Submitted:
29 December 2023
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
Obesity in Chronic Kidney Disease
Reverse Epidemiology of Renal Transplantation
Adipose Tissue
Inflammatory Mediators of Adipose Tissue
Leptin
Leptin in CKD
Leptin in Renal Transplant
Implications of Genetic Variability in Leptin Genes
Adiponectin
Adiponectin in CKD
Adiponectin in Renal Transplant
Implications of Genetic Variability in Adiponectin Genes
Arachidonic-Derived Vasoactive Eicosanoids
Eicosanoids in CKD
Eicosanoids in Renal Transplant
Implications of Genetic Variability in Eicosanoid Genes
Perspectives and Conclusions
Funding
Conflicts of Interest
References
- Chen TK, Knicely DH, Grams ME. Chronic Kidney Disease Diagnosis and Management: A Review. Jama. 2019;322(13):1294-304. [CrossRef]
- Drawz P, Rahman M. Chronic kidney disease. Annals of internal medicine. 2015;162(11):ITC1-16. [CrossRef]
- Martinez-Castelao A, Gorriz JL, Segura-de la Morena J, Cebollada J, Escalada J, Esmatjes E, et al. Consensus document for the detection and management of chronic kidney disease. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia. 2014;34(2):243-62. [CrossRef]
- Hall JE, Henegar JR, Dwyer TM, Liu J, Da Silva AA, Kuo JJ, et al. Is obesity a major cause of chronic kidney disease? Advances in renal replacement therapy. 2004;11(1):41-54. [CrossRef]
- Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. Jama. 2005;293(4):455-62. [CrossRef]
- Kopple JD, Feroze U. The effect of obesity on chronic kidney disease. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2011;21(1):66-71. [CrossRef]
- Kopple JD. Obesity and chronic kidney disease. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2010;20(5 Suppl):S29-30. [CrossRef]
- Chang A, Van Horn L, Jacobs DR, Jr., Liu K, Muntner P, Newsome B, et al. Lifestyle-related factors, obesity, and incident microalbuminuria: the CARDIA (Coronary Artery Risk Development in Young Adults) study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62(2):267-75. [CrossRef]
- Song YM, Sung J, Lee K. Longitudinal relationships of metabolic syndrome and obesity with kidney function: Healthy Twin Study. Clinical and experimental nephrology. 2015;19(5):887-94. [CrossRef]
- Lee HS, Lee KB, Hyun YY, Chang Y, Ryu S, Choi Y. DASH dietary pattern and chronic kidney disease in elderly Korean adults. European journal of clinical nutrition. 2017;71(6):755-61. [CrossRef]
- Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Seminars in nephrology. 2013;33(1):14-22. [CrossRef]
- Pinto KRD, Feckinghaus CM, Hirakata VN. Obesity as a predictive factor for chronic kidney disease in adults: systematic review and meta-analysis. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2021;54(4):e10022. [CrossRef]
- Ritz E, Koleganova N. Obesity and chronic kidney disease. Seminars in nephrology. 2009;29(5):504-11. [CrossRef]
- Arner P. Introduction: the inflammation orchestra in adipose tissue. Journal of internal medicine. 2007;262(4):404-7.
- Zoccali C. Overweight, obesity and metabolic alterations in chronic kidney disease. Prilozi. 2009;30(2):17-31.
- Decleves AE, Sharma K. Obesity and kidney disease: differential effects of obesity on adipose tissue and kidney inflammation and fibrosis. Current opinion in nephrology and hypertension. 2015;24(1):28-36. [CrossRef]
- Silva Junior GB, Bentes AC, Daher EF, Matos SM. Obesity and kidney disease. Jornal brasileiro de nefrologia. 2017;39(1):65-9. [CrossRef]
- Kalaitzidis RG, Siamopoulos KC. The role of obesity in kidney disease: recent findings and potential mechanisms. International urology and nephrology. 2011;43(3):771-84. [CrossRef]
- Navarro-Diaz M, Serra A, Lopez D, Granada M, Bayes B, Romero R. Obesity, inflammation, and kidney disease. Kidney international Supplement. 2008(111):S15-8. [CrossRef]
- Bash LD, Erlinger TP, Coresh J, Marsh-Manzi J, Folsom AR, Astor BC. Inflammation, hemostasis, and the risk of kidney function decline in the Atherosclerosis Risk in Communities (ARIC) Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;53(4):596-605. [CrossRef]
- Lin J, Hu FB, Mantzoros C, Curhan GC. Lipid and inflammatory biomarkers and kidney function decline in type 2 diabetes. Diabetologia. 2010;53(2):263-7. [CrossRef]
- Nicoletto BB, Fonseca NK, Manfro RC, Goncalves LF, Leitao CB, Souza GC. Effects of obesity on kidney transplantation outcomes: a systematic review and meta-analysis. Transplantation. 2014;98(2):167-76. [CrossRef]
- Humar A, Ramcharan T, Denny R, Gillingham KJ, Payne WD, Matas AJ. Are wound complications after a kidney transplant more common with modern immunosuppression? Transplantation. 2001;72(12):1920-3. ttps://doi.org/10.1097/00007890-200112270-00009.
- Singh D, Lawen J, Alkhudair W. Does pretransplant obesity affect the outcome in kidney transplant recipients? Transplantation proceedings. 2005;37(2):717-20. [CrossRef]
- Halme L, Eklund B, Kyllonen L, Salmela K. Is obesity still a risk factor in renal transplantation? Transplant international : official journal of the European Society for Organ Transplantation. 1997;10(4):284-8. [CrossRef]
- Chan W, Bosch JA, Jones D, McTernan PG, Phillips AC, Borrows R. Obesity in kidney transplantation. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2014;24(1):1-12. [CrossRef]
- Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. The American journal of clinical nutrition. 2005;81(3):543-54. [CrossRef]
- Herselman M, Esau N, Kruger JM, Labadarios D, Moosa MR. Relationship between serum protein and mortality in adults on long-term hemodialysis: exhaustive review and meta-analysis. Nutrition. 2010;26(1):10-32. [CrossRef]
- Park J, Jin DC, Molnar MZ, Dukkipati R, Kim YL, Jing J, et al. Mortality predictability of body size and muscle mass surrogates in Asian vs white and African American hemodialysis patients. Mayo Clinic proceedings. 2013;88(5):479-86. [CrossRef]
- Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, et al. Obesity paradox in end-stage kidney disease patients. Progress in cardiovascular diseases. 2014;56(4):415-25. [CrossRef]
- Ahmadi F, Mohebi-Nejad A. Re: Lovastatin for reduction of leptin in nondialysis patients with type 2 diabetic nephropathy. Iranian journal of kidney diseases. 2014;8(4):344-5.
