Submitted:
02 January 2024
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
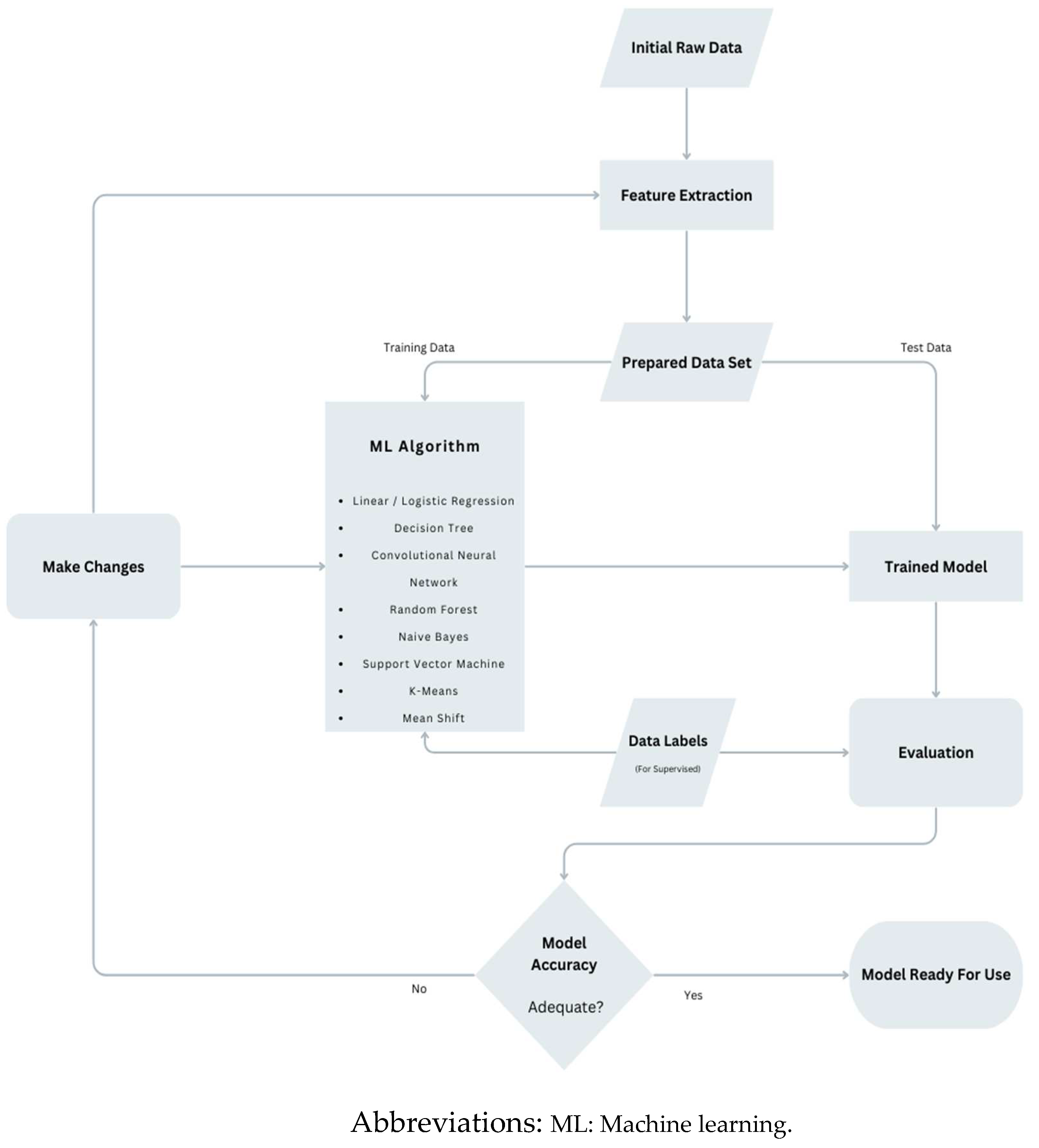
1. Introduction
2. Method
2.1. Conventionally risk prediction scores
2.2. AI based studies/risk scores
3. Discussion
4. Limitations
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008 Aug 5;52(6):428-34. [CrossRef] [PubMed]
- Francica A, Loforte A, Attisani M, Maiani M, Iacovoni A, Nisi T, et.al. Five-Year Outcome After Continuous Flow LVAD With Full-Magnetic (HeartMate 3) Versus Hybrid Levitation System (HeartWare): A Propensity-Score Matched Study From an All-Comers Multicentre Registry. Transpl Int. 2023 Sep 4;36:11675. Erratum in: Transpl Int. 2023 Oct 09;36:12088. [CrossRef] [PubMed]
- Grewal J, Tripathi N, Bortner B, Gregoski MJ, Cook D, Britt A, et.al. A multicenter evaluation of the HeartMate 3 risk score. J Heart Lung Transplant. 2023 Dec 6:S1053-2498(23)02156-3. Epub ahead of print. [CrossRef] [PubMed]
- Mehra MR, Netuka I, Uriel N, Katz JN, Pagani FD, Jorde UP, et.al; ARIES-HM3 Investigators. Aspirin and Hemocompatibility Events With a Left Ventricular Assist Device in Advanced Heart Failure: The ARIES-HM3 Randomized Clinical Trial. JAMA. 2023 Dec 12;330(22):2171-2181. [CrossRef] [PubMed]
- Kirschner M, Topkara VK, Sun J, Kurlansky P, Kaku Y, Naka Y, et.al. Comparing 3-Year Survival and Readmissions between HeartMate 3 and Heart Transplant as Primary Treatment for Advanced Heart Failure. J Thorac Cardiovasc Surg. 2023 Dec 26:S0022-5223(23)01207-2. Epub ahead of print. [CrossRef] [PubMed]
- Jorde UP, Saeed O, Koehl D, Morris AA, Wood KL, Meyer DM, et.al. The Society of Thoracic Surgeons Intermacs 2023 Annual Report: Focus on Magnetically Levitated Devices. Ann Thorac Surg. 2024 Jan;117(1):33-44. Epub 2023 Nov 8. [CrossRef] [PubMed]
- Puwanant S, Hamilton KK, Klodell CT, Hill JA, Schofield RS, Cleeton TS, et.al. Tricuspid annular motion as a predictor of severe right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant. 2008 Oct;27(10):1102-7. [CrossRef] [PubMed]
- Potapov EV, Krabatsch T, Ventura HO, Hetzer R. Advances in mechanical circulatory support: year in review. J Heart Lung Transplant. 2011 May;30(5):487-93. [CrossRef] [PubMed]
- Rich, JD. Right ventricular failure in patients with left ventricular assist devices. Cardiol Clin. 2012 May;30(2):291-302. [CrossRef] [PubMed]
- Raina A, Seetha Rammohan HR, Gertz ZM, Rame JE, Woo YJ, Kirkpatrick JN. Postoperative right ventricular failure after left ventricular assist device placement is predicted by preoperative echocardiographic structural, hemodynamic, and functional parameters. J Card Fail. 2013 Jan;19(1):16-24. [CrossRef] [PubMed]
- Kalogeropoulos AP, Kelkar A, Weinberger JF, Morris AA, Georgiopoulou VV, Markham DW, et.al. Validation of clinical scores for right ventricular failure prediction after implantation of continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2015 Dec;34(12):1595-603. Epub 2015 Jun 1. [CrossRef] [PubMed]
