Submitted:
02 January 2024
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. ECEs weight changes from the 6th do 20th day of development
2.2. Histopathological examinations
2.3. Real-time PCR
2.4. Antioxidant and lipid peroxidation markers
| HEART | control n = 16 |
HAdV-D36-infected n = 18 |
t/z* | p |
|---|---|---|---|---|
| CAT (IU/g protein) | 1584±205 | 2585±798 | 5.13 | <0.001 |
| GR (IU/g protein) | 2.6 (2.3-3.0) | 3.0 (2.2-3.9) | 0.57* | 0.569 |
| TOS (μmol/g protein) | 2.6±0.5 | 2.5±0.8 | 0.22 | 0.826 |
| SOD (NU/mg protein) | 58.0 (52.5-59.0) | 74.6 (70.9-76.2) | 4.47* | <0.001 |
| MnSOD (NU/mg protein) | 33.8±4.1 | 40.8±8.0 | 2.41 | <0.05 |
| CuZnSOD (NU/mg protein) | 22.4±5.1 | 31.6±12.9 | 2.81 | <0.05 |
| GPx (IU/g protein) | 4.0±0.9 | 3.8±0.9 | 0.62 | 0.542 |
| GST(IU/g protein) | 1.24±0.23 | 1.62±0.49 | 2.19 | <0.05 |
| MDA (μmol/g protein) | 1.6 (1.2-1.8) | 3.3(1.8-5.1) | 3.05* | <0.01 |
| control n = 16 |
HAdV-D36-infected n = 18 |
t/z* | p | |
|---|---|---|---|---|
| CAT (IU/g protein) | 4596±1255 | 4834±1487 | 0.50 | 0.617 |
| GR (IU/g protein) | 0.84±0.15 | 1,31±0,37 | 5.04 | <0.001 |
| TOS (μmol/g protein) | 2.1±0.5 | 2.9±0.9 | 2.93 | <0.01 |
| SOD (NU/mg protein) | 63.3±7.9 | 90.4±30.6 | 3.30 | <0.01 |
| MnSOD (NU/mg protein) | 40.1±7.1 | 38.0±16.8 | 0.50 | 0.623 |
| CuZnSOD (NU/mg protein) | 28.0 (18.8-31.2) | 38.0 (31.9-66.7) | 3.36* | <0.001 |
| GPx (IU/g protein) | 7.0±1.3 | 5.5±1.3 | 3.58 | <0.01 |
| GST (IU/g protein) | 5.14±0.99 | 4.72±1.14 | 1.15 | 0.258 |
| MDA (μmol/g protein) | 0.77 (0.60-1.08) | 1.35(1.16-1.92) | 2.43* | <0.05 |
3. Discussion
4. Materials and Methods
4.1. Ethical statement
4.2. Virus
4.3. Embryonated chicken eggs (ECEs)
- Ad-AC: inoculated with HAdV-D36 into the allantoic cavity (n = 105),
- Ad-YS: inoculated with HAdV-D36 into the yolk sack (n = 105),
- C-AC: inoculated with PBS into allantoic cavity (n = 105) – control group,
- C-YS: inoculated with PBS into yolk sack (n = 105 eggs) – control group.
4.4. Inoculation of ECEs
4.5. ECEs weight measurements
4.6. Sampling ECEs for histopathological and molecular studies
4.7. Histopathological examinations
4.8. Real Time PCR
4.9. Antioxidant and lipid peroxidation markers
4.9.1. Catalase (CAT) activity (EC 1.11.1.6)
4.9.2. Superoxide Dismutase (SOD) activity (EC 1.15.1.1)
4.9.3. Glutathione Peroxidase (GPx) activity (EC 1.11.1.9)
4.9.4. Glutathione Reductase (GR) activity (EC 1.8.1.7)
4.9.5. Glutathione-S Transferase (GST) activity (EC 2.5.1.18)
4.9.6. Total oxidant status (TOS)
4.9.7. Malondialdehyde (MDA) concentration
4.10. Statistical analysis
Author Contributions
Data Availability Statement
Conflicts of Interest
References
- Mitra, A. K., & Clarke, K. Viral obesity: Fact or fiction? In Obesity Reviews, 2010, 11, pp. 289–296,. [CrossRef]
- Pasarica, M., & Dhurandhar, N. V. Infectobesity: Obesity of Infectious Origin. Advances in Food and Nutrition Research, 2007, 52, 61–102. [CrossRef]
- Ponterio, E., & Gnessi, L. Adenovirus 36 and Obesity: An Overview. Viruses,1980.7, 3719–3740. [CrossRef]
- Nam, J. H., Na, H. N., Atkinson, R. L., & Dhurandhar, N. V. Genomic stability of adipogenic human adenovirus 36. International Journal of Obesity, 2014, 38(2), 321–324. [CrossRef]
- Adenoviridae ~ ViralZone. (n.d.). Retrieved October 17, 2023, from https://viralzone.expasy.org/4.
