1. Introduction
Have you ever considered the amazing journey of plastics from being a novel invention to a global environmental concern? In the past century, the use of plastic materials has increased significantly in various applications, from single-use items to engineering-grade polymers (e.g., food preservation, healthcare, transportation, and energy), thanks to their extensive range of properties such as permeability, waterproofing, hydrophobicity or hydrophilicity, stiffness, or flexibility. Plastic production has grown to an estimated annual production of over 390 million tons in 2021, with a growth rate of 4% per year [
1].
What gives them such diverse properties as permeability, waterproofing, and flexibility? It's a blend of polymeric co-partners and other additives. But here's the catch: while these additives optimize properties, they can also lead to mechanical weaknesses, compatibility issues, and even environmental and health hazards due to toxic substances like phthalate plasticizers and brominated flame retardants [
2,
3,
4]. Therefore, plastic materials are often associated with various additives, such as polymeric co-partners, to achieve specific properties. [
5,
6,
7]. These adjustments can improve processability, reduce costs, or extend the material's lifespan. However, when added in excess, certain additives can significantly affect the final material's mechanical properties and cause compatibility issues within the matrix [
8]. It is crucial to maintain a good balance between the additive's loading and their impact on the initial properties of the material, which often requires a compatibilization step. Additionally, some additives are considered as being controversial due to their toxicity during fabrication and use of plastic material. This can have harmful effects on the environment and human health, as certain compounds can migrate out of plastics [
9,
10,
11]. Examples of such additives include phthalate plasticizers and brominated flame retardants [
12,
13,
14]. On the other hand, the global plastic waste crisis requires urgent action from science and industry to manage plastic waste through reuse, recycling, or upcycling [
15]. These methodologies involve modifying thermoplastic polymers, including polymers used during the melting stage, which requires high temperatures. However, these processes can produce undesirable changes in the properties of plastics due to various factors, including thermal and oxidative degradation, as well as microstructural changes [
16,
17].
Therefore, there is a growing interest in developing efficient techniques to modify thermoplastic materials by adding desired functionalities using low-content additives and avoiding detrimental reactions during their processing [
18,
19]. Such a technique relies on solid-state modification (SSM) of polymeric materials, which exploits the mobility of the amorphous phase within semi-crystalline polymers [
20]. This method involves using mechanical forces to drive through the incorporation of monomers or oligomers into the amorphous phase of the polymer through multiple exchange reactions [
21]. The temperature plays a critical role during this process as it needs to be above the Tg of the semi-crystalline polymer and close to its Tm. The exchange reactions mainly occur in the amorphous phase, which means that the main crystalline phase and its associated properties remain unaffected. This technique can be considered a post-synthetic modification method that allows for the preservation and/or modulation of desired properties.
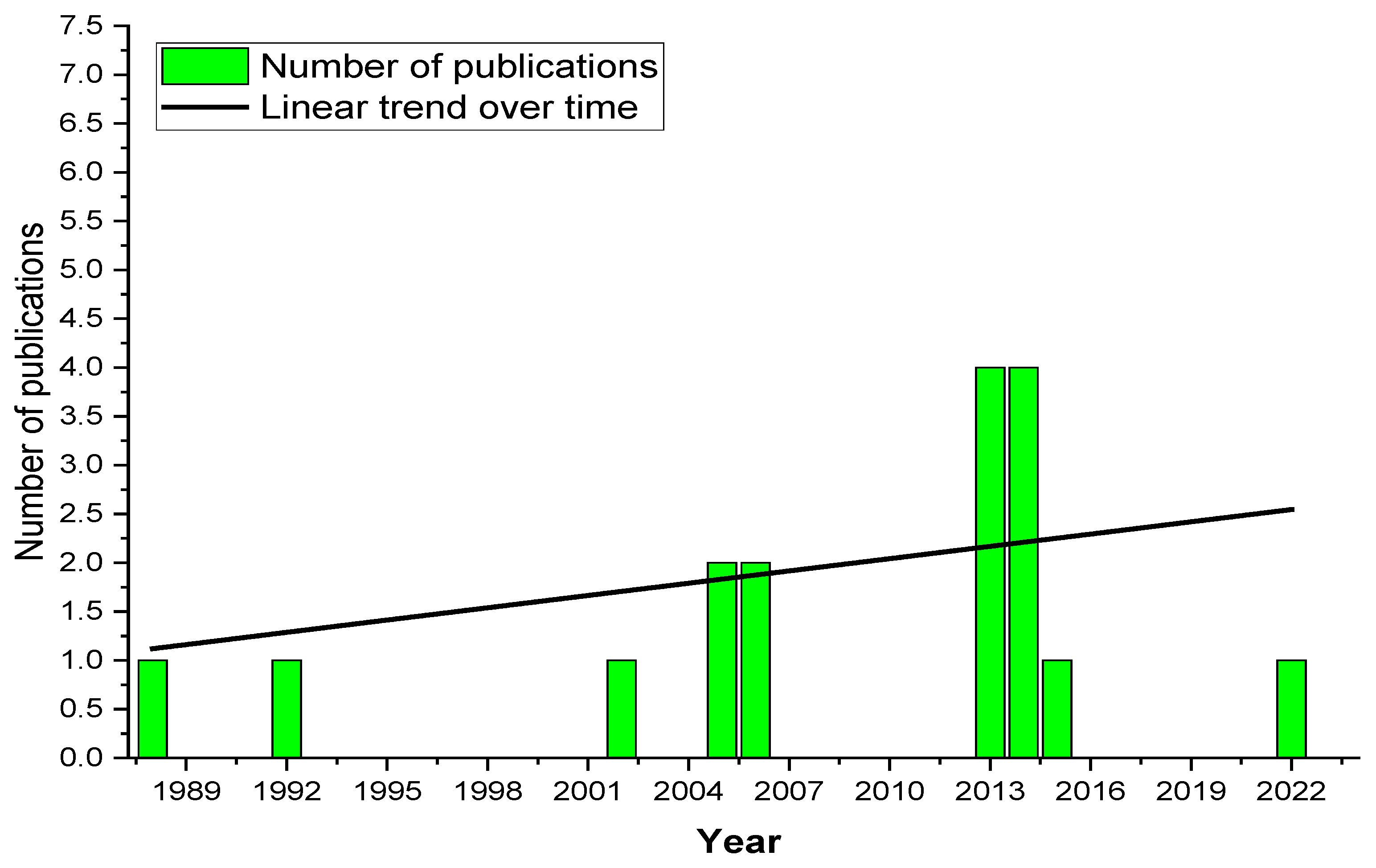
But what makes SSM unique, and why is it not widely known despite its apparent advantages? With only 17 publications on the topic over three decades, SSM remains a niche yet promising field. It holds the key to adding functionalities to thermoplastics with minimal additives and avoiding adverse reactions during processing. SSM is increasingly recognized for its potential to enhance material performance from catalysts to composites. These developments have covered some polymers, including thermoplastic and thermosets. The main purpose of these investigations was to modify inherent properties, particularly in the case of composites. Based on the SSM references histogram, the years of high publication activity belong to the period between 2012 and 2022. However, SSM has continued to investigate the topic, presenting an increasing number of articles as the years go by, especially after 2012. Additionally, SSM has expanded to different contemporary peaks research issues, such as catalysts and composites, often serving as a tool to investigate compositional effects and materials behavior, as well as to improve end-product performance.
