1. Introduction
Currently, the defense of metallic materials utilizing of conducting polymers as advanced covering materials has acquired one of the most interesting exploration fields [
1,
2,
3,
4,
5,
6,
7,
8]. The deterioration of metallic materials introduces a considerable economic and technical preoccupation. In manufacturing processes, the metallic materials surfaces used are exposed to seriously aggressive acids and alkali solutions, which induce corrosion, appreciable destruction. Some research conducted for the protection of metals in the field of technology have established that the use of composites is the most effective and simple path to prevent the damage of these metallic samples in aggressive solutions [
9,
10,
11,
12,
13,
14,
15,
16,
17,
18]. The acquirement of novel composites from distinct monomer was carried out to increase the physicochemical features of coating, to expand for long-term defense, for raised adhesion and to improve the electrochemical characteristics. One of the main aspects of using conducting polymers for corrosion protection of these materials is their permeability to water which can cause the transfer of existent corrosive constituents to the metal zone. These composite coatings frequently increase the resistance of metals to several corrosive factors. The effectiveness of these defensive layers that ensure a barrier on the surface can be determined by on different constituents: the kind of conducting polymer, electrochemical deposition practice that was employment on the electrode substrate and the corrosive media [
19,
20,
21,
22,
23,
24,
25,
26,
27]. The use of electropolymerization procedures, nanostructured coverings, implementation inorganic-organic coatings, cathodic and anodic defense are practices for the anti-corrosion protection of metals and its alloys. Metals and their alloys are used over a wide area in various zones such as: chemical production, petroleum manufacturing and refinering, building industry, engineering equipment, industrial apparatus and marine procedures; this has perfected the exploration of anticorrosion protection in various corrosive environments. While corrosion is an actual factor in the deterioration of manufacturing structures, a considerable numeral of experiments was effectuated to establish processes to diminish corrosion and “wear costs”. The protective mechanisms of conductive polymers have been provided by numerous hypotheses such as conductive polymers cause an electric field on the material substrate, preventing the passage of electrons from the metal to the oxidizing medium; conductive polymers engender a dense and short porosity layer on the specimen substrate, constituting as a barrier among the metal and the aggressive medium and they make possible the formatting of a protecting film of metal oxide onto surface of the sample. Many researches have established that conductive polymers in various situations can recover their original mechanical and electrical characteristics without important degradation [
28,
29,
30,
31,
32,
33,
34]. Conducting polymers are effective for fast doping and de-doping with high charge density and like consequently are materials with special properties for practice in various electrochemical processes. Conducting polymers are also an interesting molecular model owed to their significant ability to change characteristics whenever actuated by an electric sign. Conducting polymers like polythiophene, polypyrrole, polyaniline, and their derivatives are currently the most widely used for coating protection [
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46]. Hence, it is assumed that the integration of hydrophobic functional groups increases the protective capacity of the polymer. Dopants incorporated in conducting polymers exercise a control on the electropolymerization practice and on the features of the achieved polymeric composites [
47,
48,
49,
50,
51,
52,
53,
54]. The application of dopant ions in electrochemical polymerization can present an important result on the number- selective ion exchange of conducting polymers. In various situations, the incorporation of great hydrophobic dopant ions (surfactant), results in an efficient cation exchange with fully hydrophobic character. By exploring the efficaciousness of composite coatings, this research scope to ensure that a possible disposal to diminish corrosion and enlarge the lifetime of metals and their alloys constituents. The purpose of this paper is to obtain a novel polymer composite suitable on corrosion safety of various metallic materials. As well, the expansion of suitable electrochemical deposition practices that will provide the capability to achieve homogeneous, compact and highly adhesive composite coverings on metal surfaces. Other area of interest is the realization and optimization of new composite coatings with the best protective properties for certain materials in aggressive solutions. These new composite coatings are different from those presented in the specialized literature and have obtained better experimental determinations. This work implies the electrochemical deposition and electrochemical and spectroscopic investigation of the new composite poly (3-methyl pyrrole- sodium dodecylsulfate/2 methyl thiophene) and the corrosion behaviour of this new composite coatings. This research is a continuation of prior work on obtaining and evaluating suitable coating for corrosion protection of the metals in aggressive environments. The new polymeric composite (P3MPY-SDS/P2MT) was electrochemically deposited on cobalt- based alloy electrode by galvanostatic and potentiostatic practices from synthesis solutions of 0.1 M 3-methylpyrrole, 0.1 M 2- methyl thiophene, and 0.03 M sodium sulfate and 0.2 M sodium salicylate solution. The evaluation of the new coatings performed by FT-IR spectroscopy, cyclic voltammetry and SEM methods. Corrosion tests of (P3MPY-SDS/P2MT) coated cobalt- based alloy were examined by potentiodynamic polarization and EIS techniques in 1 M HCl solution.
2. Experimental
2.1. Materials and Methods
In this study, the cobalt- based alloy specimen was utilized as the working electrode for the corrosion consideration. The structure of the cobalt- based alloy is: Co% 67, Cr% 29, W% 4 and the aggressive medium has been 1M HCl that was achieved by diluting of AG 36% HCl (from Merck) with bidistilled water. All chemicals were of reagent grade, sodium dodecylsulfate (SDS), 3-methylpyrrole (3MPY), 2- methyl thiophene (2MT) were from Aldrich (>98%), sodium salicylate (C
7H
5NaO
3) was obtained from Sigma-Aldrich. In all experimental tests, synthesis solutions were made using bi-distilled water: 3MPY 0.1M, SDS 0.03M, 2MT 0.1M and 0.2M C
7H
5NaO
3. The electrochemical deposition and consideration of the new composite material was performed by a single –electrochemical cell of the three conventional (typical) electrodes installed (at room temperature). The working cell has been related to a VoltaLab potentiostat/galvanostat logged in the computer operating VoltaMaster software. A saturated calomel electrode (SCE) was benefited as the reference electrode and a platinum sheet as an auxiliary electrode. The working example is a cylindrical cobalt- based alloy with a surface of 0.2 cm
2. This form is preferred, as it assures an appreciable and borderless area. The cobalt alloy electrode was mechanically abraded through a sequence of sandpapers of varied sizes (200- 4000 grid) up to a mirror-shine. However, the cobalt alloy specimen was cleansed in acetone to remove all residual grease, following which, this has been rinsed in by-distilled water, dried at room temperature and put into the work cell. All tests have been performed at 25°C in atmospheric oxygen without stirring. Prior the electropolymerization of the composite, the cobalt alloy specimen has been passivated in 0.2 M sodium salicylate medium by cyclic voltammetry on a domain potential of -500mV to 1500 mV for SCE at a potential scan rate 20mV/s by applying 3 cycles. The poly (3-methylpyrrole –SDS/2 methyl thiophene) coatings have been electrochemically deposited from 0.1M 3-methylpyrrole, 0.03M SDS, 0.1 M 2- methyl thiophene and 0.2 M sodium salicylate on cobalt alloy passivated surface by galvanostatic and potentiostatic procedure (
Scheme 1).
Electrochemical deposition was performed by galvanostatic method at constant current density: 0.5 mA/cm
2 and 1mA/cm
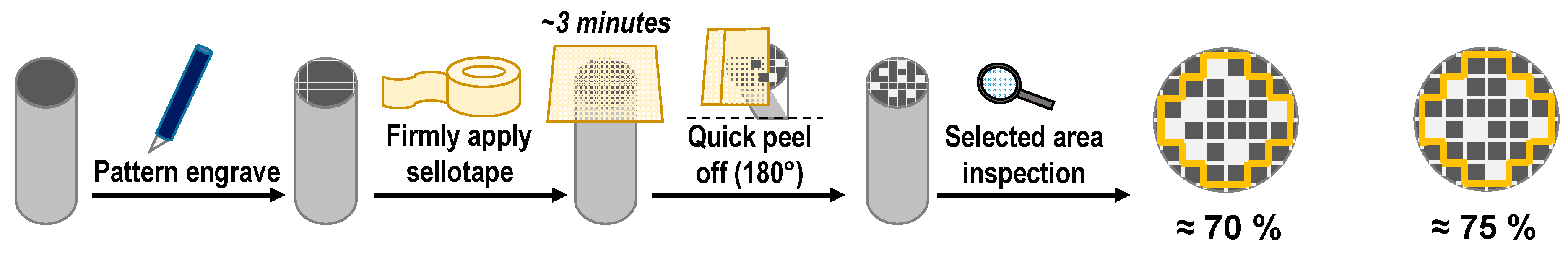
2 and by potentiostatic practice at the applied potential: 0.9 V and 1.0 V and in different molar ratio (5: 1 and 1: 5) and the electrodeposition has been left for 10 and 20 min. The adhesion of this coating has been achieved by the “standard sellotape test”, which “involves cutting the film into small squares, sticking the tape” and then detaching it. Proportional adherence has been obtained by the ratio of the number of remaining adherent cover squares to the total number of the squares (see
Scheme 2). The electrochemical behaviour of the composite coatings was explored in a 0.2 M sodium salicylate electrolyte by cyclic voltammetry technique. Protection against corrosion of the coating and bare specimens has been examined by potentiostatic and potentiodynamic polarization procedures and by electrochemical impedance spectroscopy in hydrochloric acid solution. The investigation of Tafel polarization curves has been accomplished by switching from cathodic to anodic potentials for the OCP at a scan rate of 2mV/s. EIS experiments have been realized in the frequency field from 100 KHz to 0.04 Hz and the signal amplitude was 10mV, at the OCP of uncoated and coated samples. All potentials were recorded vs. SCE. Each experimental test has been rehearsed three times to examine reproducibility.
2.2. Instruments
A VoltaLab PGZ 402 potentiostat/galvanostat configuration has been used in all electrochemical determinations. Composite coverage was effectuated with Bruker optics FT-IR spectrometer (with ATR) in the spectral domain 4000-650 cm−1 at a resolution 4 cm−1. The morphologies of the coated specimens have been examined by scanning electron microscope (SEM). Substrate morphology exploration were performed by SEM in a dual beam FEI Quanta 3D FEG model, working in high vacuum means with an accelerating voltage of 2 to 30 kV. Minimal sample’s training implicates immobilizing the specimens on a double-sided carbon tape, without coating.
3. Results and Discussion
3.1. Electrochemical deposition of P3MPY-SDS/P2MT coating on cobalt alloy
Electrochemical polymerization of 3MPY-SDS and 2MTP monomers was performed by applying the galvanostatic and potentiostatic practices on the passivated surface cobalt based alloy electrode. The passivation technique was carried out by using the voltammetry pocess in the range from −0.5V to 1.5V vs. SCE at a 20 mV/s at a scan rate in 0.2 M sodium salicylate solution by implementing 3 cycles and this proceeding has been related in prior works [
10,
40,
51]. The insoluble constituents achieved by the passivation process are complexes of cobalt oxides and cobalt salicylates that impede the metal from dissolving without complicating complicating the electrochemical polymerization technique [
51,
53,
54,
55,
56]. By perfecting the polymerization characteristics we can achieve polymeric layers of P3MPY-SDS/P2MT that have a significant anticorrosion protection impact. The electrochemical polymerization of 3MP and 2MT monomers has been accomplished on passivated cobalt alloy surfaces. After passivation, the electropolymerization of the monomers was determined without modifying the polymerization properties. A successful deposition requires getting a passivated film, which could be effective in stoping the dissolving of the oxidizable metal without hindering the permitivity of the monomer and its after oxidation. The end result is a homogeneous, compact and adhesive polymer layer at the surface electrode. P3MPY-SDS/P2MT coating was made from 0.2 M sodium salicylate, 0.1 M 3-methylpyrrole, 0.03 M sodium dodecylsulfate and 0.1 M 2 -methyl thiophene by galvanostatic process at current density: 0.5 mA/cm
2 and 1mA/cm
2 and by potentiostatic practice at: 0.9 V and 1.0 V applied potential, in differing molar ratio.
The electrochemical deposition has been enabled for 10 min and 20 min.
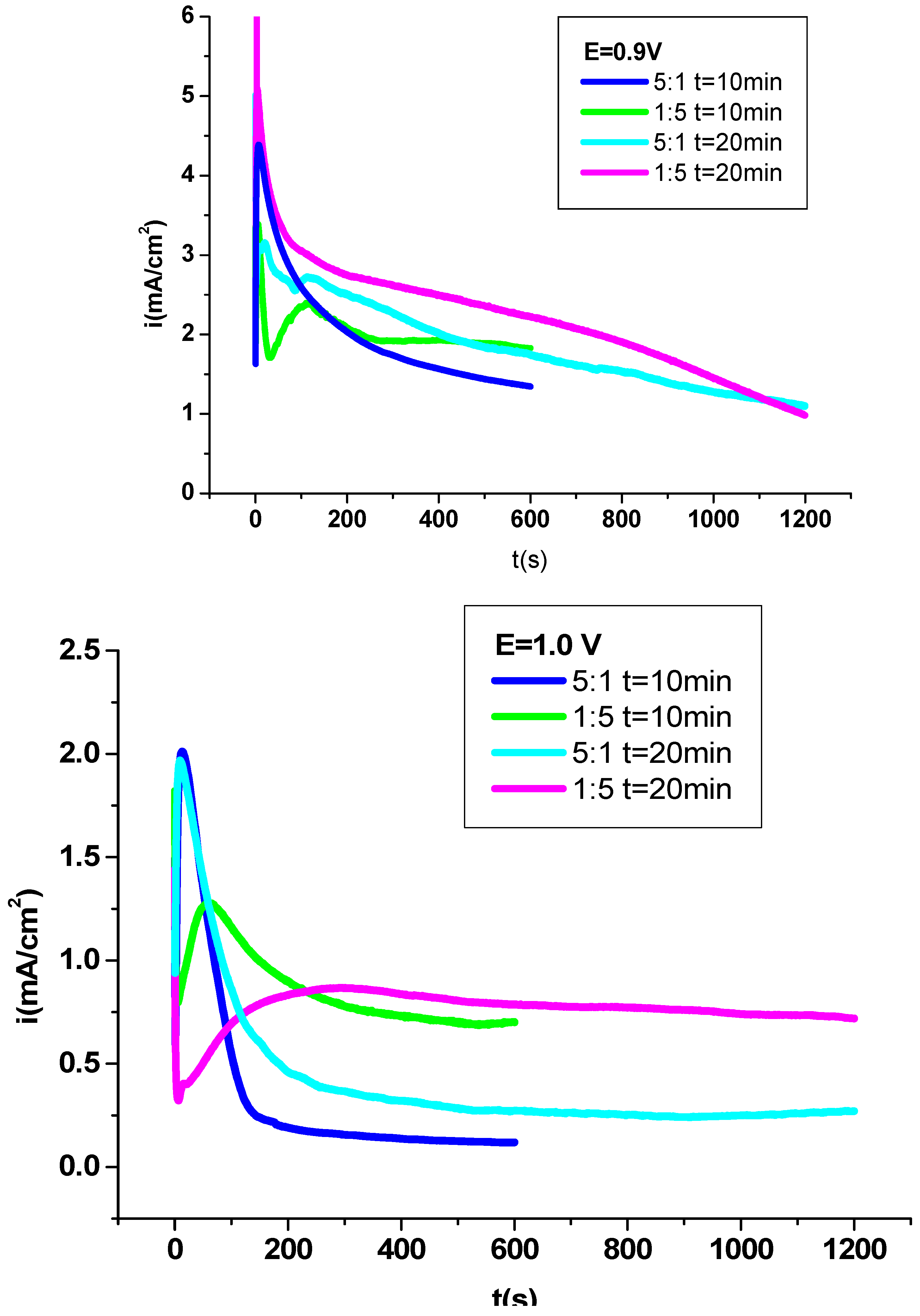
Figure 1, shows the „current density – time” curves through the formation of P3MPY-SDS/P2MT coatings on cobalt alloy at 0.9V and 1.0 V applied potential in different molar ratios. After to the oxidation time of 600 s and 1200 s, the primer shape of the „current density –time” plot throughout the estimation of the electrochemical deposition of the polymer indicates that this submission was established by “nucleation and growth” on the cobalt alloy surface [
8,
24,
33]. At first, the current was suddenly reduced due to “electro-adsorption” of the sodium salicylate and 3MPY: 2MT monomers. Following about 30 s the current rises and this is due to the dissolution of the passive film and constitution of the polymer on the cobalt alloy substrate. In the end stage, the current has been held steady as the polymer composite have been obtained on the cobalt alloy substrate. The transient amend of the higher side is linear with the gradient of the nucleation period, the electrochemical examination and the distinction in the molar ratio of 3MPY: 2MT. When the applied potential was 0.9 V at 5:1 and 1:5 molar ratio, the current is approximately constant at 1.5 mA/cm
2 and 2 mA/cm
2, the current has been upper than in another potentiostatic electrodeposition circumstances and the layers obtained were homogeneous and adhesive. From
Figure 1, the potentiostatic electrodeposition at 0.9 V applied potential in 1:5 for 3MPY: 2MT molar ratio has a longer induction period for obtaining of P3MPY-SDS/P2MT coating and has been lesser favorable for the constitution the polymer with the higher features. Electrochemical characteristics such as the applied potential have been established to possess a great impact on the “induction time”.
