1. Introduction
Due to their low cost and great nutritional profile, eggs are considered a highly nutritive food [
1] that is consumed daily. Brazilian egg production has been rapidly growing, in 2021 it totaled 54.9 billion per unit and consumption per person reached 277 units per person [
2]. Changes in the consumer eating patterns and rising prices of other proteins have contributed to the that increase. Consumers show preference for poultry products from alternative systems (cage-free, free-range, organic, and rustic free-range), with stricter safety rules and welfare concepts [
3].
Therefore, large companies became aware of market innovations and have adopted alternative egg production systems, which has led to diversification of egg categories available on the market [
4]. The semi-intensive system consists of a production model in which hens have access outdoor areas, where they are released in the morning and gathered in the afternoon, which can positively impact production performance and egg quality.
Increasing prices of commodities used in poultry feeding has had significant impact on the poultry industry, affecting production costs. Corn, which is the main energy source for poultry farming, is increasingly disputed by the food industry, making it unaffordable and expensive, with increases of about 200% from 2015 to 2021 [
2]. Therefore, one way to reduce the cost of feed is to seek alternative sources to corn that are effective, accessible, inexpensive, and suitable for the region.
Cactus pear is a widely used alternative animal feed in the Brazilian Northeast because it is considered an energy-source feed due to the high concentration of non-fiber carbohydrates [
5,
6,
7]. It also presents good adaptability characteristics and high productive potential to the edaphoclimatic conditions of this region [
8,
9], besides the wide availability which reaches approximately 600,000 ha cultivated in Brazil. The most widely used genotypes are
Opuntia with cultivars
Gigante and
Redonda and
Nopalea with cultivar
Miúda [
10]. Among the cactus pear cultivars,
Miúda (
Nopalea cochinilifera (L.) Salm Dyck) stands out for its nutritional qualities: high contents of total carbohydrates (822.1 g/kg), non-fiber carbohydrates (597.5 g/kg), mineral matter (128.8 g/kg) and low contents of neutral detergent fiber (224.6 g/kg) and acid detergent fiber (189.7 g/kg) [
6], in addition, it presents high energy content (3,653 kcal/g) [
11].
Among the forms of supply,