In this respect, this review will focus on describing the current perspective of using pre-formed thermoplastic polymers with solid-state modification (SSM) methodologies to modify thermoplastic polymers with desired functionalities. By contrast with solid-state polymerization (SSP), which relies on a kind of bulk polymerization technique, SSM goes beyond by encompassing the concept of thermomechanochemistry. Thermomechanochemistry is a highly specialized field of chemistry that delves into the interaction between thermal (heat), mechanical (force or pressure), and chemical factors in material reactions and transformations [
22,
23]. This study area is particularly significant for understanding and designing materials that undergo chemical changes due to mechanical stress or thermal conditions. It combines the principles of thermodynamics, mechanical engineering, and chemistry to analyze and manipulate the behavior of materials. Finally, the aim is to provide the reader with information not only to understand the relevant solid-state modification technique but also to encourage future research, as we believe that SSM methodologies are a continuously thrilling field.
Figure 1.
Polymer solid-state modification research article numbers over time in Scopus from 1988 until 2023. Scopus search: (TITLE-ABS-KEY (solid-state AND modification AND SSM).
Figure 1.
Polymer solid-state modification research article numbers over time in Scopus from 1988 until 2023. Scopus search: (TITLE-ABS-KEY (solid-state AND modification AND SSM).
3. Thermo-Mechanochemistry: A Scientific Field that can be Focused on Solid-State Modification Through Mechanical Means
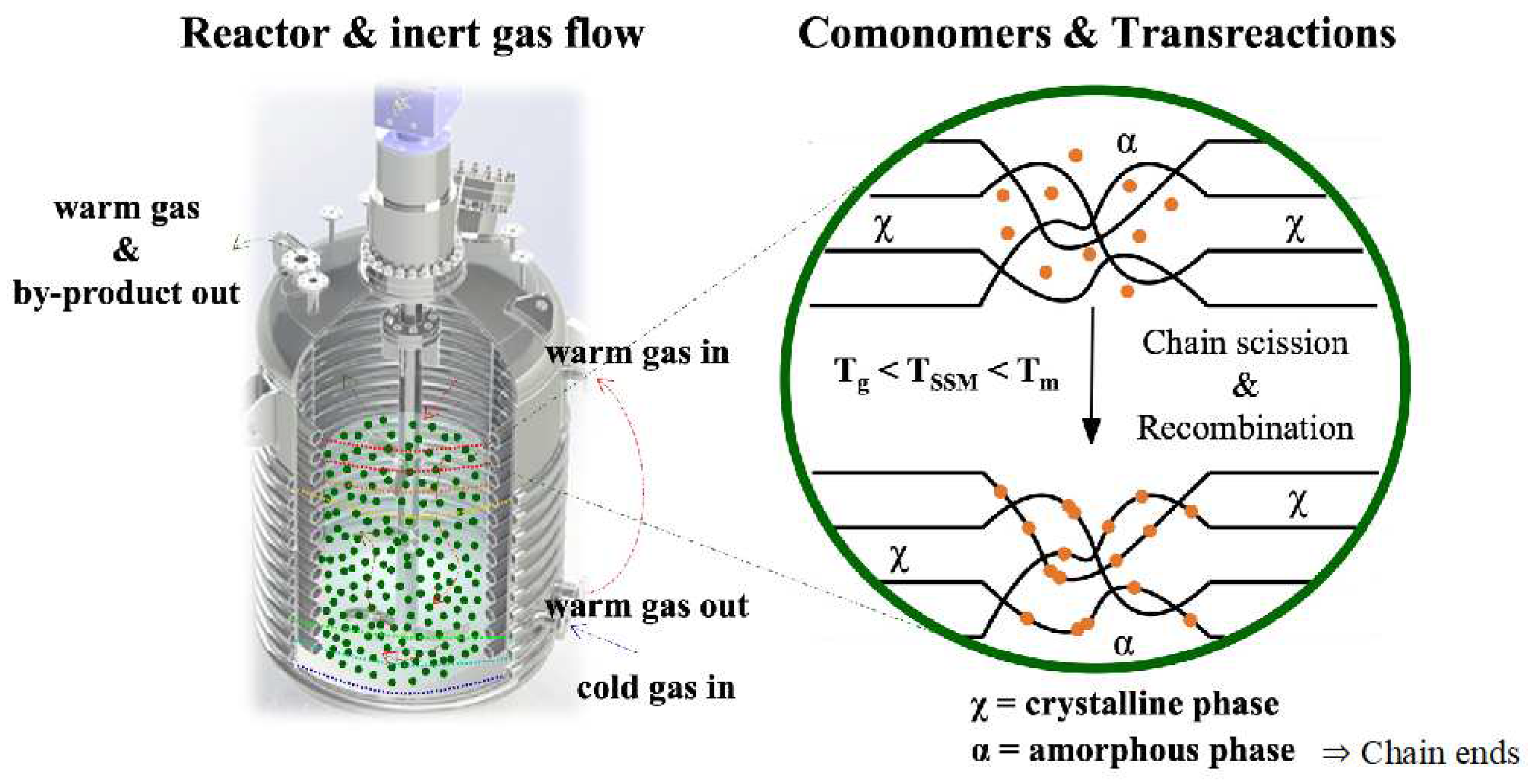
SSM is a technique that has not been widely explored in literature, despite being at the forefront of thermo-mechanochemistry. It involves modifying polymeric matrices after synthesis to extend their range of applications or improve a specific targeted property. This technique is related to solid-state polymerization (SSP), which increases the molecular weight of semi-crystalline polycondensates. SSP is commonly used in the production of glass fiber-reinforced PET grades. [
28]. This technique enables the formation of copolymers without requiring a high-temperature melt polycondensation reaction. This process demands precise control of reaction parameters and significant thermal stability. SSM involves heating the starting material in an inert atmosphere or vacuum to a temperature below its melting point. This temperature is high enough to initiate and propagate the exchange reaction in the presence of a comonomer. However, the material must be above its glass transition temperature to ensure enough mobility of the end groups in the amorphous phase. It is also essential to prevent the crystalline phase from participating in the reaction. The image of
Figure 2 illustrates this basic principle [
29].