- Neyer J, Arsanjani R, Moriguchi J, Siegel R, Kobashigawa J. Echocardiographic parameters associated with right ventricular failure after left ventricular assist device: A review. J Heart Lung Transplant. 2016 Mar;35(3):283-293. Epub 2016 Jan 6. [CrossRef] [PubMed]
- Kang G, Ha R, Banerjee D. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant. 2016 Jan;35(1):67-73. Epub 2015 Jun 17. Erratum in: J Heart Lung Transplant. 2017 Nov;36(11):1272. [CrossRef] [PubMed]
- Loforte A, Grigioni F, Marinelli G. The risk of right ventricular failure with current continuous-flow left ventricular assist devices. Expert Rev Med Devices. 2017 Dec;14(12):969-983. Epub 2017 Nov 25. [CrossRef] [PubMed]
- Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, et.al.; MOMENTUM 3 Investigators. A Fully Magnetically Levitated Left Ventricular Assist Device - Final Report. N Engl J Med. 2019 Apr 25;380(17):1618-1627. Epub 2019 Mar 17. [CrossRef] [PubMed]
- Løgstrup BB, Nemec P, Schoenrath F, Gummert J, Pya Y, Potapov E, et.al. Heart failure etiology and risk of right heart failure in adult left ventricular assist device support: the European Registry for Patients with Mechanical Circulatory Support (EUROMACS). Scand Cardiovasc J. 2020 Oct;54(5):306-314. Epub 2020 Jun 18. [CrossRef] [PubMed]
- Frankfurter C, Molinero M, Vishram-Nielsen JKK, Foroutan F, Mak S, Rao V, et.al. Predicting the Risk of Right Ventricular Failure in Patients Undergoing Left Ventricular Assist Device Implantation: A Systematic Review. Circ Heart Fail. 2020 Oct;13(10):e006994. Epub 2020 Sep 28. [CrossRef] [PubMed]
- Varshney AS, DeFilippis EM, Cowger JA, Netuka I, Pinney SP, Givertz MM. Trends and Outcomes of Left Ventricular Assist Device Therapy: JACC Focus Seminar. J Am Coll Cardiol. 2022 Mar 22;79(11):1092-1107. [CrossRef] [PubMed]
- Saeed D, Feldman D, Banayosy AE, Birks E, Blume E, Cowger J, et.al. The 2023 International Society for Heart and Lung Transplantation Guidelines for Mechanical Circulatory Support: A 10- Year Update. J Heart Lung Transplant. 2023 Jul;42(7):e1-e222. Epub 2023 May 25. [CrossRef] [PubMed]
- Takeda K, Takayama H, Colombo PC, Yuzefpolskaya M, Fukuhara S, Han J, et.al. Incidence and clinical significance of late right heart failure during continuous-flow left ventricular assist device support. J Heart Lung Transplant. 2015 Aug;34(8):1024-32. Epub 2015 Mar 26. [CrossRef] [PubMed]
- Kapelios CJ, Charitos C, Kaldara E, Malliaras K, Nana E, Pantsios C, et.al. Late-onset right ventricular dysfunction after mechanical support by a continuous-flow left ventricular assist device. J Heart Lung Transplant. 2015 Dec;34(12):1604-10. Epub 2015 Jun 11. [CrossRef] [PubMed]
- Rich JD, Gosev I, Patel CB, Joseph S, Katz JN, Eckman PM, et.al; Evolving Mechanical Support Research Group (EMERG) Investigators. The incidence, risk factors, and outcomes associated with late right-sided heart failure in patients supported with an axial-flow left ventricular assist device. J Heart Lung Transplant. 2017 Jan;36(1):50-58. Epub 2016 Aug 20. [CrossRef] [PubMed]