Miúda cactus pear meal (CPM) which is a product obtained after dehydration of the cladodes, contains 82.2% dry matter (DM), 8% crude protein (CP), 1% ether extract (EE), 25.1% neutral detergent fiber (NDF), 46.7% non-fiber carbohydrates (NFC), 18.5% mineral matter (MM), 2.3% calcium, 0.2% phosphorus [
12] and 3,647 kcal/g gross energy (GE) [
11], with great potential to be a cheap and accessible energy source.
Some available studies on cactus pear meal show promise in the feeding of broilers in free-range system [
11], industrial broiler [
13] and quails [
14]. As far as it is known, there is no published study on the effect of cactus pear meal in the feeding of laying hens, and research is needed to demonstrate its potential and to measure its value. Thus, the objective of this study, was to evaluate the use of CPM in the diet of laying hens in semi-intensive system.
4. Discussion
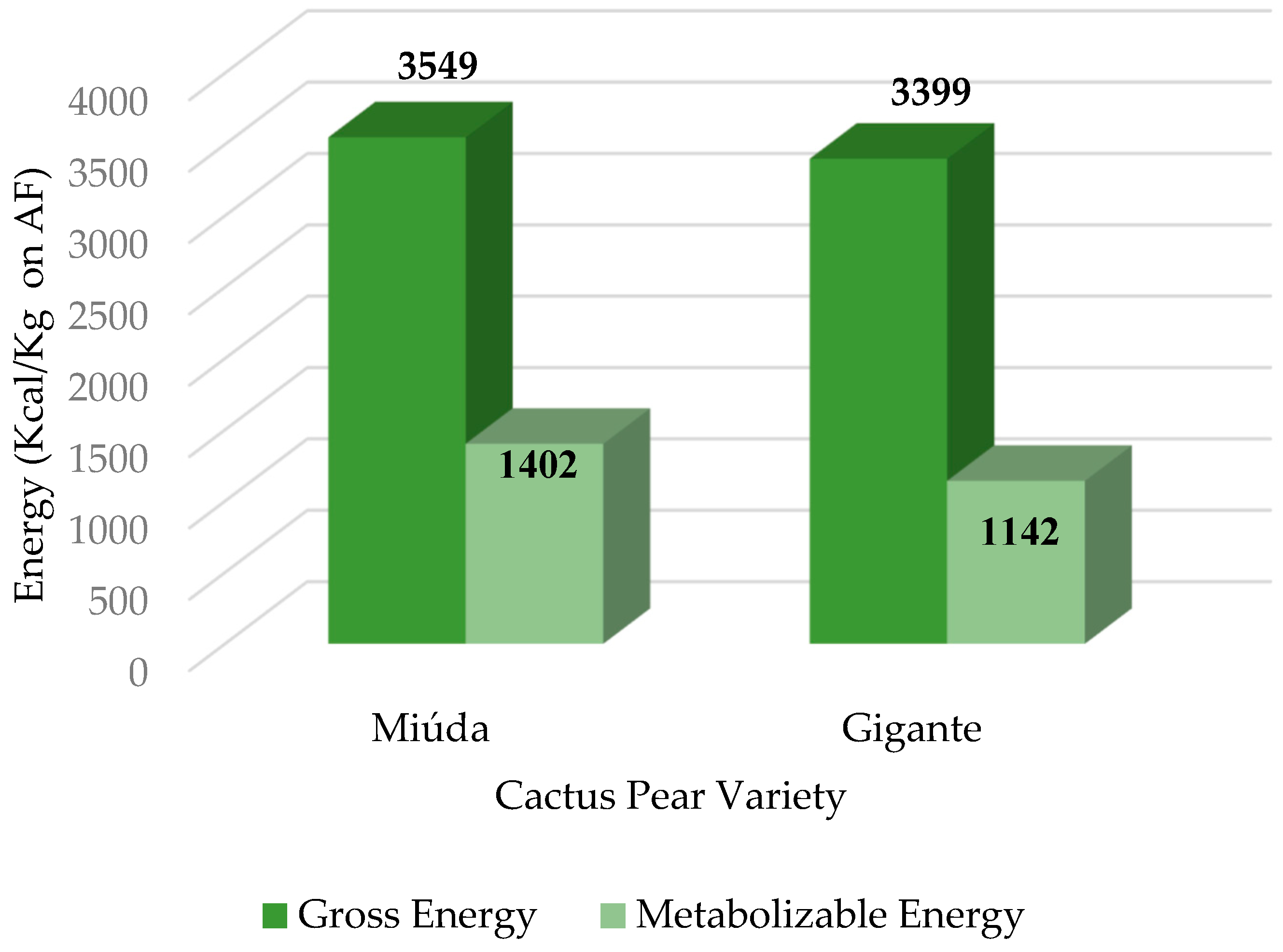
The contents of 3,549 and 3,399 kcal/kg of GE and 1,402 and 1,142 kcal/kg AME (
Figure 1) were found in the present study for the varieties of CPM and
Gigante cactus pear meal. In the study of [
32], found values of 4,009, 3,757 and 3,945 kcal/kg GE; 3,144, 3,019 and 1,624 kcal/kg AME for corn, sorghum, and wheat bran, respectively for chicks aged 26 to 33 days. The authors [
33], found new gross energy values in corn grain (3,884 kcal/kg), wheat grain (3,867 kcal/kg) and sorghum grain (3,987 kcal/kg). As for the metabolizable energy, values of 3,719 kcal/kg for corn, 3,265 kcal/kg for wheat, and 3,695 kcal/kg for sorghum were found for chicks aged 22 to 28 days. Whereas [
34] found metabolizable energy values of 1,259 kcal/kg and 1,316 kcal/kg for corn and wheat, respectively, for 7-, 14-, 21-, 28-, and 35-day-old birds. However, these differences in gross energy and metabolizable energy of those feedstuffs were expected, as there are variations in soil conditions, climate, raw material obtention, storage time, processing, age of the birds, physiological state, methodology used, and chemical composition [
33,
35,
36].
The AME value of CPM (1,402 kcal/kg) was higher than that of
Gigante cactus pear meal (1,142 kcal/kg). Such differences may be related to the species and chemical composition of the varieties of cactus pear that may interfere in the metabolizable energy. The AME value of cactus pear
Miúda stood out due to its bromatological composition that presented lower contents of soluble fiber when compared to
Gigante [
7,
37,
38].