- Ahmadi SF, Zahmatkesh G, Streja E, Molnar MZ, Rhee CM, Kovesdy CP, et al. Body mass index and mortality in kidney transplant recipients: a systematic review and meta-analysis. American journal of nephrology. 2014;40(4):315-24. [CrossRef]
- Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC. Racial and survival paradoxes in chronic kidney disease. Nature clinical practice Nephrology. 2007;3(9):493-506. [CrossRef]
- Pommer W. Preventive Nephrology: The Role of Obesity in Different Stages of Chronic Kidney Disease. Kidney Dis (Basel). 2018;4(4):199-204. [CrossRef]
- Ashby VB, Leichtman AB, Rees MA, Song PX, Bray M, Wang W, et al. A Kidney Graft Survival Calculator that Accounts for Mismatches in Age, Sex, HLA, and Body Size. Clinical journal of the American Society of Nephrology : CJASN. 2017;12(7):1148-60. [CrossRef]
- Hossain M, Woywodt A, Augustine T, Sharma V. Obesity and listing for renal transplantation: weighing the evidence for a growing problem. Clinical kidney journal. 2017;10(5):703-8. [CrossRef]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. The Journal of allergy and clinical immunology. 2005;115(5):911-9; quiz 20. [CrossRef]
- Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450-5. [CrossRef]
- Proenca AR, Sertie RA, Oliveira AC, Campana AB, Caminhotto RO, Chimin P, et al. New concepts in white adipose tissue physiology. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2014;47(3):192-205. [CrossRef]
- Roman S, Agil A, Peran M, Alvaro-Galue E, Ruiz-Ojeda FJ, Fernandez-Vazquez G, et al. Brown adipose tissue and novel therapeutic approaches to treat metabolic disorders. Translational research : the journal of laboratory and clinical medicine. 2015;165(4):464-79. [CrossRef]
- Bates R, Huang W, Cao L. Adipose Tissue: An Emerging Target for Adeno-associated Viral Vectors. Molecular therapy Methods & clinical development. 2020;19:236-49. [CrossRef]
- Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53(5):1285-92. [CrossRef]
- Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. International journal of molecular sciences. 2014;15(4):6184-223. [CrossRef]
- Cildir G, Akincilar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends in molecular medicine. 2013;19(8):487-500. [CrossRef]
- Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochimica et biophysica acta. 2014;1842(3):446-62. [CrossRef]
- Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends in endocrinology and metabolism: TEM. 2012;23(8):407-15. [CrossRef]
- AlZaim I, Hammoud SH, Al-Koussa H, Ghazi A, Eid AH, El-Yazbi AF. Adipose Tissue Immunomodulation: A Novel Therapeutic Approach in Cardiovascular and Metabolic Diseases. Frontiers in cardiovascular medicine. 2020;7:602088. [CrossRef]
- Exley MA, Hand L, O'Shea D, Lynch L. Interplay between the immune system and adipose tissue in obesity. The Journal of endocrinology. 2014;223(2):R41-8. [CrossRef]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117(1):175-84. [CrossRef]
- Cao H. Adipocytokines in obesity and metabolic disease. The Journal of endocrinology. 2014;220(2):T47-59. [CrossRef]
- Faggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15(14):2565-71. [CrossRef]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425-32. [CrossRef]
- Zhang F, Chen Y, Heiman M, Dimarchi R. Leptin: structure, function and biology. Vitamins and hormones. 2005;71:345-72. [CrossRef]
- Alix PM, Guebre-Egziabher F, Soulage CO. Leptin as an uremic toxin: Deleterious role of leptin in chronic kidney disease. Biochimie. 2014;105:12-21. [CrossRef]
- Minocci A, Savia G, Lucantoni R, Berselli ME, Tagliaferri M, Calo G, et al. Leptin plasma concentrations are dependent on body fat distribution in obese patients. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2000;24(9):1139-44. [CrossRef]
- Briley LP, Szczech LA. Leptin and renal disease. Seminars in dialysis. 2006;19(1):54-9. [CrossRef]
- Sharma K, Considine RV. The Ob protein (leptin) and the kidney. Kidney international. 1998;53(6):1483-7. [CrossRef]
- Teta D, Bevington A, Brown J, Pawluczyk I, Harris K, Walls J. Acidosis downregulates leptin production from cultured adipocytes through a glucose transport-dependent post-transcriptional mechanism. Journal of the American Society of Nephrology : JASN. 2003;14(9):2248-54. [CrossRef]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. The New England journal of medicine. 1996;334(5):292-5. [CrossRef]
- Schrauwen P, van Marken Lichtenbelt WD, Westerterp KR, Saris WH. Effect of diet composition on leptin concentration in lean subjects. Metabolism: clinical and experimental. 1997;46(4):420-4. [CrossRef]
- Leshan RL, Bjornholm M, Munzberg H, Myers MG, Jr. Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring). 2006;14 Suppl 5:208S-12S. [CrossRef]
- Zhang J, Wang N. Leptin in chronic kidney disease: a link between hematopoiesis, bone metabolism, and nutrition. International urology and nephrology. 2014;46(6):1169-74. [CrossRef]
- Hama H, Saito A, Takeda T, Tanuma A, Xie Y, Sato K, et al. Evidence indicating that renal tubular metabolism of leptin is mediated by megalin but not by the leptin receptors. Endocrinology. 2004;145(8):3935-40. [CrossRef]
- Heimburger O, Lonnqvist F, Danielsson A, Nordenstrom J, Stenvinkel P. Serum immunoreactive leptin concentration and its relation to the body fat content in chronic renal failure. Journal of the American Society of Nephrology : JASN. 1997;8(9):1423-30. [CrossRef]