- Rame JE, Pagani FD, Kiernan MS, Oliveira GH, Birati EY, Atluri P, et.al. Evolution of Late Right Heart Failure With Left Ventricular Assist Devices and Association With Outcomes. J Am Coll Cardiol. 2021 Dec 7;78(23):2294-2308. [CrossRef] [PubMed]
- Alkhunaizi FA, Azih NI, Read JM, Goldberg RL, Gulati AA, Scheel PJ 3rd, et.al. Characteristics and Predictors of Late Right Heart Failure After Left Ventricular Assist Device Implantation. ASAIO J. 2023 Mar 1;69(3):315-323. Epub 2022 Oct 2. [CrossRef] [PubMed]
- Rajapreyar I, Soliman O, Brailovsky Y, Tedford RJ, Gibson G, Mohacsi P, et.al. Late Right Heart Failure After Left Ventricular Assist Device Implantation: Contemporary Insights and Future Perspectives. JACC Heart Fail. 2023 Aug;11(8 Pt 1):865-878. Epub 2023 May 31. Erratum in: JACC Heart Fail. 2023 Aug;11(8 Pt 1):1035. [CrossRef] [PubMed]
- Shah P, Yuzefpolskaya M, Hickey GW, Breathett K, Wever-Pinzon O, Ton VK, et.al. Twelfth Interagency Registry for Mechanically Assisted Circulatory Support Report: Readmissions After Left Ventricular Assist Device. Ann Thorac Surg. 2022 Mar;113(3):722-737. Epub 2022 Jan 7. [CrossRef] [PubMed]
- Hall SA, Copeland H, Alam A, Joseph SM. The "Right" Definition for Post-Left Ventricular Assist Device Right Heart Failure: The More We Learn, the Less We Know. Front Cardiovasc Med. 2022 Apr 26;9:893327. [CrossRef] [PubMed]
- Ochiai Y, McCarthy PM, Smedira NG, Banbury MK, Navia JL, Feng J, et.al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation. 2002 Sep 24;106(12 Suppl 1):I198-202. [PubMed]
- Kavarana MN, Pessin-Minsley MS, Urtecho J, Catanese KA, Flannery M, Oz MC, et.al.. Right ventricular dysfunction and organ failure in left ventricular assist device recipients: a continuing problem. Ann Thorac Surg. 2002 Mar;73(3):745-50. [CrossRef] [PubMed]
- Dang NC, Topkara VK, Mercando M, Kay J, Kruger KH, Aboodi MS, et.al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant. 2006 Jan;25(1):1-6. Epub 2005 Dec 9. [CrossRef] [PubMed]
- Craig, ML. Management of right ventricular failure in the era of ventricular assist device therapy. Curr Heart Fail Rep. 2011 Mar;8(1):65-71. [CrossRef] [PubMed]
- Kukucka M, Potapov E, Stepanenko A, Weller K, Mladenow A, Kuppe H, et.al. Acute impact of left ventricular unloading by left ventricular assist device on the right ventricle geometry and function: effect of nitric oxide inhalation. J Thorac Cardiovasc Surg. 2011 Apr;141(4):1009-14. [CrossRef] [PubMed]
- Grant AD, Smedira NG, Starling RC, Marwick TH. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol. 2012 Aug 7;60(6):521-8. [CrossRef] [PubMed]
- Kanwar MK, Lohmueller LC, Kormos RL, Teuteberg JJ, Rogers JG, Lindenfeld J, et.al. A Bayesian Model to Predict Survival After Left Ventricular Assist Device Implantation. JACC Heart Fail. 2018 Sep;6(9):771-779. [CrossRef] [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et.al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012 Feb;31(2):117-26. [CrossRef] [PubMed]