- Dhurandhar, N. v, Kulkarni A’ J, P., Ajinkya, S. M., & Sherikar, A. Effect of adenovirus infection on adiposity in chicken. In Veterinary Microbiology, 1992, Vol. 31.
- Rathod, M. A., Rogers, P. M., Vangipuram, S. D., McAllister, E. J., & Dhurandhar, N. V. Adipogenic cascade can be induced without adipogenic media by a human adenovirus. Obesity, 2009, 17(4), 657–664. [CrossRef]
- Laron, Z. Insulin - A growth hormone. In Archives of Physiology and Biochemistry,2008, 114(1), pp. 11–16). [CrossRef]
- Pasarica, M., Shin, A. C., Yu, M., Yang, H. M. O., Rathod, M., Catherine Jen, K. L., MohanKumar, S., MohanKumar, P. S., Markward, N., & Dhurandhar, N. v. Human adenovirus 36 induces adiposity, increases insulin sensitivity, and alters hypothalamic monoamines in rats. Obesity, 2006, 14(11), 1905–1913. [CrossRef]
- Almgren, M., Atkinson, R., He, J., Hilding, A., Hagman, E., Wolk, A., Thorell, A., Marcus, C., Näslund, E., Östenson, C. G., Schalling, M., & Lavebratt, C. Adenovirus-36 is associated with obesity in children and adults in Sweden as determined by rapid ELISA. PLoS ONE, 2012, 7(7). [CrossRef]
- Zhou, Y., Pan, Q., Wang, X., Zhang, L., Xiao, F., & Guo, L. The relationship between human adenovirus 36 and obesity in Chinese Han population. Bioscience Reports, 2018,38, 20180553. [CrossRef]
- Chudnovets, A., Liu, J., Narasimhan, H., Liu, Y., & Burd, I. Role of Inflammation in Virus Pathogenesis during Pregnancy. Journal of Virology, 2020,. [CrossRef]
- Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry, 1951,193(1), 265–275. [CrossRef]
- Aebi, H. [13] Catalase in vitro. Methods in Enzymology, 1984, 105(C), 121–126. [CrossRef]
- Oyanagui, Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Analytical Biochemistry, 1984, 142(2), 290–296. [CrossRef]
- Mannervik, B. [60] Glutathione peroxidase. Methods in Enzymology, 1985, 113(C), 490–495. [CrossRef]
- Carlberg, I., & Mannervik, B. [59] Glutathione reductase. Methods in Enzymology, 1985,113(C), 484–490. [CrossRef]
- Habig, W. H., & Jakoby, W. B. [51] Assays for differentiation of glutathione S-Transferases. Methods in Enzymology,1981,77(C), 398–405. [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clinical Biochemistry, 2005 38(12), 1103–1111. [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clinical Biochemistry, 2004, 37(2), 112–119. [CrossRef]
- Ohkawa, H., Ohishi, N., & Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 1979, 95(2), 351–358. [CrossRef]
- Karandish, M., Shirani, F., Teimoori, A., Rashno, M., & Latifi, M. Using rats as a research model to investigate the effect of human adenovirus 36 on weight gain. In ARYA Atheroscler,2017, 13.
- Na, H. N., & Nam, J. H. Adenovirus 36 as an obesity agent maintains the obesity state by increasing MCP-1 and inducing inflammation. Journal of Infectious Diseases, 2012, 205(6), 914–922. [CrossRef]
- [24] Ribatti, D., Tamma, R., & Elieh Ali Komi, D. The morphological basis of the development of the chick embryo immune system. In Experimental Cell Research, 2019, 381(2) , pp. 323–329). Elsevier Inc. [CrossRef]
- Alexis, L., Romanoff, M., The Avian Embry Structural and functional development. Science, 1960,16,1305,.