The reaction mechanisms involved in SSM are similar to those in melt modification (
Figure 3) [
30]. However, a solid-state matrix is utilized as the reaction medium instead of a melt polymeric matrix. It is important to note that SSM has limitations on end-group diffusion due to restricted mobility. This is not an issue in melt or dissolution technology. These limitations become more severe over long reaction times when nearby functional groups already react. As a result, the concentration and distribution of these groups are reduced locally, making the migration of unreactive chain ends crucial for the reaction to continue. Removing condensate effectively through diffusion is crucial for achieving high SSM rates and extents. Although internal and surface diffusion are theoretically different, they are related and influenced by similar parameters. This is because both internal and surface diffusion eliminates concentration gradients of by-products in the reaction zone. This eliminates depolymerization and promotes a shift in the reaction equilibrium towards the desired products [
29].
In SSM processes, the removal of by-products is accomplished by either applying a vacuum or through the convection caused by an inert gas at atmospheric pressure. The oligomers that are formed during the reaction can sublimate into the gas phase along with the condensate. This process can also involve heating the mixture under a continuous flow of inert gas (open system), which promotes the removal of by-products. During SSM (Solid State Polymerization) processes, the elimination of by-products is achieved either by using vacuum or the convection created by an inert gas at atmospheric pressure. The oligomers that are produced during the reaction can vaporize into the gas phase alongside the condensate. The primary objectives of using an inert gas are to remove the condensate, prevent polymer oxidation by excluding oxygen from the reactor atmosphere, and to heat the reaction mixture. The most used inert gases include nitrogen, carbon dioxide, helium, superheated steam, and supercritical carbon dioxide. This process can also involve heating the mixture under a continuous flow of inert gas (open system), which helps to remove by-products.
4. Parameters that Affect the Solid-State Modification Technique
Solid-state modification (SSM) is a complex process, and several parameters can be controlled to attain the desired polymer material. These parameters include temperature, gas flow, vacuum applied, crystallinity, catalysts, and others. The factors that impact the SSM technique are listed below [
21]:
4.1. Temperature
The temperature at which the solid-state modification (SSM) process occurs is a crucial factor that impacts almost all other process stages [
29]. The reaction temperature mainly affects the reaction kinetics [
31]. Maintaining a temperature range optimal for maximizing the reaction rate while avoiding undesired reactions such as partial melting, sticking, or cyclization is crucial. However, if the monomer has a high melting temperature, the temperature range can be broader, and the impact of temperature on the process rate is minimized. It is not feasible to carry out SSM on polymers with a melting temperature that is too low [
32]. Raising the temperature of a process quickens its overall rate, the chemical reaction, and the movement of terminal functional groups and mass transport [
27,
29,
30]. However, the temperature shouldn't be too close to the melting temperature to avoid particle agglomeration. In summary, the reaction should be conducted above the glass transition temperature and between 10°C to 40°C below the melting temperature of the crystalline phase of the prepolymer to prevent particle agglomeration [
31]. [
31,
33,
34].
4.2. Atmosphere
SSM can be carried out using either a vacuum or an inert gas, such as nitrogen. The main objectives of these reaction conditions are to remove by-products, inhibit polymer oxidation by excluding oxygen from the reactor atmosphere, and heat the reaction mass (in the case of gas flow only). The difference between these two methods is that for vacuum, the rapid removal of by-products is affected by the applied pressure, while for gas flow, it depends on the flow rate of the inert gas used. In SSM processes, the commonly used inert gases include nitrogen, carbon dioxide, helium, and superheated steam [
35]. When performing the Solid-State Polymerization (SSM) process, it is important to take into account the characteristics of the inert gas being used, since they may have an impact on the process. SSM can be carried out in two different ways: either under a continuous flow of inert gas, which is referred to as an open system and is primarily focused on removing by-products, or under a stagnant inert gas atmosphere, which is known as a closed system and helps to limit the loss of monomers and oligomers [
36,
37].
