1. Introduction
The wind dispersion of toxic trace elements from mine tailings in arid and semi-arid environments is a globally recognized concern which requires attention to minimize the risk of biomagnification of these elements into food chain [1]. Conventional techniques used for the remediation of contaminated soils are not eco-friendly and are expensive, and energy intensive [2,3]. Therefore, the use of cost-effective remediation strategies involving the use of plants and the associated microbial communities to restore the ecosystem services of contaminated and/or degraded areas has emerged as a highly promising approach in the last decades [4,5].
Phytostabilization, a form of phytoremediation, employs plants and their associated microbes to effectively immobilize pollutants in belowground tissues and/or in the rhizosphere. The ultimate goal of this approach is to create a plant canopy that prevents eolian dispersion and water erosion through the immobilization of pollutants [4,6]. However, the successful implementation of phytoremediation strategies in arid and semiarid environments faces challenges due to the unfavorable climatic conditions such as high salinity, extreme temperatures, and drought, which compromise plant growth and biomass production [7,8]. Drought is the main limiting factor for plant development in these regions due to the low rainfall rates and high temperatures. Therefore, under limited soil moisture, plants show a significant reduction in photosynthesis leading to the reduction of leaf expansion and consequently premature leaf senescence [9,10]. To overcome this problem, it is crucial to use plant species tolerant to the main pollutants and well-adapted to the prevailing local climate conditions [1,7]. Previous studies have reported several plant species colonizing mining areas within arid and semiarid regions that exhibit traits similar to metallophytes. They are considered non-hyperaccumulator species, but they have high metal tolerance alongside with exceptional salt tolerance, as well as the capacity of withstanding severe droughts [12–15]. These native plants are often considered ideal candidates for phytostabilization strategies, given their inherent traits and ability to support the harsh local conditions [12,16,17].
Beside climatic factors, mine tailings are typically characterized by a poor structure, very acidic pH, high metal concentrations and low nutrient content, which adversely impacts both vegetation cover and microbial communities [18,19]. The use of organic and inorganic amendments such as biochar, compost, animal wastes and liming has been largely reported to decrease the availability of metals by increasing soil pH and physical adsorption, while improving soil physical, chemical, and biological properties [7,19]. In particular, organic amendments increase soil organic carbon which interacts with clay minerals forming stable aggregates, improving soil structure and macroporosity. Moreover, organic matter holds hygroscopic water on the surface of soil particles and capillary water in soil pores increasing thereby soil water retention, infiltration, and storage [20–22]. On the other hand, the establishment of a vegetation cover in mine tailings enhances the heterotrophic microbial community, which may, in turn, promote plant growth and contribute to metal immobilization [23,24]. Thus, exploring these beneficial interactions is a valuable tool to improve the overall effectiveness of phytoremediation strategies in metal-contaminated areas. Several authors have reported the beneficial effects of inoculating plant growth-promoting rhizobacteria (PGPR) on plant establishment and development in metal-contaminated areas [6,8,23,25–27]. Microbial inoculants are known to decrease the availability of contaminants and enhance plant growth under stressed conditions through various mechanisms, including the production indole-3-acetic acid (IAA) and siderophores, the solubilization of mineral phosphorus and the activity of the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase [8,23,26]. Furthermore, it is crucial to use drought-tolerant strains in order to confer advantages to the host plant against water deficiency, especially in arid and semiarid areas.
The present study aimed to evaluate the effects of microbial inoculants and organo-mineral amendments on the improvement of chemical and microbiological properties of mine tailings. The ability of different native metal-tolerant plants to grow in amended mine tailings under moderate and severe drought stress conditions was also evaluated.