- Holman, WL. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS): what have we learned and what will we learn? Circulation. 2012 Sep 11;126(11):1401-6. [CrossRef] [PubMed]
- Argiriou M, Kolokotron SM, Sakellaridis T, Argiriou O, Charitos C, Zarogoulidis P, et.al. Right heart failure post left ventricular assist device implantation. J Thorac Dis. 2014 Mar;6 Suppl 1(Suppl 1):S52-9. [CrossRef] [PubMed]
- Mehra MR, Park MH, Landzberg MJ, Lala A, Waxman AB; International Right Heart Failure Foundation Scientific Working Group. Right heart failure: toward a common language. J Heart Lung Transplant. 2014 Feb;33(2):123-6. [CrossRef] [PubMed]
- Kormos RL, Antonides CFJ, Goldstein DJ, Cowger JA, Starling RC, Kirklin JK, et.al. Updated definitions of adverse events for trials and registries of mechanical circulatory support: A consensus statement of the mechanical circulatory support academic research consortium. J Heart Lung Transplant. 2020 Aug;39(8):735-750. [CrossRef] [PubMed]
- Morgan JA, John R, Lee BJ, Oz MC, Naka Y. Is severe right ventricular failure in left ventricular assist device recipients a risk factor for unsuccessful bridging to transplant and post-transplant mortality. Ann Thorac Surg. 2004 Mar;77(3):859-63. [CrossRef] [PubMed]
- Takeda K, Naka Y, Yang JA, Uriel N, Colombo PC, Jorde UP, et.al. Outcome of unplanned right ventricular assist device support for severe right heart failure after implantable left ventricular assist device insertion. J Heart Lung Transplant. 2014 Feb;33(2):141-8. [CrossRef] [PubMed]
- Subramani S, Sharma A, Arora L, Hanada S, Krishnan S, Ramakrishna H. Perioperative Right Ventricular Dysfunction: Analysis of Outcomes. J Cardiothorac Vasc Anesth. 2022 Jan;36(1):309-320. [CrossRef] [PubMed]
- Ali HR, Kiernan MS, Choudhary G, Levine DJ, Sodha NR, Ehsan A, et.al. Right Ventricular Failure Post-Implantation of Left Ventricular Assist Device: Prevalence, Pathophysiology, and Predictors. ASAIO J. 2020 Jun;66(6):610-619. [CrossRef] [PubMed]
- Lo Coco V, De Piero ME, Massimi G, Chiarini G, Raffa GM, Kowalewski M, et.al. Right ventricular failure after left ventricular assist device implantation: a review of the literature. J Thorac Dis. 2021 Feb;13(2):1256-1269. [CrossRef] [PubMed]
- Bravo CA, Navarro AG, Dhaliwal KK, Khorsandi M, Keenan JE, Mudigonda P, et.al. Right heart failure after left ventricular assist device: From mechanisms to treatments. Front Cardiovasc Med. 2022 Oct 19;9:1023549. [CrossRef] [PubMed]
- Chamogeorgakis T, Toumpoulis I, Bonios MJ, Lanfear D, Williams C, Koliopoulou A, et.al. Treatment Strategies and Outcomes of Right Ventricular Failure Post Left Ventricular Assist Device Implantation: An INTERMACS Analysis. ASAIO J. 2023 Nov 27. [CrossRef] [PubMed]