- Merabet E, Dagogo-Jack S, Coyne DW, Klein S, Santiago JV, Hmiel SP, et al. Increased plasma leptin concentration in end-stage renal disease. The Journal of clinical endocrinology and metabolism. 1997;82(3):847-50. [CrossRef]
- Nordfors L, Lonnqvist F, Heimburger O, Danielsson A, Schalling M, Stenvinkel P. Low leptin gene expression and hyperleptinemia in chronic renal failure. Kidney international. 1998;54(4):1267-75. [CrossRef]
- Pecoits-Filho R, Nordfors L, Heimburger O, Lindholm B, Anderstam B, Marchlewska A, et al. Soluble leptin receptors and serum leptin in end-stage renal disease: relationship with inflammation and body composition. European journal of clinical investigation. 2002;32(11):811-7. [CrossRef]
- Shankar A, Syamala S, Xiao J, Muntner P. Relationship between Plasma Leptin Level and Chronic Kidney Disease. International journal of nephrology. 2012;2012:269532. [CrossRef]
- Aminzadeh MA, Pahl MV, Barton CH, Doctor NS, Vaziri ND. Human uraemic plasma stimulates release of leptin and uptake of tumour necrosis factor-alpha in visceral adipocytes. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(12):3626-31. [CrossRef]
- Kalbacher E, Koppe L, Zarrouki B, Pillon NJ, Fouque D, Soulage CO. Human uremic plasma and not urea induces exuberant secretion of leptin in 3T3-L1 adipocytes. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2011;21(1):72-5. [CrossRef]
- Ambarkar M, Pemmaraju SV, Gouroju S, Manohar SM, Bitla AR, Yajamanam N, et al. Adipokines and their Relation to Endothelial Dysfunction in Patients with Chronic Kidney Disease. Journal of clinical and diagnostic research : JCDR. 2016;10(1):BC04-8. [CrossRef]
- Ding N, Liu B, Song J, Bao S, Zhen J, Lv Z, et al. Leptin promotes endothelial dysfunction in chronic kidney disease through AKT/GSK3beta and beta-catenin signals. Biochemical and biophysical research communications. 2016;480(4):544-51. [CrossRef]
- D'Elia L, Manfredi M, Perna L, Iacone R, Russo O, Strazzullo P, et al. Circulating leptin levels predict the decline in renal function with age in a sample of adult men (The Olivetti Heart Study). Internal and emergency medicine. 2019;14(4):507-13. [CrossRef]
- Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, et al. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. The Journal of clinical investigation. 1996;97(9):2152-7. [CrossRef]
- Kirchgessner TG, Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. The Journal of clinical investigation. 1997;100(11):2777-82. [CrossRef]
- Silva MI, Vale BS, Lemos CC, Torres MR, Bregman R. Body adiposity index assess body fat with high accuracy in nondialyzed chronic kidney disease patients. Obesity (Silver Spring). 2013;21(3):546-52. [CrossRef]
- Leal VO, Lobo JC, Stockler-Pinto MB, Farage NE, Calixto A, Geloneze B, et al. Apelin: a peptide involved in cardiovascular risk in hemodialysis patients? Renal failure. 2012;34(5):577-81. [CrossRef]
- Souza GC, Costa C, Scalco R, Goncalves LF, Manfro RC. Serum leptin, insulin resistance, and body fat after renal transplantation. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2008;18(6):479-88. [CrossRef]
- Kayacan SM, Yildiz A, Kazancioglu R, Sahin S, Sever MS, Ark E. The changes in serum leptin, body fat mass and insulin resistance after renal transplantation. Clinical transplantation. 2003;17(1):63-8. [CrossRef]
- El Haggan W, Chauveau P, Barthe N, Merville P, Potaux L, Aparicio M. Serum leptin, body fat, and nutritional markers during the six months post-kidney transplantation. Metabolism: clinical and experimental. 2004;53(5):614-9. [CrossRef]
- Nicoletto BB, Souza GC, Goncalves LF, Costa C, Perry IS, Manfro RC. Leptin, insulin resistance, and metabolic changes 5 years after renal transplantation. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2012;22(4):440-9. [CrossRef]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394(6696):897-901. [CrossRef]
- Sanchez-Margalet V, Martin-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clinical and experimental immunology. 2003;133(1):11-9. [CrossRef]
- Garavito P, Mosquera-Heredia MI, Fang L, Payares F, Ruiz M, Arias I, et al. Polymorphisms of leptin-melanocortin system genes associated with obesity in an adult population from Barranquilla. Biomedica : revista del Instituto Nacional de Salud. 2020;40(2):257-69. Polimorfismos de los genes del sistema leptina-melanocortina asociados con la obesidad en la poblacion adulta de Barranquilla. [CrossRef]
- Mendez-Hernandez A, Gallegos-Arreola MP, Moreno-Macias H, Espinosa Fematt J, Perez-Morales R. LEP rs7799039, LEPR rs1137101, and ADIPOQ rs2241766 and 1501299 Polymorphisms Are Associated With Obesity and Chemotherapy Response in Mexican Women With Breast Cancer. Clinical breast cancer. 2017;17(6):453-62. [CrossRef]
- Mota-Zamorano S, Luna E, Garcia-Pino G, Gonzalez LM, Gervasini G. Variability in the leptin receptor gene and other risk factors for post-transplant diabetes mellitus in renal transplant recipients. Annals of medicine. 2019;51(2):164-73. [CrossRef]
- Lian Y, Tang Z, Xie Y, Chen Z. Leptin receptor gene polymorphisms and risk of hypertension: a meta-analysis. International journal of clinical and experimental medicine. 2015;8(8):14277-82.
