Submitted:
03 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
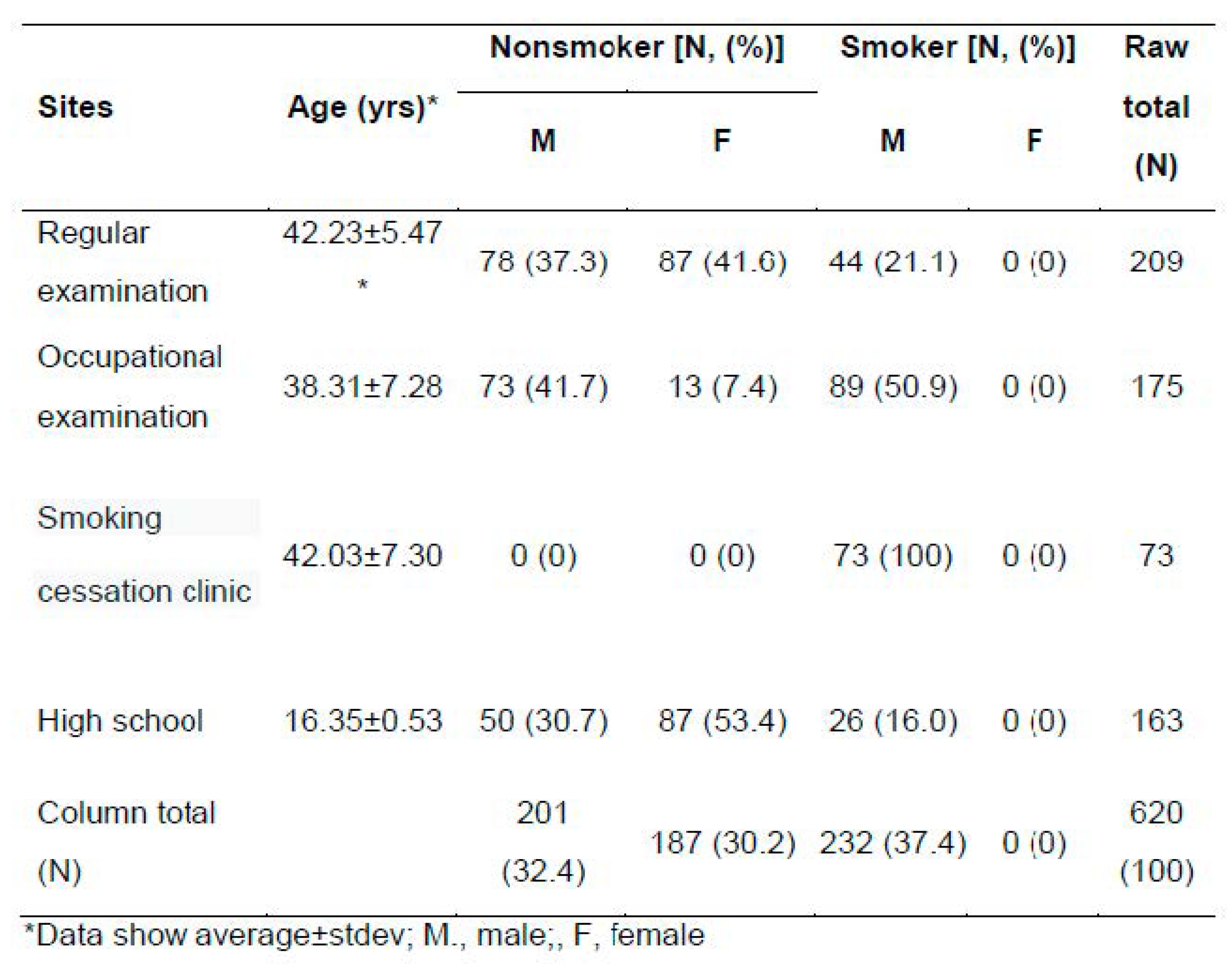
2.1. Characteristics of Subjects
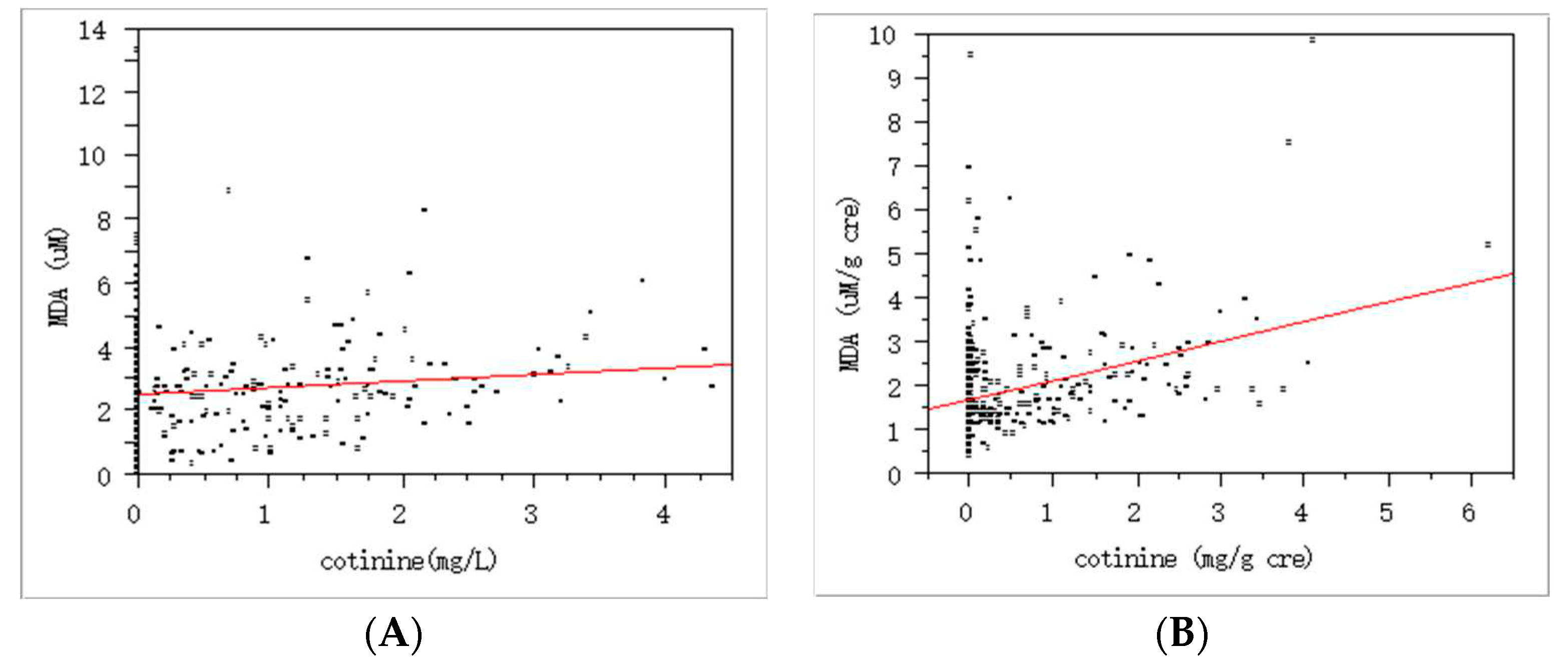
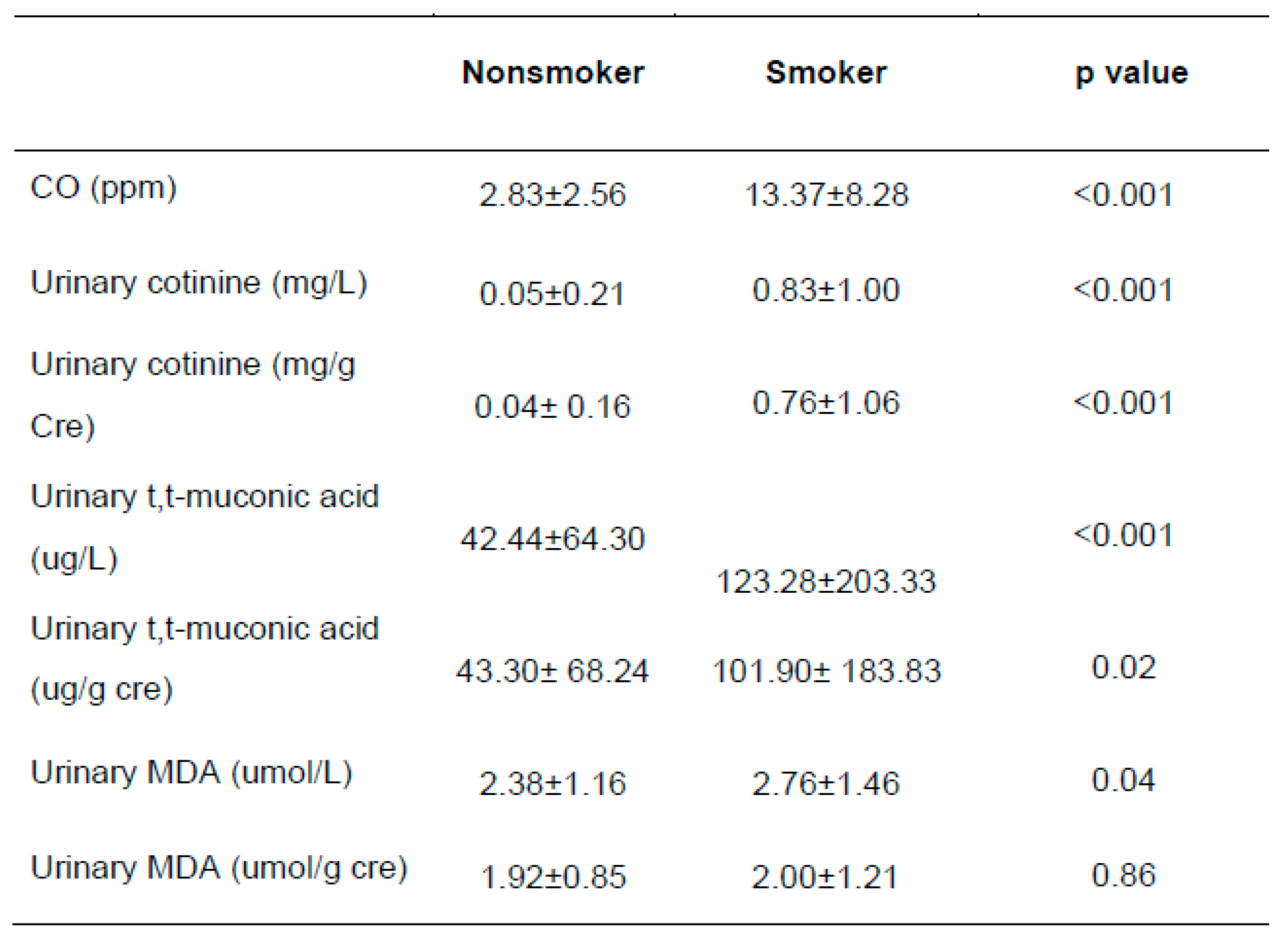
2.2. Exposure Levels of Tobacco Smoking
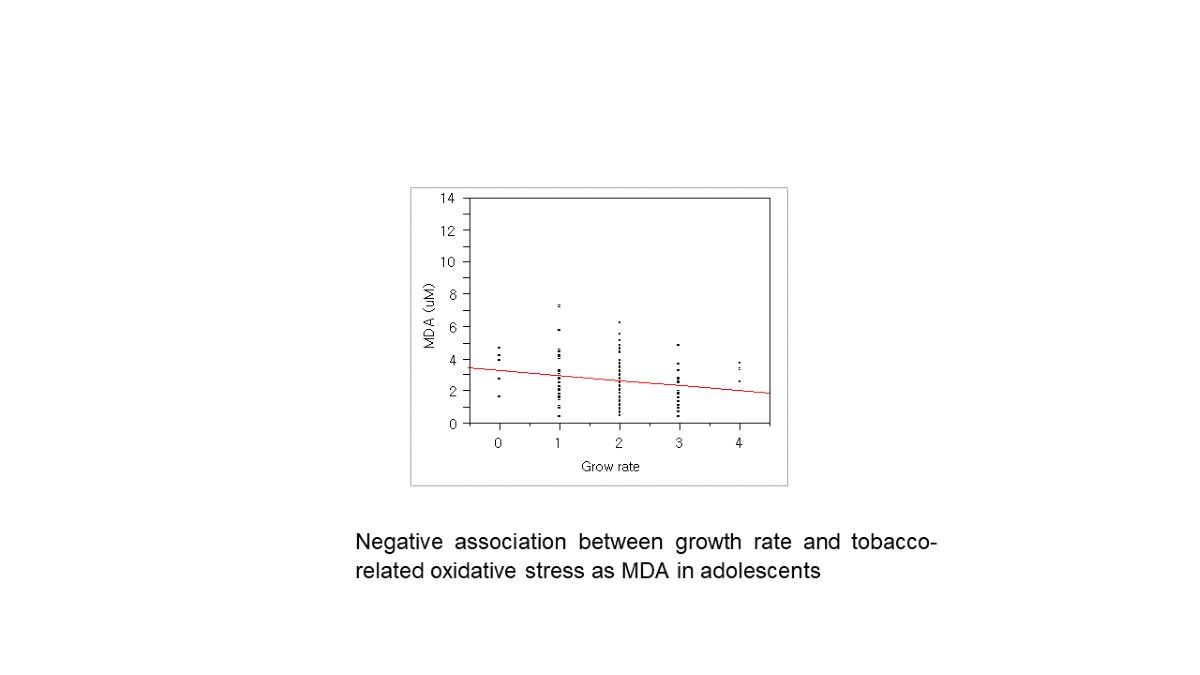
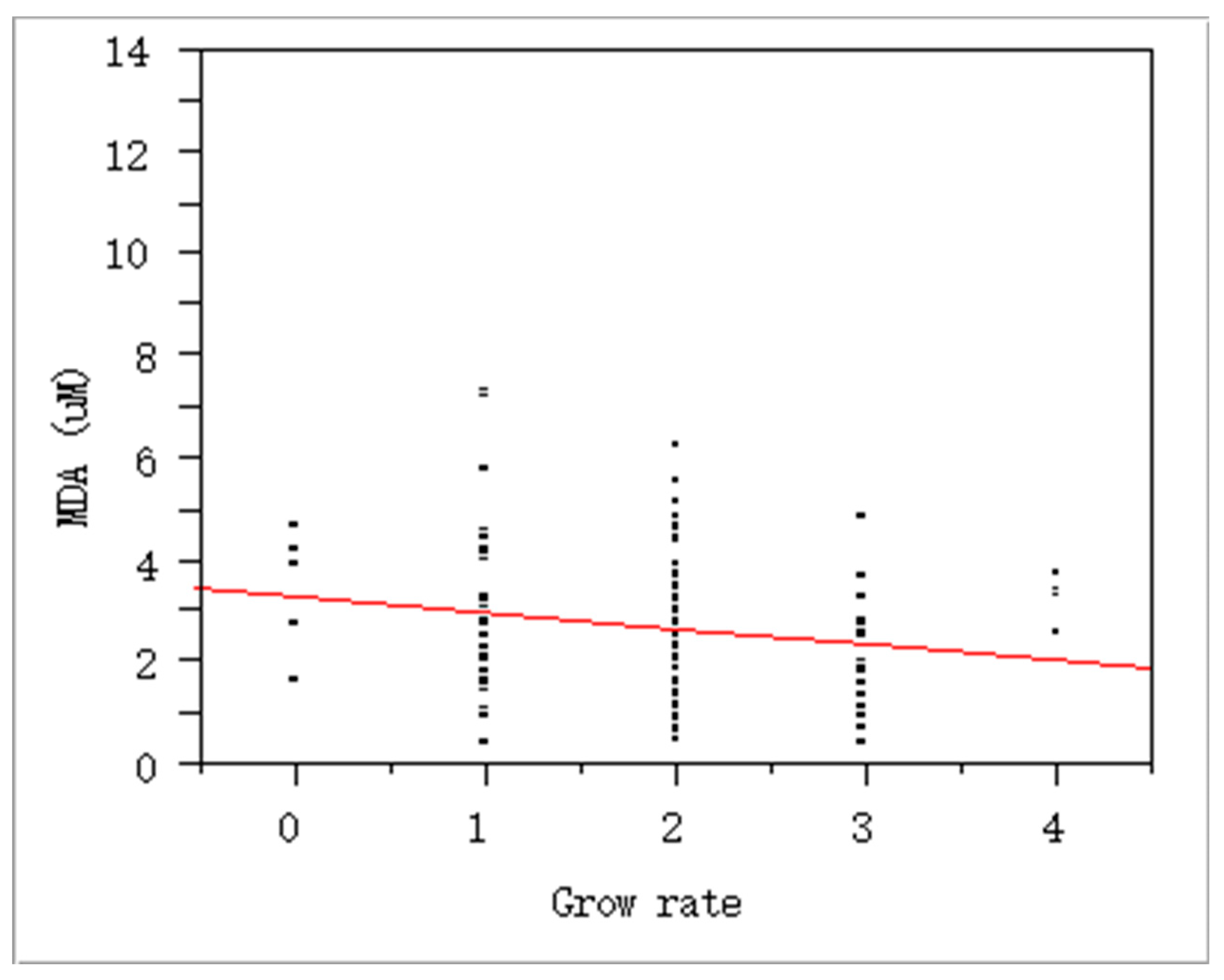
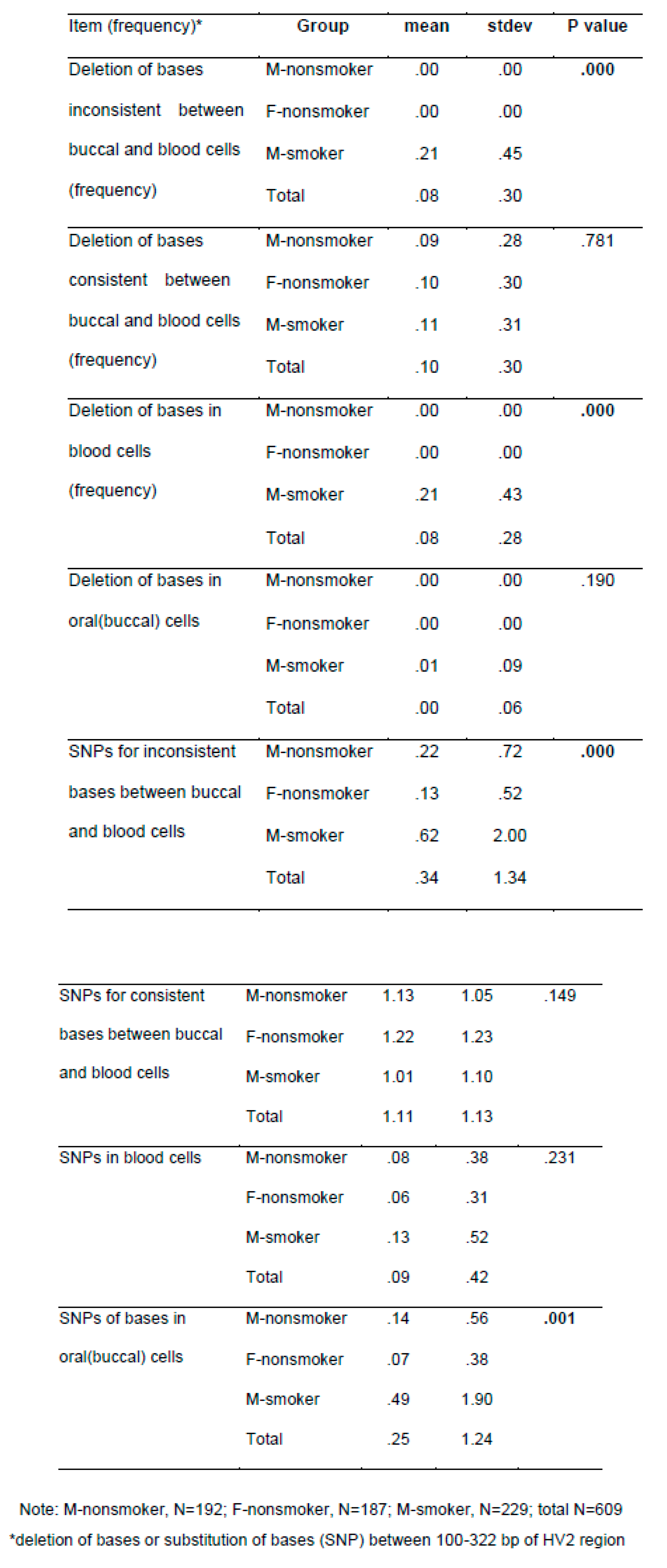
2.3. mtDNA Alteration by Tobacco Smoking
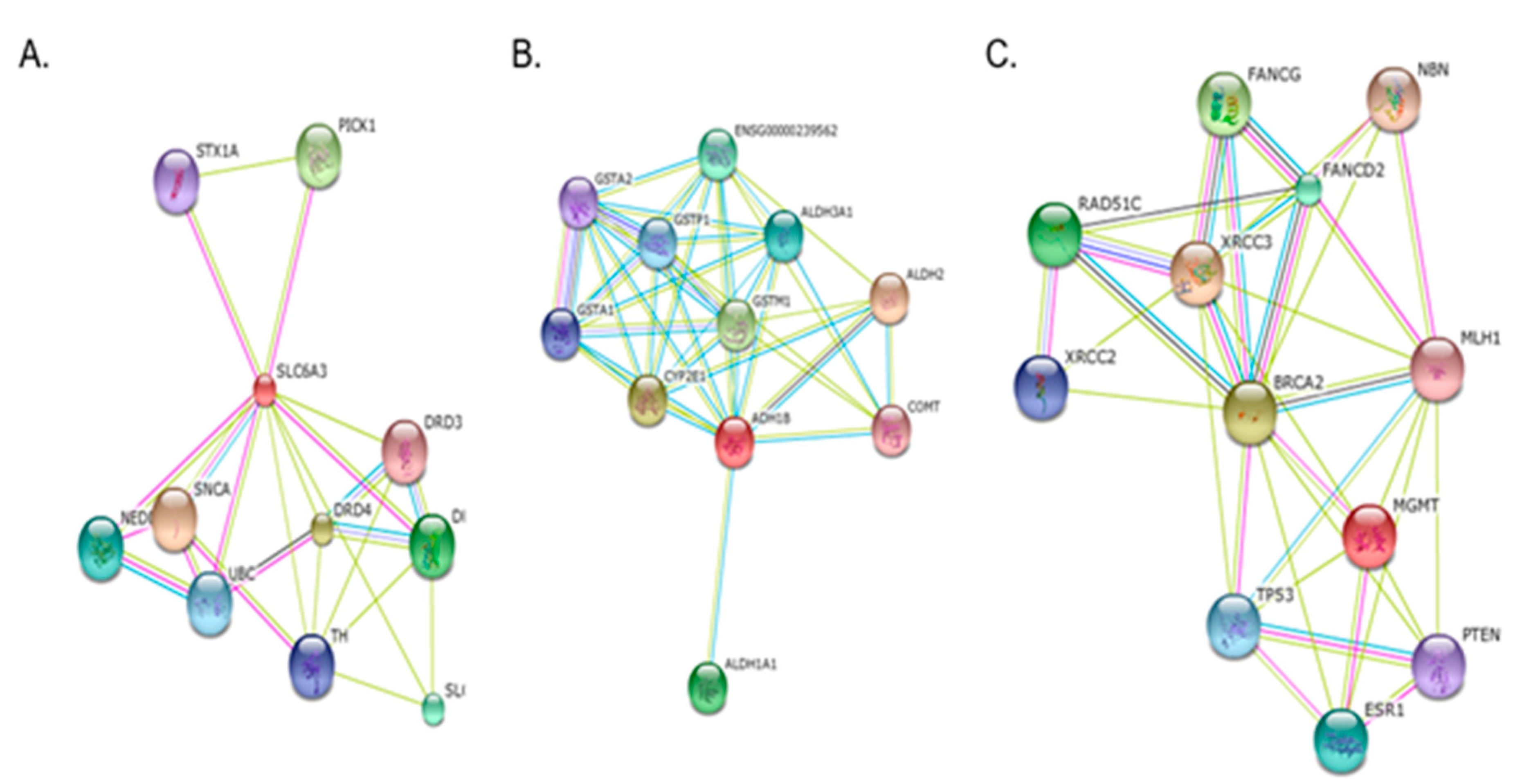
2.4. Genetic Polymorphisms Affecting Exposure Biomarkers
