Submitted:
29 December 2023
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
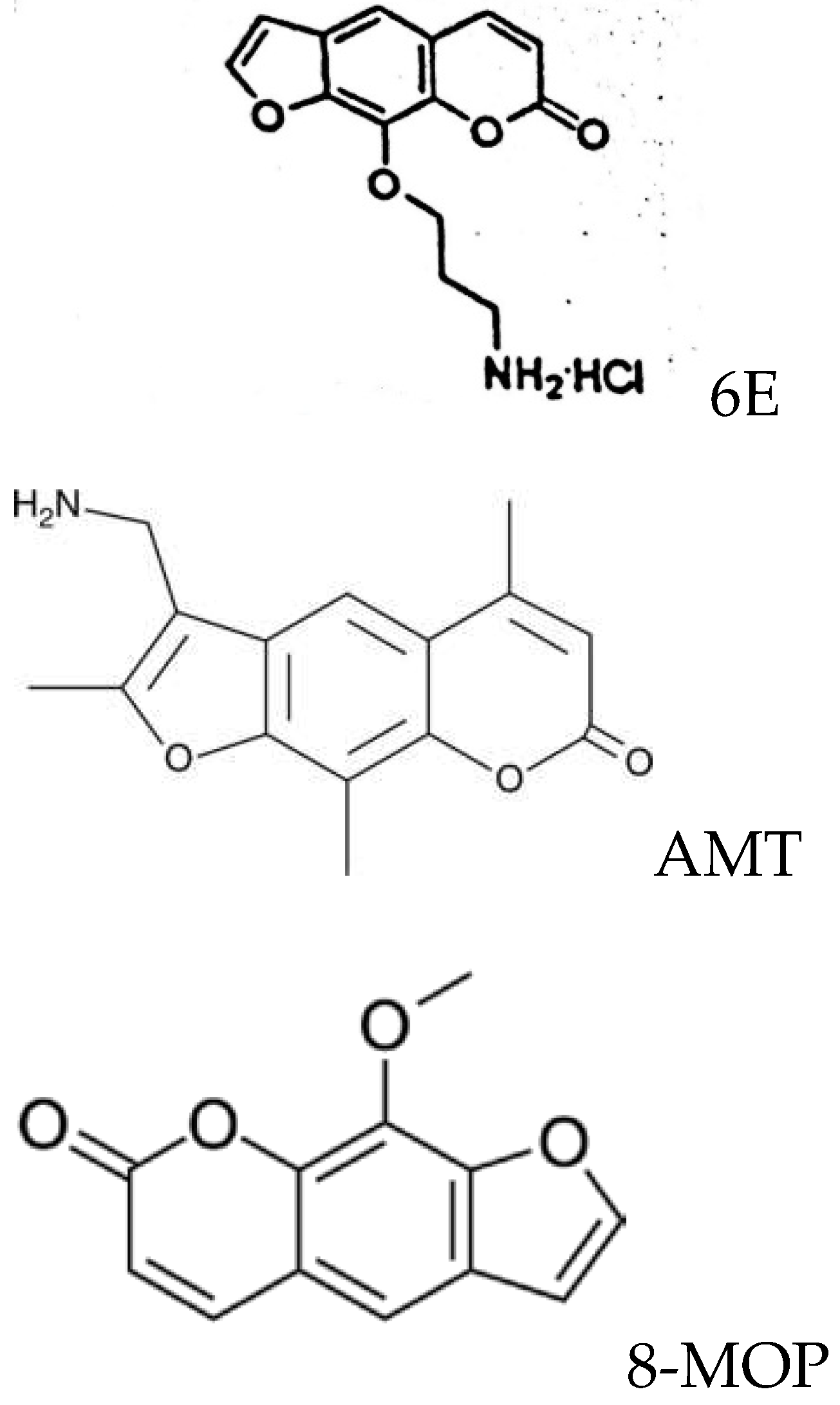
1. Introduction
2. Materials and Methods
3. Results
3.1. Values
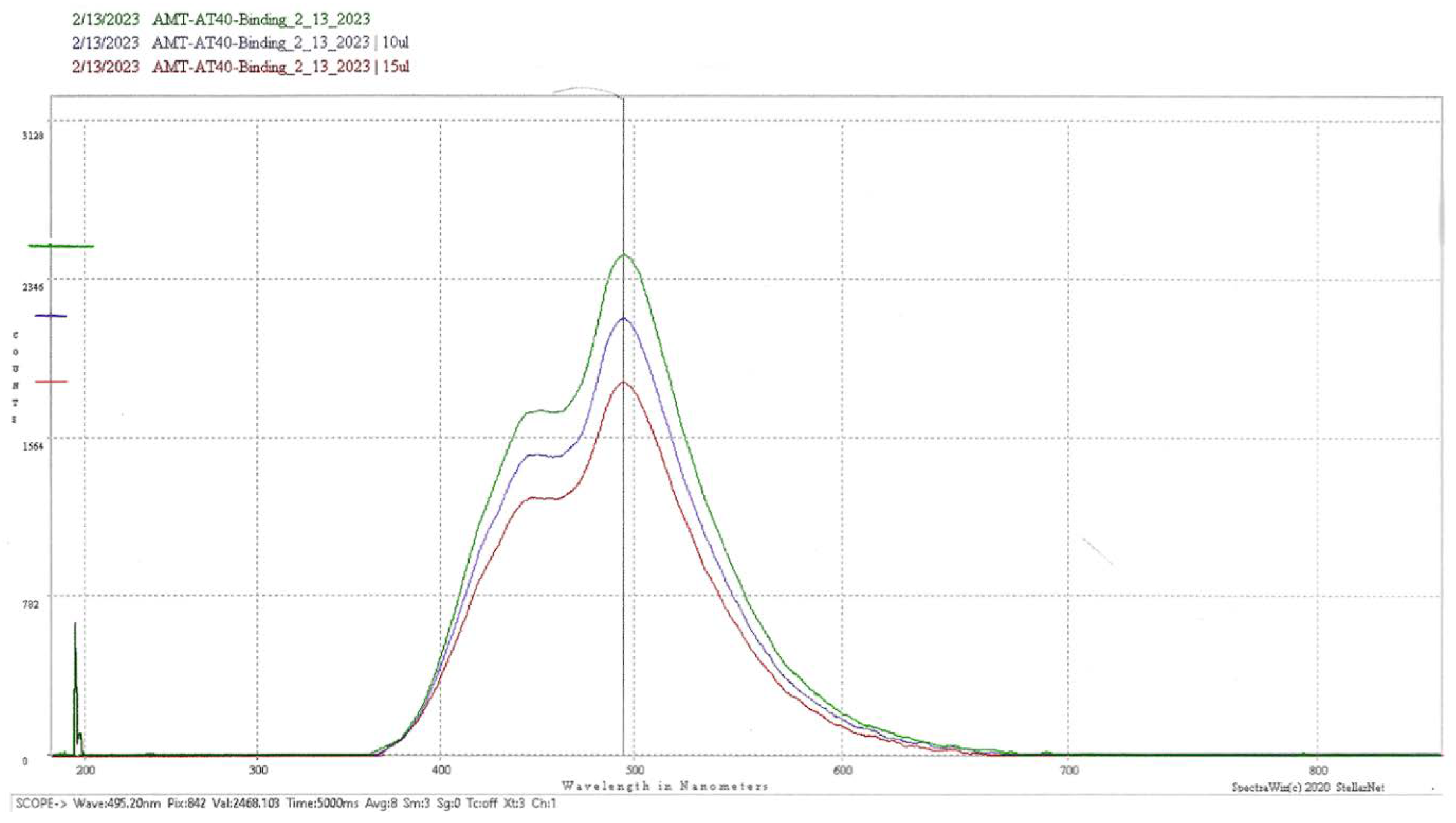
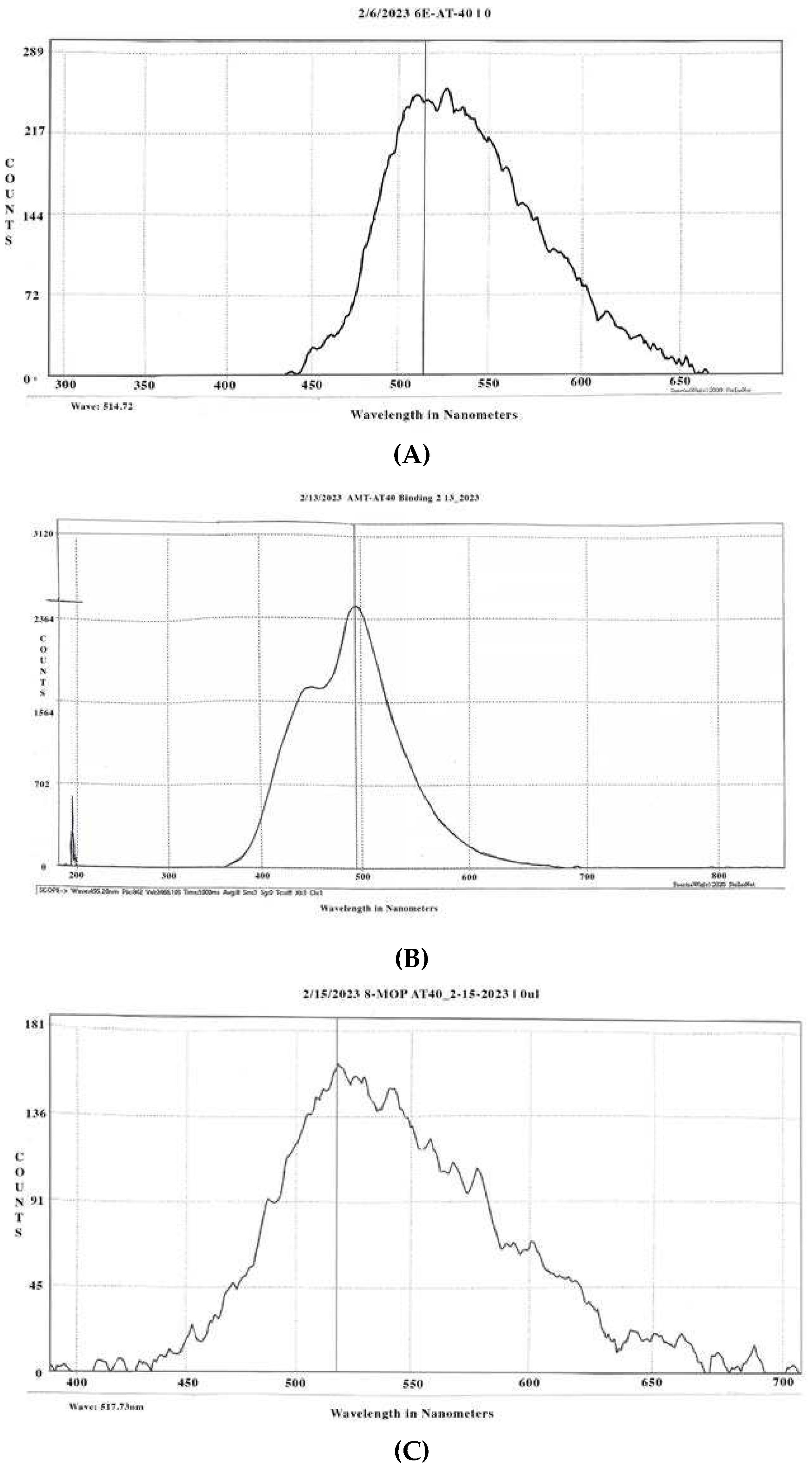
3.1.1. Psoralen Spectrofluorometry
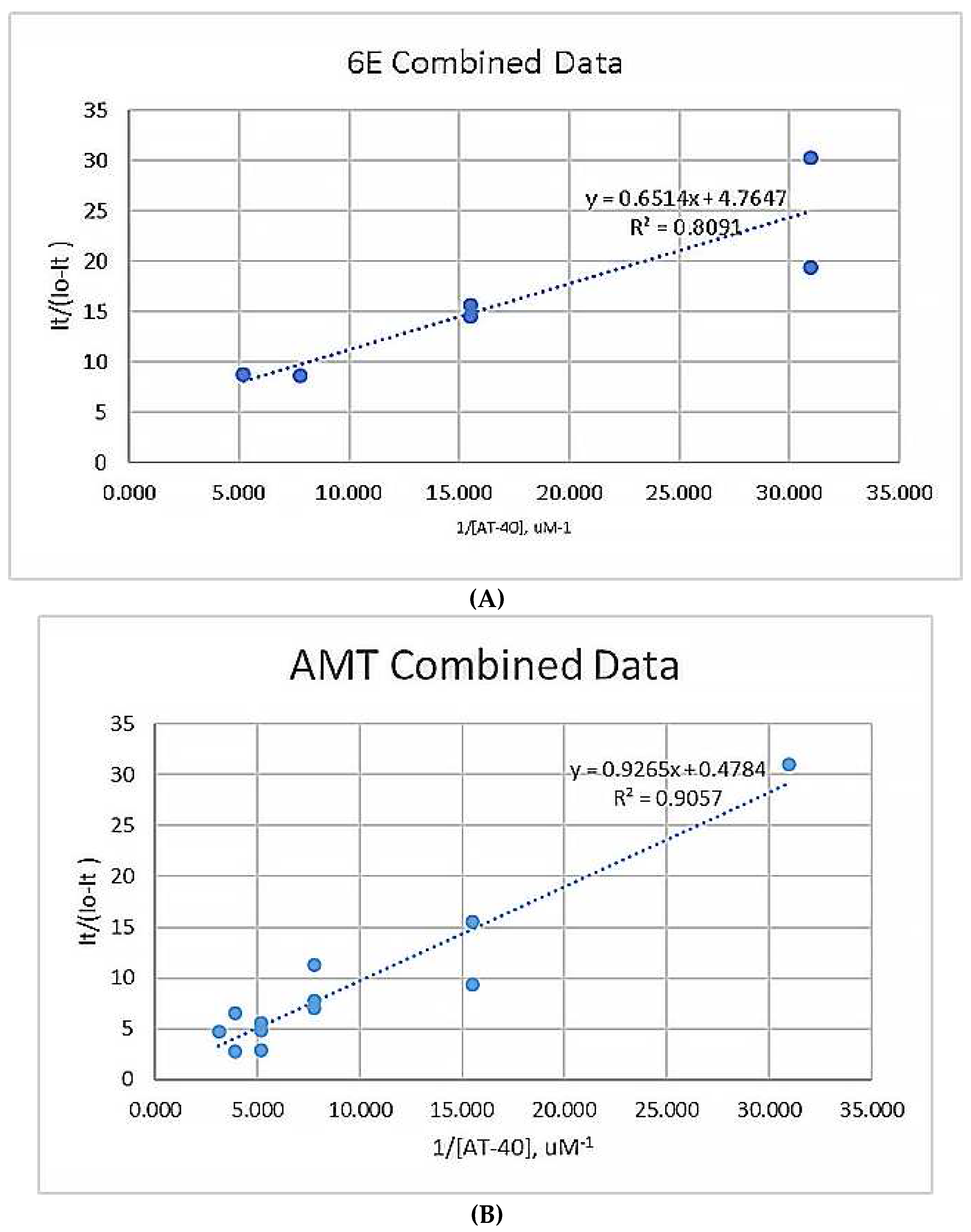
