Submitted:
02 January 2024
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
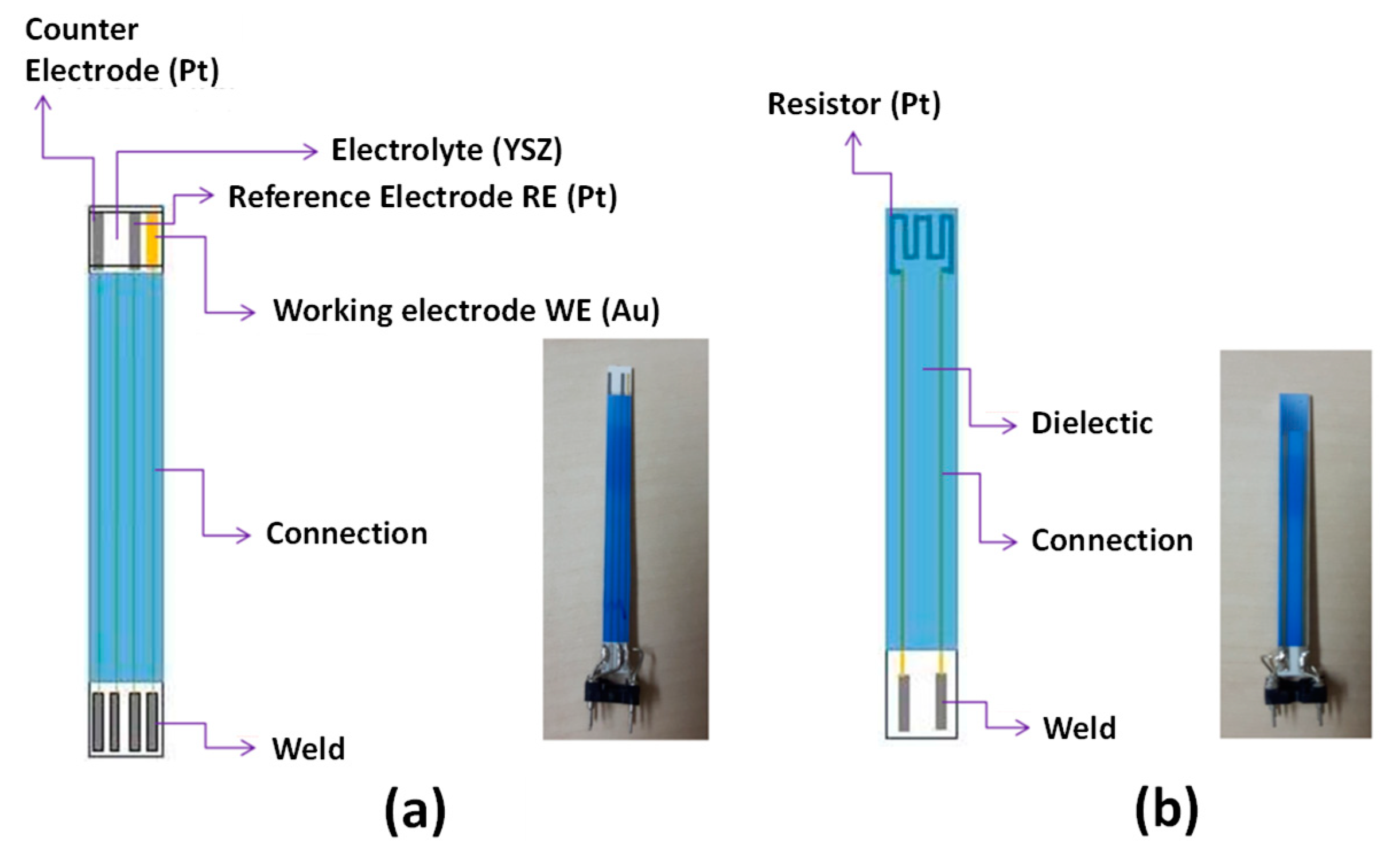
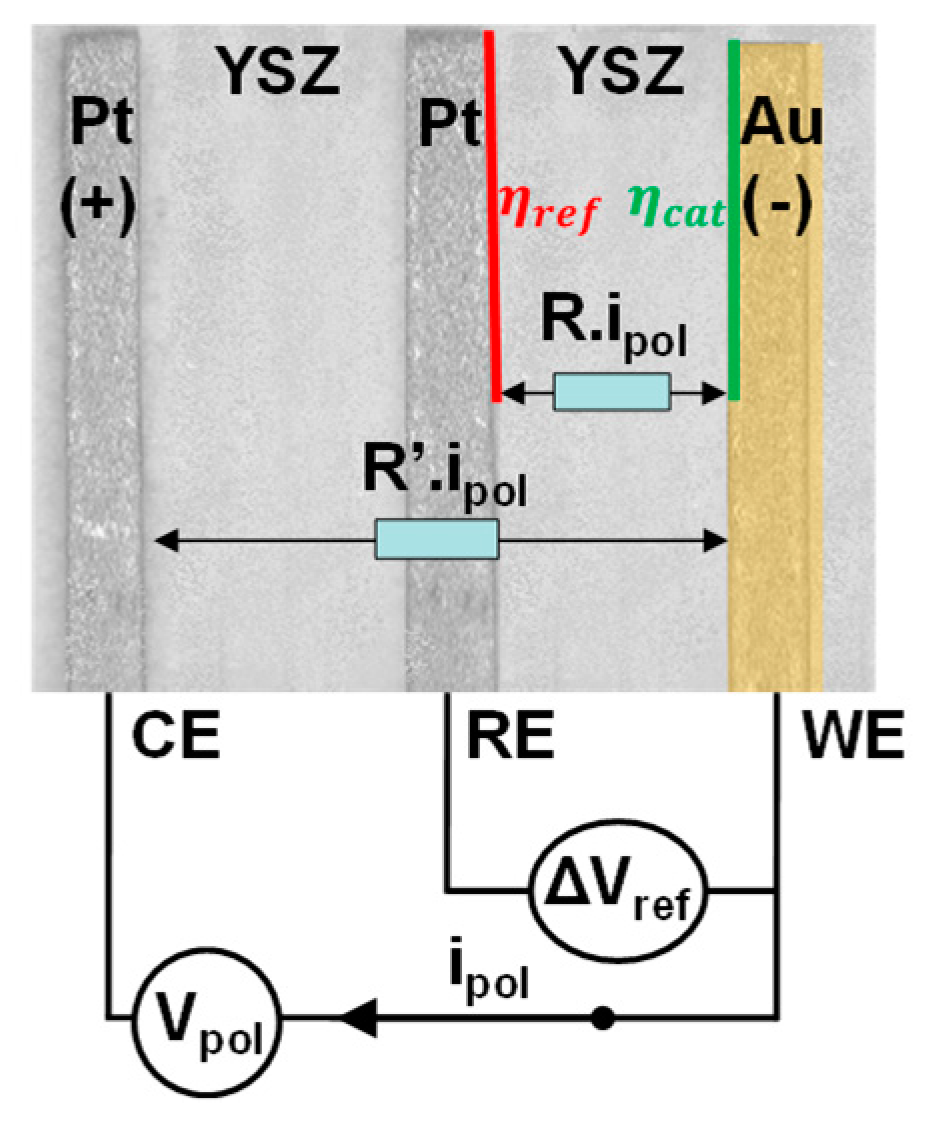
2.1. Sensor’s Physical and Electro-Chemical Description
- If is very low, the current value can be approximated by a first order Taylor series expansion :
-
f and (As it will be seen later, for our tested polarization currents and gaseous compositions, overpotential at gold cathode has been measured between -1.1V and -0.1V) :By association of equation (7) to (12), the following expression can be formulated:
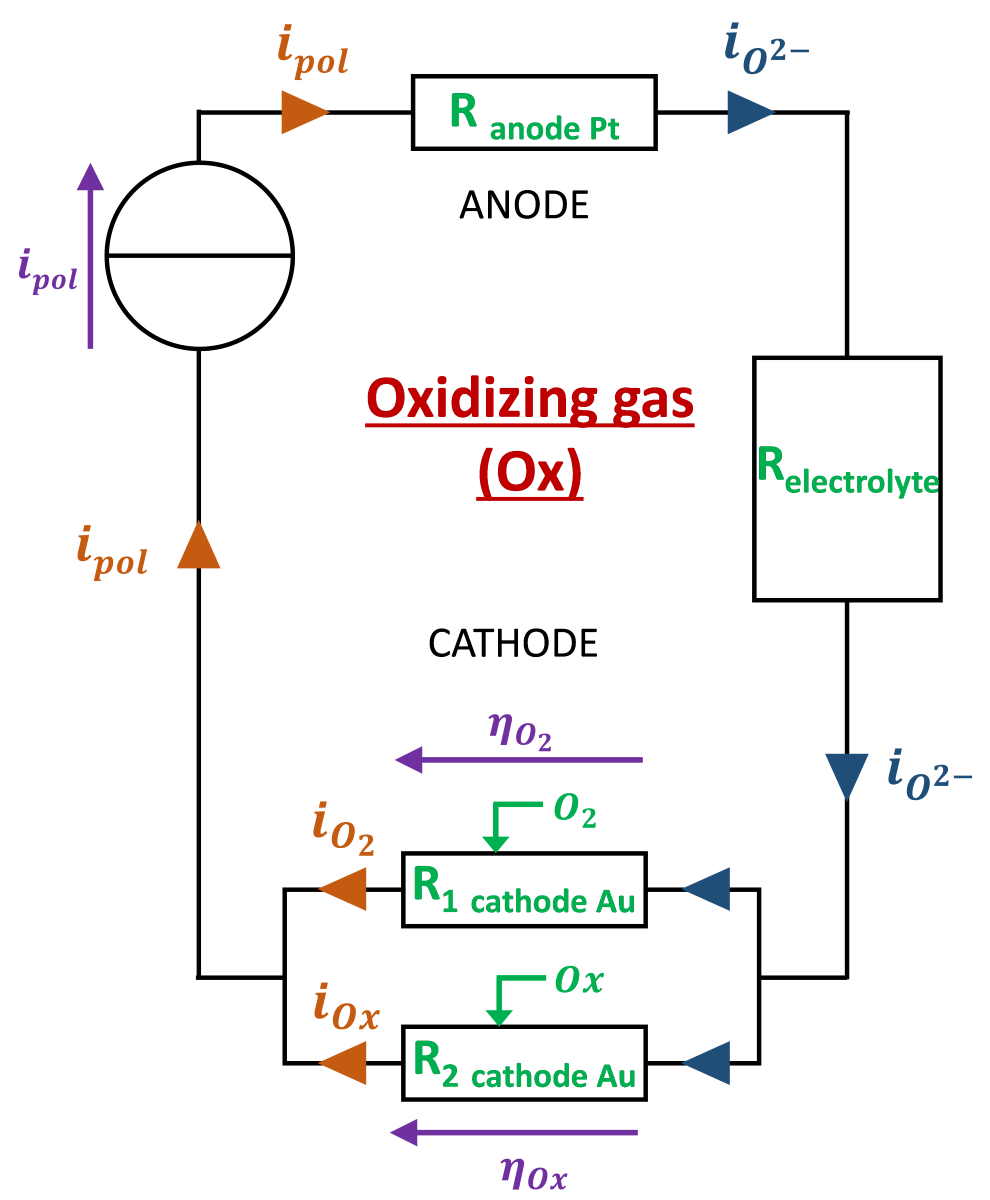
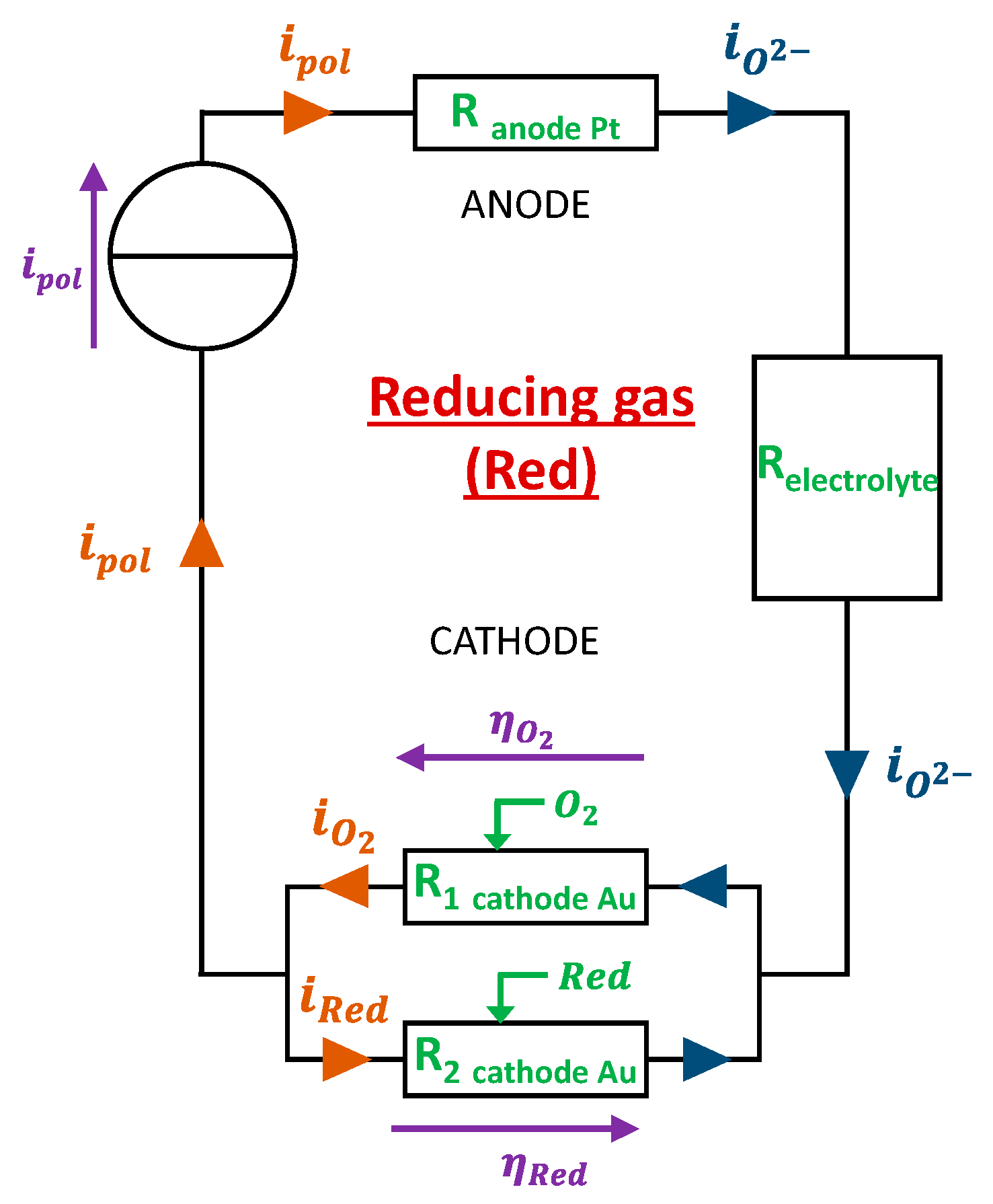
2.2. Electrochemical and Associated Electrical Models