3. Discussion
4. Materials and Methods
4.1. Subjects and Sampling
4.2. Analyses of Urinary Cotinine
4.3. Analyses of Urinary MDA
4.4. Analyses of Urinary TTMA
4.5. Targeted Genotyping
4.6. Analyses of mtDNA Alteration between Blood and Buccal Cells
4.7. Statistical Analyses
Supplementary Materials
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Papalou, O.; Kandaraki, E.A.; Papadakis, G.; Diamanti-Kandarakis, E. Endocrine Disrupting Chemicals: An Occult Mediator of Metabolic Disease. Frontiers in Endocrinology 2019, 10, 112. [CrossRef]
- Ivarsson, J.; Ferrara, F.; Vallese, A.; Guiotto, A.; Colella, S.; Pecorelli, A.; Valacchi, G. Comparison of Pollutant Effects on Cutaneous Inflammasomes Activation. International Journal of Molecular Sciences 2023, 24, 16674. [CrossRef]
- Son, E.; Kwon, K.H. How Body Burden from Exposure to Endocrine Disruptors Effects Accelerated Aging? Environmental Research and Technology 2023, 6, 383-390.
- Zhou, G.; Xiao, W.; Xu, C.; Hu, Y.; Wu, X.; Huang, F.; Lu, X.; Shi, C.; Wu, X. Chemical Constituents of Tobacco Smoke Induce the Production of Interleukin-8 in Human Bronchial Epithelium, 16HBE Cells. Tobacco induced diseases 2016, 14, 1-9. [CrossRef]
- Jabeen, K.; Akash, M.S.H.; Haider, K.; Faheem, A.; Tariq, M.; Rehman, K. Tobacco Smoking as an EDC in Metabolic Disorders. Endocrine Disrupting Chemicals-induced Metabolic Disorders and Treatment Strategies 2021, 343-355.
- Vogeley, C.; Esser, C.; Tüting, T.; Krutmann, J.; Haarmann-Stemmann, T. Role of the Aryl