- Aijala M, Santaniemi M, Bloigu R, Kesaniemi YA, Ukkola O. Leptin receptor Arg109 homozygotes display decreased total mortality as well as lower incidence of cardiovascular disease and related death. Gene. 2014;534(1):88-92. [CrossRef]
- Romanowski M, Dziedziejko V, Maciejewska-Karlowska A, Sawczuk M, Safranow K, Domanski L, et al. Adiponectin and leptin gene polymorphisms in patients with post-transplant diabetes mellitus. Pharmacogenomics. 2015;16(11):1243-51. [CrossRef]
- Gervasini G, Garcia-Pino G, Mota-Zamorano S, Luna E, Garcia-Cerrada M, Tormo MA, et al. Association of polymorphisms in leptin and adiponectin genes with long-term outcomes in renal transplant recipients. The pharmacogenomics journal. 2020;20(3):388-97. [CrossRef]
- Guadalup García-Pino,, Enrique Luna,, Sonia Mota-Zamorano,, et al. Effect of leptin concentrations and leptin receptor gene polymorphisms on the outcome of renal transplantation. Archives of medical science. 2020. [CrossRef]
- Daghestani M, Purohit R, Daghistani M, Warsy A. Molecular dynamic (MD) studies on Gln233Arg (rs1137101) polymorphism of leptin receptor gene and associated variations in the anthropometric and metabolic profiles of Saudi women. PloS one. 2019;14(2):e0211381. [CrossRef]
- Lopez-Quintero A, Garcia-Zapien AG, Flores-Martinez SE, Diaz-Burke Y, Gonzalez-Sandoval CE, Lopez-Roa RI, et al. Contribution of polymorphisms in the LEP, LEPR and RETN genes on serum leptin and resistin levels in young adults from Mexico. Cell Mol Biol (Noisy-le-grand). 2017;63(8):10-8. [CrossRef]
- Foucan L, Bassien-Capsa V, Rambhojan C, Lacorte JM, Larifla L. Influence of K656N Polymorphism of the Leptin Receptor Gene on Obesity-Related Traits in Nondiabetic Afro-Caribbean Individuals. Metabolic syndrome and related disorders. 2019;17(4):197-203. [CrossRef]
- Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, Palin MF. Adiponectin action from head to toe. Endocrine. 2010;37(1):11-32. [CrossRef]
- Sharma K. The link between obesity and albuminuria: adiponectin and podocyte dysfunction. Kidney international. 2009;76(2):145-8. [CrossRef]
- Neumann E, Frommer KW, Vasile M, Muller-Ladner U. Adipocytokines as driving forces in rheumatoid arthritis and related inflammatory diseases? Arthritis and rheumatism. 2011;63(5):1159-69. [CrossRef]
- Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. The Journal of biological chemistry. 2003;278(41):40352-63. [CrossRef]
- Achari AE, Jain SK. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. International journal of molecular sciences. 2017;18(6). [CrossRef]
- Kim Y, Park CW. Mechanisms of Adiponectin Action: Implication of Adiponectin Receptor Agonism in Diabetic Kidney Disease. International journal of molecular sciences. 2019;20(7). [CrossRef]
- Jorsal A, Tarnow L, Frystyk J, Lajer M, Flyvbjerg A, Parving HH, et al. Serum adiponectin predicts all-cause mortality and end stage renal disease in patients with type I diabetes and diabetic nephropathy. Kidney international. 2008;74(5):649-54. [CrossRef]
- Heidari M, Nasri P, Nasri H. Adiponectin and chronic kidney disease; a review on recent findings. Journal of nephropharmacology. 2015;4(2):63-8.
- Kuo IC, Wu PH, Lin HY, Niu SW, Huang JC, Hung CC, et al. The association of adiponectin with metabolic syndrome and clinical outcome in patients with non-diabetic chronic kidney disease. PloS one. 2019;14(7):e0220158. [CrossRef]
- DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J. Insulin resistance in uremia. The Journal of clinical investigation. 1981;67(2):563-8. [CrossRef]
- Martinez Cantarin MP, Waldman SA, Doria C, Frank AM, Maley WR, Ramirez CB, et al. The adipose tissue production of adiponectin is increased in end-stage renal disease. Kidney international. 2013;83(3):487-94. [CrossRef]
- Martinez Cantarin MP, Keith SW, Waldman SA, Falkner B. Adiponectin receptor and adiponectin signaling in human tissue among patients with end-stage renal disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(12):2268-77. [CrossRef]