It can be observed that the 0.9 V, and 1.0V applied potential in 5:1 and 1:5 molar ratio for 3MPY-SDS: 2MT has a short induction period for the evolution of the electrodeposited film and it has been appreciated for getting coatings high quality. The SDS surfactant as a dopant ion used in electrochemical deposition (present in 3MPY) can have a relevant result on the selectivity of ion broadening by enssuring the conductivity of polymer. Visional inspection of the cobalt alloy surface after electropolymerization denotes the formation of a black layer of P3MPY-SDS/P2MT. The coating is dense, compact and adhesion on to cobalt alloy substrate. Coating adhesion as appraised by “the standard sellotape”was estimated to be ~75% [
8,
14,
40]
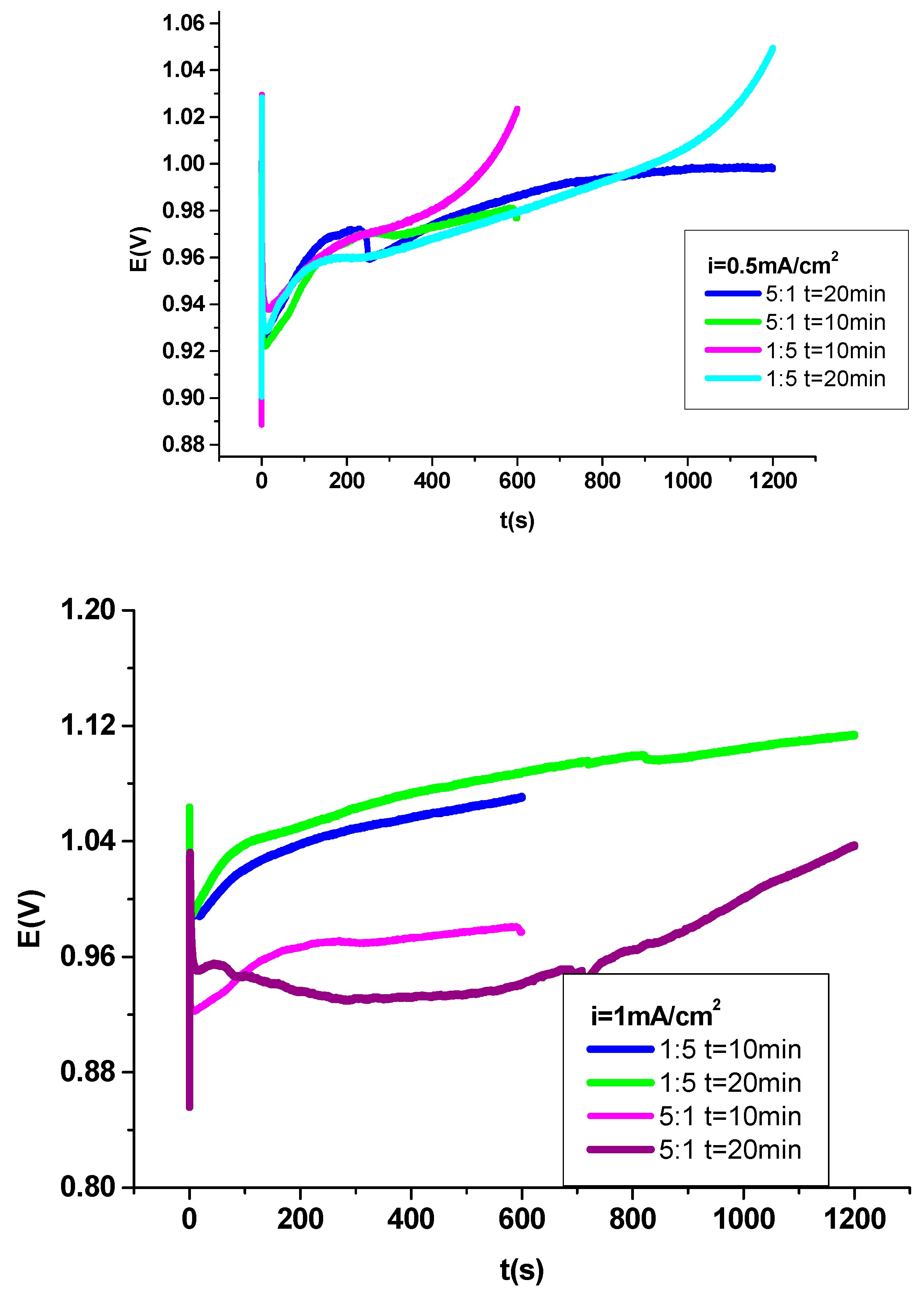
Graph 2 displays the “potential-times” curves by acquistion of the polymer film as: poly (3-methylpyrrole- sodium dodecylsulfate/2-methyl thiophene) on cobalt alloy surface (P3MPY-SDS/P2MT) at different applied current density in varying molar ratio. After the oxidation interval of 600 s and 1200 s, the primary configuration of the „potential –time” curves by the polymer electrodeposition procedure mentions that the coating has been achieved by “nucleation and growth” on cobalt alloy area [
8,
24,
33,
34,
35,
36,
40]. Presenting
Figure 2, at applied current density of 0.5 mA/cm
2 and 1mA/cm
2 at certain molar ratio, shorter induction interval appears, and induction range of lower than 5s was observed in the electropolymerization of the film composite of 3MPY-SDS: 2MT and its value diminutions by increasing the molar ratio and the rising of the “nucleation potential".
The potential of the electropolymerization is distinct between 0.92V and 1.04 V, versus SCE for applied current density: 0.5mA/cm
2, and 1mA/cm
2 in 5:1and 1:5 molar ratio for 3MPY-SDS and 2MT. Composite coatings appear most dense and adherent at applied current densities of approximately 0.5mA/cm
2. The visional exploration of the cobalt alloy specimen by deposition divulges the constitution of a black P3MPY-SDS/P2MT /cobalt alloy layer. The coating adhesion evaluation by “the standard sellotape” was approximately to be ~70-75% [
13,
14,
40]
3.2. Electrochemical exploration P3MPY-SDS/P2MT composite coating
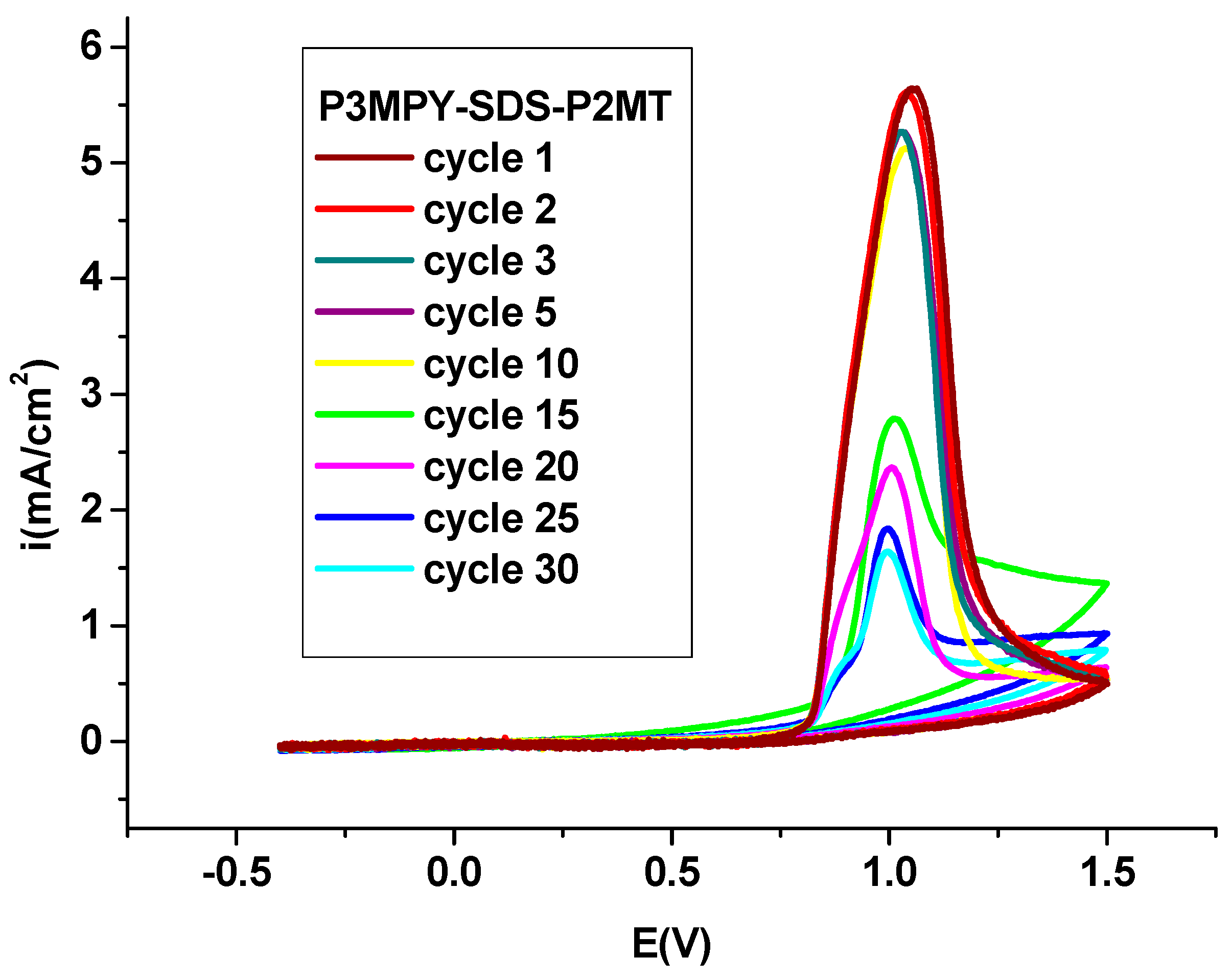
The electrochemical behaviour of modification P3MPY-SDS/P2MT/cobalt alloy specimen in 0.2 M sodium salicylate (free monomer) is display in
Figure 3 to the potential domain of -0.4 and +1.5 V vs. SCE and the scan speed of 20mV/s. Cyclic voltammetry procedure has been performed in the prolonged potential range to take into account all the “physical and electrochemical” properties of this coating. By exploring the Graph 3, it can be stated (affirmed) that the electrochemical behaviour of covering is influenced by the numeral of cycles and the attributes of the electrodeposition. The resistance of any conducting polymer under “reduced and oxidized” states is an important characteristic for numerous practices.