2. Results
2.1. Influence of Irrigation Regimes and Microbial Inoculants on Soil Chemical Properties
The addition of topsoil and organo-mineral amendments significantly improved mine tailings’ properties, since the pH increased (7.55), as well as the total nitrogen and organic carbon content which stood at 0.32% and 3.11%, respectively (Table 1). It was also observed a remarkable reduction in EC, although remaining slightly high (4.71 ± 0.04 mS cm-1). Post-harvest analyses showed that chemical properties varied across the three plant species and treatments tested (Table 1). If compared to the pre-plant values, the pH slightly decreased for all plants species. However, irrigation regimes and inoculation had a marginal influence on pH, despite the slight decrease observed in the rhizosphere of P. harmala plants with increasing water deficiency. A similar trend was observed for EC. In general, bacterial inoculation significantly increased the available concentrations of phosphorus (P-Olsen) if compared to non-inoculated treatments, with the higher increases being observed in the rhizosphere of P. setaceum. The cultivation of plants caused a remarkable increase in organic carbon content (≈1.3-fold) and in total nitrogen (≈1.5-fold) if compared to the pre-plant values. Interestingly, PGPR inoculation also enhanced total nitrogen and organic carbon content across all plants and irrigation treatments.
Table 1.
Physicochemical properties of amended mine tailings before plant establishment (Pre-plant) and at the end of the experiment for the different plant species: Atriplex halimus, Penisetum setaceum and Peganum harmala. Plants were grown under 3 water irrigation regimes: Control - watered at 80% of field capacity (FC); Moderate Stress - watered at 45% of FC; Severe Stress - watered at 30% of FC, and under non-inoculated and inoculated conditions.
Table 1.
Physicochemical properties of amended mine tailings before plant establishment (Pre-plant) and at the end of the experiment for the different plant species: Atriplex halimus, Penisetum