- Markaki A, Psylinakis E, Spyridaki A. Adiponectin and end-stage renal disease. Hormones (Athens). 2016;15(3):345-54. [CrossRef]
- Chudek J, Adamczak M, Karkoszka H, Budzinski G, Ignacy W, Funahashi T, et al. Plasma adiponectin concentration before and after successful kidney transplantation. Transplantation proceedings. 2003;35(6):2186-9. [CrossRef]
- Idorn T, Hornum M, Bjerre M, Jorgensen KA, Nielsen FT, Hansen JM, et al. Plasma adiponectin before and after kidney transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2012;25(11):1194-203. [CrossRef]
- Cortazar F, Molnar MZ, Isakova T, Czira ME, Kovesdy CP, Roth D, et al. Clinical outcomes in kidney transplant recipients receiving long-term therapy with inhibitors of the mammalian target of rapamycin. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(2):379-87. [CrossRef]
- Alam A, Molnar MZ, Czira ME, Rudas A, Ujszaszi A, Kalantar-Zadeh K, et al. Serum adiponectin levels and mortality after kidney transplantation. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(3):460-7. [CrossRef]
- Okamoto Y, Christen T, Shimizu K, Asano K, Kihara S, Mitchell RN, et al. Adiponectin inhibits allograft rejection in murine cardiac transplantation. Transplantation. 2009;88(7):879-83. [CrossRef]
- Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochemical and biophysical research communications. 2004;323(2):630-5. [CrossRef]
- Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nature medicine. 2005;11(10):1096-103. [CrossRef]
- Wu J, Liu Z, Meng K, Zhang L. Association of adiponectin gene (ADIPOQ) rs2241766 polymorphism with obesity in adults: a meta-analysis. PloS one. 2014;9(4):e95270. [CrossRef]
- Dastani Z, Hivert MF, Timpson N, Perry JR, Yuan X, Scott RA, et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS genetics. 2012;8(3):e1002607. [CrossRef]
- Canale MP, Manca di Villahermosa S, Martino G, Rovella V, Noce A, De Lorenzo A, et al. Obesity-related metabolic syndrome: mechanisms of sympathetic overactivity. International journal of endocrinology. 2013;2013:865965. [CrossRef]
- Kang ES, Magkos F, Kim BS, Zhai R, Su L, Kim YS, et al. Variants of the adiponectin and adiponectin receptor-1 genes and posttransplantation diabetes mellitus in renal allograft recipients. The Journal of clinical endocrinology and metabolism. 2012;97(1):E129-35. [CrossRef]
- Abraham NG, Drummond G. CD163-Mediated hemoglobin-heme uptake activates macrophage HO-1, providing an antiinflammatory function. Circulation research. 2006;99(9):911-4. [CrossRef]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiological reviews. 2006;86(2):583-650. [CrossRef]
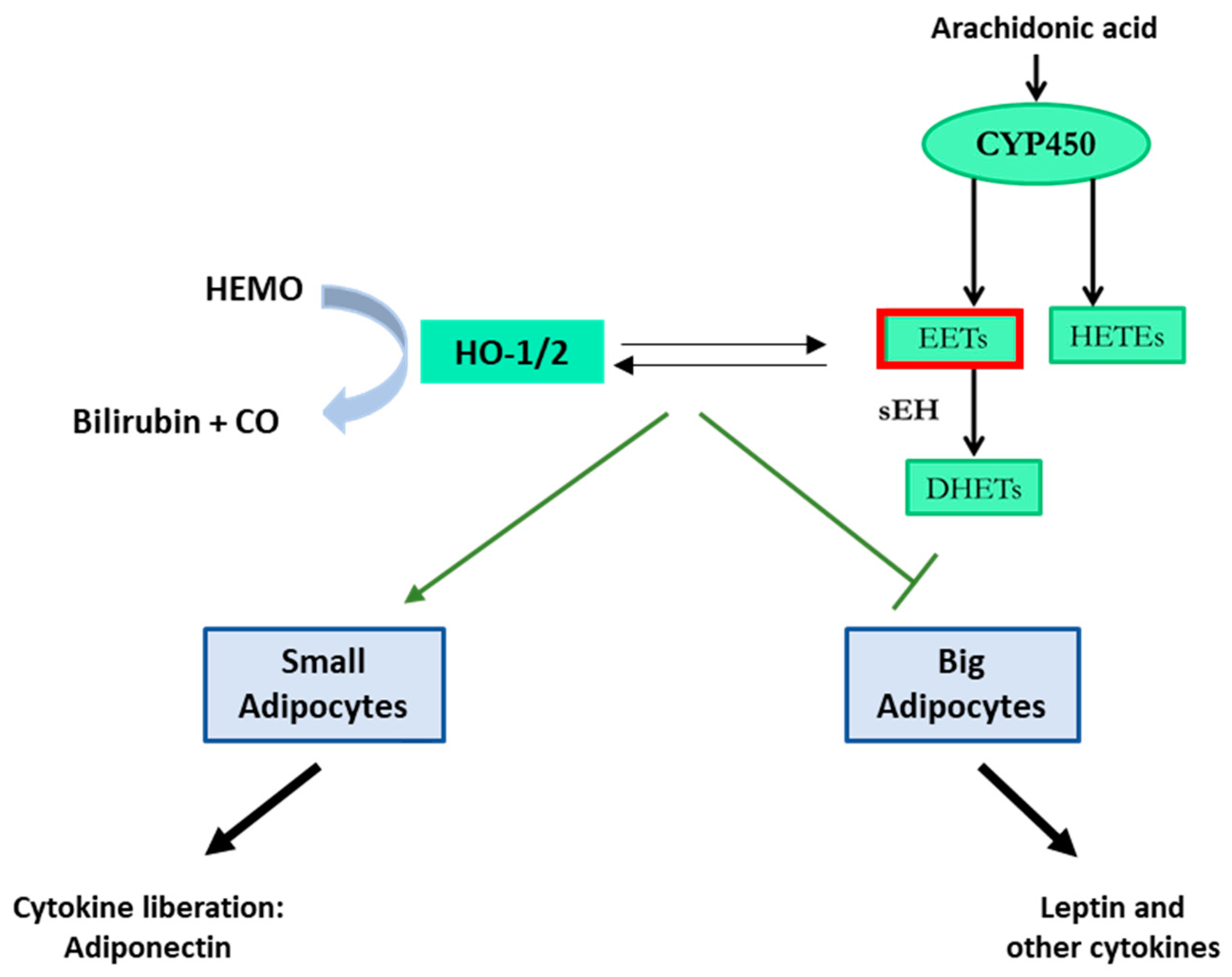
- Burgess A, Vanella L, Bellner L, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acids and heme oxygenase-1 interaction attenuates diabetes and metabolic syndrome complications. Prostaglandins & other lipid mediators. 2012;97(1-2):1-16. [CrossRef]
- Burgess AP, Vanella L, Bellner L, Gotlinger K, Falck JR, Abraham NG, et al. Heme oxygenase (HO-1) rescue of adipocyte dysfunction in HO-2 deficient mice via recruitment of epoxyeicosatrienoic acids (EETs) and adiponectin. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2012;29(1-2):99-110. [CrossRef]
- Dai M, Wu L, Wang P, Wen Z, Xu X, Wang DW. CYP2J2 and Its Metabolites EETs Attenuate Insulin Resistance via Regulating Macrophage Polarization in Adipose Tissue. Scientific reports. 2017;7:46743. [CrossRef]
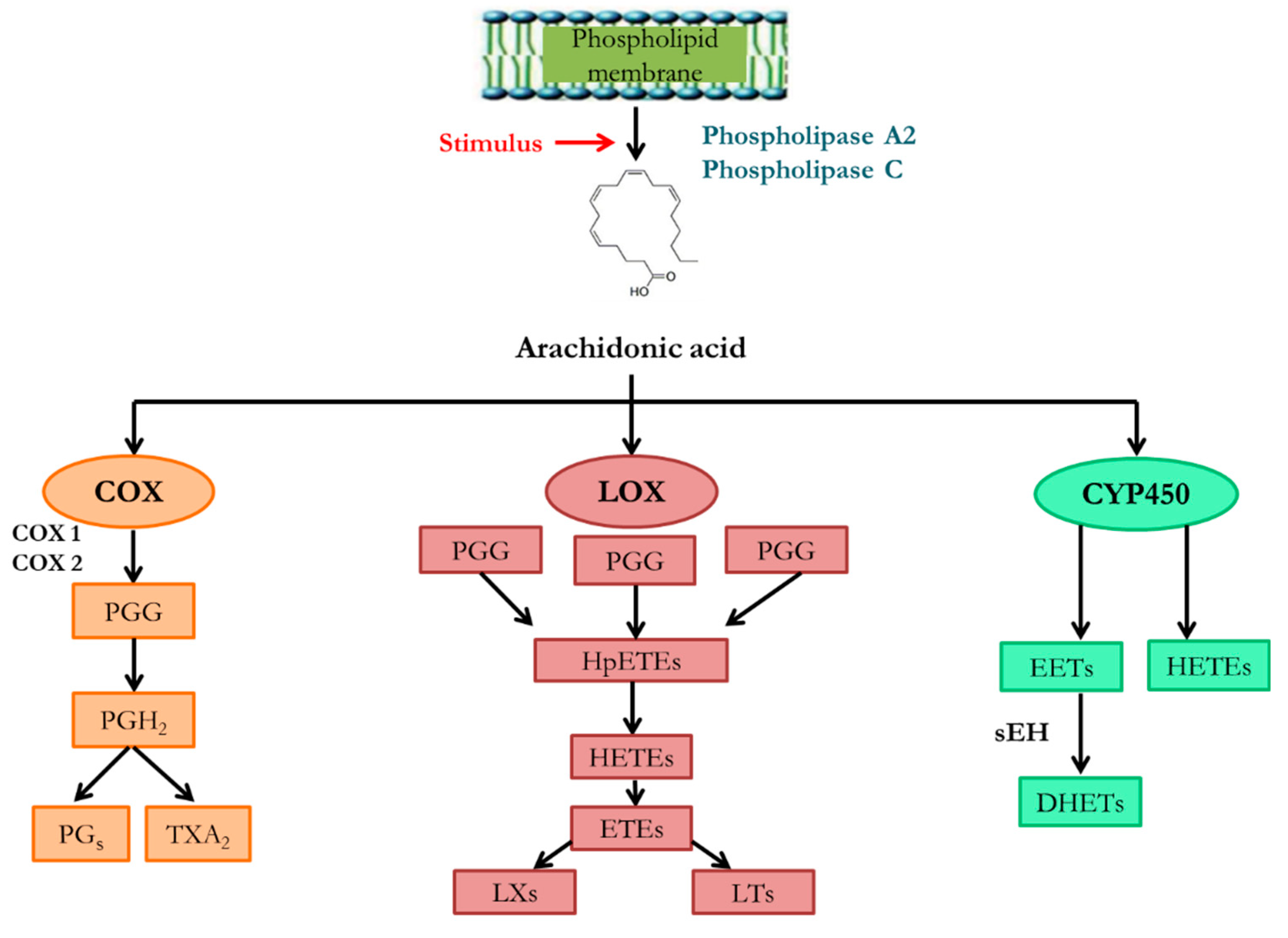
- Wang T, Fu X, Chen Q, Patra JK, Wang D, Wang Z, et al. Arachidonic Acid Metabolism and Kidney Inflammation. International journal of molecular sciences. 2019;20(15). [CrossRef]
- Capdevila J, Chacos N, Werringloer J, Prough RA, Estabrook RW. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(9):5362-6. [CrossRef]
- Lasker JM, Chen WB, Wolf I, Bloswick BP, Wilson PD, Powell PK. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. The Journal of biological chemistry. 2000;275(6):4118-26. [CrossRef]
- Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circulation research. 2000;87(11):992-8. [CrossRef]