The fundamental factor that establishes the “lifetime of a conducting polymer” is the constant chemical participation (presence) of the matrix itself [
20,
33,
38]. The resistance of the P3MPY-SDS/P2MT polymer coating has been determined by cyclic voltammetry (without monomer) in sodium salicylate medium (see Graph 3). Consequently, this composite could often be cycled from “oxidized and reduced” condition without appreciable deterioration of the composite, the current density decreases with every cycle and finally attains a steady valuable. The polymer that exhibits a minimal diminish in current density upon repeated cycling is more stable electrochemically
3.3. FT-IR Evaluations
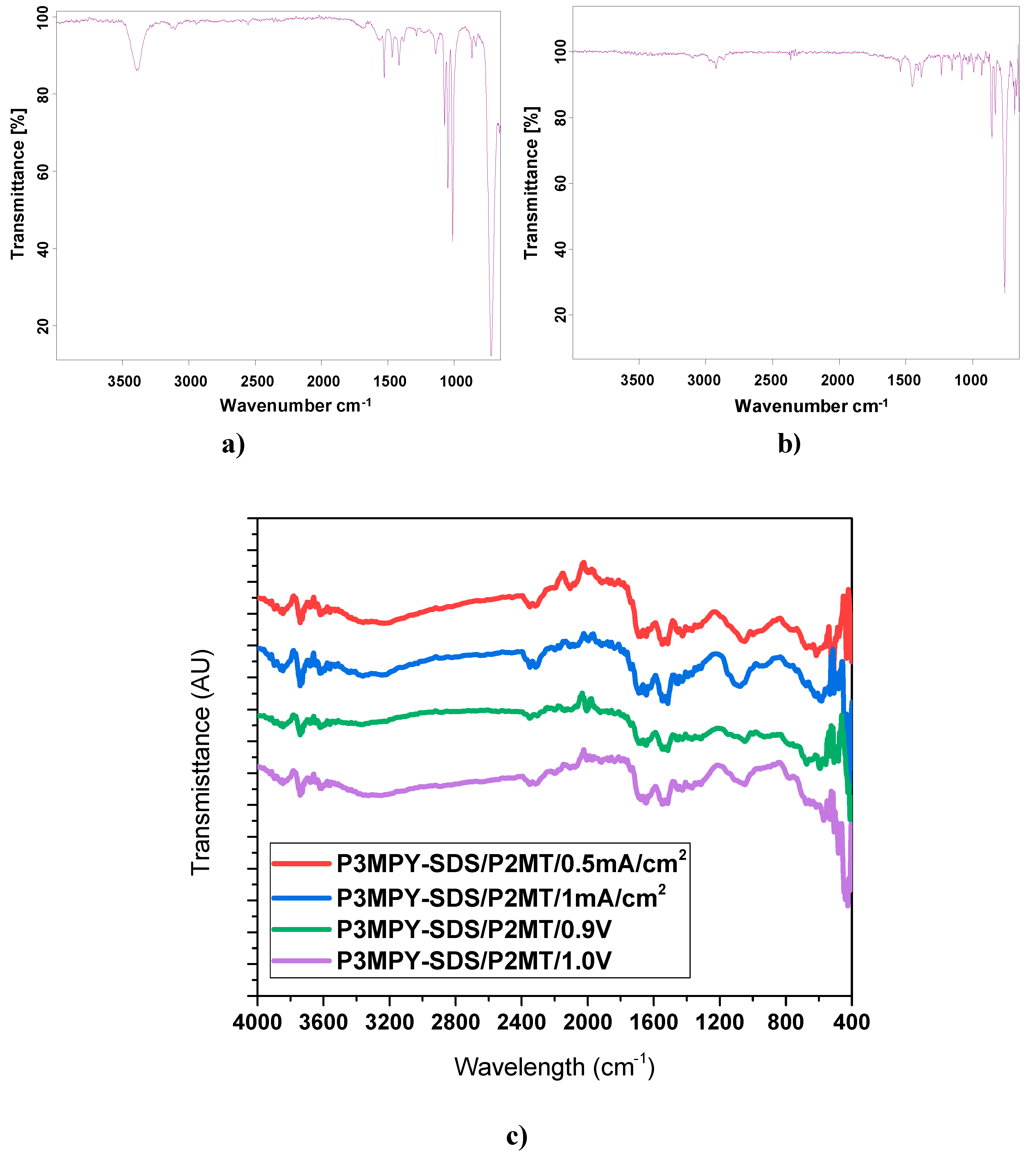
The novel composite polymeric covering was investigated by FT-IR spectroscopy (see
Figure 4) in the domain 4000–650 cm
−1 at a resolution of 4 cm
−1 (about four scans). FT-IR techniques can be utilized to emphasize the kind of bonding used to obtain a new polymer composite. Distinctive peaks in the transmittance spectra of P3MPY-SDS/P2MT/cobalt alloy covered are displayed in Graph 4. The FT-IR determinations depict the occurrence of significant “absorption bands” remarked in P3MPY and P2MT electrodeposited on the cobalt -based alloy surface. The results of FTIR examination correspond well with the views reported in [
35,
36,
37,
38,
39,
49,
51] where similar peaks were noted. The prominent band in the transmittance spectrum of 3MPY and 2MT that were presented in
Figure 4a and b are the consequence: the spectrum of 3MPY indicated in
Figure 4a, where the considerable property of band for the aromatic ring in 3MPY is positioned to 1542 and 1451 cm
−1 for C=C “stretching”, being obviously highlighted. The indicative bands at 1378 and 2941cm
−1 are ascribed to the N-H “stretching vibration” of the pyrrole ring and the CH3 stretch of the 3-methylpyrrole parts. The peaks, that can be attributed as “in- plane and out -of -plane” of the CH chains at 1107, 1071 and 673 cm
−1 are shown in the polymer. In
Figure 4b is presented the spectrum of 2MT, the peak at 2978 cm
−1 is displayed to the CH
3 stretch of 2-methylthiophene units and the peaks appropriate to the ”asymmetric and symmetric” C=C “stretching vibrations” of the 2MT ring are noticed at 1553 cm
−1 and 1421 cm
−1. The band looking at 1037 cm
−1 and 767 cm
−1 establishes the “C-S-C stretching” vibration of the “thiophene ring”.
The P3MPY-SDS/P2MT/cobalt alloy electrodeposition spectra by “potentiostatic and galvanostatic” practices are shown in
Figure 4c. The distinct peaks at 3463 and 3208 cm
−1 are attributed to the N-H stretching vibration in the polymer. The peaks displaying at 3518 and 3432 cm
−1 correlate with the OH stretching of the counterions. The peaks regarding 3100–2900 cm
−1 are allocated to the CH
3 stretching of 3-methylpyrrole constituents. The band considering at 1221 cm
−1 represents the C-N of the pyrrole ring. Important attributions of the “aromatic ring” peaks in P3MPY are evident at 1567 and 1453 cm
−1 for C=C stretch, being clearly determined. The “absorption bands” placed at 1571 and 1463 cm
−1 are depicted in the “stretching vibration of the quinoid rings” (
Figure 4c). The peaks located at 1381 and 1318 cm
−1 are assigned to N-H “stretching vibration” in methylpyrrole ring; the band at 1660 and 1645 cm
−1 is attached to the C=C stretching. In the P3MPY-SDS/P2MT coating spectrum, bands appropriate for “asymmetric and symmetric C=C stretching vibrations” of the 2-methylthiophene ring are observed at 1563 cm
−1 and 1462 cm
−1 (
Figure 4c) [
14,
35,
36,
37,
51]. The peaks revealed at approximately 1043 cm
−1 and 764 cm
−1 exhibit the C-S-C stretching vibration of the 2-methylthiophene ring and the peak at 1531 cm
−1 is associated for the C=C “stretching vibration”. The peaks allocated at 1451and 1308 cm
−1 are attached to the “stretching vibration” of the CH
2 and CH
3 elements in the SDS surfactant and the short peaks at 1071 cm
−1 and 670 cm
−1 are attributed to the S=O “stretching vibration” of SDS anion. The occurrence of the link C=O and CH is indicated by “stretching vibration” at 1678cm
−1 and 1251 cm
−1, most were assumed to be related to the enhancement of SDS in the polymer matrix. (
Figure 4c). The bands at approximately 1070 -770 cm
−1 are ascribed to the” in-plane” and “out-plane” C-H of the aromatic rings and to “out of plane” vibration of C-H doped of P3MPY in sodium salicylate electrolyte (
Figure 4 c-e.) [
35,
36,
37,
51]. By comparison
Figure 4a-b with 4c, it can be assumed that the P3MPY-SDS/P2MT coating is electrosynthesis on the cobalt alloy surface. The indicated bands of the monomer (MPY and 2MT) are presented in the spectrum of the coating (P3MPY-SDS/P2MT) on the cobalt alloy electrode.
3.4. Electrochemical investigation
3.4.1. Potentiodynamic polarization technique
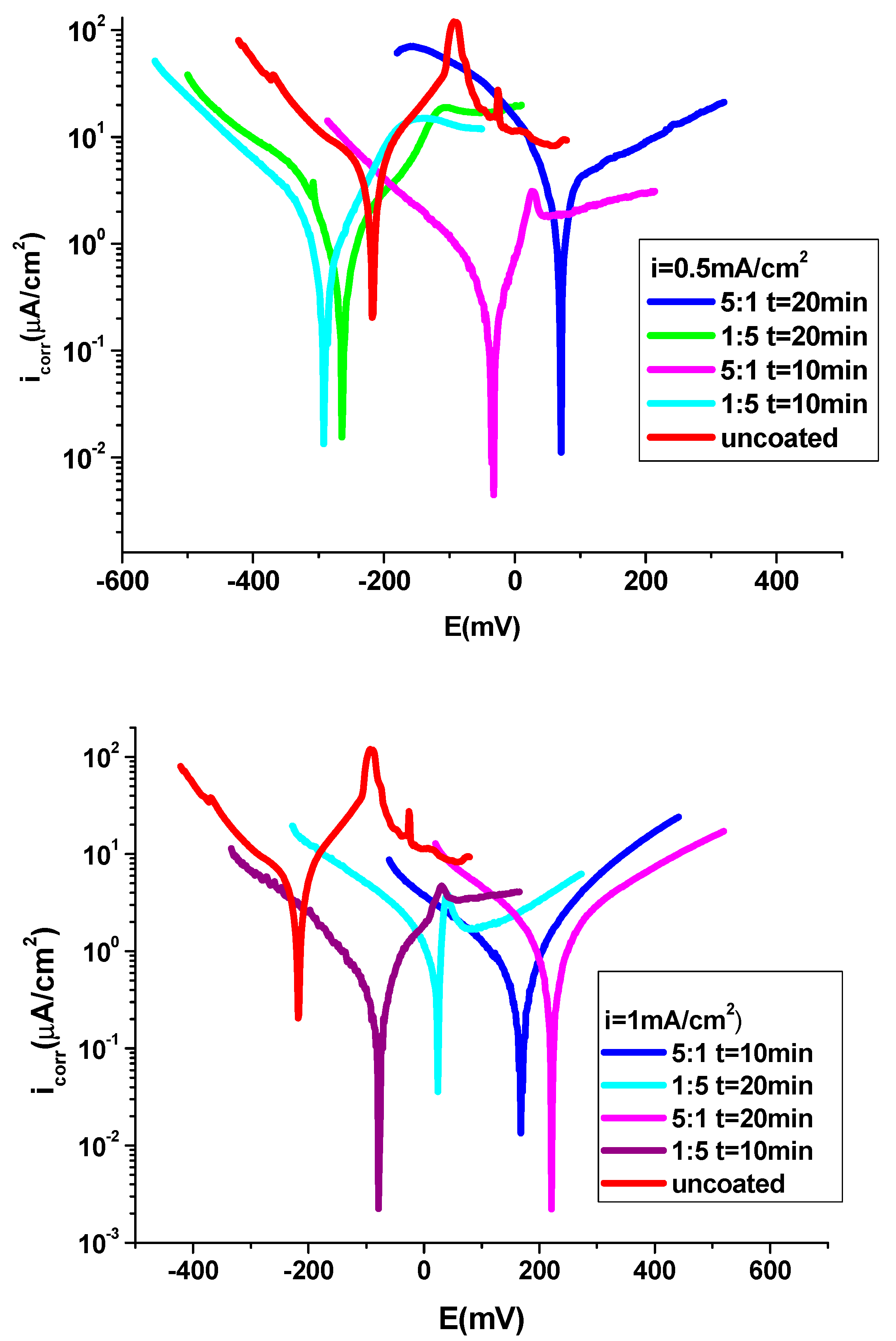
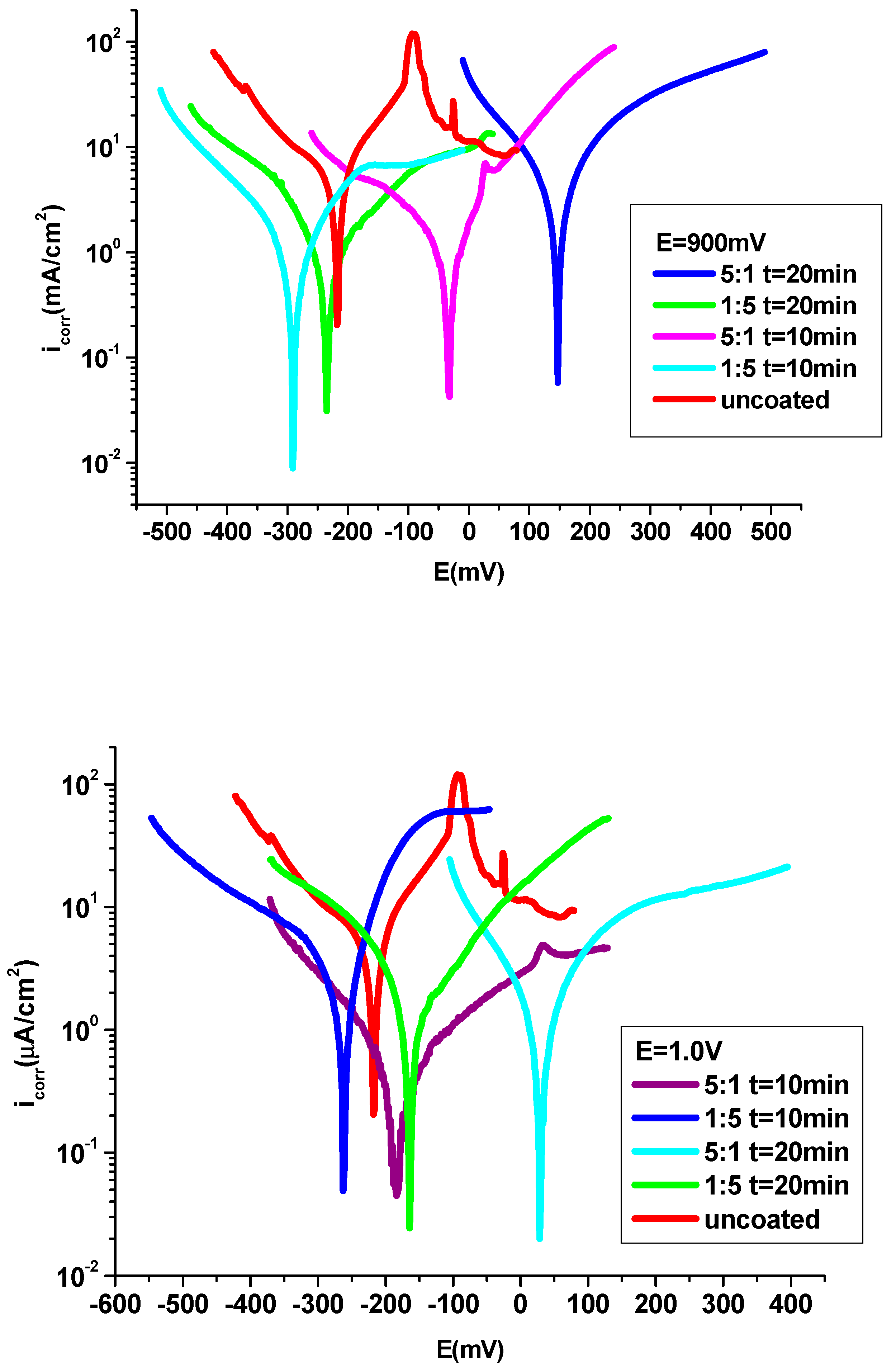
The anticorrosive properties of P3MPY-SDS/P2MT/cobalt alloy polymeric composite were evaluated in 1M HCl by potentiodynamic polarization procedure and electrochemical impedance spectroscopy. The polarization curves of uncoated and coated P3MPY-SDS/P2MT/cobalt alloy in 1 M HCl solution are presented in
Figure 5 and
Figure 6. As well, polarization comportment of the cobalt -based alloy specimen was realized by cobalt alloy coated P3MPY-SDS/P2MT made by potentiostatic and galvanostatic techniques at varied current densities and potentials in different amounts and several submission periods. In this work, one of the biggest practices for protection effect of cobalt alloy in corrosive environment is the use of the composite layers, which examinate the anodic or cathodic corrosion process and both. The surfaces covered by the P3MPY-SDS/P2MT composite expounded an appreciable mitigation of the cathodic and anodic current which revealed the reduction of the cathodic and anodic reactions. The electrochemical experiments have been performed in 1M HCl solution to estimate the protective activity by the polymeric films against corrosion. It can be noted from
Figure 5-6, that both anodic metal dissolution and cathodic hydrogen reduction processes have been impeded by the electrochemical deposition of these P3MPY-SDS/P2MT coatings in the aggressive environment. This case revealed that this covered had considerable action of cathodic and anodic processes of the electrochemical practice.
The corrosion potential (E
corr), corrosion current density (i
corr), anodic and cathodic Tafel slopes were taken into account by extrapolate the lineal side of the anodic and cathodic Tafel branches of cobalt alloy surface coating by the P3MPY-SDS/P2MT composite are shown in
Table 1,
Table 2 and
Table 3. Considering these polarization curves, it is evident that the corrosion potential of the covered cobalt alloy specimen is displaced at more positive potential comparing to the uncoated electrode. This situation may be due to the offensive of corrosive constituents that touch the pores of the film as an effect of the formatting of passive layers that prevent the attack of cobalt alloy electrode.