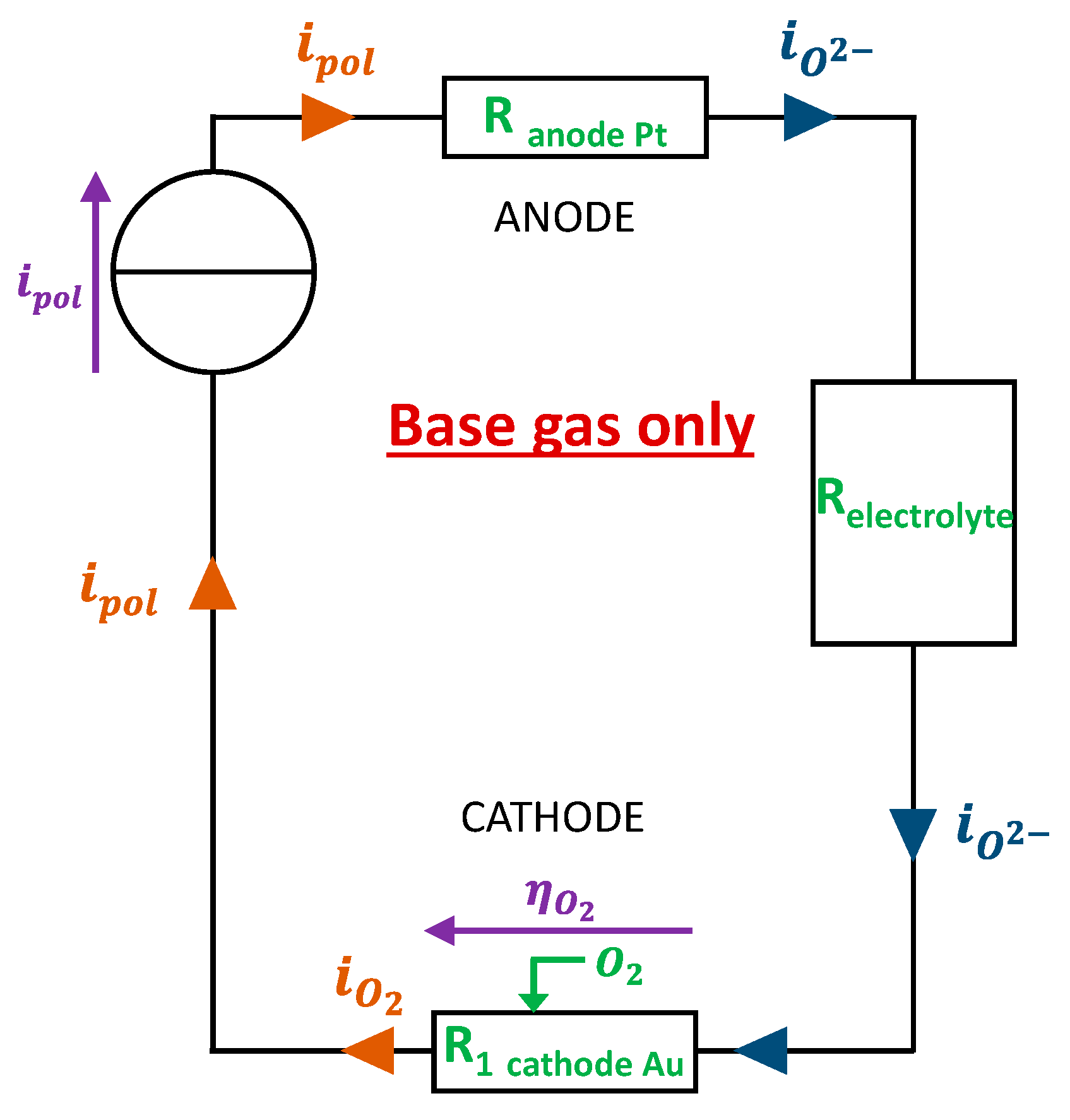
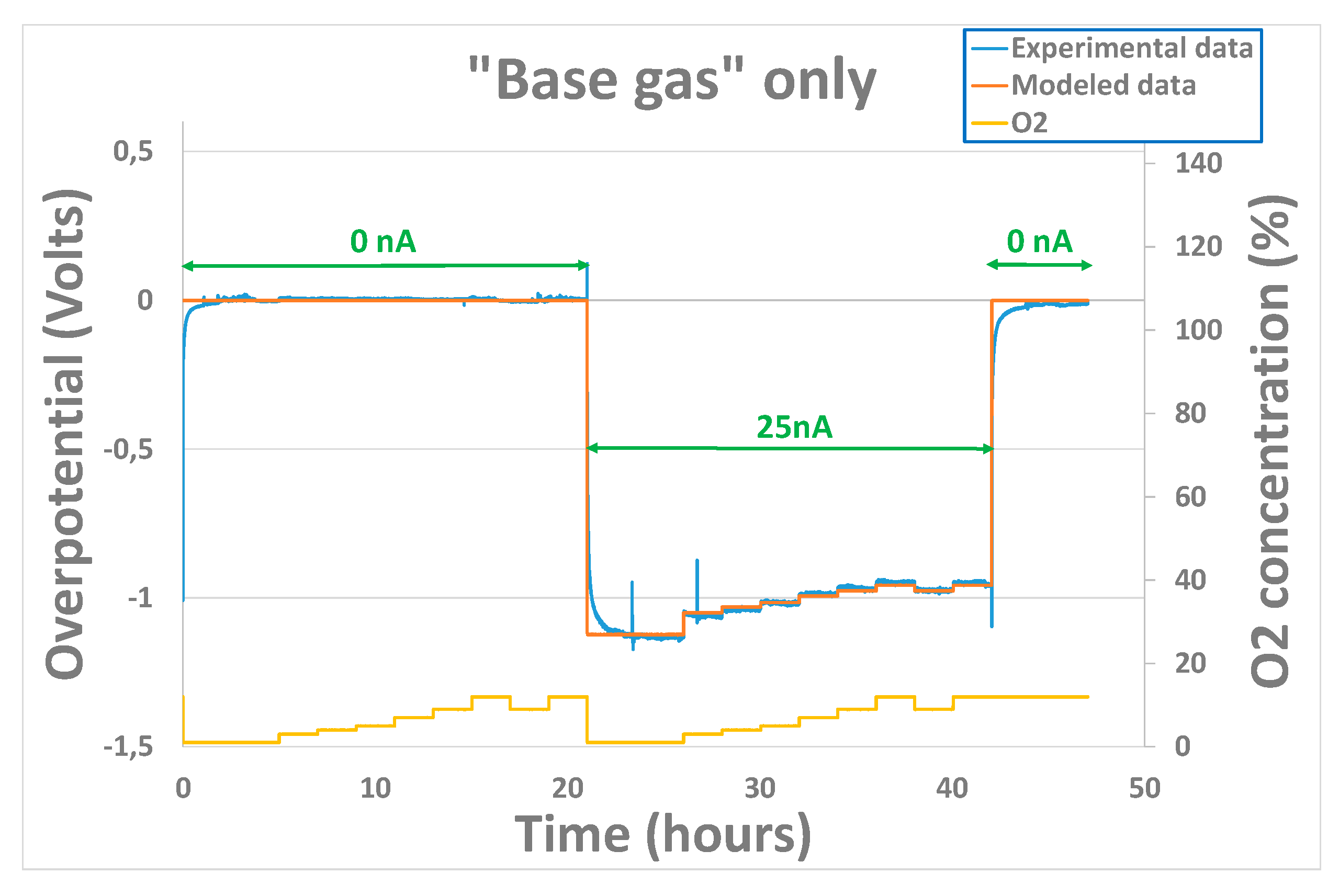
- Model 1 : “Base gas” case
- -
- at the cathode :
- -
- at the anode :
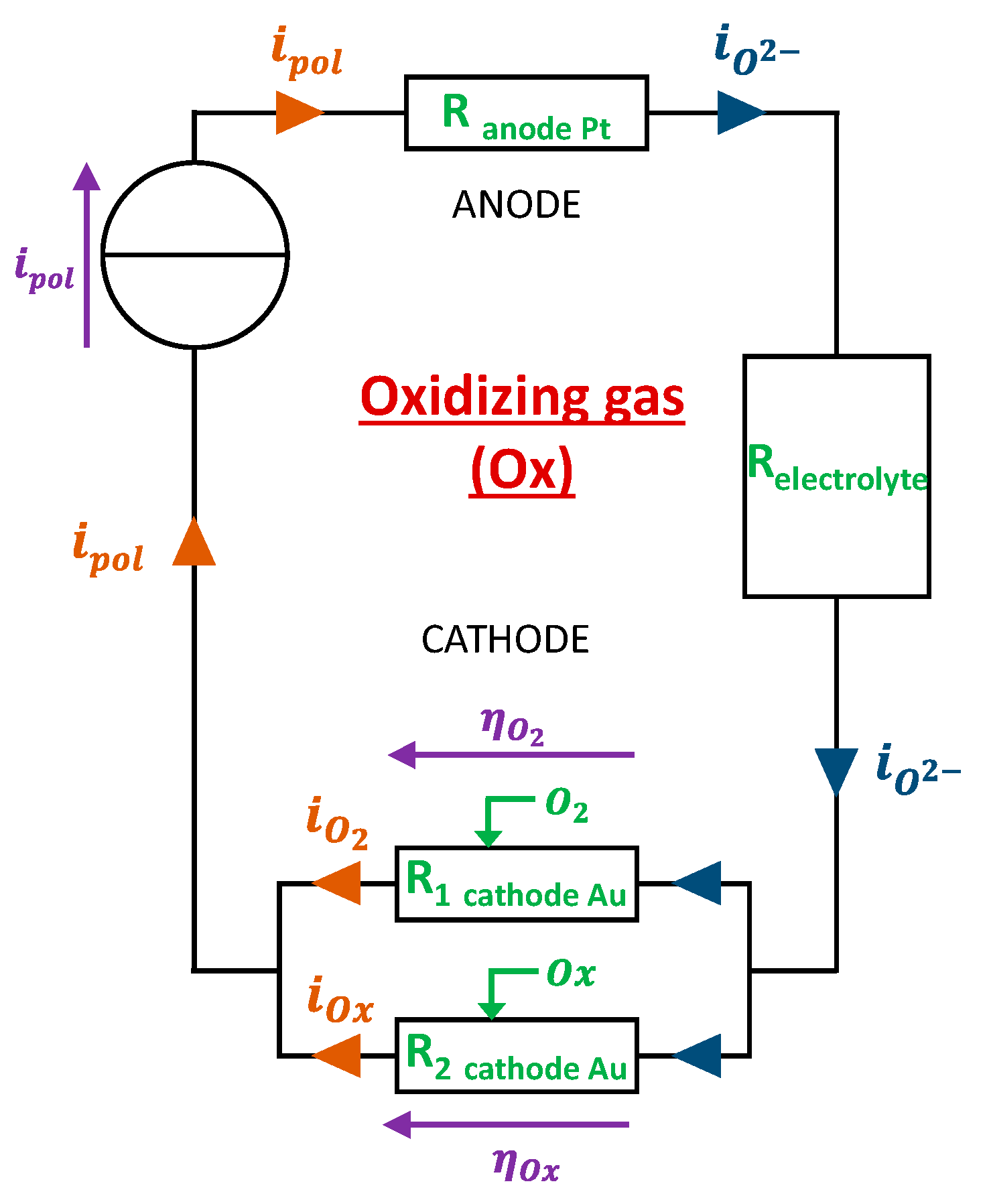
- Model 2 : Presence of an oxidizing gas (NO2, NO)
- -
- at the cathode :
- -
- at the cathode :
- Model 3 : Presence of a reducing gas (NH3, CO)
- -
- at the cathode :
- -
- at the anode :
2.3. Multivariate Fitting Methods
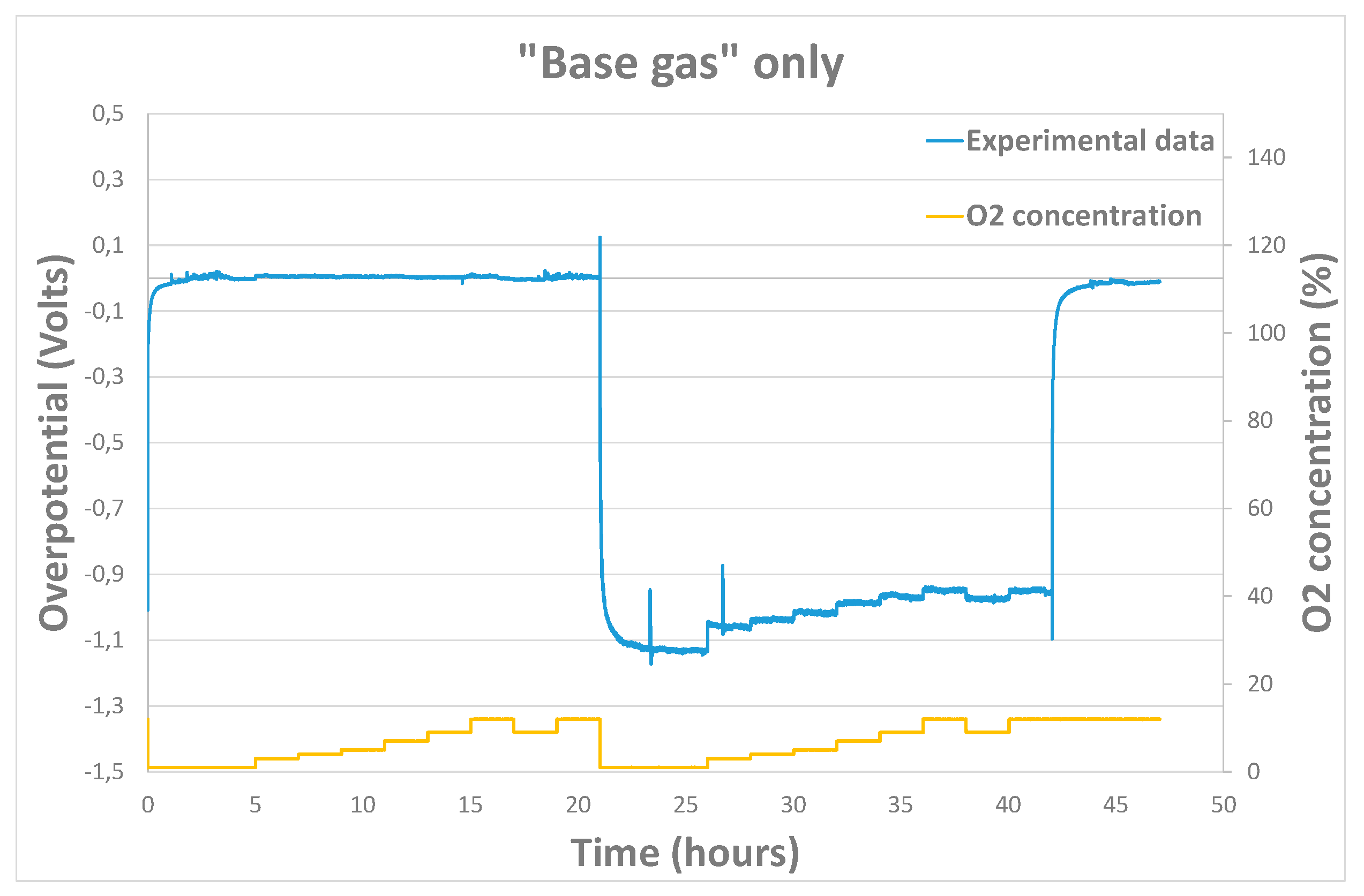
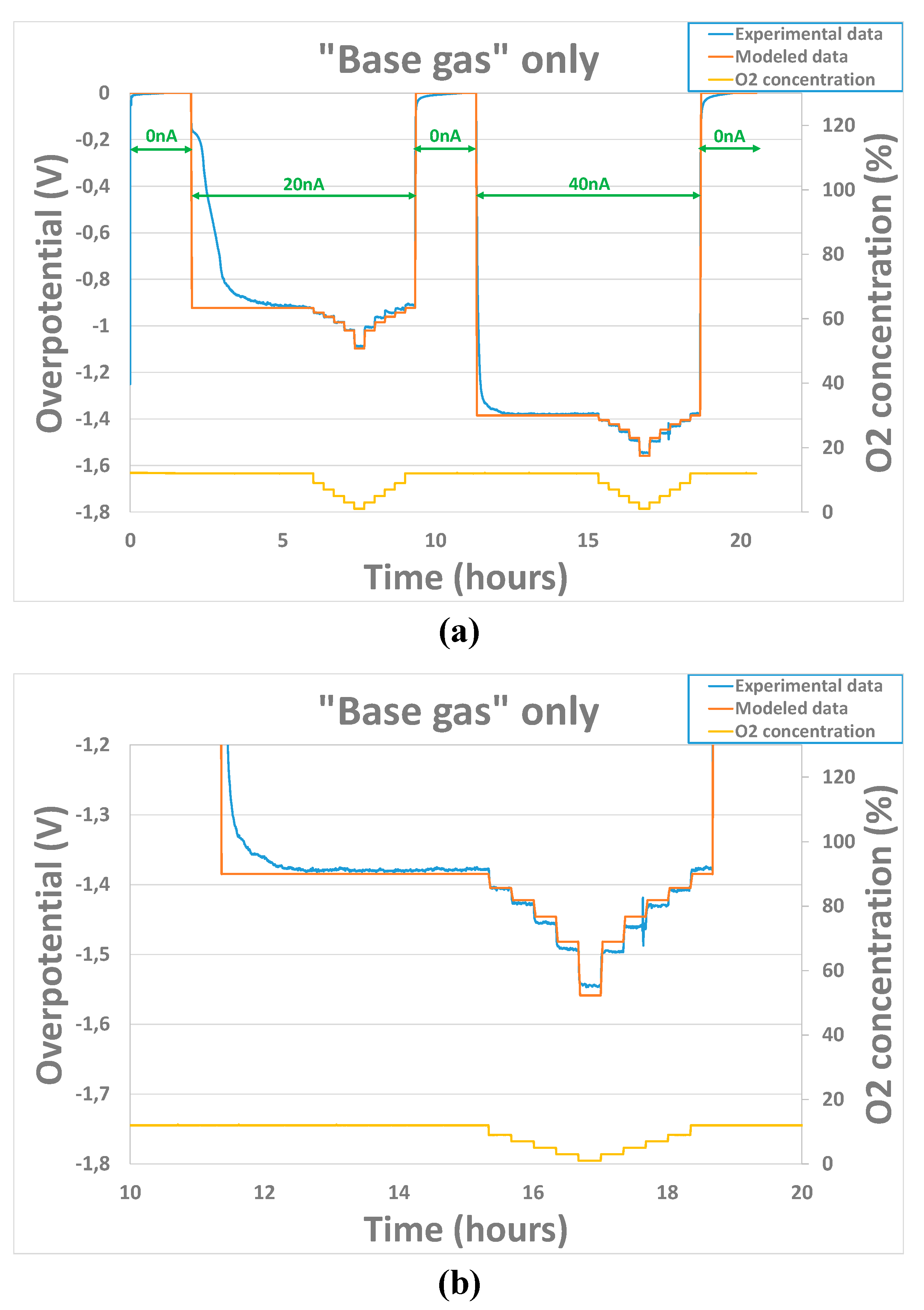
3. Results & Discussions
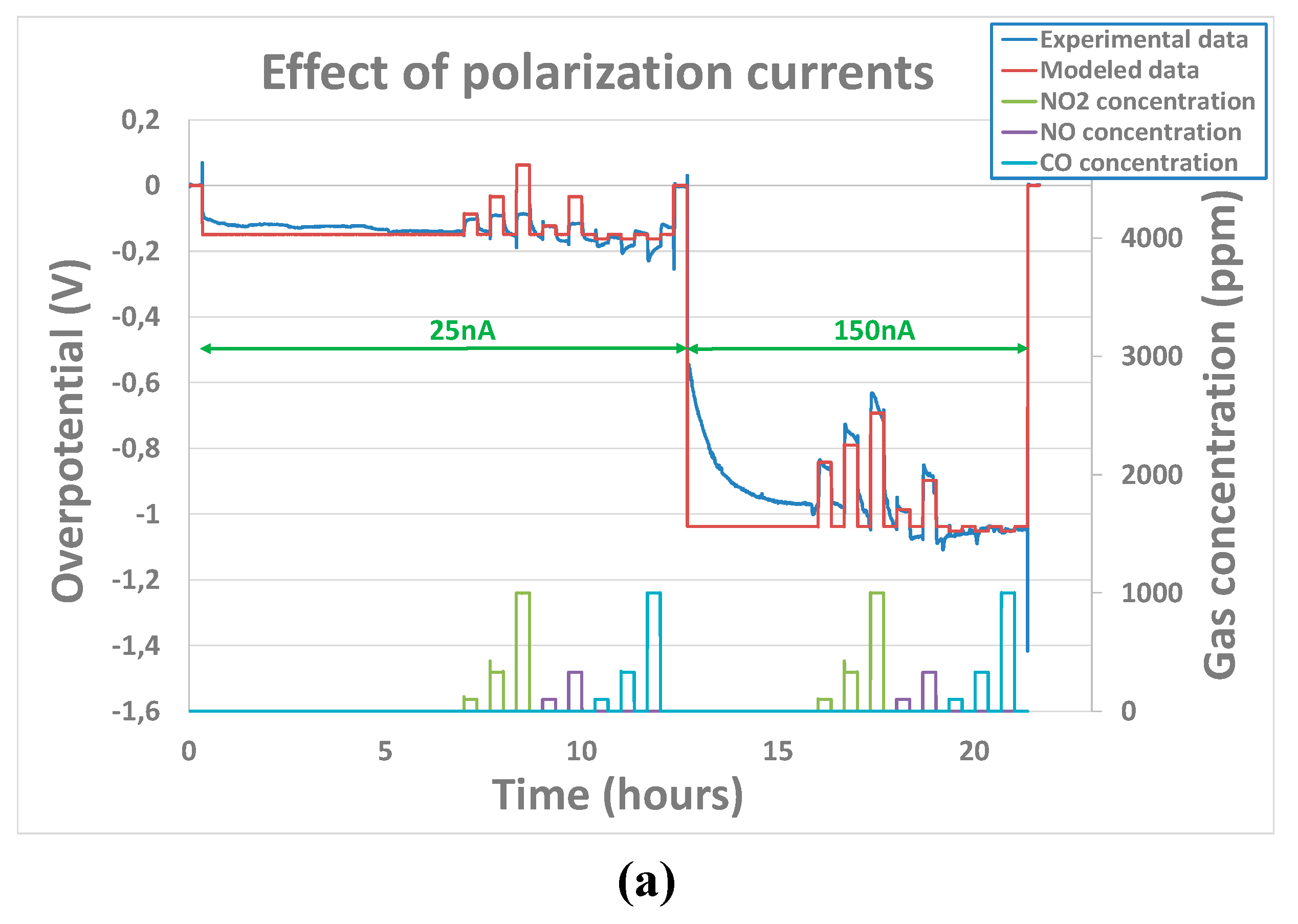
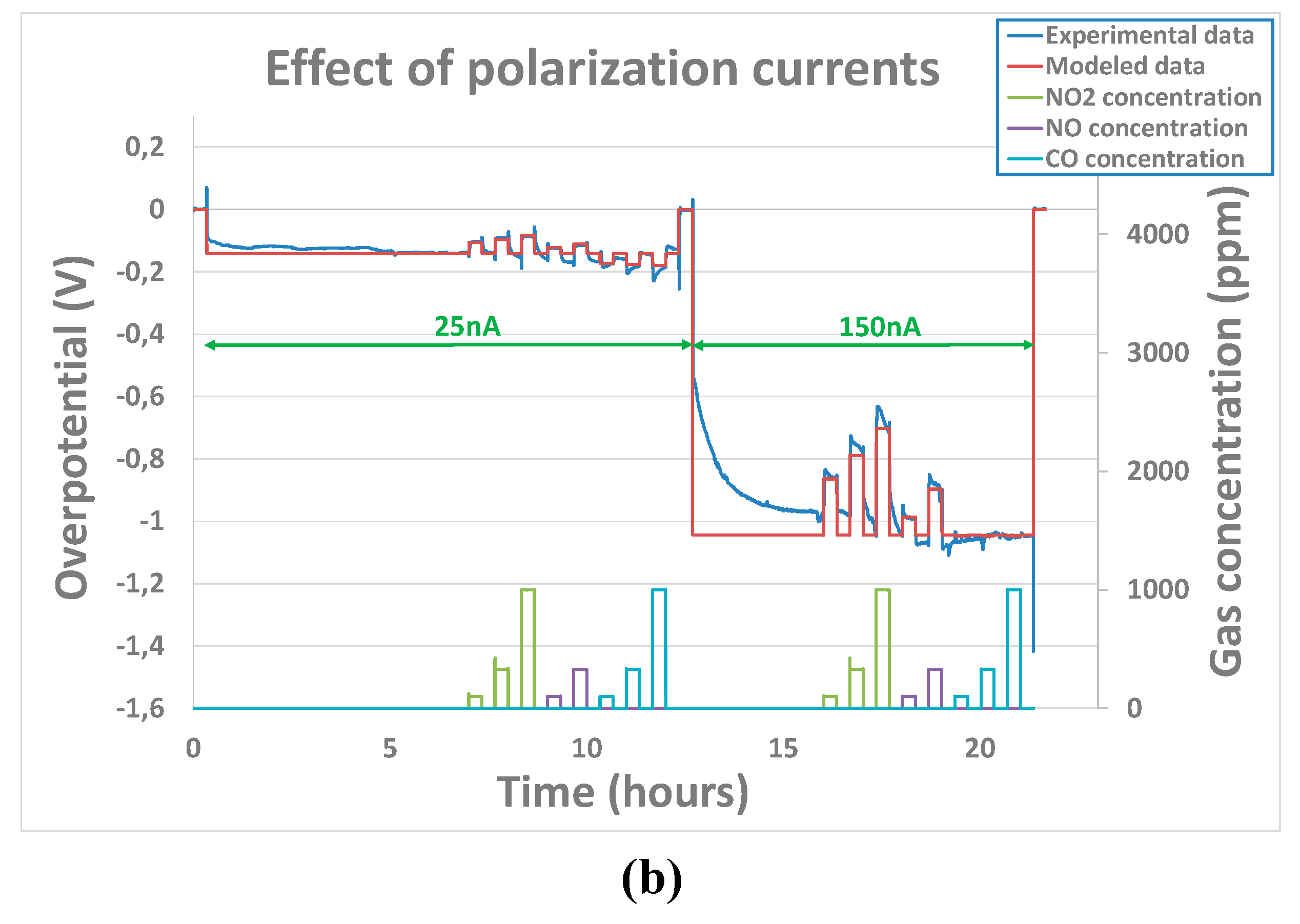
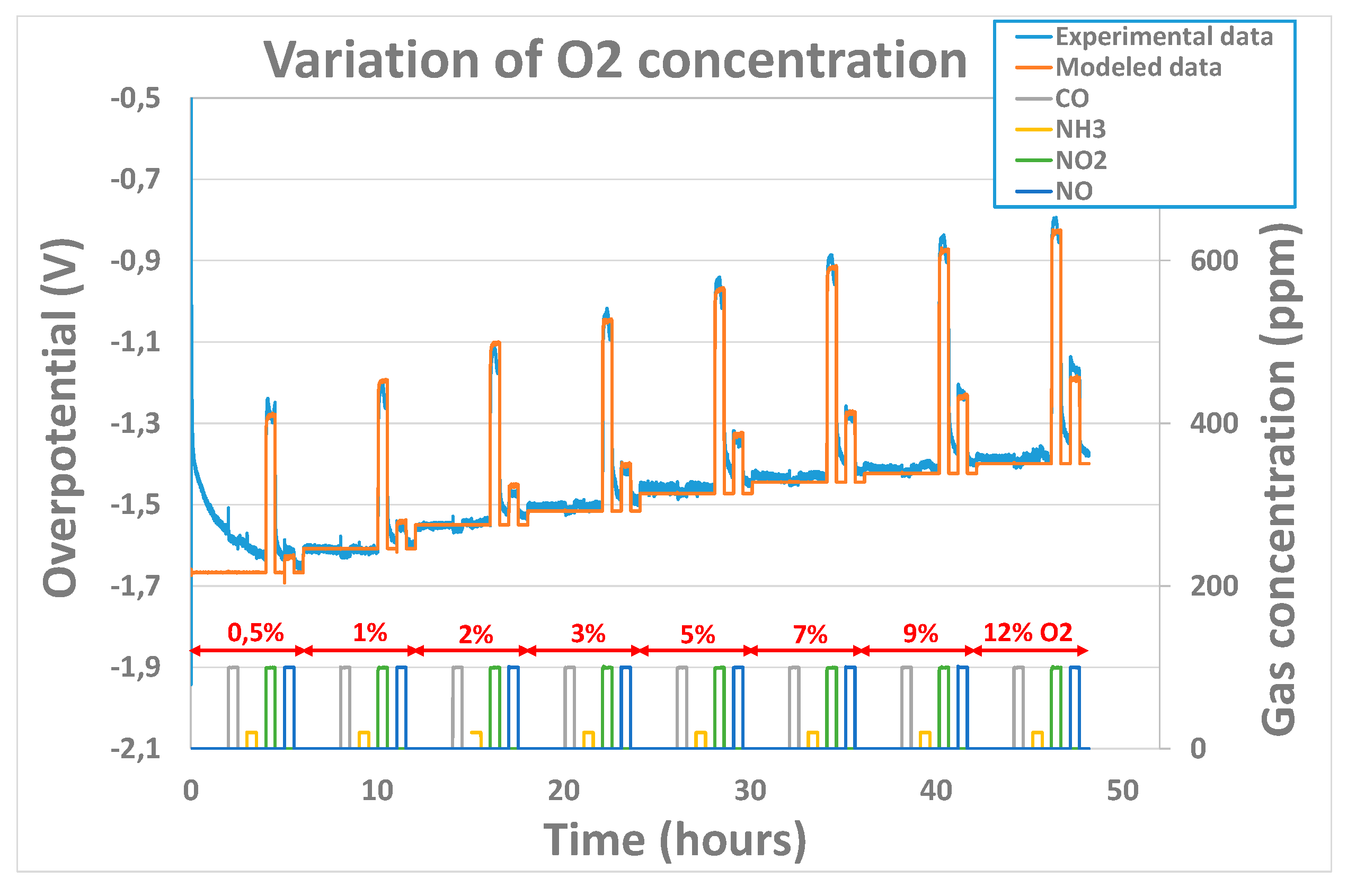