- Sporkova A, Kopkan L, Varcabova S, Huskova Z, Hwang SH, Hammock BD, et al. Role of cytochrome P-450 metabolites in the regulation of renal function and blood pressure in 2-kidney 1-clip hypertensive rats. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300(6):R1468-75. [CrossRef]
- Imig JD. Orally active epoxyeicosatrienoic acid analogs in hypertension and renal injury. Adv Pharmacol. 2022;94:27-55. [CrossRef]
- Fan F, Ge Y, Lv W, Elliott MR, Muroya Y, Hirata T, et al. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci (Landmark Ed). 2016;21(7):1427-63. [CrossRef]
- Hoopes SL, Garcia V, Edin ML, Schwartzman ML, Zeldin DC. Vascular actions of 20-HETE. Prostaglandins & other lipid mediators. 2015;120:9-16. [CrossRef]
- Stegall MD, Gaston RS, Cosio FG, Matas A. Through a glass darkly: seeking clarity in preventing late kidney transplant failure. Journal of the American Society of Nephrology : JASN. 2015;26(1):20-9. [CrossRef]
- Chapman JR. Progress in Transplantation: Will It Be Achieved in Big Steps or by Marginal Gains? American journal of kidney diseases : the official journal of the National Kidney Foundation. 2017;69(2):287-95. [CrossRef]
- Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S, et al. OPTN/SRTR 2018 Annual Data Report: Kidney. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2020;20 Suppl s1:20-130. [CrossRef]
- Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nature medicine. 2011;17(11):1391-401. [CrossRef]
- Rodrigues-Diez R, Gonzalez-Guerrero C, Ocana-Salceda C, Rodrigues-Diez RR, Egido J, Ortiz A, et al. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. Scientific reports. 2016;6:27915. [CrossRef]
- Jourde-Chiche N, Fakhouri F, Dou L, Bellien J, Burtey S, Frimat M, et al. Endothelium structure and function in kidney health and disease. Nature reviews Nephrology. 2019;15(2):87-108. [CrossRef]
- Rangaswami J, Mathew RO, Parasuraman R, Tantisattamo E, Lubetzky M, Rao S, et al. Cardiovascular disease in the kidney transplant recipient: epidemiology, diagnosis and management strategies. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2019;34(5):760-73. [CrossRef]
- Duflot T, Laurent C, Soudey A, Fonrose X, Hamzaoui M, Iacob M, et al. Preservation of epoxyeicosatrienoic acid bioavailability prevents renal allograft dysfunction and cardiovascular alterations in kidney transplant recipients. Scientific reports. 2021;11(1):3739. [CrossRef]
- Dolegowska B, Blogowski W, Domanski L. Is it possible to predict the early post-transplant allograft function using 20-HETE measurements? A preliminary report. Transplant international : official journal of the European Society for Organ Transplantation. 2009;22(5):546-53. [CrossRef]
- King LM, Ma J, Srettabunjong S, Graves J, Bradbury JA, Li L, et al. Cloning of CYP2J2 gene and identification of functional polymorphisms. Molecular pharmacology. 2002;61(4):840-52. [CrossRef]
- Smith HE, Jones JP, 3rd, Kalhorn TF, Farin FM, Stapleton PL, Davis CL, et al. Role of cytochrome P450 2C8 and 2J2 genotypes in calcineurin inhibitor-induced chronic kidney disease. Pharmacogenetics and genomics. 2008;18(11):943-53. [CrossRef]
- Bahadur N, Leathart JB, Mutch E, Steimel-Crespi D, Dunn SA, Gilissen R, et al. CYP2C8 polymorphisms in Caucasians and their relationship with paclitaxel 6alpha-hydroxylase activity in human liver microsomes. Biochemical pharmacology. 2002;64(11):1579-89. [CrossRef]
- Yu L, Shi D, Ma L, Zhou Q, Zeng S. Influence of CYP2C8 polymorphisms on the hydroxylation metabolism of paclitaxel, repaglinide and ibuprofen enantiomers in vitro. Biopharmaceutics & drug disposition. 2013;34(5):278-87. [CrossRef]
- Spiecker M, Liao J. Cytochrome P450 epoxygenase CYP2J2 and the risk of coronary artery disease. Trends in cardiovascular medicine. 2006;16(6):204-8. [CrossRef]
- Dai D, Zeldin DC, Blaisdell JA, Chanas B, Coulter SJ, Ghanayem BI, et al. Polymorphisms in human CYP2C8 decrease metabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics. 2001;11(7):597-607. [CrossRef]