4.3. Crystallinity
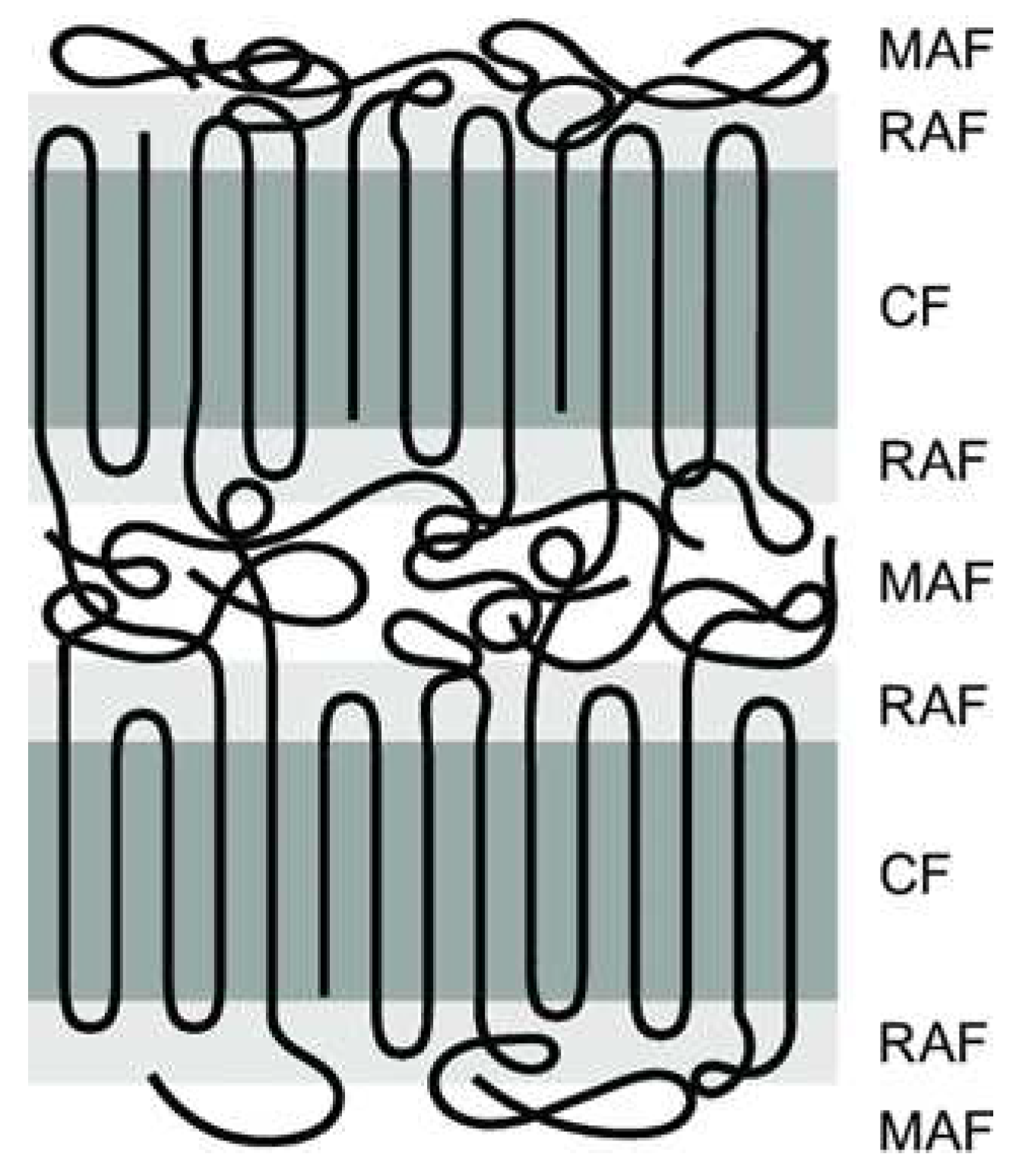
Crystallinity plays a crucial role in SSM as it impacts the movement of chain end groups and the diffusion of by-products. The effect of crystallinity on the rate of SSM is two-fold. Higher crystallinity levels result in an increased concentration of end groups released into the amorphous phase, which in turn leads to an acceleration of the reaction rate. [
28]. The polymer chains become less mobile as the reaction progresses due to increased crystallinity. This decrease in mobility slows down the rate of SSM. When reactions are limited by by-product diffusion, high crystallinity also reduces the rate of SSM. However, in processes controlled by chemical reactions, high crystallinity actually increases the rate of SSM due to the concentration of end groups in the amorphous phase [
38]. In order to prevent particles from sticking together, it is necessary for them to have a high level of crystallinity. the particle agglomeration, particles must have sufficiently high crystallinity [
39]. Furthermore, it has been shown for many years that semi-crystalline polymers can be described using a 3-phase model. This means that they contain a crystalline phase and an amorphous phase. However, the amorphous phase is divided into two zones: a mobile and a rigid amorphous zone, as shown in
Figure 3 [
40]. The exchange reactions should occur in the more mobile zones that can better tolerate changes [
41].
Figure 4.
Schematic representation of 3-phase model, i.e., mobile amorphous fraction (MAF), the rigid amorphous fraction (RAF) and the crystalline fraction (CF).
Figure 4.
Schematic representation of 3-phase model, i.e., mobile amorphous fraction (MAF), the rigid amorphous fraction (RAF) and the crystalline fraction (CF).
4.4. Catalysts
Catalysts are crucial for the rate of polymerization and modification reactions. Their role extends beyond simply accelerating reactions. They also contribute to the quality and properties of the final polymer product by influencing the chain initiation and growth, as well as the interactions among the polymer constituents. Additionally, catalysts can lead to complete conversions of specific groups, significantly impacting the polymerization rate, as observed with phase-transfer catalysts [
42]. This ability to control reaction rates is crucial, especially in processes where removing by-products like water is necessary for the desired reaction outcomes [
20]. The incorporation of catalysts into the polymerization process can be done at different stages. They can be added during the production of the prepolymer or while melting the prepolymer, providing flexibility in controlling the polymerization process. This versatility allows for a tailored approach to achieve specific properties and characteristics in the final thermoplastic polymer product [
36].
5. Thermoplastic Polymers Modified Through Solid-State Modification Technique
Solid-state modification (SSM) of post-synthetic thermoplastic polymers is a rapidly evolving technique with numerous advantages and potential applications. This approach is particularly attractive because it allows adding new functionalities to existing polymer matrices, thereby extending their utility or assigning new purposes [
43]. One of the main benefits of SSM is its ability to overcome solubility issues often encountered with certain polymers. Additionally, it offers a more environmentally friendly alternative to traditional methods, featuring greater selectivity and the potential for scalability [
44]. SSM in thermoplastic polymers is cost-effective and allows for more significant creative variability and reduced degradation reactions compared to solutions or melts. It has been applied effectively to reprocess and recycle condensation plastics, enhancing their properties without needing solid-state re-polymerization. This makes high-end recycling economically attractive, especially for high-value-added engineering applications like manufacturing higher-performance fibers and films with high tensile properties [
45]. SSM is especially beneficial due to its compatibility with a wider range of monomers, including those that are fragile or have a low degradation temperature, thanks to the milder conditions it employs, particularly in terms of temperature control. This expands the scope of modifications that can be made to thermoplastic polymers. Several research groups are exploring various modifications of polymers using SSM techniques. This includes mechanochemistry, which allows for the physical blending and alteration of polymer structures without the need for solvents or high temperatures, as typically required in other polymer processing methods. However, it is important to note that, to date, only a few thermoplastic polymers have been effectively modified using these techniques.