Hydrocarbon Receptor in Environmentally Induced Skin Aging and Skin Carcinogenesis. International Journal of Molecular Sciences 2019, 20, 6005. [CrossRef]
- Ratajczak, A.; Jankowski, P.; Strus, P.; Feleszko, W. Heat Not Burn Tobacco Product—a New Global Trend: Impact of Heat-Not-Burn Tobacco Products on Public Health, a Systematic Review. International journal of environmental research and public health 2020, 17, 409. [CrossRef]
- Scherer, G.; Scherer, M.; Rögner, N.; Pluym, N. Assessment of the Exposure to Polycyclic Aromatic Hydrocarbons in Users of various Tobacco/Nicotine Products by Suitable Urinary Biomarkers. Arch. Toxicol. 2022, 96, 3113-3126. [CrossRef]
- Zhang, Y.; Dong, S.; Wang, H.; Tao, S.; Kiyama, R. Biological Impact of Environmental Polycyclic Aromatic Hydrocarbons (ePAHs) as Endocrine Disruptors. Environmental pollution 2016, 213, 809-824. [CrossRef]
- Mitchell, C.; Schneper, L.M.; Notterman, D.A. DNA Methylation, Early Life Environment, and Health Outcomes. Pediatr. Res. 2016, 79, 212-219.