3.1.2. Scatchard Plots
3.1.3. Binding Constants
3.2. Figures
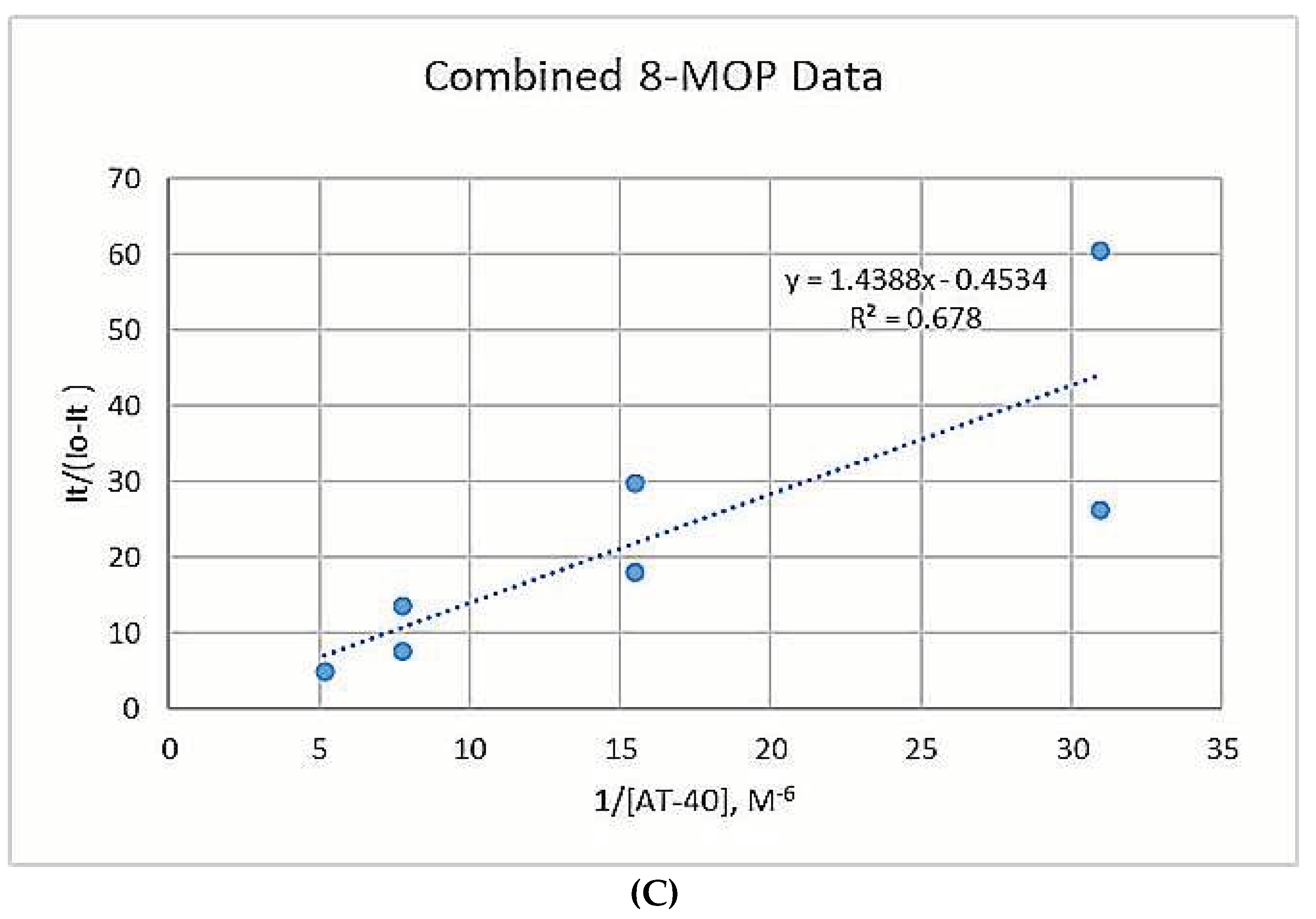
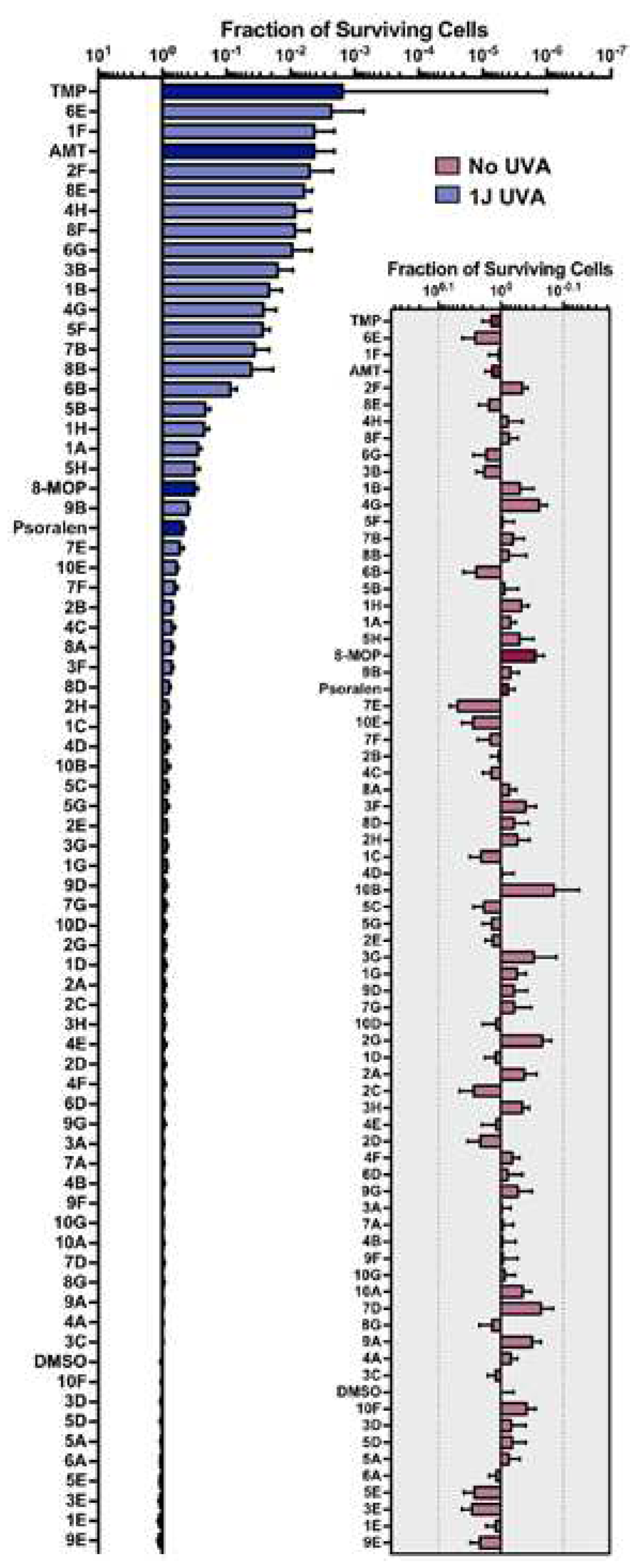
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lerner AB, Denton CR, Fitzpatrick TB. Clinical and experimental studies with 8-methoxypsoralen in vitiligo. J Invest Dermatol. 1953 Apr;20(4):299–314. [CrossRef]