- Garcia, P., Wang, Y., Viallet, J., & Macek Jilkova, Z. The Chicken Embryo Model: A Novel and Relevant Model for Immune-Based Studies. In Frontiers in Immunology ,2021, 12,. [CrossRef]
- Kindel, T. L., & Strande, J. L. Bariatric surgery as a treatment for heart failure: review of the literature and potential mechanisms. Surgery for Obesity and Related Diseases, 2018, 14(1), 117–122. [CrossRef]
- Burgoyne, J. R., Mongue-Din, H., Eaton, P., & Shah, A. M. Redox signaling in cardiac physiology and pathology. In Circulation Research, 2018, 111(8), pp. 1091–1106,. [CrossRef]
- Aimo, A., Castiglione, V., Borrelli, C., Saccaro, L. F., Franzini, M., Masi, S., Emdin, M., & Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. In European Journal of Preventive Cardiology, 2020, 27(5), pp. 494–510),. [CrossRef]
- Higuchi, Y., Otsu, K., Nishida, K., Hirotani, S., Nakayama, H., Yamaguchi, O., Matsumura, Y., Ueno, H., Tada, M., & Hori, M. Involvement of Reactive Oxygen Species-mediated NF- κ B Activation in TNF- α -induced Cardiomyocyte Hypertrophy. Journal of Molecular and Cellular Cardiology, 2002, 34(2), 233–240. [CrossRef]
- Pal, S., Polyak, S. J., Bano, N., Qiu, W. C., Carithers, R. L., Shuhart, M., Gretch, D. R., & Das, A. Hepatitis C virus induces oxidative stress, DNA damage and modulates the DNA repair enzyme NEIL1, 2010. [CrossRef]