setaceum and Peganum harmala. Plants were grown under 3 water irrigation regimes: Control - watered at 80% of field capacity (FC); Moderate Stress - watered at 45% of FC; Severe Stress - watered at 30% of FC, and under non-inoculated and inoculated conditions.
| |
|
pH |
EC (mS cm-1) |
P Olsen (mg kg-1) |
TN (%) |
OC (%) |
| |
Pre-Plant |
|
7.55 ± 0.20 |
4.71 ± 0.04 |
42.84 ± 0.61 |
0.32 ± 0.01 |
3.11 ± 0.05 |
| Atriplex halimus |
Non-inoculated |
Control |
6.72 ± 0.04a
|
4.35 ± 0.02b
|
40.86 ± 3.63b
|
0.51 ± 0.02a
|
4.70 ± 0.02a
|
| Moderate stress |
6.62 ± 0.05b
|
4.34 ± 0.01b
|
40.17 ± 5.35b
|
0.42 ± 0.03b
|
4.68 ± 0.11a
|
| Severe stress |
6.75 ± 0.03a |
4.42 ± 0.02a
|
52.68 ± 3.06a
|
0.38 ± 0.04b
|
3.68 ± 0.09b
|
| |
|
**F=8.055 |
**F=13.796 |
*F=8.604 |
*F=13.069 |
***F=134.088 |
| Inoculated |
Control |
6.57 ± 0.02a
|
4.21 ± 0.04b
|
51.30 ± 5.31a
|
0.54 ± 0.01a
|
4.71 ± 0.04a
|
| Moderate stress |
6.54 ± 0.05a
|
4.32 ± 0.05a
|
55.30 ± 5.85a
|
0.53 ± 0.04a
|
4.37 ± 0.58a
|
| Severe stress |
6.51 ± 0.02a
|
3.90 ± 0.04c
