The partition function serves as the link between thermodynamics and statistical mechanics, connecting these two fields through the framework of quantum mechanics. By utilizing the partition function, various thermodynamic functions like entropies, enthalpies, and heat capacities can be computed.
2.1. Overview of Methods Used to Calculate the Partition Function
This section presents a summary of various studies that use the partition function in different forms. It describes the simplifications and approximation methods employed, the molecular constants used, and the recommended temperature range. It is important to note that extrapolating data beyond the recommended range, despite its common occurrence, is not advisable. Our data and formulas, however, can be applied throughout the full temperature range of 100 K to 6000 K with minimal deviation.
In Tatum’s research [
23], 14 diatomic molecules were examined, and their partition function was calculated using their dissociation equilibrium constants within a temperature range of 1000 K to 8000 K. His findings revealed that only four molecular constants were utilized to estimate the partition functions. Additionally, a cut-off was implemented at
for the upper energy. These four molecular constants were (
;
;
,
).
Other study done by Irwin [
24] who used the partition function in the form of polynomials,
To calculate the partition function for numerous atomic and molecular species within the temperature range of 1000 K to 16 000 K, a least-squares fitting method was employed for each species to determine the coefficients in Equation (
1). However, for other molecular data, extrapolation was used and their weight was reduced by a factor of 10
6 prior to fitting the coefficients. Although Irwin stated that these least-squares fits have small errors, using linear extrapolation for non-linear data generates significant errors, and the magnitude of these errors was not estimated. Bohn and Wolf [
26] developed a new approximate formulation for the partition function and applied it to two diatomic molecules, H
2 and CO, in the temperature range of T = 1000-6000 K. Using a limited number of molecular constants (
,
,
,
) and accounting only for the electronic ground state, they computed specific heat capacity (c
V) and internal energy (E
int) for these two molecules. Sauval and Tatum [
28] evaluated the total internal partition functions for 300 diatomic molecules, 69 neutral atoms, and 19 positively charged ions. The approximated partition function polynomial for molecules and atoms is valid for a certain temperature range from 1000 K to 9000 K and has the following expression:
The molecular constants of all of these molecules, atoms, and ions are taken from [
27].
In 1985, Rossi et al. [
29] presented total molecular partition functions for 53 molecular species using the first few molecular constants (
,
,
,
) which are taken from Ref. [
27] in the temperature range of 1000 K to 5000 K. The authors used rigid-rotor approximation in the calculation of Q
J while they claimed that they are not. However, they allow the interaction between vibration and rotation. They used polynomial approximations in order to find the “exact” specific heat which behave near the origin and more suitable to approximation schemes. Then, by integration they can obtain the partition function as follows:
where
to
are coefficients. Accordingly, the specific heat approximation is
Kurucz [
30] commented that the approximate expressions for the partition functions used and reported in previous papers are not rigorous enough for H
2 and CO. This is because they did not properly track the number of bound levels and did not include higher-order coefficients. Data for H
2 were formulated and used from [
31]. Kurucz summed over all the energies of the three lowest electronic states for both diatomic molecules and tabulated polynomial fits for the molecular partition functions from 1000 K to 9000 K with steps of 100 K.
Irwin [
32] also studied the internal partition functions of H
2 and CO in the same temperature range. He found that the errors for H
2 at 4000 K are larger than for CO by approximately 2%. He used the electronic ground state of H
2 and evaluated Y
using the least-squares fitting method of Dunham series with the energy data from Ref. [
31]. Irwin compared his evaluations with those of Kurucz [
30] and Sauval [
28] and found that his values of Q are higher, which he attributed to the number of electronic ground states included and the higher rotational level treatments used.
Pagano et al [
33] derived internal partition functions and thermodynamic properties of Jupiter-atmosphere species in a wide range of temperatures from 50 to 50 000 K. The results are presumably self-consistent, with E(v; J
max)
for each
v, in terms of maximum rotational states for each vibrational level.