5.1. Polypropylene
The study conducted by Qiu et al. in 2004 on grafting maleic anhydride onto polypropylene presented several significant benefits. This process, known for its efficiency and eco-friendliness, particularly in the absence of solvents, has been shown to enhance the properties of polypropylene in various ways [
46]. While the exact study by Qiu et al. (2004) is not directly cited in the available literature, similar studies provide insights into the benefits of this methodology. Among its advantages it is possible to highlight: (1) the improved mechanical properties that includes better mixing in blends and composites with other polymers and fillers, as well as improved impact resistance and low-temperature brittleness in blends [
47]. (2) the process is energy-efficient, particularly important in industrial applications where cost and energy consumption are critical factors. On the other hand, the process to carry out the modification is quite complex due to achieving the optimal degree of grafting requires careful control of various parameters, which can be complex and challenging. Additionally, this methodology requires specialized equipment since the process may require specific equipment for effective grafting, potentially increasing the investment and operational costs. Finally, maintaining consistent quality in the grafting process can be challenging, especially at a larger industrial scale.
Jain at all, (2005) employed SSM for the preparation of polypropylene/silica nanocomposites with varying degrees of adhesion between the filler and matrix [
48]. The methodology combines solid-state modification with a sol-gel method to create nanocomposites with varying degrees of adhesion between the filler (silica) and the matrix (polypropylene). Interestingly, the use of solid-state modification and sol-gel reactions can enhance the mechanical and thermal properties of the resulting polypropylene-silica nanocomposites, moreover, the methodology allows for the adjustment of the degree of adhesion between the silica filler and the polypropylene matrix, enabling customization of the composite properties. However, the combination of solid-state modification and sol-gel methods can be more complex and challenging to optimize compared to more straightforward composite fabrication methods. Additionally, the complexity and potential scalability issues need to be considered when applying this method on an industrial scale.
Finally, Both reported methodologies involve combining mechanochemistry with external energy sources, which is critical for controlling local heating and enabling chemical reactions that overcome activation energy barriers unsuitable for conventional mechanochemical syntheses [
49].
5.2. Poly(butylene terephthalate) (PBT)
PBT has been effectively modified using solid-state modification, a user-friendly technique that has led to various changes in its properties. In the field of SSM, this is he thermoplastic polymer that has been widely reported [
15,
50,
51,
52,
53,
54]. The research groups of C. Koning and S. Muñoz-Guerra show only examples involving poly(butylene terephthalate) (PBT) and biobased monomers, e.g., sugar or fatty acid derivatives [
50,
51,
54]. These studies are focused on the microstructure of the copolyesters obtained and their morphology, emphasizing the influence of structures and compositions on thermal properties and crystallinity [
54]. C. Lavilla also studied the comparison of copolymers obtained in SSM with those obtained in conventional polycondensation and showed that these methods appeared to be equivalent [
53]. Although these modifications are typically carried out using batch processes on a small scale, there is great potential for continuous processes to be used for this purpose, such as reactive extrusion, as shown by several recent studies on mechanochemistry [
55,
56].
Recently, Gerbehaye et al. has advanced this field by designing a new process that transitions polyester SSM from batch to continuous, using PBT and 1,12-dodecanediol (DDO) as model compounds [
15]. Initially, a calorimetric method was developed to investigate the key characteristics of the reaction on a small scale. This helped with the selection of the appropriate catalyst using the differential scanning calorimetry (DSC) technique. In the next step, a qualitative kinetic discussion was conducted to confirm the results obtained from the calorimetric study. The study also shed light on the influence of the reaction time on the molecular and thermal features of the copolymers produced. After optimizing the reaction conditions, they were transferred to a gram-scale batch reactor. Finally, a continuous REx process was tested to minimize the reaction time and assess the shear forces in the SSM framework. Furthermore, REx makes this type of mechanical reaction more industrially viable [
55,
56]. The primary challenge of this work is rebuilding polymer chains, particularly in terms of molecular weight construction. This was evident since higher times in REx, visual inspection of the material indicated more significant degradation. Therefore, one limitation of this methodology is that longer extrusion times result in more significant molecular weight loss. Initially, DDO acts as a plasticizer aiding the exchange reaction, but prolonged exposure leads to melting and potential loss of crystallinity [
15]. REx of polyester with a diol monomer seems feasible, but improved chain building might require an additional vacuum step. Traditional melt polycondensation involves transesterification to form oligomers, followed by polycondensation under high vacuum to remove by-products. Applying a similar vacuum step to SSM might enhance the process. This study presented a new process design for recycling polymeric materials, which could make polymers more sustainable and industrial scalable.
5.3. Polyamides
In this kind of thermoplastic polymers, two studies in the field of polymer science focused on modifying polyamides through solid-state methods [
57,
58]. Jeyakumar et al. studied the modification of polyamide-6,6 (PA-6,6) to prepare a comonomer by using p-Xylylenediaminepara- and m-Xylylenediamine [
57]. This process was conducted under an inert atmosphere and initially resulted in a decrease in molecular weight due to chain scission. However, the molecular weight increases again with prolonged treatment as the comonomer is incorporated through postcondensation. Additionally, the solid-state modified copolyamides exhibit higher melting and crystallization temperatures than their melt-synthesized counterparts, indicating a non-random, block-like chemical microstructure. On the other hand, the second study investigates the structural and conformational differences in polyamide 6 (PA6) when modified with Dytek A (a semi-aromatic nylon salt of 1,5-diamino-2-methylpentane) and isophthalic acid (IPA) [
58]. The results showed no co-crystallization between the Dytek A-isophthalic acid salt and PA6 repeat units. Increasing amounts of the DyI salt in the polymer led to a steady decrease in crystallinity. Also, a transformation from trans to gauche conformers in the polymer chain was observed upon heating.