3.2. Introduction of Pollutant Gases together with “Base Gas”
3.2.1. Effect of Polarization Current
3.2.1. Effect of Oxygen Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- J. Hansen, L. Nazarenko, Soot climate forcing via snow and ice albedos, Proceedings of the National Academy of Sciences of the United States of America 2004, 101, n°2, p423-428, (2004).
- EEA report, 2013, National emissions reported to the Convention on Long-range Transboundary Air Pollution (LRTAP Convention) (http://www.eea.europa.eu).
- J.W. Fergus, Sensing mechanism of non-equilibrium solid-electrolyte-based chemical sensors, Journal of Solid State Electrochemistry 2011, 15, p971–984. [CrossRef]
- H. Ryu, D. Thompson, Y. Huang, B. Li, Y. Lei, Electrochemical sensors for nitrogen species: A review, Sensors and Actuators Reports 2020, 2, Issue 1, 100022. [CrossRef]
- Y. Tang, X. Xu, H. Du, H. Zhu, D. Li, D. Ao, Y. Guo, Y. Fu, X Zu, Cellulose nano-crystals as a sensitive and selective layer for high performance surface acoustic wave HCl gas sensors, Sensors and Actuators A: Physical 2020, 301, 111792. [CrossRef]
- S-H. Hsiao, J-X. Wu, H-I. Chen, High-selectivity NOx sensors based on an Au/InGaP Schottky diode functionalized with self-assembled monolayer of alkanedithiols, Sensors and Actuators B: Chemical 2020, 305, 127269. [CrossRef]