- Smirnova, O. A., Ivanova, O. N., Bartosch, B., Valuev-Elliston, V. T., Mukhtarov, F., Kochetkov, S. N., & Ivanov, A. v. Hepatitis C Virus NS5A Protein Triggers Oxidative Stress by Inducing NADPH Oxidases 1 and 4 and Cytochrome P450 2E1.2016,. [CrossRef]
- Duygu, F., Karsen, H., Aksoy, N., & Taskin, A. (2012). Relationship of oxidative stress in hepatitis B infection activity with HBV DNA and fibrosis. Annals of Laboratory Medicine, 2012, 32(2), 113–118. [CrossRef]
- Raza, H., John, A., & Howarth, F. C. Alterations in Glutathione Redox Metabolism, Oxidative Stress, and Mitochondrial Function in the Left Ventricle of Elderly Zucker Diabetic Fatty Rat Heart. Int. J. Mol. Sci, 2012, 13, 16241–16254. [CrossRef]
- Caetano, F., Letícia, C. , Almeida, T., Carolina, Bernardes, S., Silva, M., Lú Cia Pedrosa, M., Caldeira, D., Wanderson, C., de Lima, G., Carla, Pinto, A., César, P., Ferreira, P., & Carlos De Magalhães, J. (n.d.). Caraparu virus induces damage and alterations in antioxidant defenses in the liver of BALB/c mice after subcutaneous infection. [CrossRef]
- Menegatto, M. B. da S., Ferraz, A. C., Lima, R. L. S., Almeida, L. T., Brito, R. C. F. de, Reis, A. B., Carneiro, C. M., Lima, W. G. de, Silva, B. de M., Magalhães, J. C. de, & Magalhães, C. L. de B. Oropouche virus infection induces ROS production and oxidative stress in liver and spleen of mice. Journal of General Virology, 2023,104(5), 001857. [CrossRef]
