberg 2002;
Klingenberg and Monteiro 2005). Finally, a Canonical Variate Analysis (CVA) between sexes was done for asymmetric component. Mahalanobis distances was used for this test.
All analyses were done with MorphoJ v. 1.07a (Klingenberg 2011), computed as permutation tests with 10.000 permutation runs. For all analyses, α=0.05.
Results
Although the carapace is markedly convex, when we compared the 2D Procrustes distances to the tangent space distances (i.e., Euclidean distances in tangent space), the relation was very close to linear for all the data, e.g., it appeared an excellent correlation between the tangent and the shape space (correlation between the tangent space onto Procrustes distance=0.999034). This result proved the acceptability of the data set for further statistical analysis in the Euclidean space.
At the beginning, intraobserver error, which is associated with the placement of landmarks, was evaluated. The results showed that variation among samples (MS =0.0003500863) were higher than between double distribution of landmarks (MS = 0.0000001508); hence, high intraobserver agreement was ascertained.
The absence of clustering of vectors (as the equivalent to bimodal distributions of left-right differences) provided no support for a presence of AS (
Figure 3). Procrustes ANOVA shows the mean squares for the mixed-model ANOVA and its corresponding effects (
Table 1). The significative interaction of “individual*side” confirmed the presence of presence of FA for both sexes but side factor (indicating DA) was not detected to be statistically significant. CVA reflected statistically significative differences between sexes (p<0.0001; 10,000 permutation rounds).
Discussion
Developmental instability (DI) refers to the organism’s inability to buffer the perturbation of a developmental process in given genetic and/or environmental conditions (
Jung, Woo, and Pak 2016). Many studies have shown that DI can be caused by genetic and environmental stresses such as nutritional deficiencies, genetic diseases and parasitosis (
Jung, Woo, and Pak 2016). Fluctuating asymmetry (FA, random departures from perfect symmetry) has been established as an indicator of developmental stress, inferring a measurable DI (
Graham et al. 2010;
Jung, Woo, and Pak 2016).
The use of geometric morphometrics plus multivariate statistical techniques, which yielded a low measurement error, provided us clear information on the carapacial asymmetry of adult males and females of Chelonoidis carbonarius. Our results reflected a significative FA for both sexes.
To date, available studies of asymmetries among testudines are scarce and sometimes with contradictory conclusions. For instance, Dillard (
Dillard 2017) found no shell symmetric differences among two populations of
Pseudemys species (
P. concinna and
P. rubriventris) but Davis & Grosse found FA in
Trachemys scripta (
Davis & Grosse, 2008), as did
Băncilă et al. (2012) in
Testudo graeca ibera, while Buicǎ & Cogǎlniceanu (
Buicǎ and Cogǎlniceanu 2013) detected low levels of FA in this same species. Cherepanov (
Cherepanov 2014) described 81% of asymmetrical pattern in a sample which included
Mauremys caspica, Emys orbicularis, Testudo graeca and
Agrionemys horsfieldi and
Goessling et al. (2017) detected it in
Gopherus polyphemus. The lack of relationship between asymmetry and body size, another conclusion of our study, has been observed in studies on other species (
Băncilă et al. 2012;
Buicǎ and Cogǎlniceanu 2013;
Goessling et al. 2017).
Considering that FA is the product of cumulated effects from stresses over the development history, detected levels of FA demonstrate an inability to buffer developmental noise and achieve homeostasis. In any case, as animals were sampled from different areas in Arauca and no consanguinity -a possible developmental perturbation- was expected, our results suggest a high degree of stress and highlight that human intervention in Arauca are affecting wildlife.
Conclusions
Wild Chelonoidis carbonarius from Arauca plains, East Colombia, present a fluctuating symmetry on its carapace with sex differences. These results may suggest a high degree of stress and highlight that human intervention in Arauca are affecting differently sexes. Globally, results remark the need for increased conservation and management of red-footed tortoises in populations in Arauca that are experiencing reduced fitness. Similar studies in the future, correlated with an estimate of human impact, could provide proofs of causality between developmental instabilities and environmental stressors.