In 2011, by using the methods published in 2003 and 2000 by Fischer [
35] and Gamache [
36] respectively, laraia et al. [
34] presented molecular partition functions for certain molecules with their isotopes in the temperature range 70-3000 K. Vibrational partition functions were evaluated and determined using the approximation of the harmonic oscillator [
39], however, rotational partition function were calculated as in Ref. [
37,
38]. In this study, all state-dependent and state-independent degeneracy factors were considered and taken into account. The molecular partition function of H2 was evaluated using direct summation and ab initio energies. In addition, for the temperature range with the intervals of 25 K, four-point Lagrange interpolation was used. Colonna et al. [
40] described the statistical thermodynamics of H
2 molecules in normal ortho/para mixture and determined its internal partition function on rigorous basis. Consequently, solving directly the existing ambiguity of those quantities in the definition related to the partition functions. Moreover, thermodynamic properties obtained from internal partition function for temperature range of 5 K to 10 000 K are found.
2.2. Model
The Born-Oppenheimer approximation posits that rotational, vibrational, and electronic energies of a molecule are independent of each other. As a result, the partition function of a molecule can be expressed using the standard formula of statistical mechanics by multiplying the translational, rotational, vibrational, and electronic contributions.
where
,
,
and
stand for translational, rotational, vibrational and electronic partition functions, respectively.
This total internal partition function can be written in this form by taking the assumption that the internal rotations within molecule are ignored. Moreover, Bohn and Wolf [
26] illustrated that the electronic state for molecules is non-degenerate since the electronic state energy typically is higher than the dissociation energy. Therefore,
.
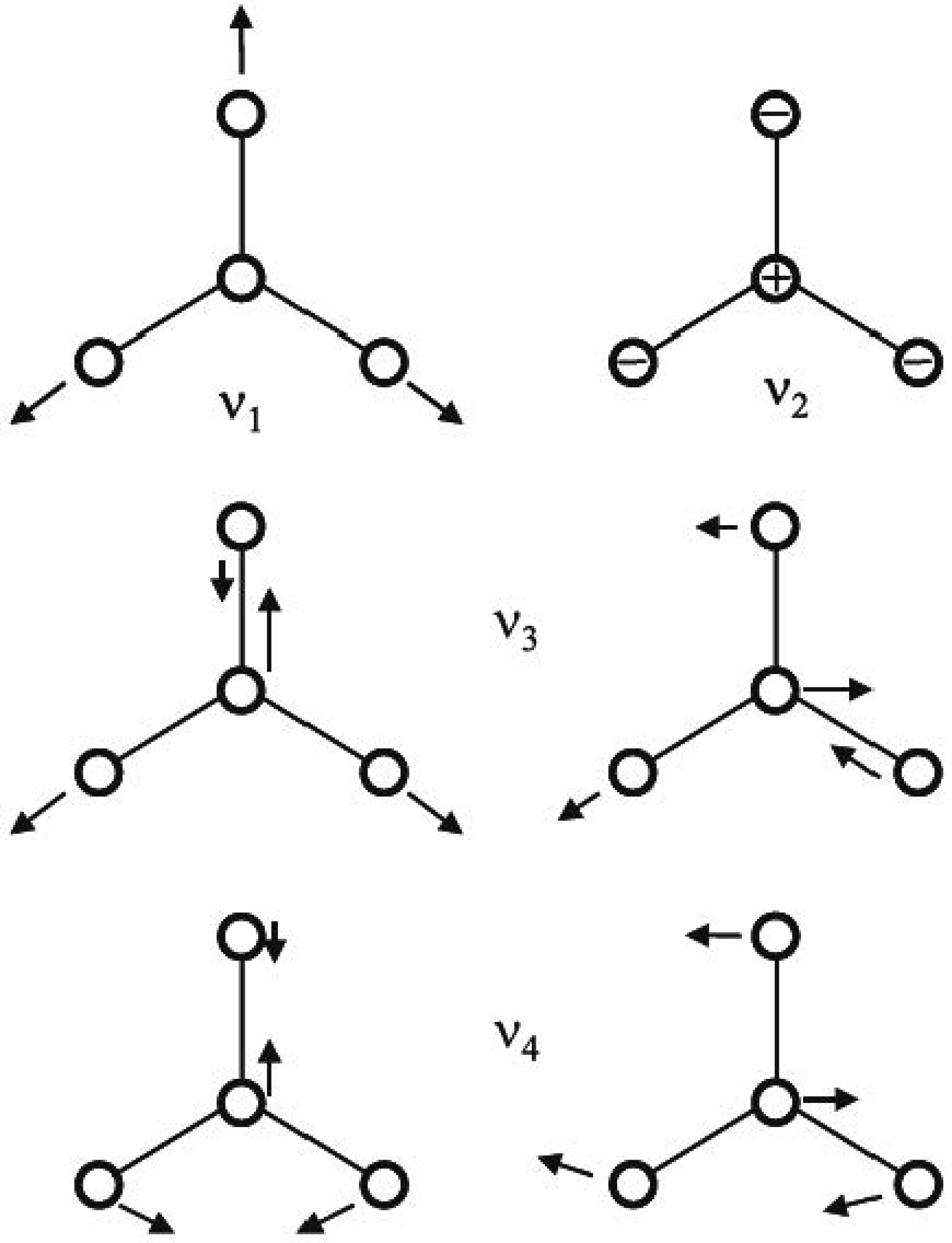
The BF3 molecule is a molecule with trigonal planar geometry where Boron atom at the center and Fluorides are peripheral atoms at the corners, all in one plane. This molecular geometry molecule’s point group is D
3h. BF3 has six possible symmetry types (six vibrations) and they are represented as follows:
B-F stretching vibrations are totally symmetric and cannot be combined with other molecular coordinates in or/and out of plane.
is the only asymmetric vibration represented in
. Consequently, the out-of-plane B-F bending vibrations belonging to this type which is clearly different and cannot be mixed with other coordinates. However, the