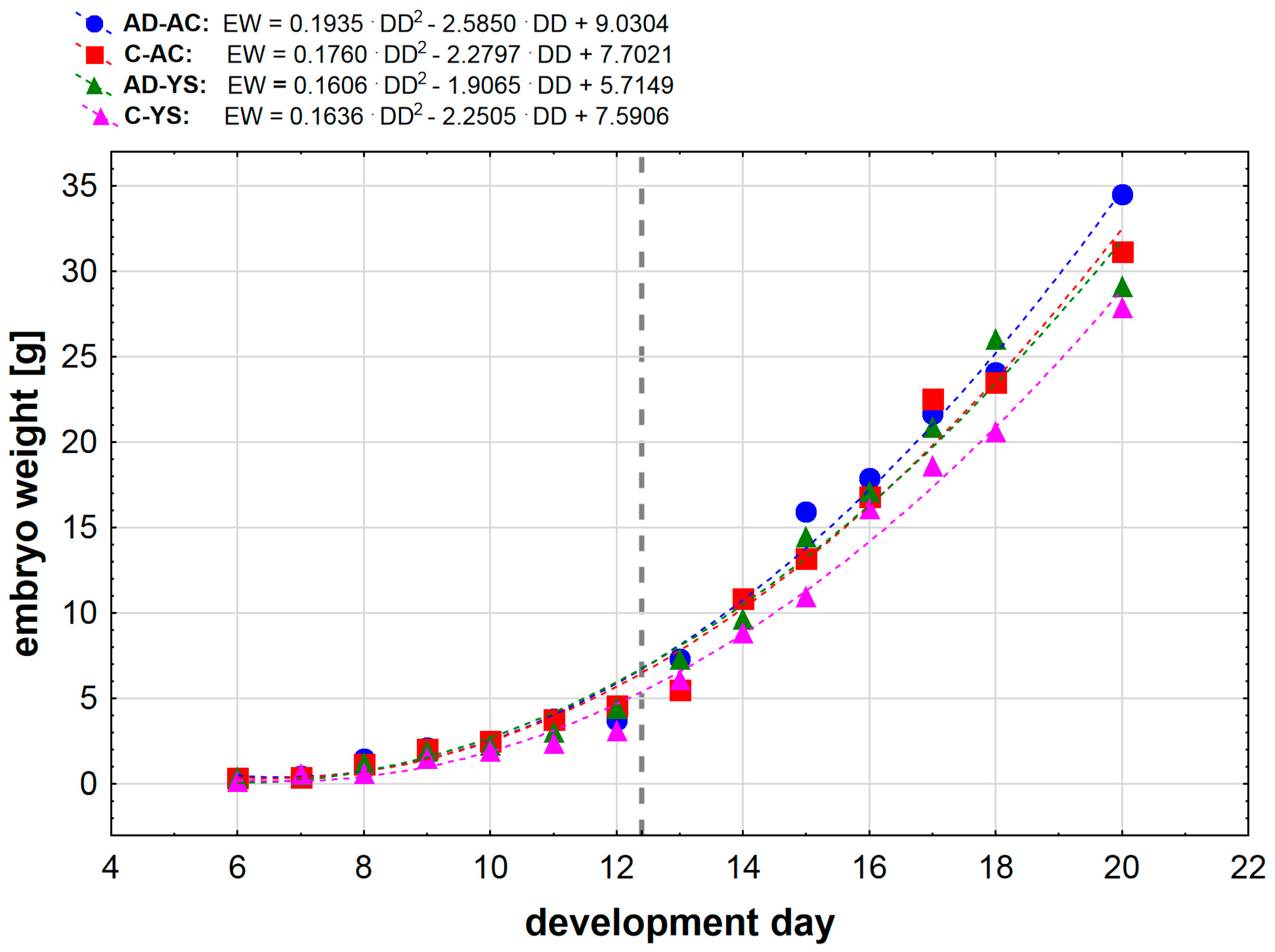
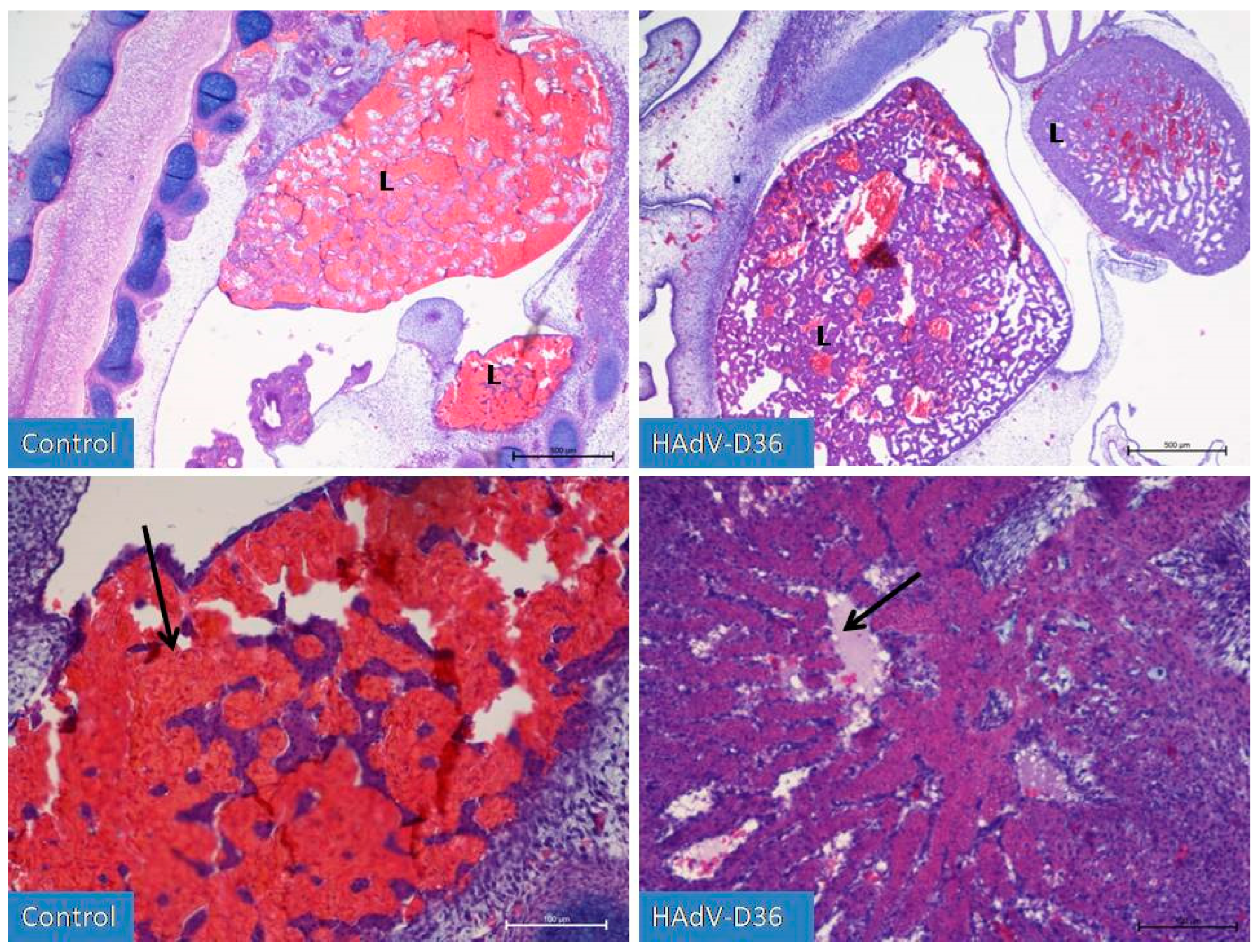
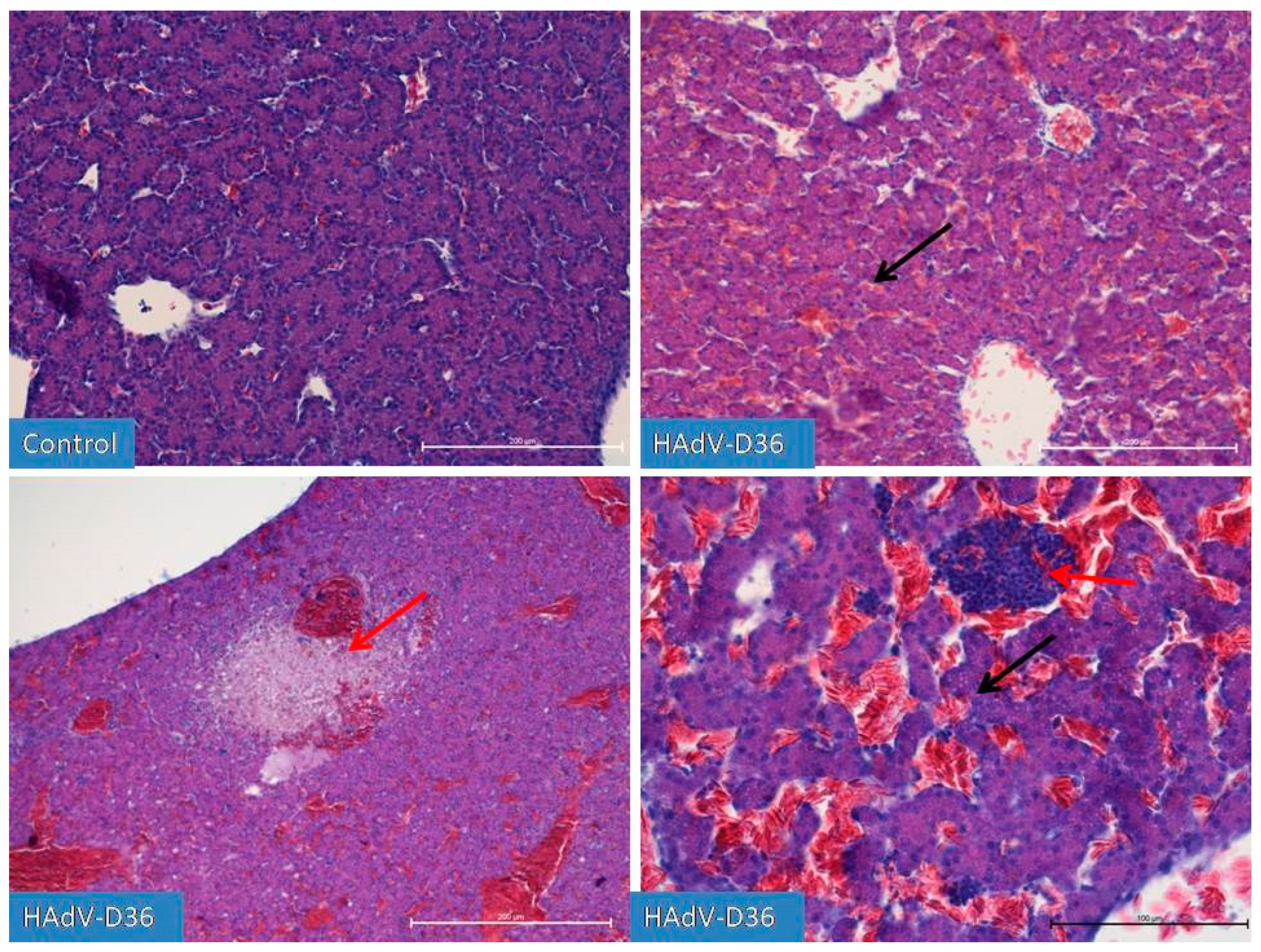
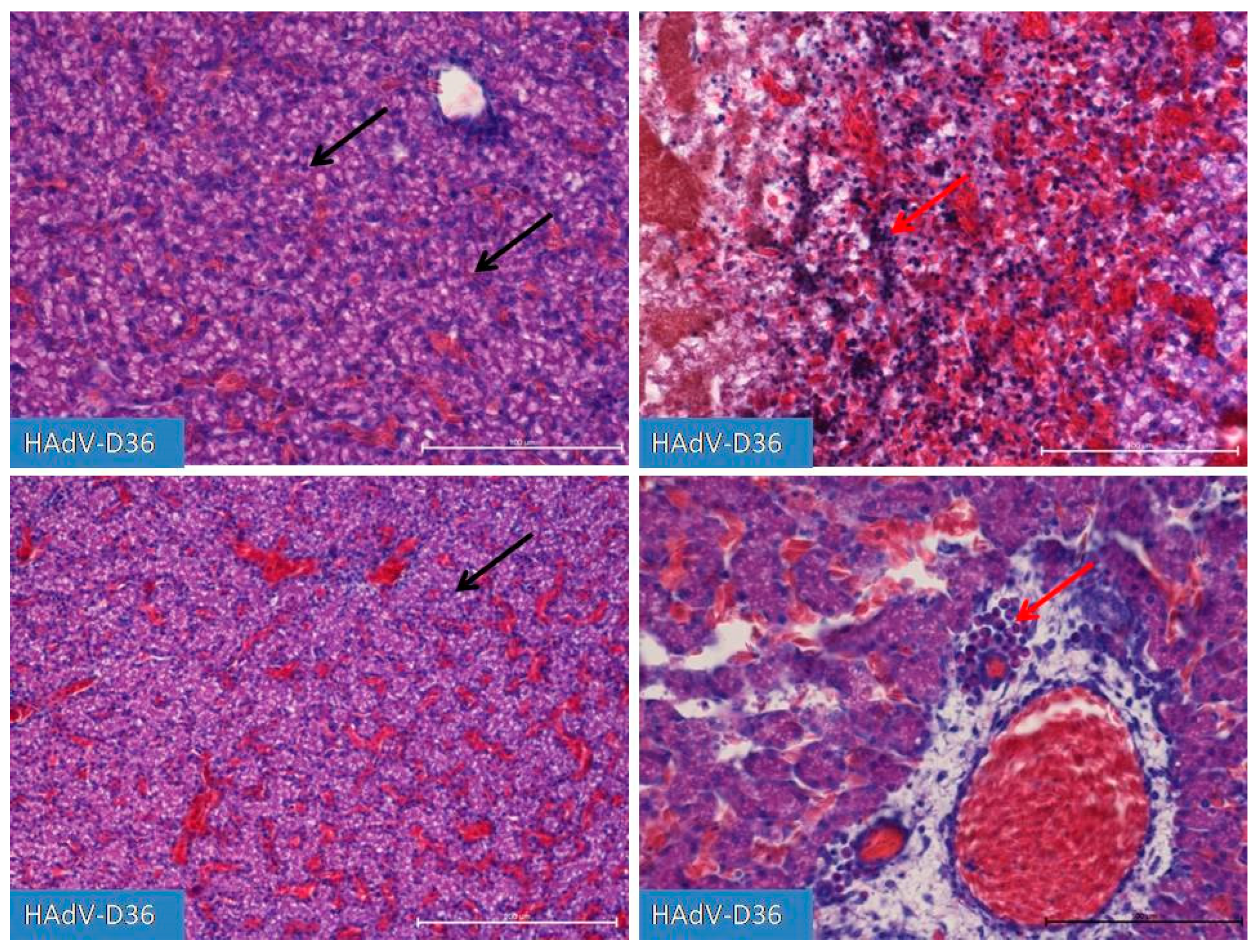
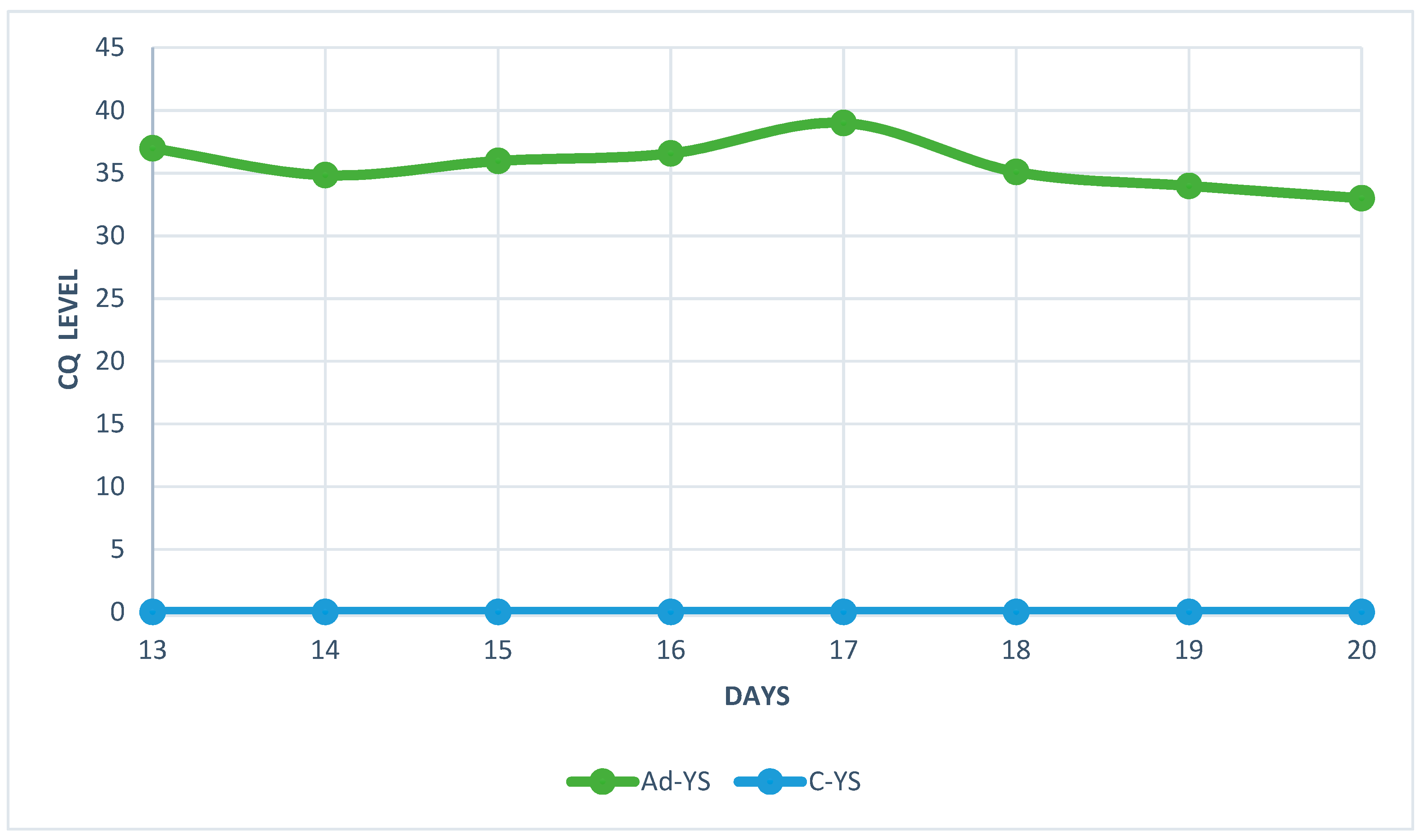
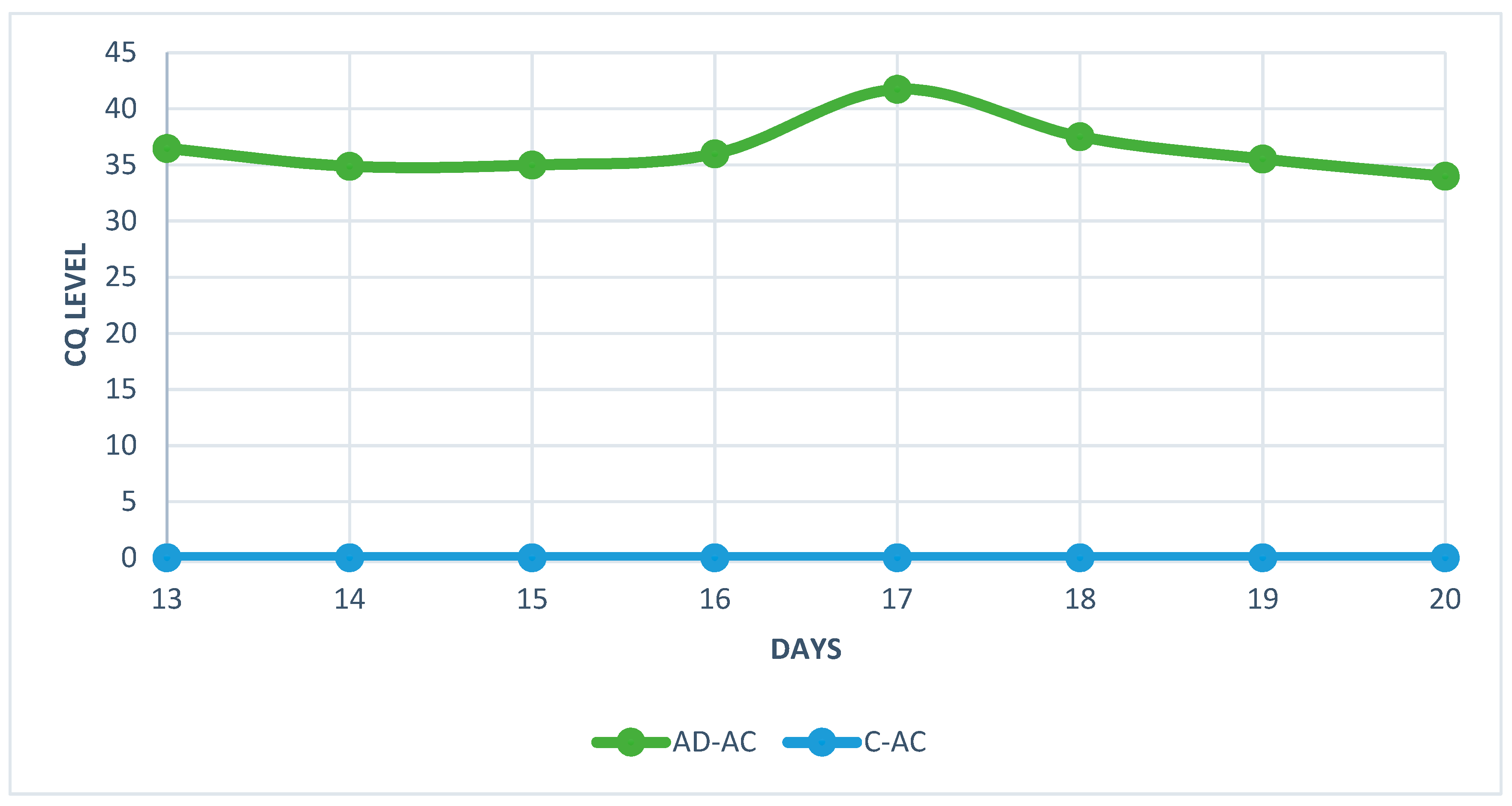
|
Day of incubation |
ECEs weight [g] | |||
| Ad-YS | C-YS | Ad-AC | C-AC | |
| 6 | 0.38 ± 0.12 | 0.15 ± 0.04 | 0.24 ± 0.10 | 0.34 ± 0.02 |
| 7 | 0.55 ± 0.17 | 0.57 ± 0.01 | 0.47 ± 0.14 | 0.40 ± 0.11 |
| 8 | 1.23 ± 0.38 | 0.63 ± 0.08 | 1.47 ± 0.51 | 1.16 ± 0.26 |
| 9 | 1.93 ± 0.39 | 1.50 ± 0.38 | 2.15 ± 0.21 | 2.03 ± 0.43 |
| 10 | 2.28 ± 0.43 | 1.93 ± 0.13 | 2.26 ± 0.45 | 2.51 ± 0.47 |