- Harnad S. (2008) The Annotation Game: On Turing (1950) on Computing, Machinery, and Intelligence (PUBLISHED VERSION BOWDLERIZED). In, Epstein, Robert, Roberts, Gary and Beber, Grace (eds.) Parsing the Turing Test: Philosophical and Methodological Issues in the Quest for the Thinking Computer. Evolving Consciousness (01/01/08) Springer, pp. 23-66.
- Russell SJ, Norvig P. (2021). Artificial Intelligence: A Modern Approach (4th ed.). Hoboken: Pearson. ISBN 9780134610993.
- Mitchell TM (1997). Machine learning. McGraw-Hill. [CrossRef]
- Bishop CM (2006). Pattern Recognition and Machine Learning. Springer New York, NY. ISBN: 978-1-4939-3843-8.
- Mohri M, Rostamizadeh A, Talwalkar A. (2012). Foundations of Machine Learning. The MIT Press. ISBN 9780262018258.
- Nichols JA, Herbert Chan HW, Baker MAB. Machine learning: applications of artificial intelligence to imaging and diagnosis. Biophys Rev. 2019 Feb;11(1):111-118. [CrossRef] [PubMed]
- Quer G, Arnaout R, Henne M, Arnaout R. Machine Learning and the Future of Cardiovascular Care: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021 Jan 26;77(3):300-313. [CrossRef] [PubMed]
- Mortazavi BJ, Downing NS, Bucholz EM, Dharmarajan K, Manhapra A, Li SX, et.al. Analysis of Machine Learning Techniques for Heart Failure Readmissions. Circ Cardiovasc Qual Outcomes. 2016 Nov;9(6):629-640. [CrossRef] [PubMed]
- Folkert W Asselbergs, Alan G Fraser, Artificial intelligence in cardiology: the debate continues, European Heart Journal - Digital Health, Volume 2, Issue 4, December 2021, Pages 721–726. [CrossRef]
- Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008;51:2163–72.
- Fitzpatrick JR 3rd, Frederick JR, Hsu VM, Kozin ED, O'Hara ML, Howell E, et.al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant. 2008 Dec;27(12):1286-92. [CrossRef] [PubMed]
- Drakos SG, Janicki L, Horne BD, Kfoury AG, Reid BB, Clayson S, et.al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol. 2010 Apr 1;105(7):1030-5. [CrossRef] [PubMed]
- Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, et.al; HeartMate II Clinical Investigators. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010 May;139(5):1316-24. [CrossRef] [PubMed]
- Atluri P, Goldstone AB, Fairman AS, MacArthur JW, Shudo Y, Cohen JE, et.al. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann Thorac Surg. 2013 Sep;96(3):857-63; discussion 863-4. [CrossRef] [PubMed]
- Aissaoui N, Salem JE, Paluszkiewicz L, Morshuis M, Guerot E, Gorria GM, Fagon JY et.al. Assessment of right ventricular dysfunction predictors before the implantation of a left ventricular assist device in end-stage heart failure patients using echocardiographic measures (ARVADE): Combination of left and right ventricular echocardiographic variables. Arch Cardiovasc Dis. 2015 May;108(5):300-9. [CrossRef] [PubMed]
- Loforte A, Montalto A, Musumeci F, Amarelli C, Mariani C, Polizzi V, et.al. Calculation of the ALMA Risk of Right Ventricular Failure After Left Ventricular Assist Device Implantation. ASAIO J. 2018 Nov/Dec;64(6):e140-e147.