Investigation from bias curves from
Figure 5 and
Figure 6 and
Table 1 and
Table 2 showed that the electrochemical characteristics of uncoated and deposition-coated cobalt alloy specimen at applied potential 0.9 V and 1.0 V and 0.5mA/cm
2 and 1mA/cm
2 current density at 10 and 20 min for 5:1 and 1:5 molar ratio of P3MPY-SDS/P2MT have been lessen like those for cobalt alloy in 1M HCl medium. This coating has an excellent protective ability because the P3MPY polymer film was doped with a sodium dodecyl sulfate surfactant. This SDS surfactant like a "dopant ion" used in electrochemical polymerization can have a substantial result by changing the ion selectivity by providing the conductivity of the polymer. The presence of the hydrocarbonate chains of the SDS that “competitively adsorb” over the cobalt alloy substrate impeding the actively centers and as a result (effect) the Cl
- corrosive agent is obstructed from attacking the cobalt alloy surface and protection process is acquired [
23,
24,
25,
26,
27,
39,
51]. The determinations (measurements) indicated that the corrosion speed of P3MPY-SDS/P2MT covered cobalt alloy has been about ~10 times lesser than that noticed for uncoated cobalt alloy It is plain that these coatings hindered the affect of the attacking element (HCl) on the cobalt alloy. The corrosion behavior of P3MPY-SDS/P2MT composite layer determined that the covered cobalt alloy has a significantly higher protective action and inferior corrosion speed than the bare cobalt alloy. The determinations of the protection performance of these coatings through periods of immersion are shown in
Figure 7 and
Table 3. The impact of rising immersion period 0-96 h about corrosion defense of P3MPY-SDS/P2MT coatings at the corrosion of cobalt alloy 1M HCl was investigated by potentiodynamic polarization. The effectiveness of the protection slowly decreases with increasing time. It can be seen that, after the immersion period of 72 h a slight growing in corrosion rate is indicated. This reason is owed to the degradation of surface morphology by increasing immersion period as an effect of the alteration of the active area and may be caused by some existing defects on the protective film that allow to the admission of the corrosive agents at the cobalt alloy/coverage interface. The data revealed that the process of the redox reaction is complicated and may be led by electrolyte diffusion. It has been determined that the cobalt alloy specimen had an important impact on the electrochemical performance of the composite layers and that with the attendance of the polymeric layer, anodization of these coated samples was achieved by simultaneously forming of a complex oxides film of and polymeric layers. Analyzing the
Figure 5,
Figure 6 and
Figure 7 and
Table 1,
Table 2 and
Table 3, it is indicated that the lowest corrosion speed and the highest protection performance have been obtained by P3MPY-SDS/P2MT composite at 0.9V and 1.0V, at 0.5 mA/cm
2 and 1 mA/cm
2 (at 5:1 molar ratios, t=20 min) applied current density (at 5:1, 1:5 molar ratio) and a very well defense achieved at 1.0V and 1 mA/cm
2 (at 1:5 molar ratio, t= 10min) versus to uncoated substrate in 1M HCl environment.
The corrosion process of bare and composite coated cobalt alloy in HCl medium can be effectuated like this [
5,
23,
24,
25,
26,
27,
35,
52,
53,
54,
55,
56,
57,
58]:
Anodic process:
-dissolution of Metal (M=Co-Cr-W) as anodic process
M→Mn++ ne-
P3MPYundoped-ne-→P3MPYdoped
P2MTundoped -ne-→P2MT doped
Cathodic process:
The oxygen reduction as cathodic reaction:
P3MPYdoped+ne-→P3MPYundoped
P2MT doped +ne-→P2MTundoped
In the acidic media, the metal is oxidized to a superior valence state by dissolving in an anodic process. Products dissolved in the medium as oxygen, hydrogen ions are reduced by electrons accepted from the metal in a cathodic process.
The porosity (P) of the polymer composite (P3MPY-SDS/P2MT) (
Table 1 and
Table 2) is a particular parameter that must be evaluated when a coating layer is adequate or not for protecting the surface versus corrosion. The porosity of the coating was estimated by the further relation
P is total porosity, Rp- the polarization resistance for uncoated and coated cobalt-based alloy ΔEcorr-the difference from corrosion potential of coated and uncoated specimens and βa is the anodic Tafel slope of uncoated cobalt-based alloy. As well, the porosity of P3MPY-SDS/P2MT coated cobalt alloy by potentiostatic and galvanostatic procedure are 0.00001, 0.00003, 0.00005, 0.00009 and 0.0001 (at 0.5mA/cm2 and 1mA/cm2 current density and at 0.9V and 1.0V potential applied in 5:1 molar ratio). The appreciable size of the porosity in the P3MPY-SDS/P2MT layer demonstrates a significant enhancement of the protection effect by obstructing the access of the aggressive agent (Cl-) to the cobalt alloy substrate and as well reduces the corrosion of the underlying cobalt alloy surface. The P3MPY-SDS/P2MT coatings were found to exhibit a lesser porosity value, indicating that the composite layer presented dense and uniform construction of the coating.
3.4.2. Electrochemical Impedance Spectroscopy (EIS) examinations
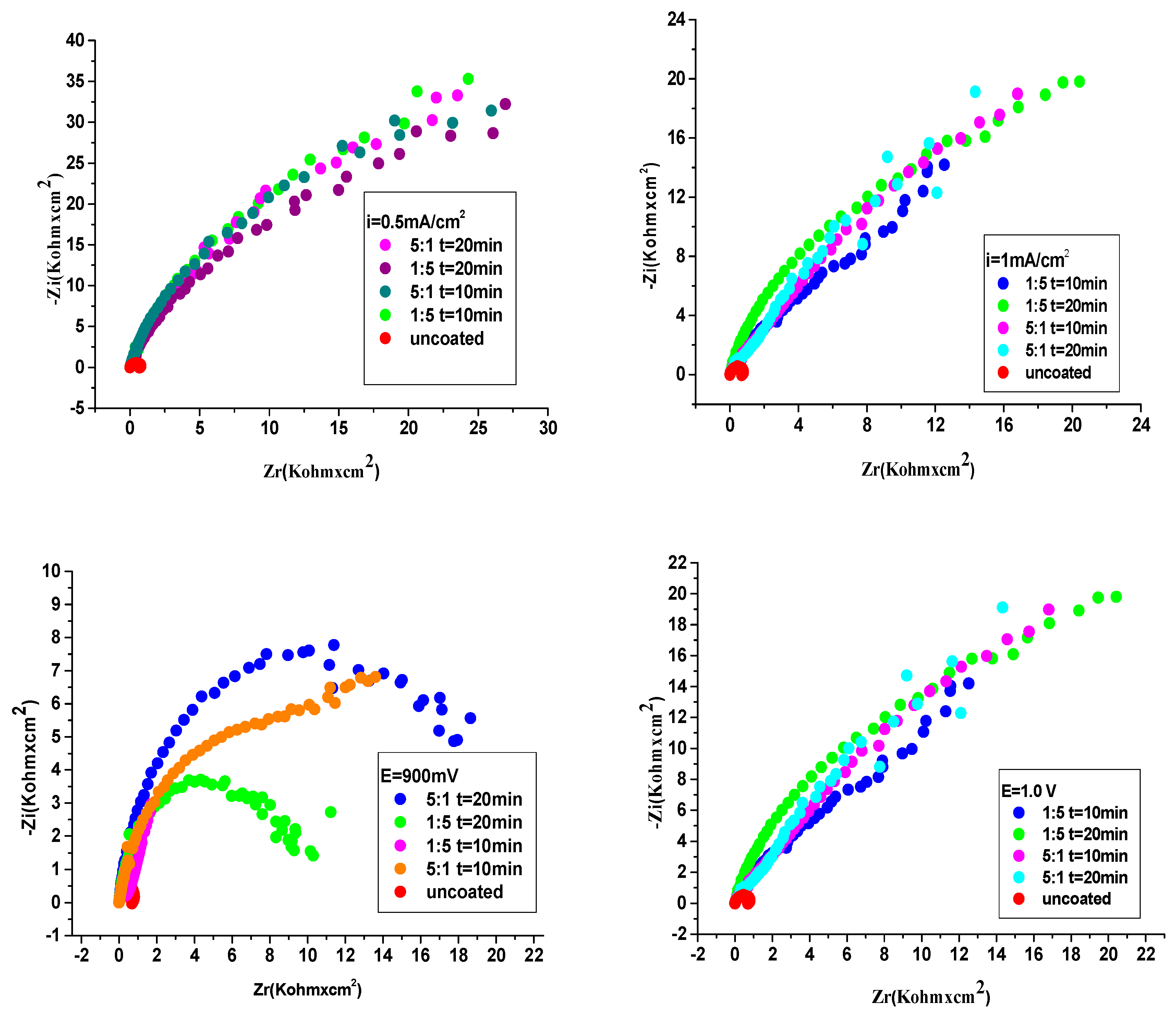
The anticorrosive activity of P3MPY-SDS/P2MT composite covered onto cobalt alloy in HCl solution was analyzed by electrochemical impedance spectroscopy (EIS). Impedance experiments have been effectuated at OCP in the frequency interval from 100 KHz to 40 mHz with an AC wave of ± 10 mV (peak-to-peak) and the impedance determinations have been accomplished at a rate of 10 points per decade of change in frequency. EIS results provide insight into the estimation of the defensive properties of the new composite as an anticorrosion safety layer.
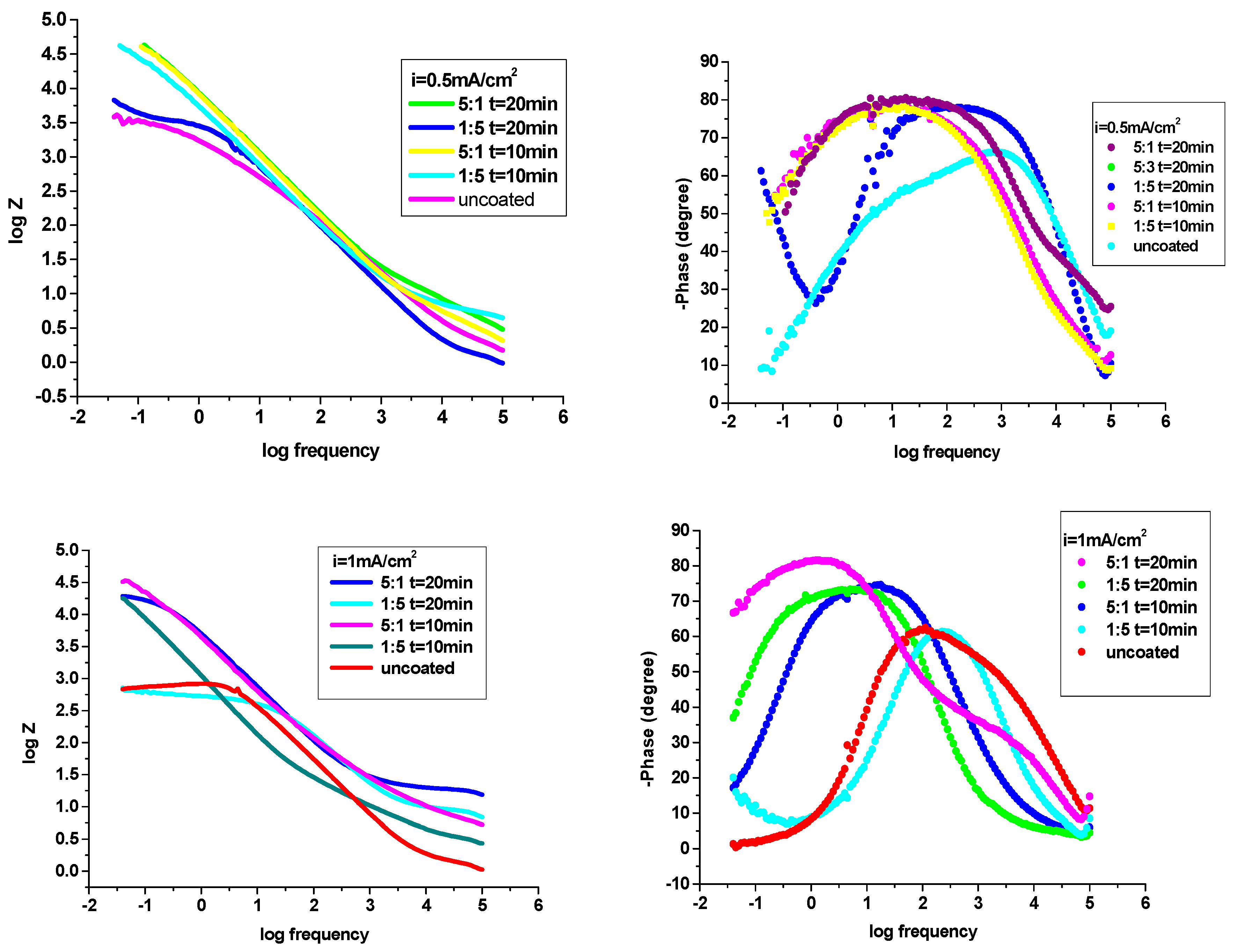
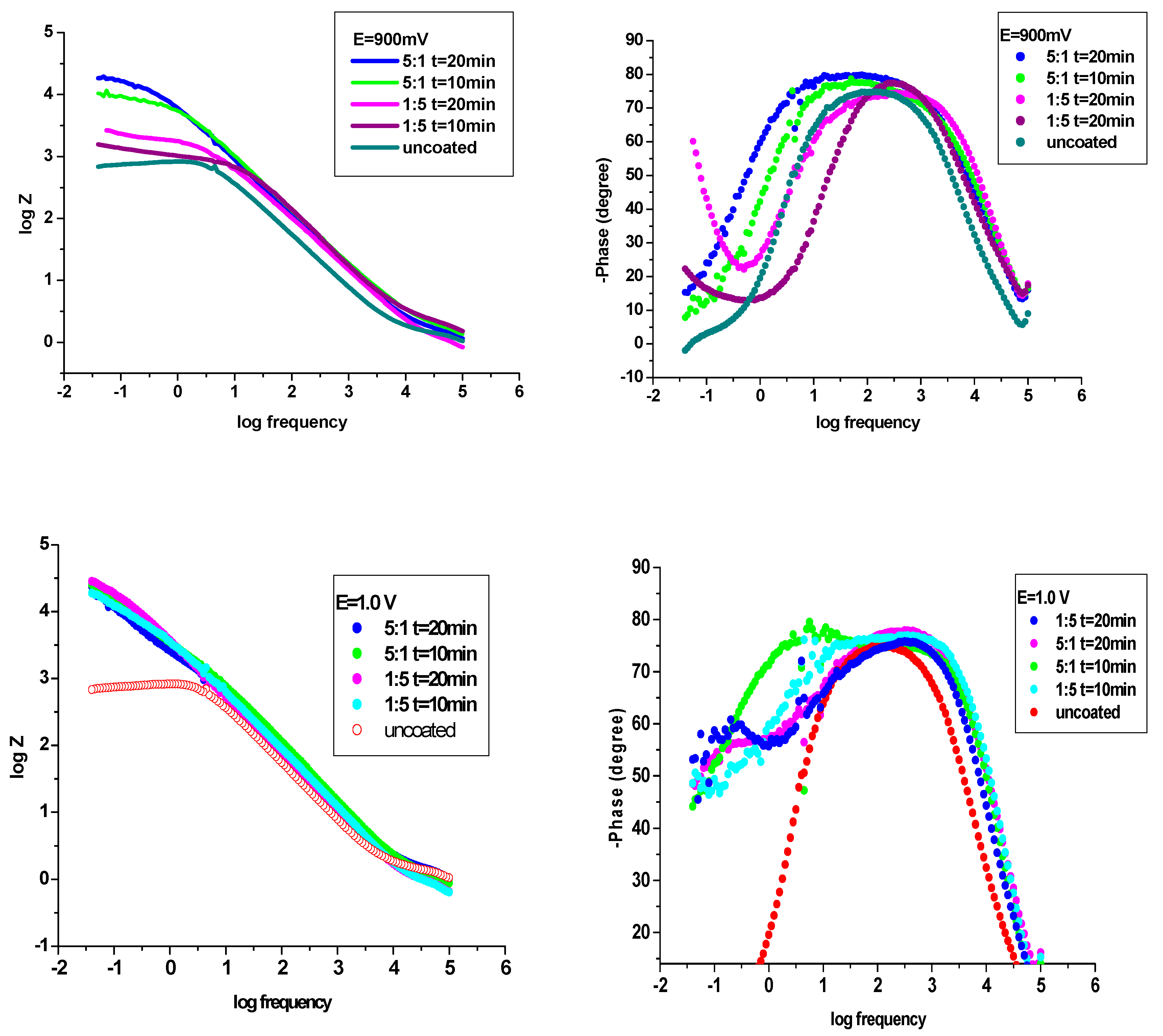
Figure 8 (a-d) depicts the Nyquist impedance data achieved for P3MPY-SDS/P2MT coatings of cobalt alloy surface and for uncoated cobalt alloy in hydrochloric acid media. It can be observed from
Figure 8, the Nyquist plots for cobalt alloy specimen showed a small capacitive loop, establishing that” charge transfer” proceeding has been prevalent during corrosion process. Nyquist plots (
Figure 8 a-d) for P3MPY-SDS/P2MT covered on cobalt alloy surface indicating one semicircle which is characteristic for a charge transfer reaction. Hence, the dimensions of the capacitance loops of the composites are bigger than that uncoated working electrode and the measures of these loops rising once the coatings are perfected, suggesting that these P3MPY-SDS/P2MT coatings have greater shielding properties on the specimen in HCl. It is noted from the Nyquist graphs that the impedance reaction of cobalt alloy has been considerably changed by the electrodeposition of the coatings, indicating that the protective layer produced was determined by the use the P3MPY-SDS/P2MT composite. As well, these capacitive loops are not precise semicircles and this shape (appearance) is ascribed to frequency dispersion, allocated in specific to the rugosity and inhomogeneity of the working electrode area.