| 11 | 3.06 ± 0.65 | 2.39 ± 0.23 | 3.82 ± 0.90 | 3.78 ± 0.33 |
| 12 | 4.41 ± 1.32 | 3.12 ± 0.54 | 3.78 ± 1.00 | 4.58 ± 0.15 |
| 13 | 7.33 ± 2.04 | 6.13 ± 0.83 | 7.30 ± 1.44 | 5.52 ± 0.12 |
| 14 | 9.64 ± 2.37 | 8.84 ± 2.78 | 10.86 ± 0.46 | 10.86 ± 2.00 |
| 15 | 14.48 ± 2.25 | 10.97 ± 2.12 | 15.93 ± 0.53 | 13.20 ± 1.60 |
| 16 | 17.12 ± 4.29 | 16.12 ± 1.51 | 17.87 ± 0.33 | 16.82 ± 0.62 |
| 17 | 20.91 ± 0.65 | 18.62 ± 2.91 | 21.66 ± 0.50 | 22.63 ± 3.62 |
| 18 | 26.04 ± 1.13 | 20.63 ± 2.65 | 24.08 ± 0.39 | 23.52 ± 3.62 |
| 19 | 27.59 ± 2.01 | 26.45 ± 2.04 | 26.19 ± 0.19 | 27.66 ± 0.98 |
| 20 | 29.13 ± 2.32 | 27.91 ± 1.83 | 34.51 ± 0.30 | 31.18 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