- Spiecker M, Darius H, Hankeln T, Soufi M, Sattler AM, Schaefer JR, et al. Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2. Circulation. 2004;110(15):2132-6. [CrossRef]
- Liu PY, Li YH, Chao TH, Wu HL, Lin LJ, Tsai LM, et al. Synergistic effect of cytochrome P450 epoxygenase CYP2J2*7 polymorphism with smoking on the onset of premature myocardial infarction. Atherosclerosis. 2007;195(1):199-206. [CrossRef]
- Genvigir FDV, Campos-Salazar AB, Felipe CR, Tedesco-Silva H, Jr., Medina-Pestana JO, Doi SQ, et al. CYP3A5*3 and CYP2C8*3 variants influence exposure and clinical outcomes of tacrolimus-based therapy. Pharmacogenomics. 2020;21(1):7-21. [CrossRef]
- Mota-Zamorano S, Robles NR, Gonzalez LM, Valdivielso JM, Lopez-Gomez J, Cancho B, et al. Genetics Variants in the Epoxygenase Pathway of Arachidonic Metabolism Are Associated with Eicosanoids Levels and the Risk of Diabetic Nephropathy. Journal of clinical medicine. 2021;10(17). [CrossRef]
- Lee CR, Pretorius M, Schuck RN, Burch LH, Bartlett J, Williams SM, et al. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with forearm vasodilator responses in humans. Hypertension. 2011;57(1):116-22. [CrossRef]
- Gervasini G, Garcia-Cerrada M, Coto E, Vergara E, Garcia-Pino G, Alvarado R, et al. A 3'-UTR Polymorphism in Soluble Epoxide Hydrolase Gene Is Associated with Acute Rejection in Renal Transplant Recipients. PloS one. 2015;10(7):e0133563. [CrossRef]
- Lee SH, Lee J, Cha R, Park MH, Ha JW, Kim S, et al. Genetic variations in soluble epoxide hydrolase and graft function in kidney transplantation. Transplantation proceedings. 2008;40(5):1353-6. [CrossRef]
- Ma L, Zhao H, Yu M, Wen Y, Zhao T, Yan M, et al. Association of Epoxide Hydrolase 2 Gene Arg287Gln with the Risk for Primary Hypertension in Chinese. International journal of hypertension. 2020;2020:2351547. [CrossRef]
- Shuey MM, Billings FTt, Wei S, Milne GL, Nian H, Yu C, et al. Association of gain-of-function EPHX2 polymorphism Lys55Arg with acute kidney injury following cardiac surgery. PloS one. 2017;12(5):e0175292. [CrossRef]
- Stec DE, Roman RJ, Flasch A, Rieder MJ. Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiological genomics. 2007;30(1):74-81. [CrossRef]
- Hu BC, Li Y, Li FH, Zhang Y, Sheng CS, Fan HQ, et al. Peripheral and central augmentation indexes in relation to the CYP4F2 polymorphisms in Chinese. Journal of hypertension. 2011;29(3):501-8. [CrossRef]
- Ward NC, Croft KD, Puddey IB, Phillips M, van Bockxmeer F, Beilin LJ, et al. The effect of a single nucleotide polymorphism of the CYP4F2 gene on blood pressure and 20-hydroxyeicosatetraenoic acid excretion after weight loss. Journal of hypertension. 2014;32(7):1495-502; discussion 502. [CrossRef]
- Mota-Zamorano S, Gonzalez LM, Luna E, Fernandez JJ, Gomez A, Nieto-Fernandez A, et al. Polymorphisms in vasoactive eicosanoid genes of kidney donors affect biopsy scores and clinical outcomes in renal transplantation. PloS one. 2019;14(10):e0224129. [CrossRef]
- Gervasini G, Luna E, Garcia-Cerrada M, Garcia-Pino G, Cubero JJ. Risk factors for post-transplant diabetes mellitus in renal transplant: Role of genetic variability in the CYP450-mediated arachidonic acid metabolism. Molecular and cellular endocrinology. 2016;419:158-64. [CrossRef]
- Fava C, Montagnana M, Almgren P, Rosberg L, Lippi G, Hedblad B, et al. The V433M variant of the CYP4F2 is associated with ischemic stroke in male Swedes beyond its effect on blood pressure. Hypertension. 2008;52(2):373-80. [CrossRef]
- Hermann M, Hellermann JP, Quitzau K, Hoffmann MM, Gasser T, Meinertz T, et al. CYP4A11 polymorphism correlates with coronary endothelial dysfunction in patients with coronary artery disease--the ENCORE Trials. Atherosclerosis. 2009;207(2):476-9. [CrossRef]
- Gervasini G, Garcia-Cerrada M, Vergara E, Garcia-Pino G, Alvarado R, Fernandez-Cavada MJ, et al. Polymorphisms in CYP-mediated arachidonic acid routes affect the outcome of renal transplantation. European journal of clinical investigation. 2015;45(10):1060-8. [CrossRef]