|
55.31 ± 6.09a
|
0.38 ± 0.43b
|
4.68 ± 0.11a
|
| |
|
NSF=2.167 |
***F=65.068 |
NSF=0.483 |
*F=15.932 |
NSF=1.503 |
| |
|
***F(I)=72.532 |
***F(I)=180.549 |
**F(I)=15.793 |
**F(I)=7.506 |
NSF(I)=0.015 |
| |
|
*F(R)=5.277 |
***F(R)=36.093 |
*F(R)=4.152 |
***F(R)=25.434 |
*F(R)=10.111 |
| |
|
*F(IxR)=6.004 |
***F(IxR)=78.615 |
NSF(IxR)=2.370 |
*F(IxR)=3.945 |
NSF(IxR)=1.393 |
| |
|
|
pH |
EC (mS cm-1) |
P Olsen (mg kg-1) |
TN (%) |
OC (%) |
| Penisetum setaceum |
Non-inoculated |
Control |
6.66 ± 0.05a
|
4.21 ± 0.04b
|
47.97 ± 1.35a
|
0.42 ± 0.02a
|
3.64 ± 0.02a
|
| Moderate stress |
6.72 ± 0.03a
|
4.32 ± 0.05a
|
48.63 ± 0.15a
|
0.45 ± 0.07a
|
3.60 ± 0.03a
|
| Severe stress |
6.72 ± 0.04a
|
3.90 ± 0.04c
|
45.31 ± 2.89a
|
0.34 ± 0.04b
|
3.71 ± 0.04a
|
| |
NSF=1.596 |
***F=65.068 |
NSF=2.707 |
*F=8.179 |
NSF=3.201 |
| Inoculated |
Control |
7.25 ± 0.01a
|
4.32 ± 0.02b
|
60.86 ± 4.38a
|
0.50 ± 0.07b,c
|
4.20 ± 0.02b
|
| Moderate stress |
6.51 ± 0.05b
|
4.34 ± 0.04b
|
63.51 ± 1.35a
|
0.54 ± 0.02b
|
4.20 ± 0.02b
|
| Severe stress |