vibration has two degeneracies which are in-plane bending B-F and asymmetric stretching vibrations with different energies and can be mixed partially. In the infrared (IR) spectrum,
and
vibration types are active, and the other two vibrations
and
are active in the Raman spectrum. Therefore, there are only four main vibrations of different frequencies that can occur in the vibrational spectrum of BF
3 molecule. These vibrations are represented in
Figure 1.
The vibrational partition function is given by
where
is the vibrational partition function corresponding to the symmetric stretching mode.
is the vibrational partition function corresponding to the antisymmetric stretching and bending modes.
Due to the anharmonicity effects, the symmetric stretching vibrational modes are dominant comparing to the other vibrational modes [
41] and in order to reduce the computational costs, we choose the improved Tietz oscillator [
43] to describe the internal symmetric stretching vibrations, and use the harmonic oscillator for the description of the antisymmetric stretching vibration and the bending vibration.
The improved Tietz oscillator is given by [
43] as:
where
is the dissociation energy,
is the equilibrium distance,
r is the internuclear separation,
is a dimensionless constant which controls the range of the interaction between atoms.
Applying the vibrational partition function given in Ref. [
44] for the improved Tietz oscillator, we formulate the vibrational partition function corresponding to the symmetric stretching mode for BF
3 as follows,
where
is the dissociation energy of BF
3 into BF and F
2,
,
is the Boltzmann’s constant, T is the temperature,
,
,
,
and
.
The potential parameter
is given by the expression:
where c is the speed of light,
denotes the symmetric stretching vibration frequency and W is the Lambert function, which satisfies
[
45].
q is a dimensionless constant given by [
46]
When , the Tietz oscillator turns to improved Rosen-Morse potential and improved Manning-Rosen potential respectively.
denotes the most vibrational quantum number and is given by
where Int stands for the integer part function. The imaginary error function, denoted erfi, is defined as [
47]
where
denotes the error function, which is a special function of sigmoid shape.
We use the harmonic oscillator to describe the antisymmetric stretching and bending vibrations of the BF
3 molecule. The vibrational partition function corresponding to these three vibration modes is expressed as follows,
where
,
and
represent the antisymmetric stretching vibrational and bending vibrational frequencies for the BF
3 molecule.