Author Contributions
PMPC and ASC designed the experiments, analysed the data, and wrote the paper. PB and DC performed the field study. All authors consent the publication of this manuscript.
Funding
The study was not funded.
Institutional Review Board Statement
The study implied a minimal and atraumatic use of animals, which were merely photographed on their normal position and immediately released. The research did imply no more manipulations.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the first author on reasonable request.
Acknowledgments
The authors wish to declare our most sincere acknowledgement to Editor and the anonymous reviewer for helpful comments on the manuscript, as well as to Araucan owners of “fincas” who allowed the access to their properties for collecting turtles.
Conflicts of Interest
Authors have no competing interests.
Abbreviations
AS: Antisymmetry; DA: Directional Asymmetry; DI: Developmental Instability; FA: Fluctuating Asymmetry.
References
- Auffray, J.C., V. Debat, and P. Alibert. 1999. “Shape Asymmetry and Developmental Stability.” In “On Growth and Form: Spatio-Temporal Pattern Formation in Biology”, ed. J.C. McLachlan Mark A.J. Chaplain, G.D. Singh. New York: John Wiley and Sons Ltd, 309–324.
- Băncilă, R.I. et al. 2012. “Fluctuating Asymmetry in the Eurasian Spur-Thighed Tortoise, Testudo graeca ibera Linneaus, 1758 (Testudines: Testudinidae).” Chelonian Conservation and Biology 11(2): 234–39.
- Barros, M.S., L.C. Resende, A.G. Silva, and P.D. Ferreira Junior. 2012. “Morphological Variations and Sexual Dimorphism in Chelonoidis carbonaria (Spix, 1824) and Chelonoidis denticulata (Linnaeus, 1766) (Testudinidae).” Brazilian Journal of Biology 72(1): 153–61. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1519-69842012000100018&lng=en&tlng=en.
- Bookstein, F.L. 1991. Morphometric Tools for Landmark Data: Geometry and Biology Morphometric Tools for Landmark Data: Geometry and Biology. ed. Cambridge University Press. Cambridge.
- Buicǎ, G., and D. Cogǎlniceanu. 2013. “Using Digital Images in the Study of Fluctuating Asymmetry in the Spur-Thighed Tortoise Testudo graeca.” Turkish Journal of Zoology 37(6): 723–29.
- Cacciali, P. et al. 2016. “The Reptiles of Paraguay: Literature, Distribution, and an Annotated Taxonomic Checklist.” Special Publication of the Museum of Southwestern Biology 11: 1–373.
- Carter, A.J.R., E. Osborne, and D. Houle. 2009. “Heritability of Directional Asymmetry in Drosophila melanogaster.” International Journal of Evolutionary Biology: 1–7. http://www.hindawi.com/journals/ijeb/2009/759159/.
- Cherepanov, G.O. 2014. “Patterns of Scute Development in Turtle Shell: Symmetry and Asymmetry.” Paleontological Journal 48(12): 1275–83. http://link.springer.com/10.1134/S0031030114120028.
- Davis, A.K., and A.M. Grosse. 2008. “Measuring Fluctuating Asymmetry in Plastron Scutes of Yellow-Bellied Sliders: The Importance of Gender, Size and Body Location.” The American Midland Naturalist 159(2): 340–48.
- Dillard, K.C. 2017. “A Comparative Analysis of Geometric Morphometrics across Two Pseudemys Turtle Species in East Central Virginia.” Virginia Commonwealth University.
- Gallego-García, N., G. Cárdenas-Arévalo, and O.V. Castaño-Mora. 2015. “Chelonoidis carbonaria (Spix 1824).” In Libro Rojo de Reptiles En Colombia, ed. B.C. Bock M.A. Morales-Betancourt, C.A. Lasso, V.P. Páez. Bogotá, Colombia: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, 406–11.
- Goessling, J.M., Rebois, K., Godwin, J.C., Birkhead, R., Murray, C.M., Hermann, S.M. 2017. Differences in Fluctuating Asymmetry Among Four Populations of Gopher Tortoises (Gopherus polyphemus). Herpetological Conservation and Biology 12(2): 548–555.