6.46 ± 0.30b
|
4.42 ± 0.02a
|
41.68 ± 3.50b
|
0.56 ± 0.03a,b
|
4.36 ± 0.03a
|
| |
*F=18.268 |
**F=13.794 |
***F=39.607 |
*F=6.581 |
*F=8.934 |
| |
|
NSF(I)=0.379 |
***F(I)=180.549 |
***F(I)=41.102 |
***F(I)=85.323 |
***F(I)=611.163 |
| |
|
***F(R)=14.706 |
**F(R)=36.093 |
***F(R)=39.541 |
*F(R)=4.316 |
*F(R)=10.866 |
| |
|
***F(IxR)=19.829 |
***F(IxR)=78.615 |
***F(IxR)=21.873 |
**F(IxR)=11.155 |
NSF(IxR)=0.891 |
| |
|
|
pH |
EC (mS cm-1) |
P Olsen (mg kg-1) |
TN (%) |
OC (%) |
| Peganum harmala |
Non-inoculated |
Control |
7.12 ± 0.02a
|
3.67 ± 0.02c
|
37.42 ± 2.50a
|
0.42 ± 0.04a
|
3.81 ± 0.01a
|
| Moderate stress |
6.44 ± 0.02b
|
4.35 ± 0.04b
|
40.29 ± 1.87a
|
0.43 ± 0.01a
|
3.77 ± 0.02a
|
| Severe stress |
6.35 ± 0.03c
|
4.46 ± 0.02a
|
38.21 ± 1.56a
|
0.45 ± 0.03a
|
3.65 ± 0.04b
|
| |
***F=814.085 |
***F=510.735 |
NSF=1.611 |
NSF=2.192 |
**F=15.624 |
| Inoculated |
Control |
7.10 ± 0.02a
|
4.23 ± 0.01a
|
54.15 ± 1.78c
|
0.53 ± 0.07a
|
4.67 ± 0.05a
|
| Moderate stress |
6.31 ± 0.02b
|
4.23 ± 0.03a
|
57.42 ± 0.94b