We made two simplifications in this model which are the intermolecular interactions are considered to be very weak and not taking into account and the second one is using the treatment of the rigid rotors of diatomic molecules. By minimizing computation costs and keeping the accuracy requirements in engineering applications, we do not take into account the effects of the nuclear spin of protons and centrifugal distortion in the treatment of rotation. It follows that the translational and rotational partition functions for BF
3 molecule can be represented as [
48]:
where
denotes the molecular mass of BF
3 and P is the atmospheric pressure.
(i = x, y, z) denote three rotational characteristic temperatures. , and represent the rotational moments of inertia for the BF3 molecule around three main axes where is the bond angle FBF. = 6, is the number of symmetry types for the BF3 molecule.
The total partition function for BF
3 is readily written as
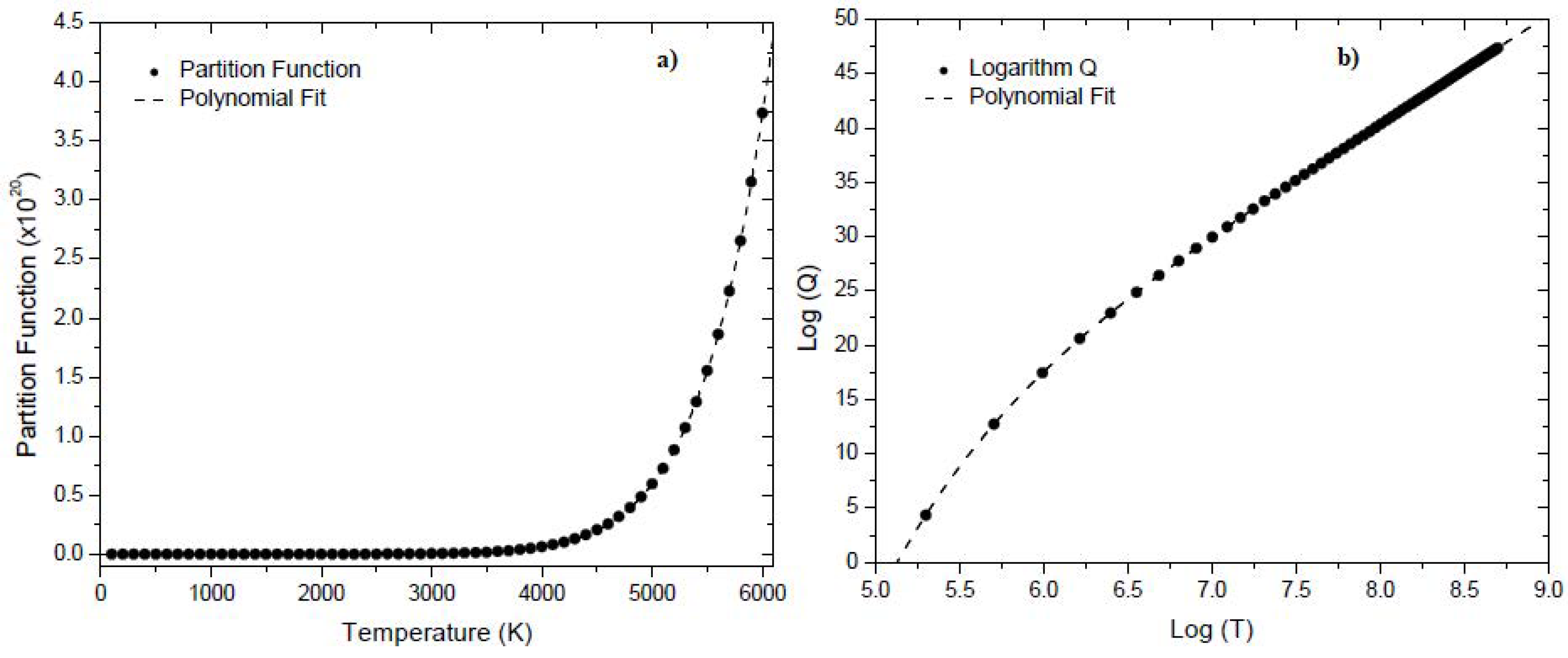
The values of the boron trifluoride partition function can be determined from analytical expressions (
17) by inputting the experimental values of
,
,
,
,
, and
. The partition function of boron trifluoride are the functions of pressure, temperature and several molecular constants, and do not depend on a large quantity of calorimetric data or theoretical and experimental spectroscopy data.