- P.A. Pushpanjali, J.G. Manjunatha, M.T. Srinivas, Highly sensitive platform utilizing poly(l-methionine) layered carbon nanotube paste sensor for the determination of voltaren, FlatChem 2020, 24, 100207. [CrossRef]
- K. Staszek, A. Szkudlarek, M. Kawa, A. Rydosz, Microwave system with sensor utilizing GO-based gas-sensitive layer and its application to acetone detection, Sensors and Actuators B: Chemical 2019, 297, 126699. [CrossRef]
- J. Gao, J-P. Viricelle, P. Breuil, C. Pijolat, P.Vernoux, A. Boreave, A. Giroir-Fendler, Improvement of the NOx selectivity for a planar YSZ, Sensors and Actuators B 2011, 154, p 106-110. [CrossRef]
- Axin Liang, B. Huipeng Hou, C. Shanshan Tang, D. Liquan Sun, E. Aiqin Luo, An advanced molecularly imprinted electrochemical sensor for the highly sensitive and selective detection and determination of Human IgG, Bioelectrochemistry 2021, 137, 107671. [CrossRef]
- Q. Wang, X. Xiao, X. Hu, L. Huang, T. Li, M. Yang, Molecularly imprinted electrochemical sensor for ascorbic acid determination based on MXene modified electrode, Materials Letters 2021, 285, 129158. [CrossRef]
- [12] K.A. Alamry, M.A. Hussein, J-W. Choi, W.A. El-Said, Non-enzymatic electrochemical sensor to detect γ-aminobutyric acid with ligand-based on graphene oxide modified gold electrode, Journal of Electroanalytical Chemistry 2020, 879, 114789. [CrossRef]
- B.S.O. Alsaedi, C.M. McGraw, T.M. Schaerf, P.W. Dillingham, Multivariate limit of detection for non-linear sensor arrays, Chemometrics and Intelligent Laboratory Systems 2020, 201, article 104016. [CrossRef]
- L.M.M. Ferro, S.G. Lemos, M. Ferreira, F. Trivinho-Strixino, Use of multivariate analysis on Fabry-Pérot interference spectra of nanoporous anodic alumina (NAA) for optical sensors purposes, Sensors and Actuators B: Chemical 2017, 248, p718-723. [CrossRef]
- L. Song, L. Yang, Z. Wang, D. Liu, L. Luo, X. Zhu, Y. Xi, Z. Yang, N. Han, F. Wang, Y. Chen, One-step electrospun SnO2/MOx heterostructured nanomaterials for highly selective gas sensor array integration, Sensors and Actuators B: Chemical 2019, 283, p793-801. 283.