Figure 8 presents that the capacitance loops sizes for coating realized at 1.0 V and 0.9 V potential applied and at 0.5 mA/cm
2 and at 1 mA/cm
2 current density at 5:1, 1:5, molar ratio, (at time 10 and 20 min) are superior in comparison with to uncoated cobalt alloy, implying a significant protective result for aggressive environments of the cobalt alloy sample. It can be seen in
Figure 8 that the capacitance loops sizes of P3MPY-SDS/P2MT coatings accomplished by galvanostatic process at 0.5 mA/cm
2 and 1mA/cm
2 current density (in molar ratio at 5:1 and 1:5) are bigger than those effectuated by potentiostatic procedure at 1.0V and 0.9 V potential applied as an effect, the protection performance of this composite layer is superior. Analyzing
Figure 8 and
Table 4 and
Table 5 it can be assumed that the P3MPY-SDS/P2MT coatings functioned as an efficacious physical barrier that prevented the accession of the aggressive agents (Cl
-) into the covering, minimizing the charge transfer and accordingly, stopping the corrosion process.
The Bode plots of the P3MPY-SDS/P2MT coating (
Figure 9) presented that the impedance modulus, at low frequencies, increases with the rising of the improvement of this composite, showing that the polymeric composite safety layer increases the anticorrosion protection of the cobalt alloy in acid solution. At
Figure 9, it is plain that cobalt alloy exhibits one time constant approximate at a phase angle of 65º at average and low frequencies, showing an inductive behaviour by weak diffusion trend. The Bode diagrams at
Figure 9, show that the presence of composite coverages (P3MPY-SDS/P2MT) on the phase angle versus the frequency logarithm exhibits a very well-defined maximum at a phase angle of 75-85º which corresponds to a relaxation time constant, what implies a capacitive behavior by the easy diffusion tendency. Hence, in these circumstances, the coated electrodes have a good capacitive behaviour according to the Nyquist data and determinations made by potentiodynamic polarization technique. Increasing in Zmod indicates an important protective ability and it is obvious that Zmod raises when composie layer is improved. A greater Zmod leads a higher inhibition efficacy.
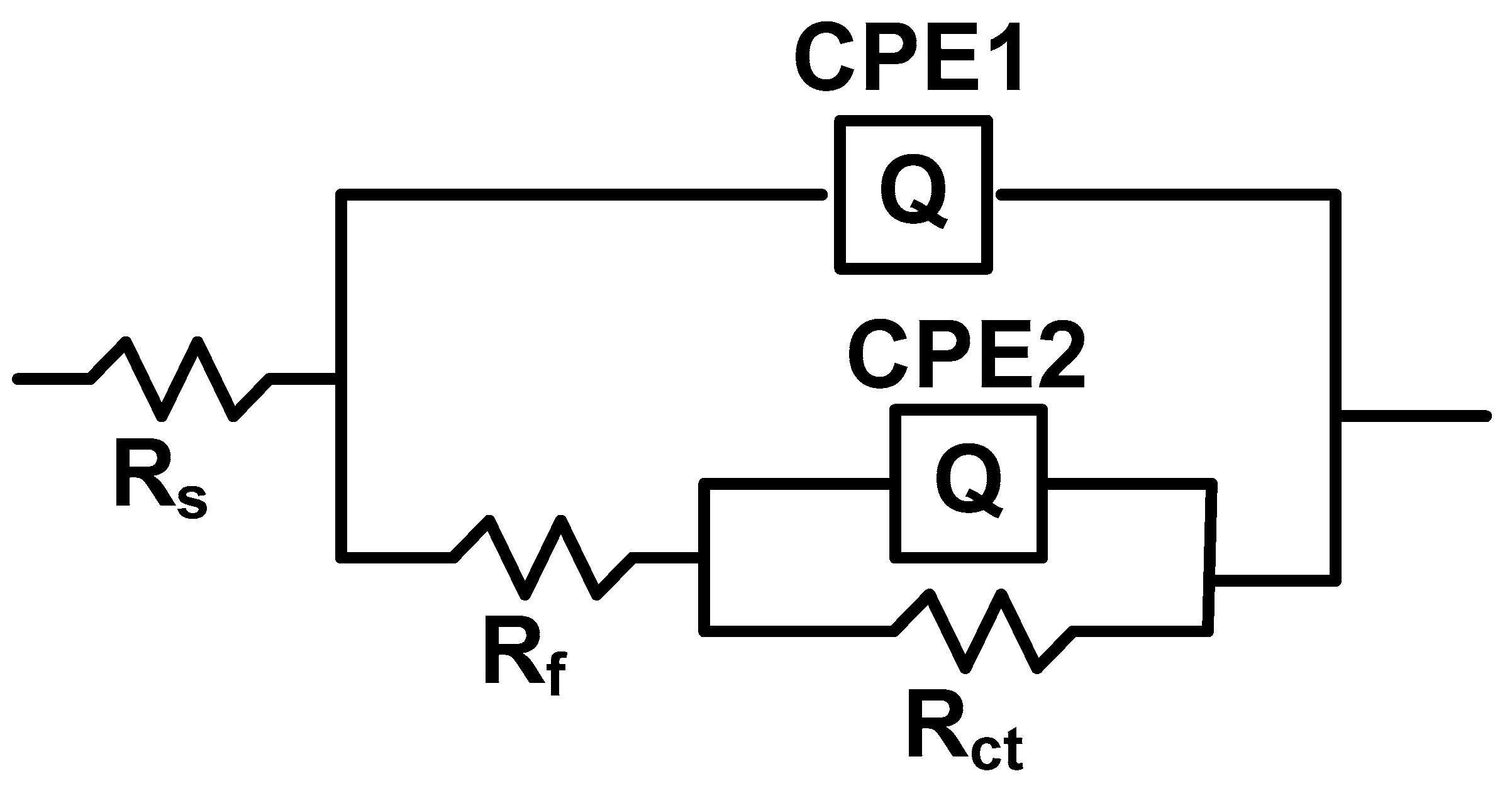
The evaluation of the impedance experiments was evidenced by matching the data with the suitable equivalent circuit indicated in
Figure 10 and the various impedance characteristics such as the solution resistance (R
s), the resistance of coating film (R
f), the charge transfer resistance (R
ct), the capacitance of coating film (C
f), the capacitance of double layer (C
dl) and protection efficiency were shown in
Table 4,
Table 5 and Table 6 In this paper, a frequency domain equivalent circuit model developed to fit and account for the acquired EIS results was proposed. In this situation the constant phase element, CPE, is revealed in the circuit instead of a pure double layer capacitor (Cdl) to provide, a more accurate match. The CPE is utilized to determine the deformation of the capacitance loop, which attributes the heterogeneity of the zone from surface roughness and impurities. The impedance of CPE can be analyzed, as follows: Z
CPE= Y
0−1 (jω)
-n, where ω is the “angular frequency”, “j” is the “imaginary number” (j
2=−1), Y
0 is the corresponding amplitude at a capacitance and “n” is the “phase shift”. The evaluation of the “n” ensures attributes about the inhomogeneity stadium of the metal area [
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37]. The higher rating of the “n” is connected to the lower roughness of the area i.n., the inhomogeneity of the zone is diminished The CPE can be resistance when n=0, Y
0=R), capacitance as n=1 (Y
0=C) and inductance when n=−1 (Y
0=1/L) or Warburg impedance as n=0.5 (Y
0=W) established for the evaluation of n [
8,
13,
23,
24,
25,
26,
27,
51].
The EIS results indicate that the charge transfer resistance Rct raised and the double layer capacitance Cdl decreased by coating. Determinations demonstrate that the increasing of Rct by the P3MPY-SDS/P2MT reinforced coating, the protective ability enhances substantially which denotes that the composite discovered the remarkable anticorrosion effect of the cobalt alloy. Lowering of Cdl may be practicable, by lowering the local dielectric constant and/or raising the thickness of the electrical double layer, as an effect of the case that the aspect of the coating reacts by adsorption at the specimen/solution interface.
In this work, the P3MPY-SDS/P2MT coating deposited by electrochemical polymerization on the surface of cobalt alloy electrode exemplifies a protective layer on cobalt alloy sample. The Nyquist and Bode plots (
Figure 8 and
Figure 9,
Table 4 and
Table 5) suggest that the mechanism of corrosion was hindered by the deposition of P3MPY-SDS/P2MT coating and this occurrence is realized as a “diffusion barrier” through a charge transfer process.