The authors [
39] states that the metabolizable energy is directly and positively affected by the composition of the feed in starch, fat and protein and negatively affected by the structural carbohydrates of the plant. The AME value of
Gigante cactus pear meal may have been negatively affected by the soluble fiber content (NSPs and pectin) mainly the high content of pectin [
38] and its high water solubility [
40]. These physicochemical characteristics of the soluble fiber fraction result in increasing viscosity of the digest. High viscosity decreases the diffusion rate of endogenous enzymes in the digest, which will reduce nutrient digestion. In addition, the highly viscous digesta will have less interaction with enzymes in the brush border membrane, which also decreases digestibility and nutrient utilization [
40,
41].
On the other hand, the AME value of CPM was positively affected by the high contents of non-fiber carbohydrates [
7,
38,
42,
43] mainly starch [
7], being the main source of energy for birds. These results are attributed to the higher intake of non-fiber carbohydrates, which consequently provided higher energy intake [
44].
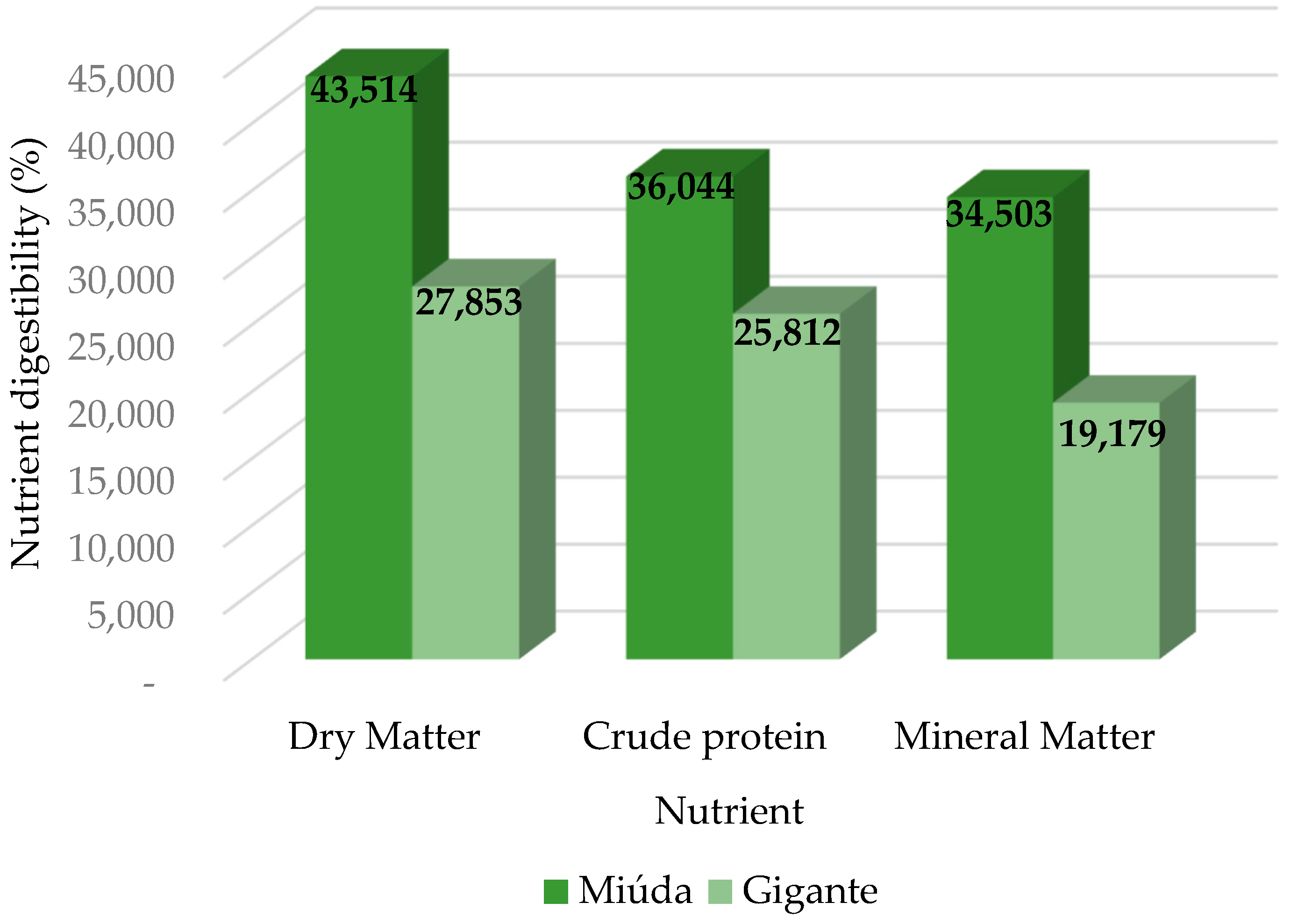
CMDC, CPDC and MMDC of variety CPM were higher when compared to
Gigante cactus pear meal (
Figure 2), and this is presumably due to the nutritive value that varies between cultivars [
45]. The DMDC value of CPM (43.5%) was higher than
Gigante’s cactus pear meal (27.9%). The reduced dry matter digestibility of the cactus pear varieties, mainly for the
Gigante cactus pear meal may be attributed to considerable amounts of NSP that cannot be digested by birds because they lack endogenous enzymes. Soluble NSPs can increase the viscosity of the digestate and reduce nutrient digestibility [
46]. The superiority in DMDC of CPM can be attributed to the sugar and starch contents [
7,
38], since the concentration of these carbohydrates contributes considerably to high palatability, which explains the higher dry matter digestibility of that variety, corroborating the results represented in
Figure 2.