These studies demonstrate the intricate control of polymer properties through solid-state modification techniques. The non-random structures in the first study and the altered crystallinity in the second highlight how subtle changes in polymer processing can significantly impact the resulting material's properties. However, the scalability of these approaches is limited because they were performed in a glass tube reactor with a batch process.
6. Outlooks, and Perspective in the Future
Versatility and Customization through SSM: SSM is recognized for its adaptability in modifying thermoplastic polymers. This flexibility is crucial for creating custom-designed materials suitable for various applications, such as high-performance fibers, films, and advanced composites. The future of SSM lies in expanding the range of functionalities that can be incorporated into these polymers, enhancing material properties like strength, flexibility, and heat resistance.
6.1. Eco-Friendly and Cost-Effective
SSM's eco-friendly and cost-effective nature makes it a valuable method, particularly for high-end recycling and upcycling processes. Future developments may include further reducing environmental impacts and optimizing the process for large-scale industrial applications, focusing on energy-efficient recycling processes.
6.2. Process Simplification and Scalability
Although SSM processes can be complex and require meticulous control of parameters, ongoing research aims to simplify these processes. Innovations may involve combining mechanochemistry with external energy sources to overcome activation energy barriers, thereby making SSM more accessible and scalable.
6.3. Polymer-Specific Innovations
SSM has been used to modify various polymers such as polypropylene, poly(butylene terephthalate) (PBT), and polyamides. Future innovations could include more efficient grafting techniques for polypropylene, optimizing continuous processes in PBT, and scaling up modification processes for polyamides like PA-6,6 and PA6.
7. Conclusions
In terms of potential for innovation and progress, the solid-state modification of thermoplastic polymers holds significant potential for both innovation and progress. This includes environmental benefits, creating tailored materials, and overcoming process optimization and scalability challenges.
Regarding scaling up for industrial applications, this is one of the primary challenges for the future is scaling up SSM processes for industrial use. This will require advancements in technology, considerations of economic viability, and maintaining quality control at a larger scale.
Finaly, in advancements in recycling and circular economy, the potential of SSM in recycling and enhancing the properties of recycled polymers presents an exciting area for future research. Developing more efficient recycling methods using SSM could contribute significantly to a circular economy in the polymer industry.
Author Contributions
Writing—original draft preparation, J.P., C.G., J.R. and R.M. Funding acquisition, J.R. and R.M., J.P., C.G., J.R. and R.M. contributed equally to this review. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This research was funded by Walloon Excellence in Technology, grant CONSOLID.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We gratefully acknowledge the support from the Wallonia and the European Commission “FSE and FEDER” for the research FEDER program entitled “UP_PLASTIC. J.-M.R. is a senior research associate at F.R.S.-F.N.R.S. (Belgium) and under the WET-T research program entitled “CONSOLID”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Science Advances 2017, 3, 25–29. [Google Scholar] [CrossRef]
- Zabihzadeh Khajavi, M.; Mohammadi, R.; Ahmadi, S.; Farhoodi, M.; Yousefi, M. Strategies for Controlling Release of Plastic Compounds into Foodstuffs Based on Application of Nanoparticles and Its Potential Health Issues. Trends in Food Science & Technology 2019, 90, 1–12. [Google Scholar] [CrossRef]
- Luo, H.; Liu, C.; He, D.; Sun, J.; Li, J.; Pan, X. Effects of Aging on Environmental Behavior of Plastic Additives: Migration, Leaching, and Ecotoxicity. Science of The Total Environment 2022, 849, 157951. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liao, S.; Li, Q.; Guan, Q.; Jia, P.; Zhou, Y. Physical and Chemical Modifications of Poly(Vinyl Chloride) Materials to Prevent Plasticizer Migration—Still on the Run. Reactive and Functional Polymers 2020, 147, 104458. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Srinivas, S.; Narain, R. Modification of Polymers. In Polymer Science and Nanotechnology: Fundamentals and Applications; Elsevier Inc., 2020; pp. 95–104. ISBN 9780128168066.
- Pillay, V.; Seedat, A.; Choonara, Y.E.; Du Toit, L.C.; Kumar, P.; Ndesendo, V.M.K. A Review of Polymeric Refabrication Techniques to Modify Polymer Properties for Biomedical and Drug Delivery Applications. AAPS PharmSciTech 2013, 14, 692–711. [Google Scholar] [CrossRef]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface Modification of Polymers: Methods and Applications. Advanced Materials Interfaces 2018, 5, 1–26. [Google Scholar] [CrossRef]
- Meer, M.; Mohd, S.; Itam, Z.; Beddu, S.; Zahari, N.M.; Liyana, N.; Kamal, M.; Mohamad, D.; Zulkepli, N.A.; Shafiq, M.D.; et al. Flame Retardant Coatings : Additives, Binders, and Fillers. Polymers 2022, 14, 2911. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; Saal, F.S.V.; Swan, S.H. Plastics, the Environment and Human Health: Current Consensus and Future Trends. Philosophical Transactions of the Royal Society B: Biological Sciences 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, K.; Sablani, S.S.; Tang, J.; Rasco, B. Migration of Chemical Compounds from Packaging Polymers during Microwave, Conventional Heat Treatment, and Storage. Comprehensive Reviews in Food Science and Food Safety 2013, 12, 523–545. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. Journal of Hazardous Materials 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Kemmlein, S.; Herzke, D.; Law, R.J. Brominated Flame Retardants in the European Chemicals Policy of REACH-Regulation and Determination in Materials. Journal of Chromatography A 2009, 1216, 320–333. [Google Scholar] [CrossRef] [PubMed]
- European Commission Laying down Ecodesign Requirements for Electronic Displays Pursuant to Directive 2009/125/EC of the European Parliament and of the Council, Amending Commission Regulation (EC) No 1275/2008 and Repealing Commission Regulation (EC) No642/2009. Official Journal of the European Union 2019, 241–266.