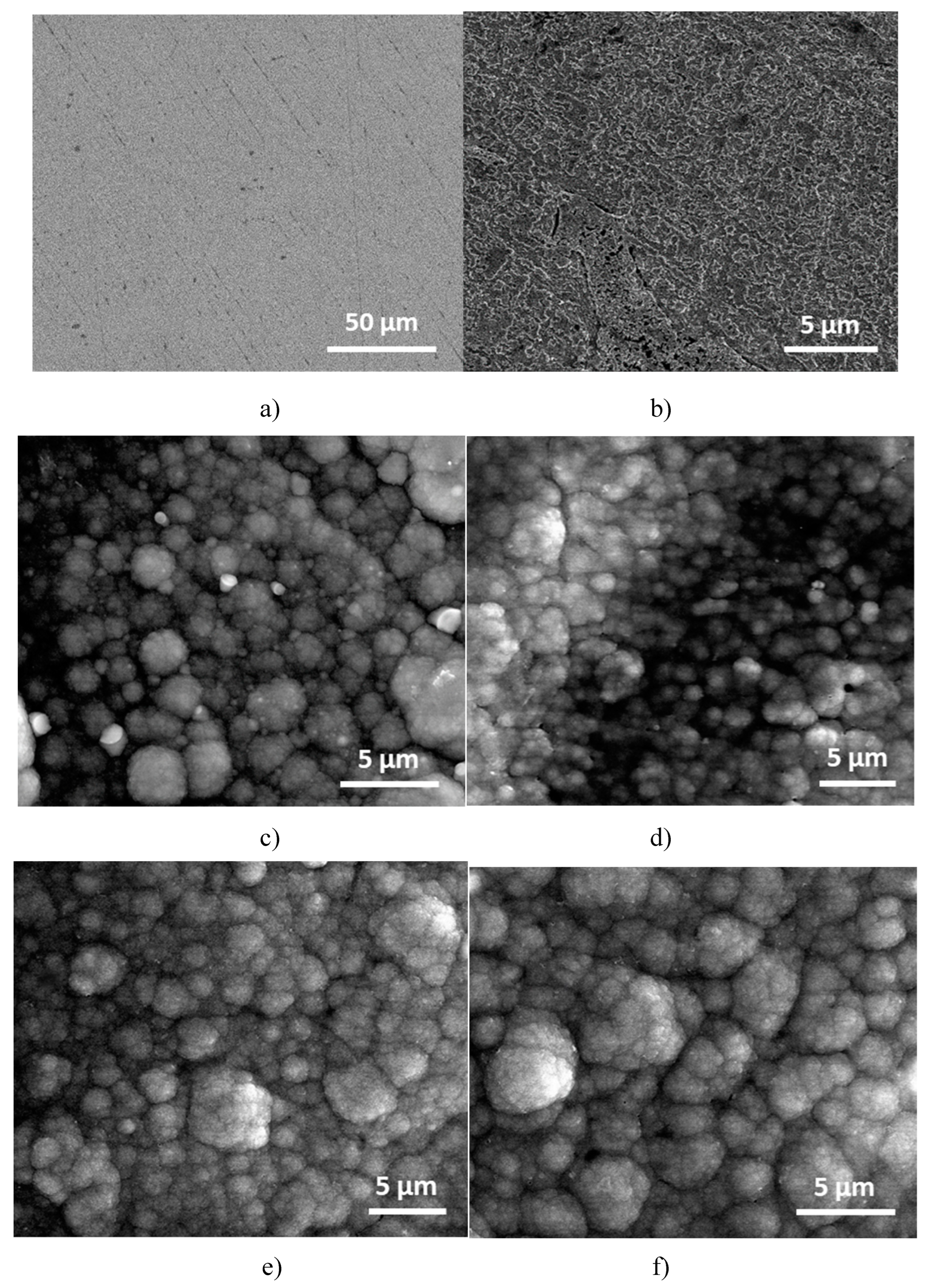
3.5. SEM investigations
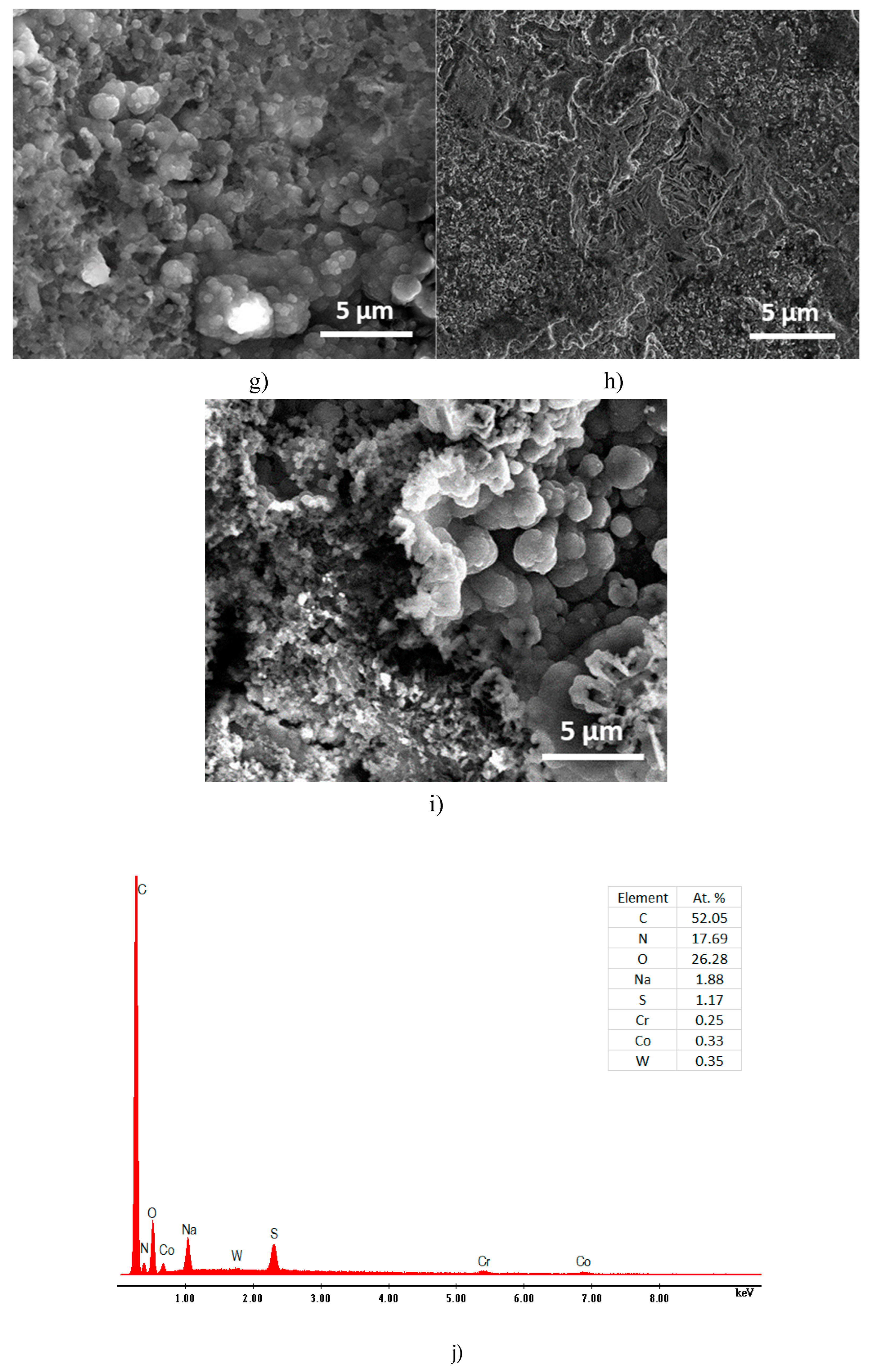
The morphological construction of the P3MPY-SDS/P2MT composite layers made on the surface cobalt alloy was investigated by scanning electron microscopy (SEM). SEM micrographs of the deposition of P3MPY-SDS/P2MT coatings under certain situations on the cobalt alloy specimen are exhibited in
Figure 11. These micrographs show a black layer of the P3MPY-SDS/P2MT made by potentiostatic and galvanostatic techniques showing that the composite covered the cobalt alloy substrate. Investigating the covered and uncovered cobalt alloy in 1 M HCl media from Figure 13 c-i it can be said that the P3MPY-SDS/P2MT covering has a uniform “cauliflower”-shaped configuration by a small “globular microstructure” throughout the surface, suggesting that the film deposited on the Co alloy substrate is dense and homogeneous as previoulsy reported in literature [
20,
33,
38,
39,
40,
51,
57,
58,
59]. These micrographs showed a uniform film to be made on the cobalt alloy electrode and the characteristics of this coating are so qualitative that no cracks are remarked on the covering. The SDS surfactant dopant integrated into polymers has relevant effect on both electropolymerization practice and also the characteristics of the obtained coating [
20,
33,
38,
39,
40,
51,
57,
58,
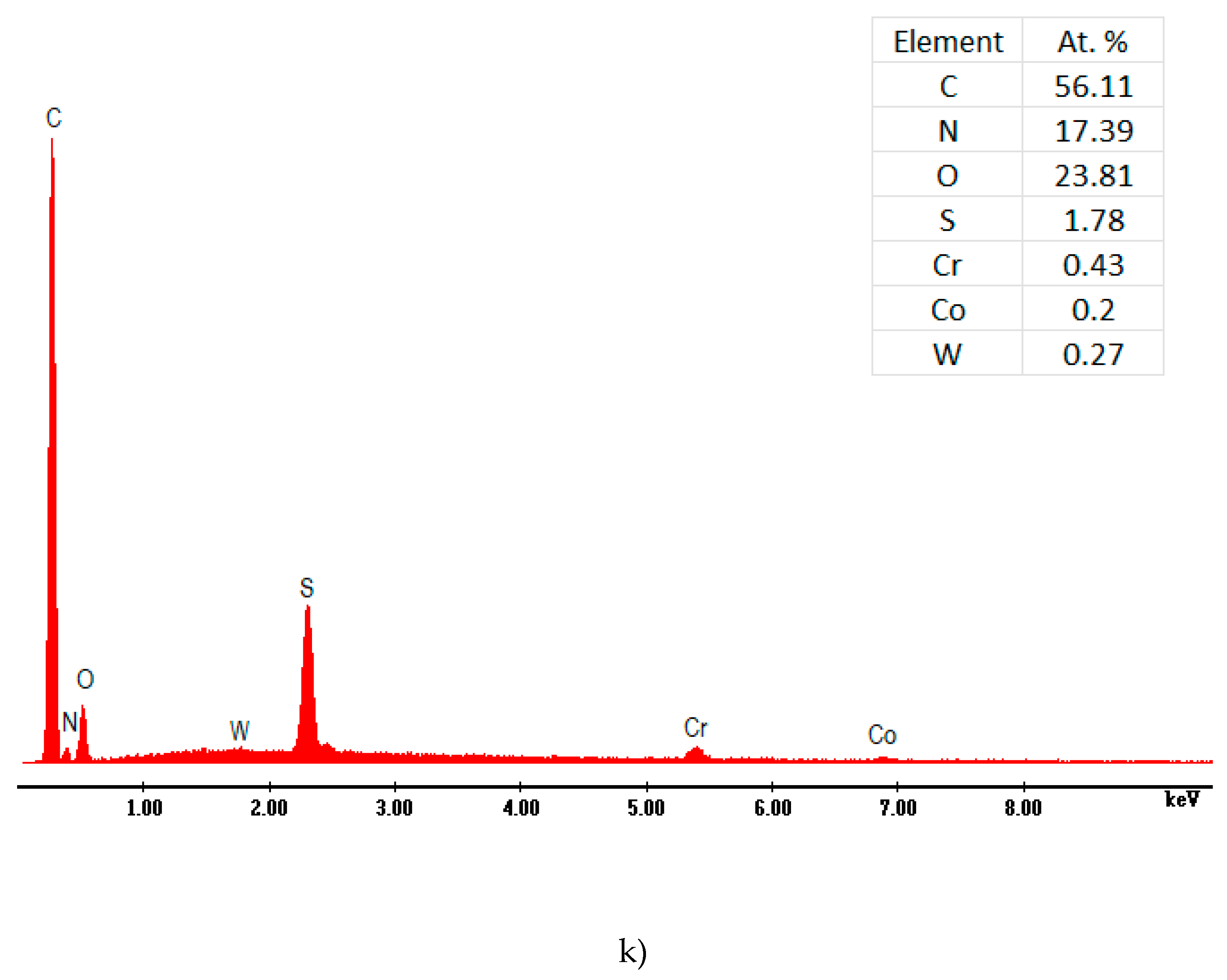
59]. The better coated substrate and superior adsorption result likewise explicated the excellent anticorrosion performance of this composite. The EDS investigation of the P3MPY-SDS/P2MT covered cobalt alloy has been effectuated and the spectra have been designated in
Figure 11 (j-k). The attendant of composite layers on cobalt alloy electrode is distinguished from the peaks of C, N, O and S constituents in EDS spectra. These data are in accordance by the FTIR results of coating, where the anionic SDS surfactant and salicylate ions are accompanied in the polymer matrix. After an immersion time between 0 and 96 h in 1M HCl medium a clear change in the substrate morphology of the covering was observed according to electrochemical determinations. It can be seen at the images:11j-k which reveals the diffusion of corrosive ions Cl
- into the composite layer.
4. Conclusions
The P3MPY-SDS/P2MT polymeric composite coatings were successfully electrochemically deposited homogeneous, dense, uniform, and adherent on cabalt based alloy specimen were obtained using the galvanostatic and potentiostatic techniques at certain current density and potential in sodiun salycilate solution.
The electrochemical determinations show that the P3MPY-SDS/P2MT involves as a protective layer onto cobalt alloy in hydrocloric acid environment.
The electrochemical polymerization technique utilized to acquire of composite coating is the most adequate, completely simple and low-priced.
The anticorrosion performance of P3MPY-SDS/P2MT polymer composite electrodeposited under optimal circumstances was evaluated in 1M HCl medium by potentiodynamic polarization and EIS practices.
The corrosion ratings of this P3MPY-SDS/P2MT composite covered cobalt- based alloy surface is about ~10 times less than the uncoated cobalt alloy and the protective efficiency of this layer is more than 90%.
Anticorrosion protection characteristics follow the sequence: P3MPY-SDS/P2MT to: 0.5mA/cm2 >1 mA/cm2 > 0.9V > 1.0V since the appearance of these coatings determines a substantial diminution in corrosion mechanism.
Examination of FT-IR spectra demonstrates that the P3MPY-SDS/P2MT coating is established onto the cobalt alloy substrate.
The SEM images of P3MPY-SDS/P2MT covering over cobalt-based alloy are compact, homogeneous and adherent on the cobalt- based alloy surface and the attribution of the coating is the best feature.
The superior substrate coating and superior adsorption action also implied the higher anticorrosion defense effect of the composite layers.
It was determined that the cobalt alloy specimen had an important impact on the electrochemical performance of the composite layers, and these coated electrodes were obtained by the simultaneous constitution of a complex oxides film and polymeric layers.
Due to the perfected physical barrier impact the coating substrate is uniform, compact with low porosity and superior protective effect, the P3MPY-SDS/P2MT coatings were stronger in corrosion.
P3MPY-SDS/P2MT coatings were effectuated at 0.9 V and 1.0V potential applied, and to 0.5 mA/cm2 and 1 mA/cm2 current density in molar ratio at 5:1 at 20 min and10 min allowed polymer revealed superior corrosion protection efficiency than the coating obtained at 1.0V potential applied and 1 mA/cm2 current density in molar ratio at 1:5 under same circumstances.