- Yousefi-Darani, M. Babor, O. Paquet-Durand, B. Hitzmann, Model-based calibration of a gas sensor array for on-line monitoring of ethanol concentration in Saccharomyces cerevisiae batch cultivation, Biosystems Engineering 2020, 198, p.198-209.
- J. Chu, W. Li, X. Yang, Y. Wu, D. Wang, A. Yang, H. Yuan, X. Wang, Y. Li, M. Rong, Identification of gas mixtures via sensor array combining with neural networks, Sensors and Actuators B: Chemical 2020, 329, 129090. [CrossRef]
- V. Singh, Quantum dot decorated multi-walled carbon nanotube modified electrochemical sensor array for single drop insulin detection, Materials Letters 2019, 254, p.415-418. [CrossRef]
- D.D. Gornall, S.D. Collyer, S.P.J. Higson, Investigations into the use of screen-printed carbon electrodes as templates for electrochemical sensors and sonochemically fabricated microelectrode arrays, Sensors and Actuators B: Chemical 2009, 141, Issue 2, p.581-591. [CrossRef]
- J. Guo, G. Fang, S. Wang, J. Wang, Quartz crystal microbalance sensor based on 11-mercaptoundecanoic acid self-assembly and amidated nano-titanium film for selective and ultrafast detection of phosphoproteins in food, Food Chemistry 2020, 344, 128656. [CrossRef]
- S. Sri Surya Srikanth, B. Rajesh Kumar, V. Suresh, V. Jyothi, I. Sreenivasa Rao, G. Aswani Kumar, Design of MEMS Cantilever Sensors for Identification of VOCs Using IntelliSuite, Materials Today: Proceedings 2020, 22, Part 4, p.3162-3170. [CrossRef]
- L. Iglesias, M.T. Boudjiet, I. Dufour, Discrimination and concentration measurement of different binary gas mixtures with a simple resonator through viscosity and mass density, Sensors and Actuators B: Chemical 2019, 285, p.487-494. [CrossRef]
- T. Ritter, J. Lattus, G. Hagen, R. Moos, On the influence of the NOx equilibrium reaction on mixed potential sensor signals: A comparison between FE modelling and experimental data, Sensors and Actuators B: Chemical 2019, 296, p. 126627.
- T. Liu, H. Guan, T. Wang, X. Liang, F. Liu, C. Zhang, G. Lu, Mixed potential type acetone sensor based on GDC used for breath analysis, Sensors and Actuators B: Chemical 2021, 326, 128846. [CrossRef]
- Eric L. Brosha, Rangachary Mukundan, David R. Brown, Fernando H. Garzon, J.H. Visser, Development of ceramic mixed potential sensors for automotive applications, Solid State Ionics 2002, 148, Issues 1–2, p.61-69. [CrossRef]
- A.J. Bard, L.R. Faulkner, Electrochemical Methods. Fundamentals and Applications, 2nd ed., John Wiley & Sons, Inc. : Hoboken, New Jersey, United States, 2001 p. 92-105.
- Romanytsia, J-P. Viricelle, P. Vernoux, C. Pijolat, Application of advanced morphology Au–X (X=YSZ, ZrO2) composites as sensing electrode for solid state mixed-potential exhaust NOx sensor, Sensors and Actuators B: Chemical 2015, 207, Part A, p.391-397. [CrossRef]
- J-P. Viricelle, P. Vernoux, J. Gao, I. Romanytsia, P. Breuil, NO2-selective electrochemical sensors for Diesel exhausts, EUROSENSORS 2016, Budapest, Procedia Engineering 2016, 168, p.7-10. [CrossRef]
- J-P. Viricelle, P Vernoux, C. Pijolat, P. Breuil, Capteur NOx à filtre catalytique et polarisation, Patent FR3037655 (B1) 2017-07-28 , US US2018188202 (A1) 2018-07-05; Canada CA2989484 (A1) 2016-12-22;; WO2016202997 (A1) 2016-12-22.
- J. Cui, Z. Hao, Y. Wang, L. Xue, H. Xue, L. Tu, L. Hao, M. Tian, J. Guo, D. Zhao, G. Kevin Li, H. Ding, Mechanism of ozone-assisted catalytic oxidation of isopropanol over single-atom platinum catalysts at ambient temperature, Chemical Engineering Journal 2022, 446, Part 2, p.136989. [CrossRef]
- H. Wei, T. Wei, L. Li, T. Zhang, F. Seidi, Y. Jin, H. Xiao, Morphological effect of ceria-supported platinum catalyst on low-temperature ethylene oxidation, Applied Catalysis B: Environmental 2023, 324, p.122242. [CrossRef]
- C. Krause, V.M. Mirsky, and K.D. Heckmann, Capacitive Detection of Surfactant Adsorption on Hydrophobized Gold Electrodes, Langmuir 1996, 12 (25), p.6059-6064. [CrossRef]
- Y. Shi and N. Cai, A general mechanistic model of solid oxide fuel cells, Tsinghua Science and Technology 2006, 11(6), p.701-711. [CrossRef]
- M. Aliramezani, C.R. Koch, M. Secanell, R.E. Hayes, R. Patrick, An electrochemical model of an amperometric NOx sensor, Sensors and Actuators B: Chemical 2019, 290, p.302-311. [CrossRef]
- V. S. Bagotzky, Fundamentals of electrochemistry, 2nd ed.; John Wiley & Sons, Inc. : Hoboken, New Jersey, United States, 2006.
| Sensor | Temperature (°C) | |||
|---|---|---|---|---|
| 1 | 450 | 3.2 | 0.026 | 0.09 |
| 500 | 11 | 0.025 | 0.13 | |
| 550 | 25 | 0.027 | 0.13 | |
| 2 | 450 | 3.5 | 0.025 | 0.10 |
| 500 | 5.5 | 0.034 | 0.10 | |
| 550 | 14.8 | 0.027 | 0.09 |
| Gas | Polarization current (nA) | |||
|---|---|---|---|---|
| Base gas | 25 - 50 - 150 | [5 – 12].10-9 | 0.03 – 0.04 | 0.1 – 0.15 |
| 25 | [3 – 5].10-9 | 0.03 - 0.04 | 0.25 - 0.3 | |
| 50 | [3 – 7].10-8 | 0.03 - 0.04 | 0.4 - 0.6 | |
| 150 | [3 - 13].10-8 | 0.03 - 0.04 | 0.4 - 0.7 | |
| 25 | [4 – 8].10-9 | 0.06 – 0.08 | 0.5 | |
| 50 | [7 – 17].10-8 | 0.06 – 0.08 | 0.5 - 0.7 | |
| 150 | [12 - 40].10-8 | 0.06 – 0.08 | 0.5 - 0.8 | |
| 25 | [7 – 25].10-9 | 0.03 - 0.04 | 0.09 | |
| 50 | < 10-10 | Not assessable | Not assessable | |
| 150 | < 10-10 | Not assessable | Not assessable |
| Gas | ||||
|---|---|---|---|---|
| Base gas | 0.02 – 0.035 | 0.09 - 0.16 | ||
| Oxidant gas | NO2 | 0.0014 – 0.015 | 0.3 - 0.4 | |
| NO | 0.003 – 0.05 | 0.4 – 0.5 | ||
| Reductant gas | CO | 0.02 – 0.035 | 0.09 - 0.16 | |
| NH3 | < 10-10 | Not assessable | Not assessable | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