- Shea, K.M. Pediatric Exposure and Potential Toxicity of Phthalate Plasticizers. Pediatrics 2003, 111, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Gerbehaye, C.; Bernaerts, K.V.; Mincheva, R.; Raquez, J.-M. Solid-State Modification of Poly(Butylene Terephthalate): Design of Process from Calorimetric Methods for Catalyst Investigation to Reactive Extrusion. European Polymer Journal 2022, 166, 111010. [Google Scholar] [CrossRef]
- Mohammed, M.; Jawad, A.J.M.; Mohammed, A.M.; Oleiwi, J.K.; Adam, T.; Osman, A.F.; Dahham, O.S.; Betar, B.O.; Gopinath, S.C.B.; Jaafar, M. Challenges and Advancement in Water Absorption of Natural Fiber-Reinforced Polymer Composites. Polymer Testing 2023, 124, 108083. [Google Scholar] [CrossRef]
- Boey, J.Y.; Lee, C.K.; Tay, G.S. Factors Affecting Mechanical Properties of Reinforced Bioplastics: A Review. Polymers 2022, 14, 3737. [Google Scholar] [CrossRef]
- Zhao, H.B.; Wang, Y.Z. Design and Synthesis of PET-Based Copolyesters with Flame-Retardant and Antidripping Performance. Macromolecular Rapid Communications 2017, 38, 1–14. [Google Scholar] [CrossRef]
- Mincheva, R.; Guemiza, H.; Hidan, C.; Moins, S.; Coulembier, O.; Dubois, P.; Laoutid, F. Development of Inherently Flame-Retardant Phosphorylated Pla by Combination of Ring-Opening Polymerization and Reactive Extrusion. Materials 2020, 13, 1–13. [Google Scholar] [CrossRef]
- Papaspyrides, C.D.; Vouyiouka, S.N. Fundamentals of Solid State Polymerization. In Solid State Polymerization; John Wiley & Sons, Ltd., 2009; pp. 1–37. ISBN 9780470451830.
- Vouyiouka, S.N.; Karakatsani, E.K.; Papaspyrides, C.D. Solid State Polymerization. Progress in Polymer Science 2005, 30, 10–37. [Google Scholar] [CrossRef]
- Willocq, B.; Odent, J.; Dubois, P.; Raquez, J.-M. Advances in Intrinsic Self-Healing Polyurethanes and Related Composites. RSC Adv. 2020, 10, 13766–13782. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Wu, F.; Mincheva, R.; Hakkarainen, M.; Raquez, J.-M.; Mielewski, D.F.; Narayan, R.; Netravali, A.N.; Misra, M. Sustainable Polymers. Nat Rev Methods Primers 2022, 2, 46. [Google Scholar] [CrossRef]
- Howard, J.L.; Cao, Q.; Browne, D.L. Mechanochemistry as an Emerging Tool for Molecular Synthesis: What Can It Offer? Chemical Science 2018, 9, 3080–3094. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Hernández, J.G. Sustainability Assessment of Mechanochemistry by Using the Twelve Principles of Green Chemistry. ChemSusChem 2021, 14, 2145–2162. [Google Scholar] [CrossRef]
- Colacino, E.; Delogu, F.; Hanusa, T. Advances in Mechanochemistry. ACS Sustainable Chemistry and Engineering 2021, 9, 10662–10663. [Google Scholar] [CrossRef]
- Martinez, V.; Stolar, T.; Karadeniz, B.; Brekalo, I.; Užarević, K. Advancing Mechanochemical Synthesis by Combining Milling with Different Energy Sources. Nature Reviews Chemistry 2023, 7, 51–65. [Google Scholar] [CrossRef]
- Chang, S.; Sheu, M. -F; Chen, S. -M Solid-state Polymerization of Poly(Ethylene Terephthalate). Journal of Applied Polymer Science 1983, 28, 3289–3300. [Google Scholar] [CrossRef]
- Papaspyrides, C.D.; Vouyiouka, S.N. Fundamentals of Solid State Polymerization. In Solid State Polymerization; John Wiley & Sons, Inc, 2009; pp. 1–37. ISBN 9783540741824.
- Vouyiouka, S.N.; Karakatsani, E.K.; Papaspyrides, C.D. Solid State Polymerization. Progress in Polymer Science 2005, 30, 10–37. [Google Scholar] [CrossRef]
- Yoon, K.H.; Kwon, K.H.; Jeon, M.H.; Park, O.O. Diffusion of Ethylene Glycol in Solid State Poly(Ethylene Terephthalate). Polymer Journal 1993, 25, 219–226. [Google Scholar] [CrossRef]
- Bamford, C.H.; Wayne, R.P. Polymerization in the Solid Phase : A Polycondensation Reaction. Polymer 1969, 10, 661–681. [Google Scholar] [CrossRef]
- Chang, S.; Sheu, M.-F.; Chen, S.-M. Solid-State Polymerization of Poly(Ethylene Terephthalate). Journal of Applied Polymer Science 1983, 28, 3289–3300. [Google Scholar] [CrossRef]
- Wang, X.Q.; Deng, D.C. A Comprehensive Model for Solid-State Polycondensation of Poly(Ethylene Terephthalate): Combining Kinetics with Crystallization and Diffusion of Acetaldehyde. Journal of Applied Polymer Science 2002, 83, 3133–3144. [Google Scholar] [CrossRef]
- Culbert, B.; Christel, A. Continuous Solid-State Polycondensation of Polyesters. In Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Scheirs, J., Long, T.E., Eds.; John Wiley & Sons, Ltd., 2003; pp. 143–194. ISBN 9780470090688.