- Graham, J.H., D.C. Freeman, and J.M. Emlen. 1993. “Antisymmetry, Directional Asymmetry, and Dynamic Morphogenesis.” Genetica 89(1–3): 121–37.
- Graham, J.H., S. Raz, H. Hel-Or, and E. Nevo. 2010. “Fluctuating Asymmetry: Methods, Theory, and Applications.” Symmetry 2: 466–540.
- Jung, H., E.J. Woo, and S. Pak. 2016. “A Comparison of Cranial Fluctuating Asymmetry between the Two Sexes in a Joseon Dynasty Population of Korea.” Anthropologischer Anzeiger 73(3): 215–23.
- Kharlamova, A. et al. 2010. “Directional Asymmetry in the Limbs, Skull and Pelvis of the Silver Fox (V. vulpes).” Journal of Morphology 271: 1501–8.
- Klingenberg, C.P. 2002. “Morphometrics and the Role of the Phenotype in Studies of the Evolution of Developmental Mechanisms.” Gene 287(1–2): 3–10.
- ———. 2011. “MorphoJ: An Integrated Software Package for Geometric Morphometrics.” Molecular Ecology Resources 11(2): 353–57.
- Klingenberg, C.P., and G.S. McIntyre. 1998. “Geometric Morphometrics of Developmental Instability: Analyzing Patterns of Fluctuating Asymmetry with Procrustes Methods.” Evolution 52(5): 1363.
- Klingenberg, C.P., G.S. McIntyre, and S.D. Zaklan. 1998. “Left-Right Asymmetry of Fly Wings and the Evolution of Body Axes.” Proceedings of the Royal Society B: Biological Sciences 265(February): 1255–59.
- Klingenberg, C.P., and L.R. Monteiro. 2005. “Distances and Directions in Multidimensional Shape Spaces: Implications for Morphometric Applications.” Systematic Biology 54(4): 678–88.
- Leśniak, K. 2018. “Directional Asymmetry of Facial and Limb Traits in Horses and Ponies.” Veterinary Journal 198(1): 46–51.
- Lotto, F., and M. Béguelin. 2014. “Asimetría Direccional Del Postcráneo En Poblaciones Prehispánicas Del Sur de Sudamérica.” Antropología Biológica 7(1): 133–42.
- Mancini, S., S.L. Sally, and R. Gurnsey. 2005. “Detection of Symmetry and Anti-Symmetry.” Vision Research 45(16): 2145–60.
- Palmer, A.R. 1994. “Fluctuating Asymetry Analysis: A Primer.” In Developmental Instability: Its Origins and Evolutionary Implications, Kluwer: T. A. Markow (ed.).
- ———. 1996. “From Symmetry to Asymmetry: Phylogenetic Patterns of Asymmetry Variation in Animals and Their Evolutionary Significance.” Proceedings of the National Academy of Sciences of the United States of America 93(25): 14279–86.
- Rhodin, A.G.J., Iverson, J.B., Bour, R., Fritz, U., Geogres, A.R., Shaffer, B., van Dijk, P.P. 2017. Turtles of the World: Annotated Checklist and Atlas of Taxonomy, Synonomy, Distribution, and Conservation Status. New York: Chelonian Research Foundation and Turtle Conservancy.
- Rohlf, F.J. 2010. Digitalized Landmarks and Outlines. New York: Stony Brook: Department of Ecology and Evolution, State University of New York.
- ———. 2015a. “The Tps Series of Software.” Hystrix 26(1): 9–12. http://life.bio.sunysb.edu/morph/.
- ———. 2015b. “TpsSmall v. 1.33.” http://life.bio.sunysb.edu/morph/.
- Sforza, C. et al. 1998. “Foot Asymmetry in Healthy Adults: Elliptic Fourier Analysis of Standardized Footprints.” Journal of Orthopaedic Research 16(6): 758–65.
- Webster, M., and H.D. Sheets. 2010. “A Practical Introduction to Landmark-Based Geometric Morphometrics.” In Quantitative Methods in Paleobiology, ed. John Alroy and Gene Hunt. The Paleontological Society, 163–88.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).