- Justice, A.E.; Winkler, T.W.; Feitosa, M.F.; Graff, M.; Fisher, V.A.; Young, K.; Barata, L.; Deng, X.; Czajkowski, J.; Hadley, D. Genome-Wide Meta-Analysis of 241,258 Adults Accounting for Smoking Behaviour Identifies Novel Loci for Obesity Traits. Nature communications 2017, 8, 14977. [CrossRef]
- Minicã, C.C.; Mbarek, H.; Pool, R.; Dolan, C.V.; Boomsma, D.I.; Vink, J.M. Pathways to Smoking Behaviours: Biological Insights from the Tobacco and Genetics Consortium Meta-Analysis. Mol. Psychiatry 2017, 22, 82-88. [CrossRef]
- Brunzell, D.H.; Stafford, A.M.; Dixon, C.I. Nicotinic Receptor Contributions to Smoking: Insights from Human Studies and Animal Models. Current addiction reports 2015, 2, 33-46. [CrossRef]
- Murphy, S.E. Nicotine Metabolism and Smoking: Ethnic Differences in the Role of P450 2A6. Chem. Res. Toxicol. 2017, 30, 410-419. [CrossRef]
- Na, H.; Kim, M.; Chang, S.; Kim, S.; Park, J.Y.; Chung, M.W.; Yang, M. Tobacco Smoking-Response Genes in Blood and Buccal Cells. Toxicol. Lett. 2015, 232, 429-437. [CrossRef]
- Haines, D.A.; Saravanabhavan, G.; Werry, K.; Khoury, C. An Overview of Human Biomonitoring of Environmental Chemicals in the Canadian Health Measures Survey: 2007–2019. Int. J. Hyg. Environ. Health 2017, 220, 13-28. [CrossRef]
- Chang, C.M.; Edwards, S.H.; Arab, A.; Del Valle-Pinero, A.Y.; Yang, L.; Hatsukami, D.K. Biomarkers of Tobacco Exposure: Summary of an FDA-Sponsored Public Workshop. Cancer Epidemiology, Biomarkers & Prevention 2017, 26, 291-302. [CrossRef]
- Kim, S.; Jo, K. Multiple Tobacco Product use among Adolescents with Asthma in Korea. International Journal of Environmental Research and Public Health 2022, 19, 9633.
- Korean Statistical Information Service, https://kosis.kr/search.
- Pollack, A.Z.; Krall, J.R.; Swan, S.H.; Louis, G.M.B. Does Older Age Modify Associations between Endocrine Disrupting Chemicals and Fecundability? International Journal of Environmental Research and Public Health 2022, 19, 8074.
- Resendiz M, Watkins DS, Öztürk NC, Zhou FC. Environmental Influence on Epigenetics in Handbook of Epigenetics, 2023; pp. 639-668.
- Kennedy, M.J. Hormonal Regulation of Hepatic Drug-metabolizing Enzyme Activity during Adolescence. Clinical Pharmacology & Therapeutics 2008, 84, 662-673. [CrossRef]
- Corton, J.C.; Lee, J.S.; Liu, J.; Ren, H.; Vallanat, B.; DeVito, M. Determinants of Gene Expression in the Human Liver: Impact of Aging and Sex on Xenobiotic Metabolism. Exp. Gerontol. 2022, 169, 111976. [CrossRef]
- Bono, R.; Squillacioti, G.; Ghelli, F.; Panizzolo, M.; Comoretto, R.I.; Dalmasso, P.; Bellisario, V. Oxidative Stress Trajectories during Lifespan: The Possible Mediation Role of Hormones in Redox Imbalance and Aging. Sustainability 2023, 15, 1814. [CrossRef]
- Nucera, F.; Mumby, S.; Paudel, K.R.; Dharwal, V.; Di Stefano, A.; Casolaro, V.; Hansbro, P.M.; Adcock, I.M.; Caramori, G. Role of Oxidative Stress in the Pathogenesis of COPD. Minerva Med. 2022. [CrossRef]
- Fetterman, J.L.; Sammy, M.J.; Ballinger, S.W. Mitochondrial Toxicity of Tobacco Smoke and Air Pollution. Toxicology 2017, 391, 18-33. [CrossRef]
- Pirini, F.; Guida, E.; Lawson, F.; Mancinelli, A.; Guerrero-Preston, R. Nuclear and Mitochondrial DNA Alterations in Newborns with Prenatal Exposure to Cigarette Smoke. International journal of environmental research and public health 2015, 12, 1135-1155. [CrossRef]
- Ding, T.; Yan, W.; Zhou, T.; Shen, W.; Wang, T.; Li, M.; Zhou, S.; Wu, M.; Dai, J.; Huang, K. Endocrine Disrupting Chemicals Impact on Ovarian Aging: Evidence from Epidemiological and Experimental Evidence. Environmental Pollution 2022, 305, 119269. [CrossRef]
- Oladipupo, I.; Ali, T.; Hein, D.W.; Pagidas, K.; Bohler, H.; Doll, M.A.; Mann, M.L.; Gentry, A.; Chiang, J.L.; Pierson, R.C. Association between Cigarette Smoking and Ovarian Reserve among Women Seeking Fertility Care. Plos one 2022, 17, e0278998. [CrossRef]
- Ning, K.; Zhao, L.; Matloff, W.; Sun, F.; Toga, A.W. Association of Relative Brain Age with Tobacco Smoking, Alcohol Consumption, and Genetic Variants. Scientific reports 2020, 10, 10. [CrossRef]
- Papaccio, F.; D′ Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121.