It was evident that this coating prevents the attack of the corrosive agent (HCl) on the cobalt-based alloy substrate and the new P3MPY-SDS/P2MT coating made by this method is promising and may determine the technological applications for the corrosion protection of materials
References
- Wang, T.; Xu, Y.; Liu, Z.; Li, G.; Ren, L. A chitosan/polylactic acid composite coating enhancing the corrosion resistance of the bio-degradable magnesium alloy. Prog. Org. Coat 2023, 178, 107469. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Q.; Wang, C.; Cao, G.; Liu, Y. Preparation and performance of PANI/CNTs composite coating on 316 stainless steel bipolar plates by pulsed electrodeposition. Prog. Org. Coat 2023, 182, 107611. [Google Scholar] [CrossRef]
- Song, L.; Gao, Z.; Sun, Q.; Chu, G.; Shi, H.; Xu, N.; Li, Z.; Hao, N.; Zhang, X.; Wang, M.L. Corrosion protection performance of a coating with 2-aminino-5-mercato-1,3,4-thiadizole-loaded hollow mesoporous silica on copper. Prog. Org. Coat 2023, 175, 107331. [Google Scholar] [CrossRef]
- Liao, F.-Q.; Chen, Y.-C. Siloxane-based epoxy coatings through cationic photopolymerization for corrosion protection. Prog. Org. Coat 2023, 174, 107235. [Google Scholar] [CrossRef]
- Gallegos-Melgar, A.; Serna, S.A.; Lázaro, I.; Gutiérrez-Castañeda, J.; Mercado-Lemus VArcos-Gutierrez, H.H.; Hernández-Hernández, M.; Porcayo-Calderón Jan Mayen, J.; Del Angel Monroy, M. Potentiodynamic Polarization Performance of a Novel Composite Coating System of Al2O3/Chitosan-Sodium Alginate, Applied on an Aluminum AA6063 Alloy for Protection in a Chloride Ions Environment. Coatings 2020, 10, 45. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Z.; Zhou, K.; Li, C.; Ma, J.; Wang, B.; Xu, K.; Cui, H.; Liu, J. Researches on corrosion behaviors of carbon steel/copper alloy couple under organic coating in static and flowing seawater. Progress in Organic Coatings 2022, 166, 106793. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, A. Study on the synthesis of PANI/CNT nanocomposite and its anticorrosion mechanism in waterborne coatings. Prog. Org. Coat. 2021, 159, 106447. [Google Scholar] [CrossRef]
- Patil, D.; Patil, P.P. Electrodeposition of poly (o-toluidine) on brass from aqueous salicylate solution and its corrosion protection performance. J. Appl. Polym. Sci 2010, 118, 2084–2091. [Google Scholar] [CrossRef]
- Yılmaz, S.M.; Atun, G. Corrosion protection efficiency of the electrochemically synthesized polypyrrole-azo dye composite coating on stainless steel. Prog. Org. Coat. 2022, 169, 106942. [Google Scholar] [CrossRef]
- Branzoi, F.; Branzoi, V. Investigation of some nonionic surfactants as corrosion inhibitors for carbon steel in sulfuric acid medium. Int. J. Electrochem. Sci 2017, 12, 7638–7658. [Google Scholar] [CrossRef]
- Zhang, Y. Strengthening, Corrosion and Protection of High-Temperature Structural Materials. Coatings 2022, 12, 1136. [Google Scholar] [CrossRef]
- Zhai, Y.; Pan, K.; Zhang, E. Anti-Corrosive Coating of Carbon-Steel Assisted by Polymer-Camphor sulfonic Acid Embedded within Graphene. Coatings 2020, 10, 879. [Google Scholar] [CrossRef]
- Shinde, V.; Gaikwad, A.B.; Patil, P.P. Synthesis and characterization of corrosion protective poly(2,5-dimethylaniline) coatings on copper. Applied Surface Science 2006, 253, 1037–1045. [Google Scholar] [CrossRef]
- Branzoi, F.; Branzoi, V. Investigation of Protective Effect of Polymeric Film Coatings on Carbon Steel in Aggressive Solutions. Int. J. Electrochem. Sci 2016, 11, 6564–6579. [Google Scholar] [CrossRef]
- Davoodi, A.; Honarbakhsh, S.; Farzi, G.A. Evaluation of corrosion resistance of polypyrrole/functionalized multi-walled carbon nanotubes composite coatings on 60Cu–40Zn brass alloy. Prog. Org. Coat. 2015, 88, 106–115. [Google Scholar] [CrossRef]
- Liu, A.; Tian, H.; Li, S.; XiaodanJu Yang, H.; Sun, Y.; Wang, L.; Li, W. Bioinspired layered hybrid coatings with greatly enhanced barrier effect and active corrosion protection performance. Prog. Org. Coat. 2021, 152, 106131. [Google Scholar] [CrossRef]
- Ozyılmaz, T.; Colak, N.; Ozyilmaz, G.; Sangun, M.K. Protective properties of polyaniline and poly(aniline-co-o-anisidine) films electrosynthesized on brass A. Prog. Org. Coat. 2007, 60, 24–32. [Google Scholar] [CrossRef]
- Bazzaoui, M.; Martins, J.I.; Bazzaoui, E.A.; Martins, L.; Machnikova, E. Sweet aqueous solution for electrochemical synthesis of polypyrrole part 1B: On copper and its alloys. Electrochim. Acta 2007, 52, 3568–3581. [Google Scholar] [CrossRef]
- González-Tejera, M.J.; García, M.V.; de la Blanca, E.S.; Redondo, M.I.; Raso, M.A.; Carrillo, I. , Electrochemical synthesis of N-methyl and 3-methyl pyrrole perchlorate doped copolymer films. Thin Solid Films 2007, 515, 6805–6811. [Google Scholar] [CrossRef]
- Branzoi, F.; Branzoi, V.; Musina, A. Fabrication and characterisation of conducting composite films based on conducting polymers and functionalised carbon nanotubes. Surface and Interface Anal. 2012, 44, 1076–1081. [Google Scholar] [CrossRef]
- Duran, B.; Bereket, G. Cyclic Voltammetric Synthesis of Poly(N-methyl pyrrole) on Copper and Effects of Polymerization Parameters on Corrosion Performance. Ind. Eng. Chem. Res. 2012, 51, 5246–5255. [Google Scholar] [CrossRef]
- Dararatana, N.; Seidi, F.; Crespy, D. Polymer conjugates for dual functions of reporting and hindering corrosion. Polymer 2020, 194, 122346. [Google Scholar] [CrossRef]
- Luo, Y.; Li, X.; Luo, Z.; Chen, L.; Han, G. Enhanced adhesive and anti-corrosive performances of polymer composite coating for rusted metallic substrates by capillary filling. Prog. Org. Coat 2023, 178, 107467. [Google Scholar] [CrossRef]
- Redondo, M.I.; de la Blanca, E.S.; García, M.V.; González-Tejera, M.J. Poly(N-methylpyrrole) Electrodeposited on copper: Corrosion protection properties. Prog. Org. Coat. 2009, 65, 386–39. [Google Scholar] [CrossRef]
- Ren, S.; Barkey, D. Electrochemically Prepared Poly (3-methylthiophene) Films for Passivation of 430 Stainless Steel. J. Electrochem. Soc. 1992, 139, 4–1021. [Google Scholar] [CrossRef]
- Çakmakci, I.; Duran, B.; Duran, M.; Bereket, G. Experimental and theoretical studies on protective properties of poly (pyrrole-co-N-methyl pyrrole) coatings on copper in chloride media. Corrosion Science 2013, 69, 252–261. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, K.; Zhang, K.; Wang, Z.; Liu, Z.; Hu, S.; Fang, Y.; He, C. Studies on Corrosion Behaviors of Q235 Steel Coated by the Polypyrrole Films Doped with different dopants. Int. J. Electrochem. Sci. 2020, 15, 2594–2603. [Google Scholar] [CrossRef]
- Rui, M.; Aiping Zhu, A. The synthesis and corrosion protection mechanisms of PANI/CNT nanocomposite doped with organic phosphoric acid. Prog.Org.Coat 2021, 153, 106134. [Google Scholar] [CrossRef]
- Marti, M.; Armelin, E.; Iribarren, J.I.; Aleman, C. Soluble polythiophenes as anticorrosive additives for marine epoxy paints. Mater. Corros 2015, 66, 23–30. [Google Scholar] [CrossRef]
- Anaya-Garza, K.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Moreno-Palmerín, J.; Rodríguez-Salazar, A.E. Corrosion resistance improvement of the Ti6Al4V/UHMWPE systems by the assembly of ODPA molecules by dip coating technique. Prog. Org. Coat 2022, 171, 107013. [Google Scholar] [CrossRef]
- Asan, G.; Asan, A.; Çelikkan, H. The effect of 2D-MoS2 doped polypyrrole coatings on brass corrosion. Journal of Molecular Structure 2020, 1203, 127318. [Google Scholar] [CrossRef]
- Guptad, K.; Neupane, S.; Singh, S.; Karki, N.; Yadav, A.P. The effect of electrolytes on the coating of polyaniline on mild steel by electrochemical methods and its corrosion behavior. Prog. Org. Coat 2021, 152, 106127. [Google Scholar] [CrossRef]
- Branzoi, V.; Branzoi, F.; Pilan, L. Characterization of electrodeposited polymeric and composite modified electrodes on cobalt based alloy. Mat. Chem. Phys. 2009, 118, 197–202. [Google Scholar] [CrossRef]
- Yagan, A.; Pekmez, N.Ö.; Yıldız, A. Poly (N-methylaniline) coatings on stainless steel by electropolymerization. Corros. Sci. 2007, 49, 2905–2919. [Google Scholar] [CrossRef]
- Zeybek, B.; Aksun, E. Electrodeposition of poly(N-methylpyrrole) on stainless steel in the presence of sodium dodecylsulfate and its corrosion performance. Prog. Org. Coat. 2015, 81, 1–10. [Google Scholar] [CrossRef]
- Pekmeza, N.Ö.; Cınkıllı, K.; Zeybekba, B. The electrochemical copolymerization of pyrrole and bithiophene on stainless steel in the presence of SDS in aqueous medium and its anticorrosive performance. Prog. Org. Coat. 2014, 77, 1277–1287. [Google Scholar] [CrossRef]
- Redondo, M.I.; de la Blanca, E.S.; García, M.V.; González-Tejera, M.J. Poly(N-methylpyrrole) Electrodeposited on copper: Corrosion protection properties. Prog. Org. Coat. 2009, 65, 386–39. [Google Scholar] [CrossRef]
- Branzoi, F.; Branzoi, V. Enzymatic electrode obtained by immobilizing of urease into a nanocomposite film based on conducting polymers and different additives. Int. J. Polym. Mater. Polym. Biomater 2014, 63, 549–556. [Google Scholar] [CrossRef]
- Su, W.; Iroh, J.O. Electrodeposition mechanism, adhesion and corrosion performance of polypyrrole and poly (N-methylpyrrole) coatings on steel substrates. Synthetic Metals 2000, 114, 225–234. [Google Scholar] [CrossRef]
- Branzoi, F.; Pahom, Z.; Nechifor, G. Corrosion protection of new composite polymer coating for carbon steel in sulfuric acid medium by electrochemical methods. Journal of Adhesion Science and Technology 2018, 2364–2380. [Google Scholar] [CrossRef]
- Gopi, D.; Saraswathy, R.; Kavitha, L.; Kim, D.-K. Electrochemical synthesis of poly(indole-co-thiophene) on low-nickel stainless steel and its anticorrosive performance in 0.5 mol L−1H2SO4. Polym. Int. 2014, 63, 280–289. [Google Scholar] [CrossRef]
- Yagan, A.; Pekmez, O.N.; Yildiz, A. Poly(N-ethylaniline) coatings on 304 stainless steel for corrosion protection in aqueous HCl and NaCl solutions. Electrochim. Acta. 2008, 53, 2474–2482. [Google Scholar] [CrossRef]
- Chou, T.P.; Chandrasekaran, C.; Limmer, S.J.; Seraji, S.; Wu, Y.; Forbess, M.J.; Nguyen, C.; Cao, G.Z. Organic–inorganic hybrid coatings for corrosion protection. Journal of Non-Crystalline Solids 2001, 290, 153–162. [Google Scholar] [CrossRef]
- Fuseini, M.; Zaghloul, M.M.Y. Investigation of Electrophoretic Deposition of PANI Nano fibers as a Manufacturing Technology for corrosion protection. Prog. Org. Coat 2022, 171, 107015. [Google Scholar] [CrossRef]
- Yan, Q.; Pan, W.; Zhong, S.; Guo, R.Z.; Li, X. Effect of solvents on the preparation and corrosion protection of polypyrrole. Prog. Org. Coat 2019, 132, 298–304. [Google Scholar] [CrossRef]
- ARamírez, M.R.; Mieres, F.; Pineda, F.; Grez, P.; Heyser, C. Electrosynthesis of polyindole-carboxylic acids on stainless steel and their corrosion protection at different temperatures in acidic solution. Prog. Org. Coat 2022, 177, 107075. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, Y.; Zhang, Z.; Yu, L. Corrosion protection of a novel SiO2/PANI coating for Q235 carbon steel. Prog. Org. Coat. 2019, 132, 227–234. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, C.; Cui, J.; Shen, S.; Qiu, H.; Li, J. Electrodeposition of polypyrrole coatings doped by benzenesulfonic acid-modified graphene oxide on metallic bipolar plates. Prog. Org. Coat 2022, 170, 106995. [Google Scholar] [CrossRef]
- Menkuer, M.; Ozkazanc, H. Anticorrosive polypyrrole/zirconium-oxide composite film prepared in oxalic acid and dodecylbenzene sulfonic acid mix electrolyte. Prog. Org. Coat. 2020, 147, 105815. [Google Scholar] [CrossRef]
- Branzoi, F.; Mihai, M.A.; Petrescu, S. Corrosion Protection Efficacy of the Electrodeposit of Poly (N-Methyl Pyrrole-Tween20/3-Methylthiophene) Coatings on Carbon Steel in Acid Medium. Coatings 2022, 12, 1062. [Google Scholar] [CrossRef]
- Pekmeza, N.Ö.; Cinkillia, K.; Zeybekba, B. The electrochemical copolymerization of pyrrole and bithiophene on stainless steel in the presence of SDS in aqueous medium and its anticorrosive performance. Prog. Org. Coat. 2014, 77, 1277–1287. [Google Scholar] [CrossRef]
- Vernickaite, E.; Tsyntsaru, N.; Cesiulis, E.H. Electrodeposition and corrosion behaviour of nanostructured cobalt–tungsten alloys coatings. Transactions of the IMF 2016, 94, 313–321. [Google Scholar] [CrossRef]
- Hong, J.H.; Yeoh, F.Y. Mechanical properties and corrosion resistance of cobalt-chrome alloy fabricated using additive manufacturing. Materials Today Proceedings 2020, 29, 196–201. [Google Scholar] [CrossRef]
- Ma, T.; Tan, B.; Guo, L.; Wang, W.; Li, W.; Ji, J.; Yan, M.; Kaya, S. Experimental and theoretical investigation on the inhibition performance of disulfide derivatives on cobalt corrosion in alkaline medium. Journal of Molecular Liquids 2021, 341, 116907. [Google Scholar] [CrossRef]
- Hong, J.H.; Yeoh, F.Y. Mechanical properties and corrosion resistance of cobalt-chrome alloy fabricated using additive manufacturing. Materials Today Proceedings 2020, 29, 196–201. [Google Scholar] [CrossRef]
- Nadolski, M.; Golański, G.; Klimas, J.; Szota, M.; Szymański, J. Microstructure and functional properties of prosthetic cobalt alloys COCRW. Institute of Metallurgy and Materials Science of Polish Academy of Sciences 2015, 3. [Google Scholar] [CrossRef]
- Güven, N.C.; Ozkazanc, H. Corrosion protection behavior of poly(N-methylpyrrole)/boron nitride composite film on aluminum-1050. Progress in Organic Coatings 2022, 164, 106696. [Google Scholar] [CrossRef]
- Hernández-Martínez, D.; León-Silva, U.; Nicho, M.E. Corrosion protection of steel by poly(3-hexylthiophene) polymer blends. Anti-Corrosion Methods and Materials 2015, 62, 229–240. [Google Scholar] [CrossRef]
- Branzoi, F.; Branzoi, V.; Musina, A. Coatings based on conducting polymers and functionalized carbon nanotubes obtained by electropolymerization. Prog. Org. Coat. 2013, 76, 632–638. [Google Scholar] [CrossRef]
Scheme 1.
Chart of the deposition practice (Ref. = SCE reference electrode, WE = cobalt alloy specimen, CE = platinum plate counter electrode).
Scheme 1.
Chart of the deposition practice (Ref. = SCE reference electrode, WE = cobalt alloy specimen, CE = platinum plate counter electrode).
Scheme 2.
Scheme for estimation of layer coating adhesion by “standard sellotape test”.
Scheme 2.
Scheme for estimation of layer coating adhesion by “standard sellotape test”.
Figure 1.
Potentiostatic electrochemical depositions of P3MPY-SDS/P2MT /cobalt alloy at potential 0.9V and 1.0V vs. SCE for 600-1200 s in various molar ratio.
Figure 1.
Potentiostatic electrochemical depositions of P3MPY-SDS/P2MT /cobalt alloy at potential 0.9V and 1.0V vs. SCE for 600-1200 s in various molar ratio.
Figure 2.
Galvanostatic electrodepositions of P3MPY-SDS/P2MT /cobalt alloy at 0.5mA/cm2 and 1mA/cm2 current density for 600–1200 s in varied molar ratio.
Figure 2.
Galvanostatic electrodepositions of P3MPY-SDS/P2MT /cobalt alloy at 0.5mA/cm2 and 1mA/cm2 current density for 600–1200 s in varied molar ratio.
Figure 3.
Cyclic voltammograms of P3MPY-SDS/P2MT / cobalt alloy coating in 0.2M C7H5NaO3 media at potential domain -0.4 and 1.5V vs. SCE and scan rate of 20mV/s.
Figure 3.
Cyclic voltammograms of P3MPY-SDS/P2MT / cobalt alloy coating in 0.2M C7H5NaO3 media at potential domain -0.4 and 1.5V vs. SCE and scan rate of 20mV/s.
Figure 4.
The FT-IR plots of (a)3MPY), (b) 2MT and c) P3MPY-SDS/P2MT/cobalt alloy electrodeposition on cobalt alloy specimen under potentiostatic and galvanostatic practice (0.5 mA/cm2, 1 mA/cm2, 0.9V and 1.0 V in 5:1 molar ratio).
Figure 4.
The FT-IR plots of (a)3MPY), (b) 2MT and c) P3MPY-SDS/P2MT/cobalt alloy electrodeposition on cobalt alloy specimen under potentiostatic and galvanostatic practice (0.5 mA/cm2, 1 mA/cm2, 0.9V and 1.0 V in 5:1 molar ratio).
Figure 5.