The CPDC values of CPM (36.0%) and
Gigante cactus pear meal (25.8%) found in this study were lower than the values found by [
47], who found protein digestibility coefficient 92.69% for corn and 91.41% for sorghum for Isa Label chickens, from 28 to 35 days. In other research, [
48] found protein digestibility coefficient of 75.24% for corn and 87.84% for sorghum for Label Rouge birds. These significant differences in the CPDC of cactus pear meal, corn and sorghum are related to the chemical composition of the feed (antinutritional factors and the amount of fiber), in addition to the strain of the birds that can influence the digestibility of nutrients. The lower CPDC may be because the cactus pears are from the genera
Opuntia (
Gigante) and
Nopalea (
Miúda), that is, the genus influences composition and the composition influence nutrient utilization. Cactus pear variety
Gigante presents a higher concentration of soluble fibers [
7,
37,
38]. Soluble fibers impair protein digestibility because they increase the viscosity of the intestinal contents, reducing the action of proteolytic enzymes, and consequently causing endogenous nitrogen losses [
49]. Another possible explanation is that lignin is a substance of the insoluble fraction of fiber, and its binding with proteins makes them unavailable for animal absorption [
37].
For MMDC, the value found for CPM in this study was higher than the value found by [
50], while the variety
Gigante cactus pear meal responded inferiorly, but very close to the value found of digestibility coefficient of 27.66 and 21.42% for young and adult Label Rouge birds, respectively, fed with feed based on corn and soybean meal. Cactus pear is considered a good source of minerals, regardless of the species (
Opuntia and
Nopalea), with the highest concentrations found for Ca, K, Mg and P and the lowest for Cu, Fe, Sr and Zn [
51,
52]. However, cacti possess the antinutritional factor calcium oxalate, which binds to calcium and possibly other minerals in a nutritionally unavailable form, thus interfering with the bioavailability of calcium for animal absorption [
37,
53]. The researchers [
54], observed that the morphology of calcium oxalate crystals was different, since the crystals were larger (ranging from 30 to 100 ÿm) and more abundant in fresh cladode tissues of the three Opuntia fícus-indica cultivars (argelina, morado e gymno-carpo) than in Opuntia robusta, which were smaller (ranging from 6 ÿm to 35 ÿm), more rounded, very sparse and observed mainly near the epidermis. This caused a reduction in calcium concentration in Opuntia robusta. Possibly, the lower mineral matter digestibility coefficient of Gigante cactus pear meal (19.2%) may be associated with calcium oxalate crystals.
Diets containing levels of CPM did not compromise the variables of productive performance, despite mainly the presence of NSPs and oxalic acid. In the scientific literature there are no studies available on the use of cactus pear meal for laying hens, but many studies are found with good alternatives to corn for these birds, but with problems that limit their use. Rice bran, which is alternative to corn, has in its composition a high percentage of phytic acid and NSPs [
55], making it a similar feed to cactus pear meal due to its chemical composition and presence of antinutritional factors. Knowing about the presence of the antinutritional factors in rice bran, [
56] tested the inclusion of rice bran in laying hens’ feed and found that it had no significant effect on egg production, feed intake, feed conversion and egg mass, as did the present study.