- Article, Peterseim, U. M., Küster, W., Gebauer, H. J., Meschig, R., & Plewig, G. (1991). Cytogenetic effects during extracorporeal photopheresis treatment of two patients with cutaneous t-cell lymphoma. Archives of Dermatological Research, 283(2), 81-85. [CrossRef]
- Oldham, M., Yoon, P., Fathi, Z., Beyer, W. F., Adamson, J., Liu, L., Alcorta, D., Xia, W., Osada, T., Liu, C., Yang, X. Y., Dodd, R. D., Herndon, J. E., Meng, B., Kirsch, D. G., Lyerly, H. K., Dewhirst, M. W., Fecci, P., Walder, H., & Spector, N. L. (2016). X-Ray psoralen activated cancer therapy (X-PACT). PLoS ONE, 11(9), e0162078. [CrossRef]
- Diekmann J, Theves I, Thom KA, Gilch P. Tracing the Photoaddition of Pharmaceutical Psoralens to DNA. Molecules. 2020 Nov 10;25(22):5242. [CrossRef]
- Krasnovskiĭ AA, Sukhorukov VL, Potapenko AIa. Fotogeneratsiia singletnogo kisloroda psoralenami [Photogeneration of singlet oxygen by psoralens]. Biull Eksp Biol Med. 1983 Sep;96(9):59-61. Russian. [PubMed]
- Dall'Acqua, F., Vedaldi, D., Bordin, F., & Rodighiero, G. (1979). New studies on the interaction between 8-Methoxypsoralen and DNA in vitro. Journal of Investigative Dermatology, 73(2), 191-197. [CrossRef]
- Gasparro, F. P. (1994). Extracorporeal Photochemotherapy: Clinical Aspects and the Molecular Basis for Efficacy. (p.18) R.G. Landes.