- Soliman OII, Akin S, Muslem R, Boersma E, Manintveld OC, Krabatsch T, et.al.; EUROMACS Investigators. Derivation and Validation of a Novel Right-Sided Heart Failure Model After Implantation of Continuous Flow Left Ventricular Assist Devices: The EUROMACS (European Registry for Patients with Mechanical Circulatory Support) Right-Sided Heart Failure Risk Score. Circulation. 2018 Feb 27;137(9):891-906.
- Loghmanpour NA, Kormos RL, Kanwar MK, Teuteberg JJ, Murali S, Antaki JF. A Bayesian Model to Predict Right Ventricular Failure Following Left Ventricular Assist Device Therapy. JACC Heart Fail. 2016 Sep;4(9):711-21. [CrossRef] [PubMed]
- Samura T, Asanoi H, Toda K, Miyagawa S, Yoshikawa Y, Hata H, et.al. Prediction of Right Ventricular Failure After Left Ventricular Assist Device Implantation Using Machine Learning of Preoperative Hemodynamics. Circulation.2018;138,15318.
- Bellavia D, Iacovoni A, Agnese V, Falletta C, Coronnello C, Pasta S, et.al. Usefulness of regional right ventricular and right atrial strain for prediction of early and late right ventricular failure following a left ventricular assist device implant: A machine learning approach. Int J Artif Organs. 2020 May;43(5):297-314. [CrossRef] [PubMed]
- Shad R, Quach N, Fong R, Kasinpila P, Bowles C, Castro M, et.al. Predicting post-operative right ventricular failure using video-based deep learning. Nat Commun. 2021 Aug 31;12(1):5192. [CrossRef] [PubMed]
- Kilic A, Dochtermann D, Padman R, Miller JK, Dubrawski A. Using machine learning to improve risk prediction in durable left ventricular assist devices. PLoS One. 2021 Mar 10;16(3):e0247866. [CrossRef] [PubMed]
- Kilic A, Macickova J, Duan L, Movahedi F, Seese L, Zhang Y, at.al. Machine Learning Approaches to Analyzing Adverse Events Following Durable LVAD Implantation. Ann Thorac Surg. 2021 Sep;112(3):770-777. [CrossRef] [PubMed]
- Nayak A, Hu Y, Patel KJ, Ko Y, Okoh AK, Wang J, et.al. Machine Learning Algorithms Identify Distinct Phenotypes of Right Heart Failure After Left Ventricular Assist Device Implant. J Heart Lung Transplant. 2022:41(4), S39.