- Morita, A.; Torii, K.; Maeda, A.; Yamaguchi, Y. Molecular Basis of Tobacco Smoke-Induced Premature Skin Aging. In Journal of Investigative Dermatology Symposium Proceedings; pp. 53-55.
- Kaur, G.; Begum, R.; Thota, S.; Batra, S. A Systematic Review of Smoking-Related Epigenetic Alterations. Arch. Toxicol. 2019, 93, 2715-2740. [CrossRef]
- Oyston, J. A Fresh Approach to Tobacco Control: Raising the Minimum Legal Age for Access. CMAJ 2017, 189, E293-E294. [CrossRef]
- Do, H.P.; Nguyen, L.H.; Nguyen, N.P.T.; Ngo, C.; Nguyen, H.L.T.; Le, G.T.; Nguyen, L.K.; Nguyen, C.T.; Tran, B.X.; Le, H.T. Factors Associated with Nicotine Dependence during Methadone Maintenance Treatment: Findings from a Multisite Survey in Vietnam. BMJ open 2017, 7, e015889. [CrossRef]
- Lee, H.; Isse, T.; Kawamoto, T.; Baik, H.W.; Park, J.Y.; Yang, M. Effect of Korean Pear (Pyruspyrifolia Cv. Shingo) Juice on Hangover Severity Following Alcohol Consumption. Food and chemical toxicology 2013, 58, 101-106. [CrossRef]
- Gagné, S. Determination of Trans, Trans-muconic Acid in Workers' Urine through Ultra-performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Biomedical Chromatography 2013, 27, 664-668. [CrossRef]
- Hasan, F.; Yadav, V.; Katiyar, T.; Yadav, S.; Pandey, R.; Mehrotra, D.; Hadi, R.; Singh, S.; Bhatt, M.L.; Parmar, D. Validation of Gene Expression Profiles of Candidate Genes using Low Density Array in Peripheral Blood of Tobacco Consuming Head and Neck Cancer Patients and Auto/Taxi Drivers with Preneoplastic Lesions. Genomics 2020, 112, 513-519. [CrossRef]
- Del Casale, A.; Paolini, M.; Gentile, G.; Borro, M.; Zocchi, C.; Fiaschè, F.; Padovano, A.; Zoppi, T.; Modesti, M.N.; De Luca, O. Dopamine DRD2 and DRD3 Polymorphisms Involvement in Nicotine Dependence in Patients with Treatment-Resistant Mental Disorders. Journal of Personalized Medicine 2022, 12, 565. [CrossRef]
- Yang, M.; Choi, Y.; Hwangbo, B.; Lee, J.S. Combined Effects of Genetic Polymorphisms in Six Selected Genes on Lung Cancer Susceptibility. Lung Cancer 2007, 57, 135-142. [CrossRef]
- Alsagaby, S.; Ahmed, A.A.; Rasheed, Z.; Althwab, S.A.; Aljohani, A.S.; Alhumaydhi, F.A.; Alhomaidan, H.T.; Alkhamiss, A.S.; Alkhowailed, M.; Alaqeel, A. Association of Genetic Polymorphisms in DNA Repair Genes ERCC2 Asp312Asn (rs1799793), ERCC2 Lys 751 Gln (rs13181), XRCC1 Arg399 Gln (rs25487) and XRCC3 Thr 241Met (rs861539) with the Susceptibility of Lung Cancer in Saudi Population. Nucleosides Nucleotides Nucleic Acids 2022, 41, 530-554.
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