- Steinborn-Rogulska, I.; Rokicki, G. Solid-State Polycondensation (SSP) as a Method to Obtain High Molecular Weight Polymers: Part II. Synthesis of Polylactide and Polyglycolide via SSP. Polimery 2013, 58, 85–92. [Google Scholar] [CrossRef]
- Ma, Y.; Agarwal, U.S.; Sikkema, D.J.; Lemstra, P.J. Solid-State Polymerization of PET: Influence of Nitrogen Sweep and High Vacuum. Polymer 2003, 44, 4085–4096. [Google Scholar] [CrossRef]
- Gantillon, B.; Spitz, R.; McKenna, T.F. The Solid State Postcondensation of PET, 1: A Review of the Physical and Chemical Processes Taking Place in the Solid State. Macromolecular Materials and Engineering 2004, 289, 88–105. [Google Scholar] [CrossRef]
- Yao, Z.; Harmon Ray, W. Modelling and Analysis of New Processes for Polyester and Nylon Production. AIChE Journal 2001, 47, 401–412. [Google Scholar] [CrossRef]
- Heidrich, D.; Gehde, M. The 3-Phase Structure of Polyesters (PBT, PET) after Isothermal and Non-Isothermal Crystallization. Polymers 2022, 14. [Google Scholar] [CrossRef]
- Ma, Y.; Agarwal, U.S.; Sikkema, D.J.; Lemstra, P.J. Solid-State Polymerization of PET: Influence of Nitrogen Sweep and High Vacuum. Polymer 2003, 44, 4085–4096. [Google Scholar] [CrossRef]
- N’Guyen, T.D.; Deffieux, A.; Boileau, S. Phase-Transfer Catalysis in the Chemical Modification of Polymers: 1. Polymer 1978, 19, 423–426. [Google Scholar] [CrossRef]
- Gauthier, M.A.; Gibson, M.I.; Klok, H.A. Synthesis of Functional Polymers by Post-Polymerization Modification. Angewandte Chemie - International Edition 2009, 48, 48–58. [Google Scholar] [CrossRef]
- Rätzsch, M.; Bucka, H.; Hesse, A.; Arnold, M. Basis of Solid-Phase Grafting of Polypropylene. Journal of Macromolecular Science, Part A 1996, 33, 913–926. [Google Scholar] [CrossRef]
- Villalobos, M.; Awojulu, A.; Greeley, T.; Turco, G.; Deeter, G. Oligomeric Chain Extenders for Economic Reprocessing and Recycling of Condensation Plastics. Energy 2006, 31, 3227–3234. [Google Scholar] [CrossRef]
- Qiu, W.; Hirotsu, T. A New Method to Prepare Maleic Anhydride Grafted Poly(Propylene). Macromolecular Chemistry and Physics 2005, 206, 2470–2482. [Google Scholar] [CrossRef]
- Hudec, I.; Sain, M.M.; Šúňová, V. Improved Interaction Effected by Maleic Anhydride-grafted Polypropylene in PRP Triblock Polymer–EVA Blend. J of Applied Polymer Sci 1993, 49, 425–433. [Google Scholar] [CrossRef]
- Jain, S.; Goossens, H.; Picchioni, F.; Magusin, P.; Mezari, B.; Van Duin, M. Synthetic Aspects and Characterization of Polypropylene–Silica Nanocomposites Prepared via Solid-State Modification and Sol–Gel Reactions. Polymer 2005, 46, 6666–6681. [Google Scholar] [CrossRef]
- G.A Bowmaker Solvent-Assisted Mechanochemistry. Chemical Communications 2013, 334–348.
- Gubbels, E.; Jasinska-Walc, L.; Merino, D.H.; Goossens, H.; Koning, C. Solid-State Modification of Poly(Butylene Terephthalate) with a Bio-Based Fatty Acid Dimer Diol Furnishing Copolyesters with Unique Morphologies. Macromolecules 2013, 46, 3975–3984. [Google Scholar] [CrossRef]
- Gubbels, E.; Jasinska-Walc, L.; Hermida-Merino, D.; Hansen, M.R.; Noordover, B.; Spoelstra, A.; Goossens, H.; Koning, C. Phase Separation in Poly(Butylene Terephthalate)-Based Materials Prepared by Solid-State Modification. Polymer 2014, 55, 3801–3810. [Google Scholar] [CrossRef]
- Gubbels, E.; Lavilla, C.; De Ilarduya, A.M.; Noordover, B.A.J.; Koning, C.E.; Muñoz-Guerra, S. Partially Renewable Copolyesters Prepared from Acetalized d -glucitol by Solid-state Modification of Poly(Butylene Terephthalate). J. Polym. Sci. Part A: Polym. Chem. 2014, 52, 164–177. [Google Scholar] [CrossRef]
- Lavilla, C.; Gubbels, E.; Alla, A.; Martínez De Ilarduya, A.; Noordover, B.A.J.; Koning, C.E.; Muñoz-Guerra, S. Carbohydrate-Based PBT Copolyesters from a Cyclic Diol Derived from Naturally Occurring Tartaric Acid: A Comparative Study Regarding Melt Polycondensation and Solid-State Modification. Green Chem. 2014, 16, 1789–1798. [Google Scholar] [CrossRef]
- Lavilla, C.; Gubbels, E.; Martínez De Ilarduya, A.; Noordover, B.A.J.; Koning, C.E.; Muñoz-Guerra, S. Solid-State Modification of PBT with Cyclic Acetalized Galactitol and d -Mannitol: Influence of Composition and Chemical Microstructure on Thermal Properties. Macromolecules 2013, 46, 4335–4345. [Google Scholar] [CrossRef]
- Bolt, R.R.A.; Leitch, J.A.; Jones, A.C.; Nicholson, W.I.; Browne, D.L. Continuous Flow Mechanochemistry: Reactive Extrusion as an Enabling Technology in Organic Synthesis. Chemical Society Reviews 2022, 51, 4243–4260. [Google Scholar] [CrossRef]
- Gaudino, E.C.; Grillo, G.; Manzoli, M.; Tabasso, S.; Maccagnan, S.; Cravotto, G. Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Jeyakumar, A.; Goossens, H.; Noordover, B.; Prusty, M.; Scheibitz, M.; Koning, C. Polyamide-6,6-based Blocky Copolyamides Obtained by Solid-state Modification. J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 5118–5129. [Google Scholar] [CrossRef]
- Cakir, S.; Jasinska-Walc, L.; Villani, M.; Hansen, M.R.; Koning, C.E. Morphology and Local Chain Structure of Polyamide 6 Modified in the Solid State with a Semi-Aromatic Nylon Salt. Materials Today Communications 2015, 2, e62–e69. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).