- Bahl A, Qureshi B, Zhang K, Bravo C, Mahr C, Li S. Explainable Machine Learning Analysis of Right Heart Failure After Left Ventricular Assist Device Implantation. ASAIO J. 2023 ;69(5):417-423. [CrossRef] [PubMed]
- Just IA, Schoenrath F, Roehrich L, Heil E, Stein J, Auer TA, et.al. Artificial intelligence-based analysis of body composition predicts outcome in patients receiving long-term mechanical circulatory support. J Cachexia Sarcopenia Muscle. 2023 Dec 26. [CrossRef] [PubMed]
- Gautam N, Ghanta SN, Clausen A, Saluja P, Sivakumar K, Dhar G, et.al. Contemporary Applications of Machine Learning for Device Therapy in Heart Failure. JACC Heart Fail. 2022 Sep;10(9):603-622. [CrossRef] [PubMed]
- Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008 Mar 18;117(11):1436-48. [CrossRef] [PubMed]
- Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant. 2015 Sep;34(9):1123-30. [CrossRef] [PubMed]
- Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, et.al; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; and Council on Cardiovascular Surgery and Anesthesia. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2018 May 15;137(20):e578-e622. [CrossRef] [PubMed]
- El Hajj MC, Viray MC, Tedford RJ. Right Heart Failure: A Hemodynamic Review. Cardiol Clin. 2020 May;38(2):161-173. [CrossRef] [PubMed]
- Wang TS, Cevasco M, Birati EY, Mazurek JA. Predicting, Recognizing, and Treating Right Heart Failure in Patients Undergoing Durable LVAD Therapy. J Clin Med. 2022 ;11(11):2984. 25 May. [CrossRef] [PubMed]
- Cheng RK, Deng MC, Tseng CH, Shemin RJ, Kubak BM, MacLellan WR. Risk stratification in patients with advanced heart failure requiring biventricular assist device support as a bridge to cardiac transplantation. J Heart Lung Transplant. 2012 Aug;31(8):831-8. [CrossRef] [PubMed]
- Lo C, Murphy D, Summerhayes R, Quayle M, Burrell A, Bailey M, et.al. Right ventricular failure after implantation of continuous flow left ventricular assist device: analysis of predictors and outcomes. Clin Transplant. 2015 Sep;29(9):763-70. [CrossRef] [PubMed]
- Shiga T, Kinugawa K, Imamura T, Kato N, Endo M, Inaba T, et.al. Combination evaluation of preoperative risk indices predicts requirement of biventricular assist device. Circ J. 2012;76(12):2785-91. [CrossRef] [PubMed]
- Kurihara C, Critsinelis AC, Kawabori M, Sugiura T, Loor G, Civitello AB, et.al. Frequency and Consequences of Right-Sided Heart Failure After Continuous-Flow Left Ventricular Assist Device Implantation. Am J Cardiol. 2018 Feb 1;121(3):336-342. [CrossRef] [PubMed]
- Farrar DJ, Chow E, Wood JR, Hill JD. Anatomic interaction between the right and left ventricles during univentricular and biventricular circulatory support. ASAIO Trans. 1988 Jul-Sep;34(3):235-40. [PubMed]
- Moon MR, Bolger AF, DeAnda A, Komeda M, Daughters GT 2nd, Nikolic SD, et.al. Septal function during left ventricular unloading. Circulation. 1997 Mar 4;95(5):1320-7. [CrossRef] [PubMed]
- Feneley MP, Gavaghan TP, Baron DW, Branson JA, Roy PR, Morgan JJ. Contribution of left ventricular contraction to the generation of right ventricular systolic pressure in the human heart. Circulation. 1985 Mar;71(3):473-80. [CrossRef] [PubMed]
- Starling MR, Walsh RA, Dell'Italia LJ, Mancini GB, Lasher JC, Lancaster JL. The relationship of various measures of end-systole to left ventricular maximum time-varying elastance in man. Circulation. 1987 Jul;76(1):32-43. [CrossRef] [PubMed]
- Brown KA, Ditchey RV. Human right ventricular end-systolic pressure-volume relation defined by maximal elastance. Circulation. 1988 Jul;78(1):81-91. [CrossRef] [PubMed]
- Goldstein JA, Barzilai B, Rosamond TL, Eisenberg PR, Jaffe AS. Determinants of hemodynamic compromise with severe right ventricular infarction. Circulation. 1990 Aug;82(2):359-68. [CrossRef] [PubMed]
- Morgan JA, Paone G, Nemeh HW, Murthy R, Williams CT, Lanfear DE, et.al. Impact of continuous-flow left ventricular assist device support on right ventricular function. J Heart Lung Transplant. 2013 Apr;32(4):398-403. [CrossRef] [PubMed]
- Sunagawa K, Sagawa K, Maughan WL. Ventricular interaction with the loading system. Ann Biomed Eng. 1984;12(2):163-89. [CrossRef] [PubMed]