Yolk percentage, shell percentage, shell thickness, specific gravity, Haugh Unit, yolk strength and yolk brightness values had no significant effects from the increasing levels of CPM. Cactus pear meal has a high concentration of non-fiber carbohydrates [
12], which makes it a good alternative source to corn, but on the other hand there is limitation of use due to the concentration of NSPs [
6]. There are no reports in the scientific environment of its use in laying hens, so it is acceptable to compare results with similar feedstuffs in terms of energy and fiber (NSPs). Wheat bran is widely used for laying hens due to its availability and energy, but it is limited due to the amount of NSPs. They [
57] found that hens responded similarly to the present study, that is, there was no significant effect of adding 3 and 6% wheat bran and beet pulp in the diets of 90-week-old laying hens on egg shape index, yolk percentage, shell percentage, shell thickness, Haugh unit, and specific gravity.
Eggs from birds that received CPM levels showed lower yolk diameter values, while birds fed corn and soybean meal-based diet had larger yolk diameters, but no studies with similar feeds were found for this trait. Yolk diameter is an important variable, since it is directly related to the reactions that occur in the albumen, where the water from the albumen crosses the yolk membrane by osmosis and is retained in the yolk. Excess water in the yolk determines the increase of its volume, leading to the weakening of the yolk membrane. This makes the yolk appear larger and flattened when the egg is observed on a flat surface after it is broken [
58].
The percent of albumen increased according to the increasing use of CPM levels. The response of the albumen percentage had an opposite effect to the yolk percentage, mainly when using 9% CPM, because they are inversely proportional, that is, as the albumen percentage increased, the yolk percentage decreased. Presumably this increase in albumen percentage must be related to the linoleic acid in the birds’ diets (Table II). The experimental diets were formulated to contain the same metabolizable energy, so as the level of cactus pear increased it was necessary to increase the amount of soybean oil in the feed to standardize the metabolizable energy. Soybean oil has a reasonable amount of linolenic acid [
59], and this acid promotes increased concentrations of estrogen, which is important in controlling egg weight since dietary fats influence egg weight [
60,
61]. The authors [
60] found that diets with supplemental fat and linoleic acid increased albumen weight of eggs of Isabrow hens from the 22
nd to 65
th week of age.
Alternative feeds to corn are well explored to reduce the cost of poultry production. Understanding the importance of exploring the effects of these feeds on egg quality, [
62] evaluated a combination of alternative ingredients and found that the percentage of albumen was higher in group 4 (64.06) than in the other groups (1 - 63.24, 2 - 63.27 and 3 - 63.56), these values are close to those found in the present study.
The shell strength decreased as the level of CPM increased. Hens fed 9% CPM had lower shell strength when compared to the control feed. This reduction may be due to the effect of oxalic acid present in the cactus pear, since it is an organic compound that binds to calcium or other minerals in an unavailable nutritional form, affecting the availability for absorption by the animal [
37,
45] thus causing deficiency of important minerals for the formation of the shell, since about 94-95% of the dry eggshell is composed of calcium carbonate (CaCO
3).
For yolk coloration, brightness had no significant effect among the experimental diets, but hens fed with 9% CPM had significantly lower values in the red to green region and coloration in the yellow to blue range. However, the intensity of the yolk color was higher in the control diet, which may be due to the reduced amount of corn in the experimental diets 3, 6, and 9%. A possible reason for this result is that corn is the ingredient source of carotenoids in poultry feeds, and these carotenoids are classified into xanthophylls and carotenes [
63,
64] added 15% almond shell in the feed of laying hens and found decrease in the values of a (greener) and b (less yellow) in yolk coloration.
The method of texture profile analysis is based on compressing the food for at least two times, simulating the action of two bites on the food. There was no significant effect on Conversion Indicator, which deals with changes suffered (weight increase or reduction) by the cooking process. The hardness property, which is the force required to achieve a deformation of the sample, had no significant effect. Regarding the cohesiveness property, there was linear increase according to the levels of CPM used. Cohesivity is defined as the degree to which a material is deformed before it breaks (physical) or between the teeth before it breaks (sensory) [
65]. Probably this significant effect for cohesiveness is related to the amount of fat present in the yolk (Table VIII) since [
66] reported that fat in the yolk increases cohesiveness. No significant effect was observed on the elasticity property, which is defined as the degree to which the deformed material returns to its original condition after a force was applied (physical) or pressed between the teeth (sensory). No effects were found for the parameter gumminess either. This is a parameter defined as the energy required to disintegrate a food to a swallowable state. Regarding chewability, which is the number of chews required, at a constant force, for the food to be swallowed [
66] there was no significant effect either.