Polarization curves of P3MPY-SDS/P2MT covered and uncovered onto cobalt alloy surface in 1M HCl by galvanostatic technique at a) 0.5 mA/cm2 and b) 1mA/cm2 current density at different molar ratios and the electrodeposition has been submitted for 10 and 20 min.
Figure 5.
Polarization curves of P3MPY-SDS/P2MT covered and uncovered onto cobalt alloy surface in 1M HCl by galvanostatic technique at a) 0.5 mA/cm2 and b) 1mA/cm2 current density at different molar ratios and the electrodeposition has been submitted for 10 and 20 min.
Figure 6.
Polarization curves of P3MPY-SDS/P2MT coated and uncoated on cobalt alloy specimen in 1M HCl by potentiostatic technique at a) 0.9V and b) 1.0 V potential applied at different molar ratios and the electrodeposition has been permitted for 10 and 20 min.
Figure 6.
Polarization curves of P3MPY-SDS/P2MT coated and uncoated on cobalt alloy specimen in 1M HCl by potentiostatic technique at a) 0.9V and b) 1.0 V potential applied at different molar ratios and the electrodeposition has been permitted for 10 and 20 min.
Figure 7.
Polarization curves of P3MPY-SDS/P2MT coated onto cobalt alloy substrate in 1M HCl by galvanostatic process at 0.5 mA/cm2 at different immersion times.
Figure 7.
Polarization curves of P3MPY-SDS/P2MT coated onto cobalt alloy substrate in 1M HCl by galvanostatic process at 0.5 mA/cm2 at different immersion times.
Figure 8.
Nyquist diagrams for uncovered and covered cobalt alloy sample by P3MPY-SDS/P2MT by galvanostatic and potentiostatic techniques at different molar ratio.
Figure 8.
Nyquist diagrams for uncovered and covered cobalt alloy sample by P3MPY-SDS/P2MT by galvanostatic and potentiostatic techniques at different molar ratio.
Figure 9.
Bode graphs for uncovered and covered cobalt alloy specimen by P3MPY-SDS/P2MT by galvanostatic and potentiostatic techniques at different molar ratio.
Figure 9.
Bode graphs for uncovered and covered cobalt alloy specimen by P3MPY-SDS/P2MT by galvanostatic and potentiostatic techniques at different molar ratio.
Figure 10.
Equivalent circuit.
Figure 10.
Equivalent circuit.
Figure 11.
SEM micrographs of cobalt alloy substrate coated by P3MPY-SDS/P2MT, a) polished cobalt alloy, b) cobalt alloy in 1 M HCl, c) at 0.9V, d) 1.0 V, e) at 0. 5mA/cm2, f) 1mA/cm2 in 5:1, 1:5 molar ratio, and (g–i) after 48 h, 72 h and 96 h immersion time in 1 M HCl, j-k) the EDS spectra P3MPY-SDS/P2MT / cobalt alloy.
Figure 11.
SEM micrographs of cobalt alloy substrate coated by P3MPY-SDS/P2MT, a) polished cobalt alloy, b) cobalt alloy in 1 M HCl, c) at 0.9V, d) 1.0 V, e) at 0. 5mA/cm2, f) 1mA/cm2 in 5:1, 1:5 molar ratio, and (g–i) after 48 h, 72 h and 96 h immersion time in 1 M HCl, j-k) the EDS spectra P3MPY-SDS/P2MT / cobalt alloy.
Table 1.
Kinetic parameters of covered and uncovered of cobalt alloy specimen in 1M HCl solutions at 25ºC.
Table 1.
Kinetic parameters of covered and uncovered of cobalt alloy specimen in 1M HCl solutions at 25ºC.
| The system P3MPY-SDS/P2MT /CoCrW |
Ecorr
(mV) |
icorr
(µA/cm2
|
Rp
kΩcm2
|
Rmpy
|
Pmm/year
|
Kg
(g/m2h)
|
ba
(mV/
decade |
-bc
(mV/
decade |
E (%) |
%P |
| CoCrW + 1 M HCl |
-230 |
21 |
0.390 |
9.91 |
0.255 |
0.234 |
86 |
-90 |
- |
|
| P3MPY-SDS/P2MT 1mA/cm2 1:5 molar ratio, t=10min |
-146 |
0.67 |
41 |
0.316 |
0.008 |
0.0074 |
108 |
-102 |
95 |
0.0009 |
| P3MPY-SDS/P2MT 1mA/cm2 5:1 molar ratio, t=10min |
160 |
0.39 |
49 |
0.180 |
0.0045 |
0.0042 |
80 |
-117 |
98 |
0.00003 |
| P3MPY-SDS/P2MT 1mA/cm2 1:5 molar ratio, t=20min |
57 |
0.46 |
44 |
0.217 |
0.0055 |
0.0051 |
91 |
-93 |
97 |
0.00004 |
| P3MPY-SDS/P2MT 1mA/cm2 5:1 molar ratio, t=20min |
180 |
0.33 |
59 |
0.155 |
0.0039 |
0.0036 |
119 |
-102 |
98 |
0.00003 |
| P3MPY-SDS/P2MT 0.5mA/cm2 1:5 molar ratio, t=10min |
-232 |
0.63 |
37 |
0.297 |
0.0075 |
0.0070 |
94 |
-111 |
96 |
0.0001 |
| P3MPY-SDS/P2MT 0.5mA/cm2 5:1 molar ratio, t=10min |
-60 |
0.36 |
41 |
0.169 |
0.0042 |
0.0040 |
121 |
-87 |
98 |
0.00009 |
| P3MPY-SDS/P2MT 0.5mA/cm2 1:5 molar ratio, t=20min |
-236 |
0.43 |
46 |
0.202 |
0.0051 |
0.0047 |
85 |
-88 |
97 |
0.0059 |
| P3MPY-SDS/P2MT 0.5mA/cm2 5:1 molar ratio, t=20min |
80 |
0.29 |
60 |
0.136 |
0.0034 |
0.0032 |
118 |
-94 |
98.5 |
0.00001 |
Table 2.
Kinetic parameters of covered and uncovered of cobalt alloy specimen in 1M HCl solutions at 25ºC.
Table 2.
Kinetic parameters of covered and uncovered of cobalt alloy specimen in 1M HCl solutions at 25ºC.
| The system P3MPY-SDS/P2MT /CoCrW |
Ecorr
(mV) |
icorr
(µA/cm2
|
Rp
kΩcm2
|
Rmpy
|
Pmm/year
|
Kg
(g/m2h)
|
ba
(mV/
decade |
-bc
(mV/
decade |
E (%) |
%P |
| CoCrW + 1 M HCl |
-230 |
21 |
0.390 |
9.91 |
0.255 |
0.234 |
86 |
-90 |
- |
|
| P3MPY-SDS/P2MT 0.9V 1:5 molar ratio, t=10min |
-242 |
0.97 |
36 |
0.451 |
0.011 |
0.010 |
109 |
-78 |
94 |
0.0007 |
| P3MPY-SDS/P2MT 0.9V 5:1 molar ratio, t=10min |
-65 |
0.49 |
41 |
0.231 |
0.005 |
0.0045 |
107 |
-88 |
97 |
0.000095 |
| P3MPY-SDS/P2MT 0.9V 1:5 molar ratio, t=20min |
-225 |
0.56 |
39 |
0.264 |
0.007 |
0.0066 |
83 |
-81 |
97 |
0.000016 |
| P3MPY-SDS/P2MT 0.9V 5:1 molar ratio, t=20min |
80 |
0.37 |
44 |
0.174 |
0.0044 |
0.0041 |
77 |
-88 |
98 |
0.00063 |
| P3MPY-SDS/P2MT 1.0V 1:5 molar ratio, t=10min |
-220 |
0.73 |
27 |
0.344 |
0.0088 |
0.0081 |
111 |
-91 |
95 |
0.0058 |
| P3MPY-SDS/P2MT 1.0V 5:1 molar ratio, t=10min |
-130 |
0.47 |
39 |
0.22 |
0.0056 |
0.0052 |
76 |
-78 |
97 |
0.000054 |
| P3MPY-SDS/P2MT 1.0V 1:5 molar ratio, t=20min |
-143 |
0.63 |
37 |
0.30 |
0.007 |
0.0069 |
69 |
79 |
97 |
0.0015 |
| P3MPY-SDS/P2MT 1.0V 5:1 molar ratio, t=20min |
62 |
0.35 |
47 |
0.165 |
0.0041 |
0.0039 |
78 |
82 |
98 |
0.000033 |
Table 3.
Kinetic parameters of covered and uncovered of cobalt alloy specimen in 1M HCl solutions at 25ºC at different immersion times.
Table 3.
Kinetic parameters of covered and uncovered of cobalt alloy specimen in 1M HCl solutions at 25ºC at different immersion times.
| The system P3MPY-SDS/P2MT /CoCrW |
Ecorr
(mV) |
icorr
(µA/cm2
|
Rmpy
|
Pmm/year
|
Kg
(g/m2h)
|
ba
(mV/
decade |
-bc
(mV/
decade |
E (%) |
| P3MPY-SDS/P2MT 0.5mA/cm2 5:1 molar ratio, t=10 min 0h |
70 |
0.46 |
0.169 |
0.0042 |
0.0040 |
121 |
-87 |
98 |
| P3MPY-SDS/P2MT 0.5mA/cm2 5:1 molar ratio, t=10 min 24h |
38 |
1.16 |
0.547 |
0.0138 |
0.0129 |
74 |
-68 |
95 |
| P3MPY-SDS/P2MT 0.5mA/cm2 5:1 molar ratio, t=10min 48h |
15 |
2.43 |
1.146 |
0.029 |
0.027 |
99 |
-86 |
88 |
| P3MPY-SDS/P2MT 0.5mA/cm2 5:1 molar ratio, t=10min 72h |
24 |
3.29 |
1.469 |
0.037 |
0.034 |
93 |
-88 |
84 |
| P3MPY-SDS/P2MT 0.5mA/cm2 5:1 molar ratio, t=10min 96h |
-2 |
4.96 |
2.341 |
0.059 |
0.055 |
95 |
-91 |
77 |
Table 4.
Electrochemical parameters of covered and uncovered of cobalt alloy electrode in 1M hCl solutions at 25ºC.
Table 4.
Electrochemical parameters of covered and uncovered of cobalt alloy electrode in 1M hCl solutions at 25ºC.
| The system P3MPY-SDS/P2MT/cobalt alloy |
Rs ohm⋅cm2
|
Q-Yo
S⋅s-n⋅cm-2
|
Q-n |
Rf ohm⋅cm2
|
Q-Yo
S⋅s-n⋅cm-2
|
Q-n |
Rct
ohm⋅cm2
|
χ |
| CoCrW + 1 M HCl |
1.30 |
4.69E-5 |
0.89 |
13 |
1.378E-5 |
0.86 |
809 |
8.25e-03 |
| P3MPY-SDS/P2MT 1mA/cm2 1:5 molar ratio, t=10min |
2.53 |
1.13E-5 |
0.93 |
64 |
5.14E-5 |
0.57 |
7.576E4 |
7.523e-04 |
| P3MPY-SDS/P2MT 1mA/cm2 5:1 molar ratio, t=10min |
2.66 |
1.91E-5 |
0.98 |
64.73 |
6.73E-5 |
0.84 |
8.921E4 |
3.733e-03 |
| P3MPY-SDS/P2MT 1mA/cm2 1:5 molar ratio, t=20min |
4.34 |
6.38E-4 |
0.61 |
49 |
4.96E-5 |
0.83 |
4.956E4 |
1.647e-03 |
| P3MPY-SDS/P2MT 1mA/cm2 5:1 molar ratio, t=20min |
2.63 |
7.43E-4 |
0.63 |
65 |
1.66E-4 |
0.93 |
4.976E4 |
3.935e-04 |
| P3MPY-SDS/P2MT 0.5mA/cm2 1:5 molar ratio, t=10min |
1.76 |
3.58E-5 |
0.79 |
94.8 |
2.26E-5 |
0.89 |
8.018E4 |
8.016e-04 |
| P3MPY-SDS/P2MT 0.5mA/cm2 5:1 molar ratio, t=10min |
3.22 |
1.16E-4 |
0.61 |
94.5 |
3.48E-5 |
0.84 |
6.963E4 |
6.653e-04 |
| P3MPY-SDS/P2MT 0.5mA/cm2 1:5 molar ratio, t=20min |
5.912 |
3.32E-4 |
0.64 |
65.2 |
4.94E-5 |
0.89 |
7.846E4 |
1.021e-03 |
| PNMPY-1SSD/P2MT 0.5mA/cm2 5:1 molar ratio, t=20min |
2.789 |
1.02E-5 |
0.84 |
174 |
1.24E-5 |
0.91 |
9.228E4 |
7.428e-04 |
Table 5.
Electrochemical parameters of coated and uncoated of cobalt alloy specimen in 1M hCl solutions at 25ºC
Table 5.
Electrochemical parameters of coated and uncoated of cobalt alloy specimen in 1M hCl solutions at 25ºC
| The system P3MPY-SDS/P2MT/cobalt alloy |
Rs ohm⋅cm2
|
Q-Yo
S⋅s-n⋅cm-2
|
Q-n |
Rf
ohm⋅cm2
|
Q-Yo
S⋅s-n⋅cm-2
|
Q-n |
Rct
ohm⋅cm2
|
χ |
| CoCrW + 1 M HCl |
1.30 |
4.69E-5 |
0.89 |
13 |
1.378E-5 |
0.86 |
809 |
8.25e-03 |
| P3MPY-SDS/P2MT 0.9V 1:5 molar ratio, t=10min |
0.79 |
3.88E-5 |
0.88 |
362 |
7.35E-4 |
0.74 |
1.71E4 |
5.10e-03 |
| P3MPY-SDS/P2MT 0.91V 5:1 molar ratio, t=10min |
0.68 |
3.52E-5 |
0.61 |
64 |
2.81E-5 |
0.89 |
2.15E4 |
1.87e-03 |
| P3MPY-SDS/P2MT 0.9V 1:5 molar ratio, t=20min |
1.06 |
4.33E-5 |
0.84 |
304 |
2.576E-5 |
0.89 |
8639 |
1.16e-03 |
| P3MPY-SDS/P2MT 0.9V 5:1 molar ratio, t=20min |
1.44 |
2.85E-5 |
0.61 |
3805 |
5.456E-5 |
0.88 |
2.77E5 |
1.86e-03 |
| P3MPY-SDS/P2MT 1.0V 1:5 molar ratio, t=10min |
0.681 |
7.16E-5 |
0.61 |
88.5 |
3.305E-5 |
0.89 |
8.53E5 |
3.88e-03 |
| P3MPY-SDS/P2MT 1.0V 5:1 molar ratio, t=10min |
1.03 |
2.79E-5 |
0.89 |
2180 |
6.789E-5 |
0.63 |
1.1E5 |
1.89e-03 |
| P3MPY-SDS/P2MT 1.0V 1:5 molar ratio, t=20min |
0.806 |
3.72E-5 |
0.89 |
237 |
1.752E-5 |
0.78 |
3.539E4 |
6.12e-03 |
| P3MPY-SDS/P2MT 1.0V 5:1 molar ratio, t=20min |
2.51 |
3.76E-5 |
0.87 |
1778 |
9.026E-5 |
0.66 |
7.24E5 |
2.59e-03 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).