- Meineri M, Van Rensburg AE, Vegas A. Right ventricular failure after LVAD implantation: prevention and treatment. Best Pract Res Clin Anaesthesiol. 2012 Jun;26(2):217-29. [CrossRef] [PubMed]
- Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, et.al; HeartMate II Investigators. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009 Jul 21;54(4):312-21. [CrossRef] [PubMed]
- Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et.al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009 Dec 3;361(23):2241-51. Erratum in: N Engl J Med. 2018 Aug 16;379(7):697. [CrossRef] [PubMed]
- Van Meter CH Jr. Right heart failure: best treated by avoidance. Ann Thorac Surg. 2001 Mar;71(3 Suppl):S220-2. [CrossRef] [PubMed]
- Patlolla B, Beygui R, Haddad F. Right-ventricular failure following left ventricle assist device implantation. Curr Opin Cardiol. 2013 Mar;28(2):223-33. [CrossRef] [PubMed]
- Fitzpatrick JR 3rd, Frederick JR, Hiesinger W, Hsu VM, McCormick RC, Kozin ED, et.al. Early planned institution of biventricular mechanical circulatory support results in improved outcomes compared with delayed conversion of a left ventricular assist device to a biventricular assist device. J Thorac Cardiovasc Surg. 2009 Apr;137(4):971-7. [CrossRef] [PubMed]
- Tedford RJ, Hemnes AR, Russell SD, Wittstein IS, Mahmud M, Zaiman AL, et.al, Champion HC. PDE5A inhibitor treatment of persistent pulmonary hypertension after mechanical circulatory support. Circ Heart Fail. 2008 Nov;1(4):213-9. [CrossRef] [PubMed]
- Baumwol J, Macdonald PS, Keogh AM, Kotlyar E, Spratt P, Jansz P, et.al. Right heart failure and "failure to thrive" after left ventricular assist device: clinical predictors and outcomes. J Heart Lung Transplant. 2011 Aug;30(8):888-95. [CrossRef] [PubMed]
- Deswarte G, Kirsch M, Lesault PF, Trochu JN, Damy T. Right ventricular reserve and outcome after continuous-flow left ventricular assist device implantation. J Heart Lung Transplant. 2010 Oct;29(10):1196-8. [CrossRef] [PubMed]
- Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, et.al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007 Jul 31;116(5):497-505. [CrossRef] [PubMed]
| Authors (et. al.) | Year | Title | Data Source | Findings |
| Loghmanpour [64] | 2016 | A Bayesian Model to Predict RVF following LVAD Therapy | INTERMACS data | Systolic PAP, pre-albumin, LDH and RV EF most predictive preoperative variables. AUC for acute, early, late RHF prediction between 0.83–0.90 sensitivity of 90% |
| Samura [65] | 2018 | Prediction of RVF after left LVAD implantation using ML of preoperative hemodynamics | Preoperative clinical and hemodynamic parameters | Prediction accuracy 95%, AUC 0.85 |
| Bellavia [66] | 2020 | Usefulness of regional RV and right atrial strain for prediction of early and late RVF following a LVAD implant: A ML approach | Biomarkers, Echocardiography, cath-lab measurements | Significant Predictors: Michigan risk score, CVP, systolic strain of RV free wall. ROC AUC 0.95 |
| Shad [67] | 2021 | Predicting post-operative RVF using video-based deep learning | Preoperative Echocardiography video | ML AUC 0.729, CRITT AUC 0.616, Penn AUC 0.605 |
| Kilic [68] | 2021 | Using ML to improve risk prediction in durable LVAD | INTERMACS data | 48.8% and 36.9% in 90-day and 1-year mortality prediction improvement with ML compared to usual logistic regression data analysis |
| Kilic [69] | 2021 | ML Approaches to Analyzing Adverse Events Following Durable LVAD Implantation | ENDURANCE trials | Bleeding, infection and RHF most common postoperative adverse events. RHF has strong transitive relationship with bleeding and infection |
| Nayak [70] | 2022 | ML Algorithms Identify Distinct Phenotypes of RHF After LVAD Implant | IMACS data | 4 post LVAD RHF phenotypes identified Clinical outcomes evaluated |
| Bahl [71] | 2023 | Explainable ML Analysis of RHF After LVAD Implantation | INTERMACS data | 5 best predictors identified Non-linear relationships identified |
| Just [72] | 2023 | AI-based analysis of body composition predicts outcome in patients receiving long-term MCS | Preoperative CT Scan | Adipose tissue as indicator for postoperative major complications. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