The cooked yolk coloration parameters a* and b* were affected by CPM levels, since as the level of CPM increased, the yolk color reduced. The reduction in yolk color intensity may be related to the presence of natural pigments (lutein, zeaxanthin, and β-carotene) [
67]. Possibly this variation is because corn is the main carotenoid source in poultry feed [
63], which means that the presence of pigments is higher in corn than in cactus pear [
68] showed that the average contents of lutein, zeaxanthin, and beta-carotene, in green corn kernels is 0.71, 9.85, and 0.88 µg/g in the fresh sample, respectively.
The mineral matter of eggs from hens fed with CPM reduced, however dry matter and crude protein did not differ between treatments. This possibly occurred because cactus pear has the antinutritional factor calcium oxalate, which is an organic compound that binds to calcium or other minerals in an unavailable nutritional form, affecting availability for animal absorption [
37,
45].
According to [
69], chromatographic analyses of total lipids extracted from cactus pear cladodes show that palmitic acid (C16:0), oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3) contribute in 13.87, 11.16, 34.87 and 32.83% of the total fatty acid content, respectively. These four fatty acids therefore represent over 90% of the total fatty acids with linoleic and linolenic acids being the main polyunsaturated ones, totaling 67.7%.
The saturated fatty acids identified were myristic, palmitic, and stearic. Myristic and palmitic acids reduced as the level of CPM increased, however, stearic acid behaved inversely proportional to myristic and palmitic, which increase in the yolk as the level of CPM increased in the diet.
The monounsaturated fatty acids identified were myristoylic, palmitoleic, oleic, and vaccenic. All monounsaturated fatty acids showed higher concentration in the yolk of eggs from hens fed with the control diet (0% CPM) and reduced as the level of CPM increased. Regarding the oleic acid, a possible explanation for the reduction is the presence of soybean oil in the feed, since in the study of [
59] the incorporation of soybean oil reduced oleic acid in yolks from chickens fed with corn.
The polyunsaturated fatty acids linoleic and α-linolenic increased as the level of CPM increased, possibly due to the incorporation of soybean oil in the feed. As the level of CPM increased, the amount of soybean oil also increased. Soybean oil is rich in linoleic acid and has a fair amount of linolenic acid. The inclusion of soybean oil in the diet increased the linoleic and linolenic acid contents and consequently increased the linoleic and α-linolenic fatty acids in the yolk [
59]. Linoleic acid promotes increased estrogen concentrations and thus stimulates protein synthesis in the oviduct, causing greater protein deposition in the albumen, resulting in a heavier egg [
61]. In addition, linoleic acid has long been accepted as having a hypocholesterolemia effect and inhibitory properties against metastatic colon cancer cells. Omega-3 linolenic acid is known to be beneficial to health, cardiovascular disease, inflammatory conditions, autoimmune disorders, and diabetes [
69]. Arachidonic acid which is the precursor of linoleic acid was detected in the yolks and the lowest concentration was found in eggs from hens receiving the control diet. As the CPM level increased in the diet, the concentration of this fatty acid also increased.
The cholesterol content in the yolk increased linearly with the use of CPM (TC= 397.4 + 20.37CPM, R
2=0.77). This can be explained by the increase in the polyunsaturated fatty acids linoleic and α-linolenic. The lipid composition of egg yolk can be altered, especially regarding the the fatty acid profile, including the content of n-3 polyunsaturated fatty acids (PUFA) [
70]. The cholesterol content in the yolk has become an important issue for consumers, as cholesterol is synthesized by the human body and consumers have been advised to avoid dietary cholesterol intake to prevent chronic diseases, including coronary heart disease. More recently, it has been determined that exogenous cholesterol actually represents a very small amount of hematic cholesterol [
71].
Although feed intake was higher for hens receiving the diet with 6% CPM, the price of feed was higher in the 9% CPM diet in comparison to the others, which may be due to the increase in soybean oil in the diets to keep them isoenergetic. Consequently, feeding cost was also higher for the diet containing 6% CPM, due to the higher feed intake. Egg dozen production (dozen/bird) was higher when hens consumed diets containing 6% CPM. A plausible explanation is that with 6% of cactus pear meal in the diet there was higher feed intake, consequently more money was spent to produce the 6% CPM diet. In contrast, the ratio of Feeding Cost/Egg Dozen, R$/dozen was higher for the control diet, while for the diet with 6% CPM the feeding cost to produce a dozen eggs was higher than the diets with 3% and 9%, respectively.