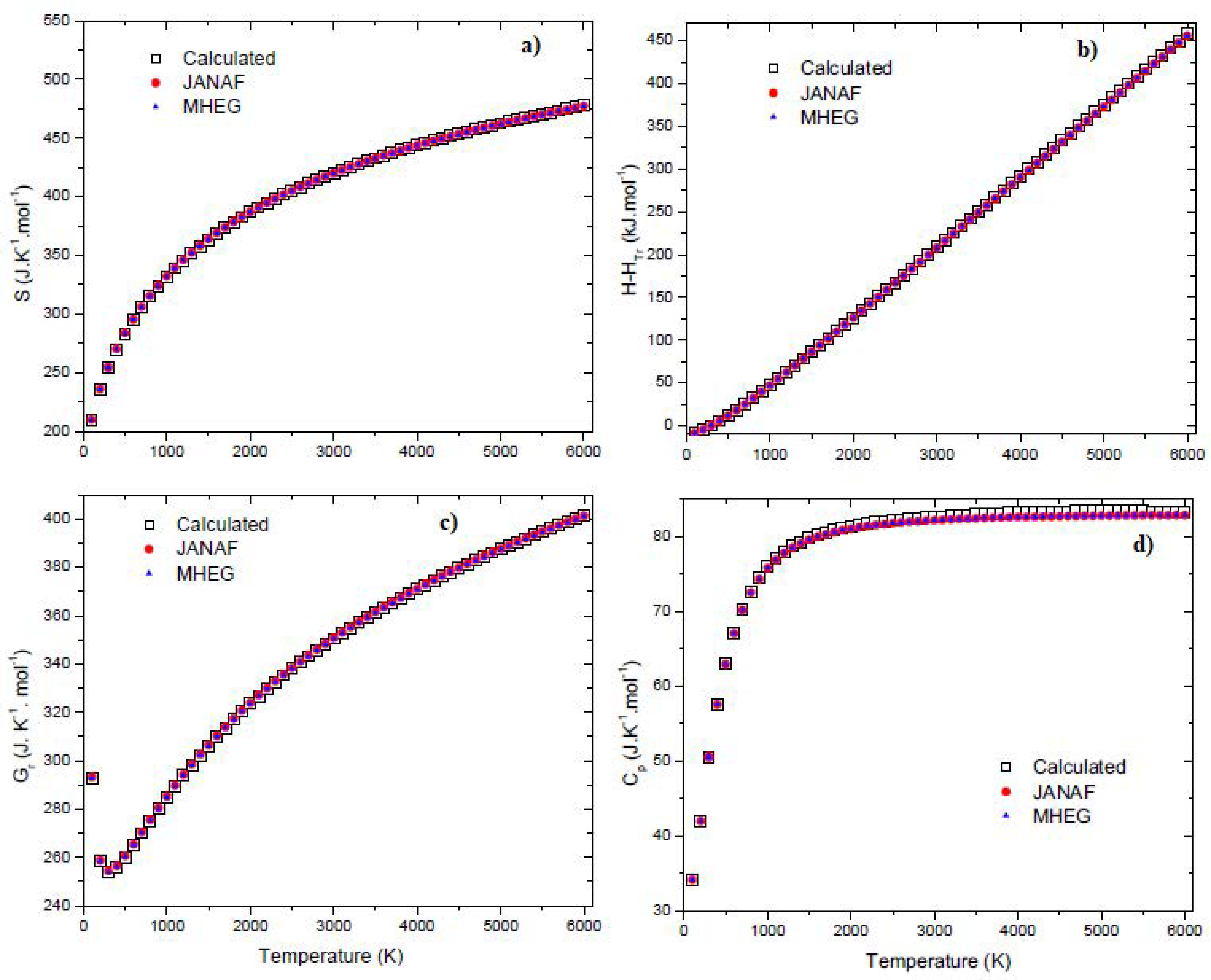
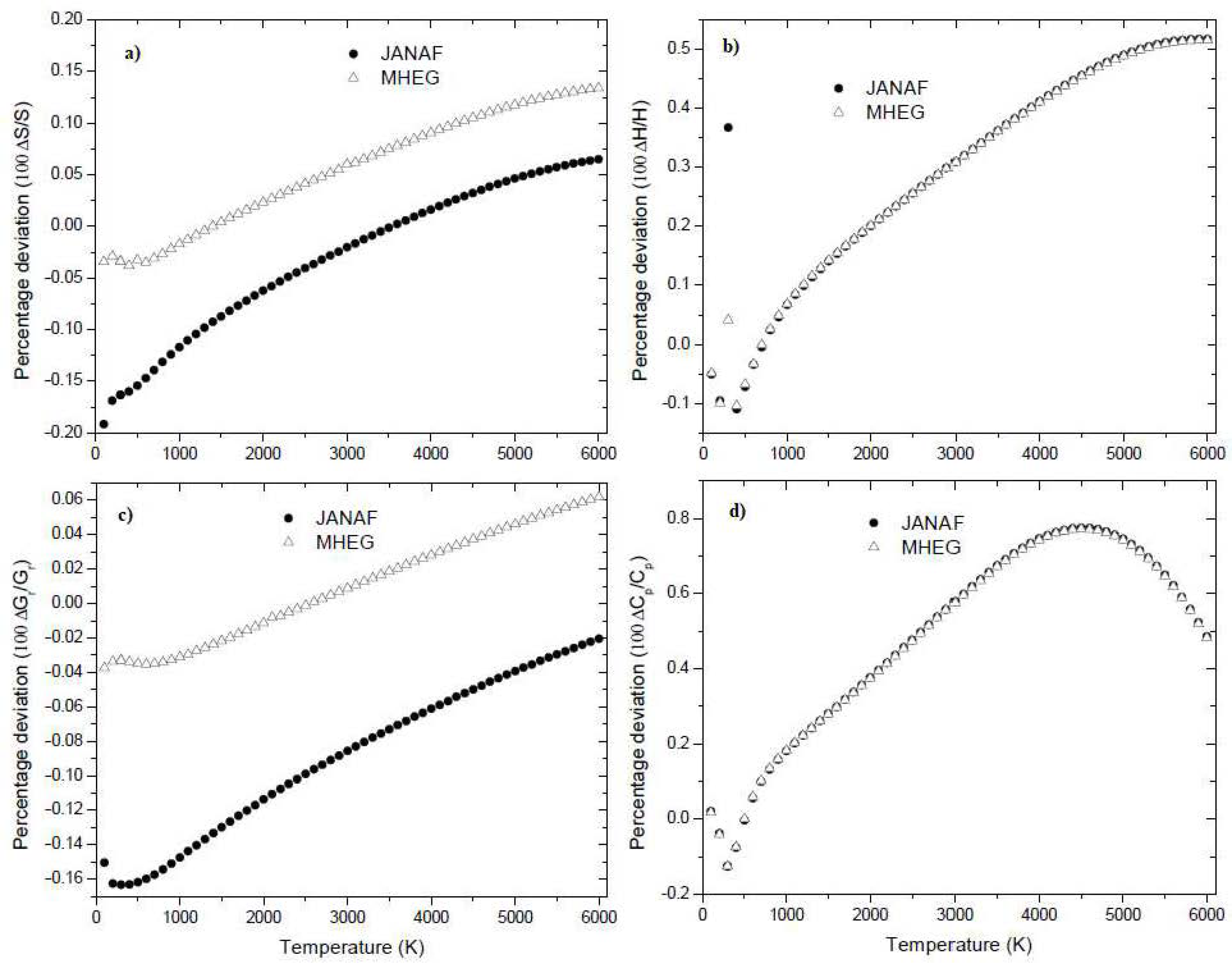
Applying the following basic thermodynamic relationship, we can obtain the expressions of the molar entropy, the molar enthalpy, the molar Gibbs free energy and the isobaric specific heat
where
V is the spatial volume and
R is the molar gas constant.
The final expressions
18,
19,
20 and
21 do not contain any adjustable coefficient determined from fitting experimental spectroscopic or calorimetric data points, and only involve direct input of few physical constants which can be very easily found in literature.