- Gordon, I. L. (1995). Scatchard analysis of fluorescent concanavalin a binding to lymphocytes. Cytometry, 20(3), 238-244. [CrossRef]
- Healy, E. (2007). Quantitative Determination of DNA–Ligand Binding Using Fluorescence Spectroscopy. Journal of Chemical Education. [CrossRef]
- Buhimschi, A. D., Gooden, D. M., Jing, H., Fels, D. R., Hansen, K. S., Beyer, W. F., Dewhirst, M. W., Walder, H., & Gasparro, F. P. (2020). Psoralen derivatives with enhanced potency. Photochemistry and Photobiology, 96(5), 1014-1031. [CrossRef]
- Buhimschi, A. D., Gooden, D. M., Jing, H., Fels, D. R., Hansen, K. S., Beyer, W. F., Dewhirst, M. W., Walder, H., & Gasparro, F. P. (2020). Psoralen derivatives with enhanced potency. Photochemistry and Photobiology, 96(5), 1014-1031. 5. [CrossRef]
- Buhimschi, A. D., Gooden, D. M., Jing, H., Fels, D. R., Hansen, K. S., Beyer, W. F., Dewhirst, M. W., Walder, H., & Gasparro, F. P. (2020). Psoralen derivatives with enhanced potency. Photochemistry and Photobiology, 96(5), 1014-1031. 5. [CrossRef]
- Bravo-Anaya, L., Rinaudo, M., & Martínez, F. (2016). Conformation and rheological properties of calf-thymus DNA in solution. Polymers, 8(2), 51. [CrossRef]
- Martinez, M., Jones, J., Bruck, I., & Kaplan, D. (2017). Origin DNA melting—an essential process with divergent mechanisms. Genes, 8(1), 26. [CrossRef]
| Compound | Kbinding (M-1) this study | Kbinding (M-1) previously measured |
|---|---|---|
| 8-MOP | 714 | |
| 0.325x106 | 12,000 | |
| 10,000 736 |
||
| AMT | 150,000 | |
| 0.516x106 | 180,000 28,400 |
|
| 6E | 7.30x106 | Not previously measured |
| AT-40 aliquots | 8-MOP Trial 1 | 8-MOP Trial 2 | AMT Trial 1 | AMT Trial 2 | 6E Trial 1 | 6E Trial 2 |
|---|---|---|---|---|---|---|
| 0 uL | 174.6 | 190.0 | 2454 | 2090 | 247.4 | 207.0 |
| 2.5 uL | 163.8 | 182.0 | 2217 | 2034 | 239.5 | 178.0 |
| 5 uL | — | 180.4 | 2217 | 1874 | 231.5 | 174.0 |
| 10 uL | 168.8 | 171.6 | 2150 | 1731 | 222.0 | 166.0 |
| 15 uL | 168.8 | 185.8 | 1834 | 1771 | 222.1 | 150.0 |
| 20 uL | 187.8 | 183.2 | 1797 | 1681 | 226 | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