- Greco M, De Santo M, Comande A, Belsito EL, Ando S, Liguori A, et al. Leptin-Activity Modulators and Their Potential Pharmaceutical Applications. Biomolecules. 2021;11(7). [CrossRef]
- Jonas M, Simon AJ, Gilburd B, Schneiderman J. Intrarenal Anti-Leptin Treatment Attenuates Ischemia and Reperfusion Injury. American journal of nephrology. 2023;54(7-8):337-48. [CrossRef]
- Cheung WW, Ding W, Gunta SS, Gu Y, Tabakman R, Klapper LN, et al. A pegylated leptin antagonist ameliorates CKD-associated cachexia in mice. Journal of the American Society of Nephrology : JASN. 2014;25(1):119-28. [CrossRef]
- Gholamin S, Razavi SM, Taghavi-Garmestani SM, Ghorbanihaghjo A, Rashtchizadeh N, Safa J, et al. Lovastatin for reduction of leptin in nondialysis patients with type 2 diabetic nephropathy. Iranian journal of kidney diseases. 2014;8(3):201-6.
- Villasis-Keever MA, Zurita-Cruz JN, Serret-Montoya J, Barbosa-Cortes L, Zepeda-Martinez CDC, Alegria-Torres G, et al. The leptin/adiponectin ratio as prognostic marker for dyslipidemia during 1 year of follow-up in pediatric patients receiving kidney replacement therapy. Nutricion hospitalaria. 2022;39(5):977-87. Indice leptina/adiponectina como indicador pronostico de dislipidemia durante 1 ano de seguimiento en pacientes pediatricos que reciben terapia de reemplazo renal. [CrossRef]
- Coimbra S, Rocha S, Valente MJ, Catarino C, Bronze-da-Rocha E, Belo L, et al. New Insights into Adiponectin and Leptin Roles in Chronic Kidney Disease. Biomedicines. 2022;10(10). [CrossRef]
- Tian M, Tang L, Wu Y, Beddhu S, Huang Y. Adiponectin attenuates kidney injury and fibrosis in deoxycorticosterone acetate-salt and angiotensin II-induced CKD mice. American journal of physiology Renal physiology. 2018;315(3):F558-F71. [CrossRef]
- Zha D, Wu X, Gao P. Adiponectin and Its Receptors in Diabetic Kidney Disease: Molecular Mechanisms and Clinical Potential. Endocrinology. 2017;158(7):2022-34. [CrossRef]
- HC. Soluble epoxide hydrolase inhibitors: a patent review. Expert opinion on therapeutic patents. 2010;20(7):941-56. [CrossRef]
- Noh MR, Jang HS, Salem FE, Ferrer FA, Kim J, Padanilam BJ. Epoxyeicosatrienoic acid administration or soluble epoxide hydrolase inhibition attenuates renal fibrogenesis in obstructive nephropathy. American journal of physiology Renal physiology. 2023;324(2):F138-F51. [CrossRef]
- Papiashvili N, Gongadze N, Bakuridze A, Bakuridze K. Antihypertensive and Cardioprotective Effects of Epoxyeicosatrienoic Acid Analogs and Soluble Epoxide Hydrolase Inhibitors (Review). Georgian medical news. 2021(312):125-32.
- Chen G, Xu R, Wang Y, Wang P, Zhao G, Xu X, et al. Genetic disruption of soluble epoxide hydrolase is protective against streptozotocin-induced diabetic nephropathy. American journal of physiology Endocrinology and metabolism. 2012;303(5):E563-75. [CrossRef]
- Sun CP, Zhang XY, Morisseau C, Hwang SH, Zhang ZJ, Hammock BD, et al. Discovery of Soluble Epoxide Hydrolase Inhibitors from Chemical Synthesis 180and Natural Products. Journal of medicinal chemistry. 2021;64(1):184-215. [CrossRef]
- Burmistrov V, Morisseau C, Danilov D, Harris TR, Dalinger I, Vatsadze I, et al. 1,3-Disubstituted and 1,3,3-trisubstituted adamantyl-ureas with isoxazole as soluble epoxide hydrolase inhibitors. Bioorganic & medicinal chemistry letters. 2015;25(23):5514-9. [CrossRef]
- Zhang G, Panigrahy D, Hwang SH, Yang J, Mahakian LM, Wettersten HI, et al. Dual inhibition of cyclooxygenase-2 and soluble epoxide hydrolase synergistically suppresses primary tumor growth and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(30):11127-32. [CrossRef]
- Oliveira CS, Giuffrida FM, Crispim F, Saddi-Rosa P, Reis AF. ADIPOQ and adiponectin: the common ground of hyperglycemia and coronary artery disease? Arquivos brasileiros de endocrinologia e metabologia. 2011;55(7):446-54. [CrossRef]
- Hao JQ, Zhang QK, Zhou YX, Chen LH, Wu PF. Association between circulating leptin concentration and G-2548A gene polymorphism in patients with breast cancer: a meta-analysis. Archives of medical science : AMS. 2019;15(2):275-83. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