|
0.53 ± 0.08a
|
4.67 ± 0.01a
|
| Severe stress |
6.32 ± 0.01b
|
3.87 ± 0.07a
|
60.29 ± 1.87a
|
0.51 ± 0.02a
|
4.43 ± 0.02b
|
| |
***F=1773.414 |
***F=60.310 |
**F=11.178 |
NSF=2.294 |
*F=17.448 |
| |
|
***F(I)=119.102 |
*F(I)=6.258 |
***F(I)=472.278 |
***F(I)=152.581 |
***F(I)=1293.901 |
| |
|
***F(R)=2258.193 |
***F(R)=110.180 |
*F(R)=6.516 |
NSF(R)=0.070 |
***F(R)=31.168 |
| |
|
NSF(IxR)=2.261 |
***F(IxR)=309.705 |
*F(IxR)=4.021 |
*F(IxR)=4.395 |
NSF(IxR)=2.806 |
2.2. Influence of Irrigation Regimes and Microbial Inoculants on Soil Microbial Activity and Structure
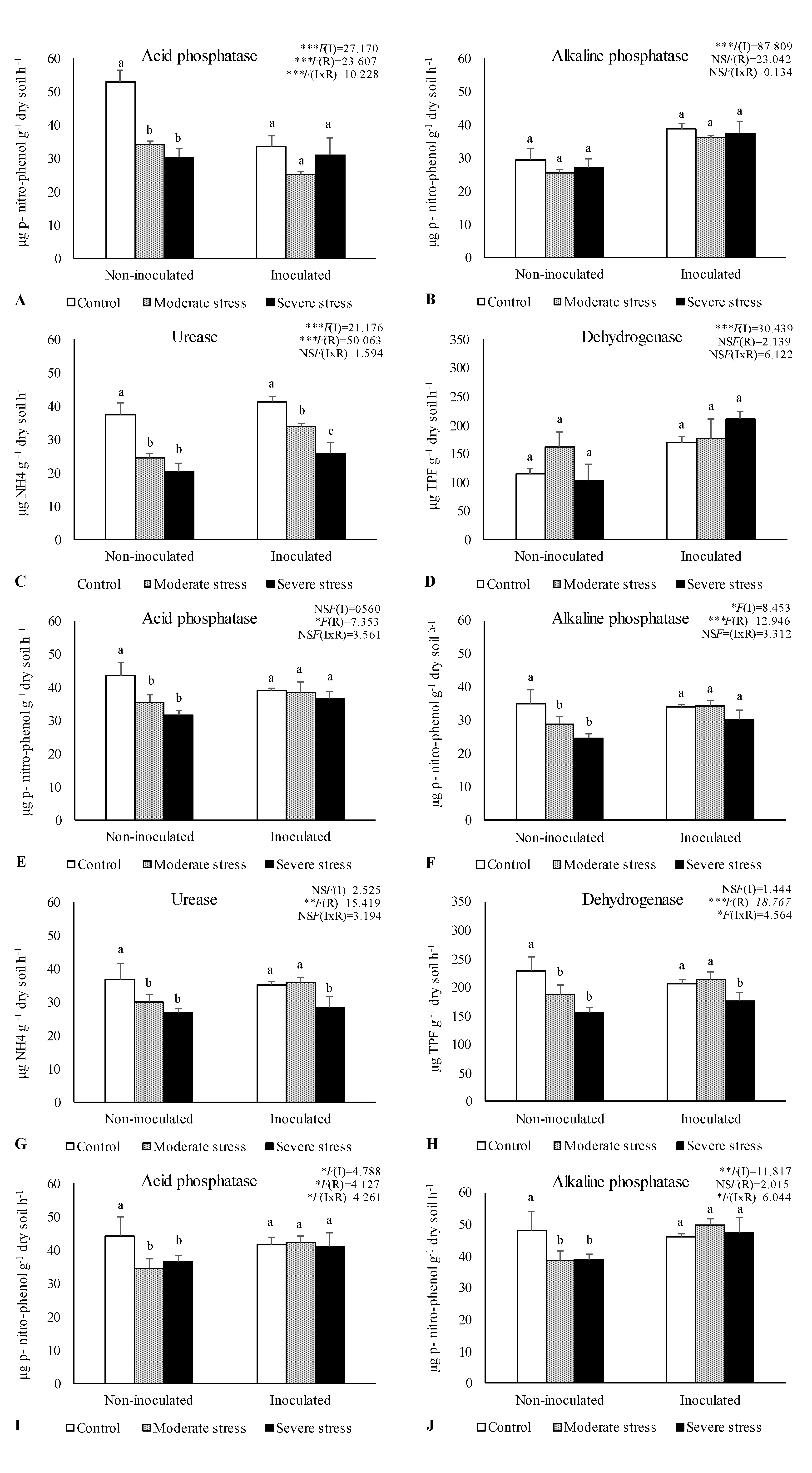
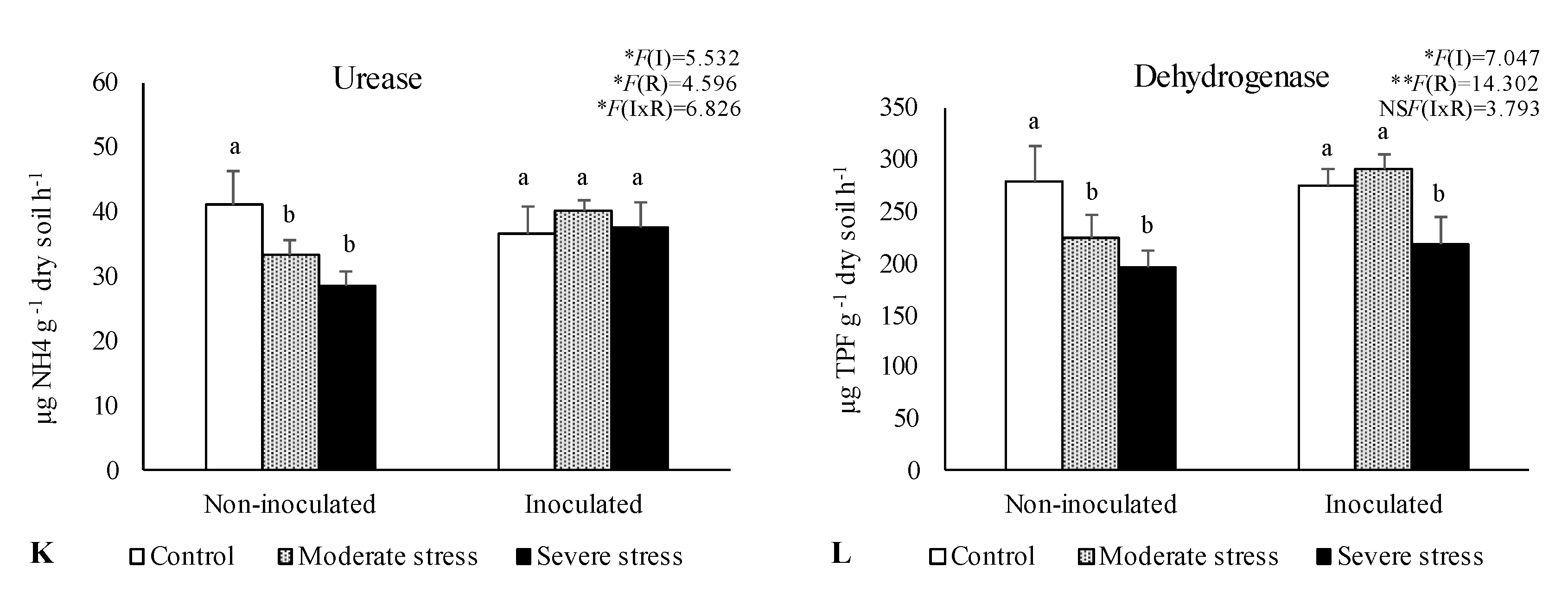
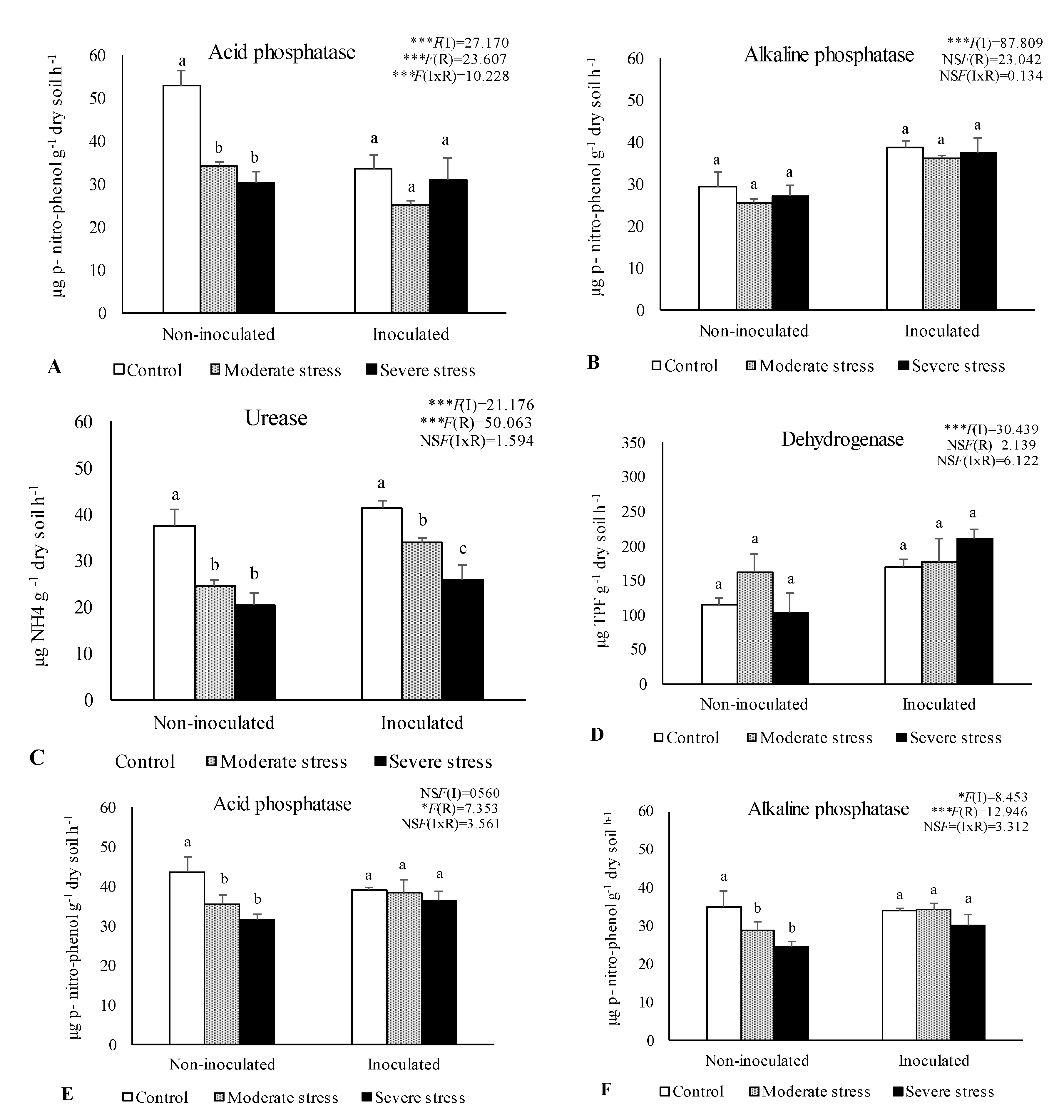
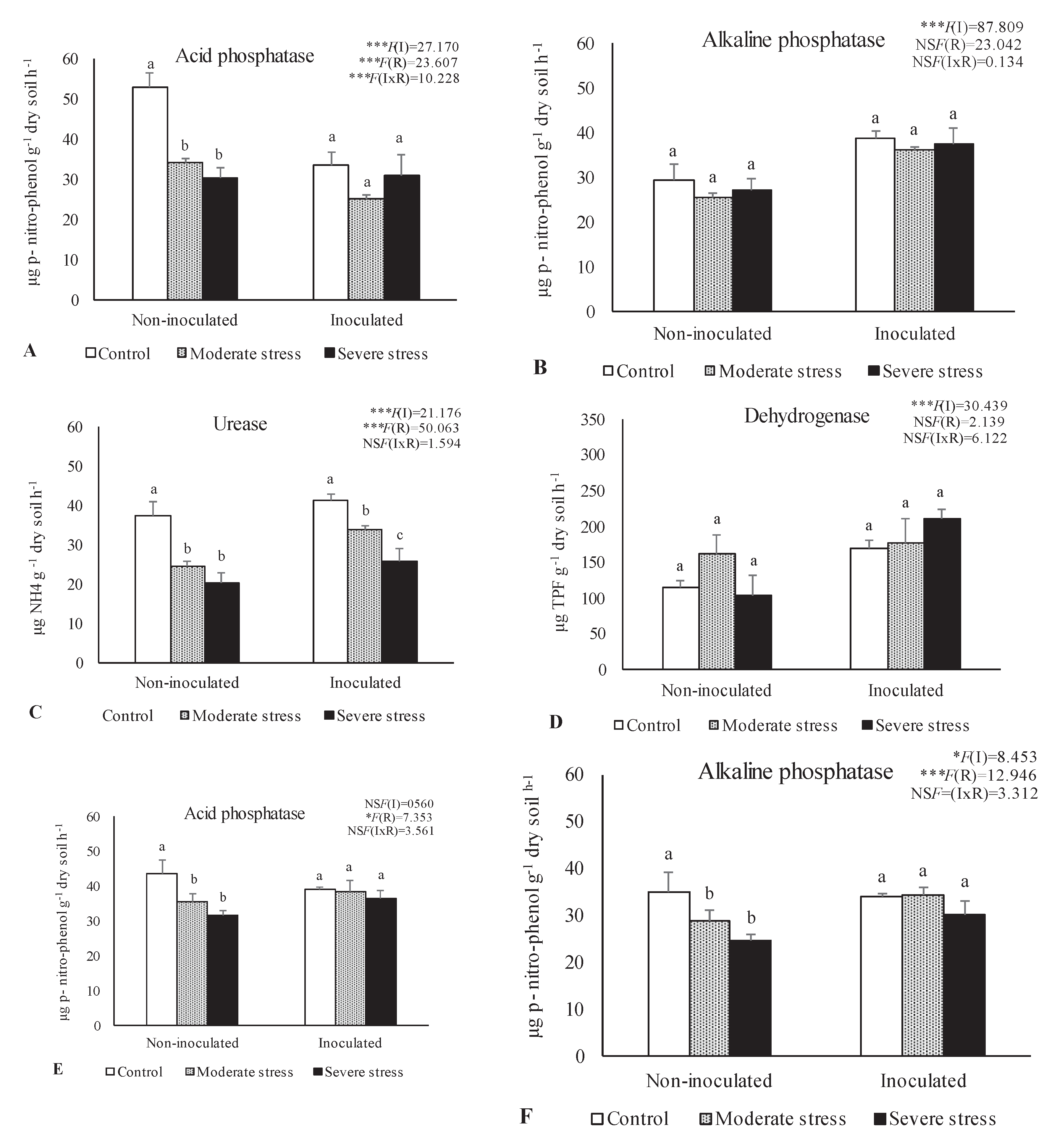
The activities of acid and alkaline phosphatases, dehydrogenase, and urease determined in the rhizosphere of the three plants species cultivated under different irrigation regimes and with and without inoculation at the end of the experiment are shown in
Figure 1. The activity of acid phosphatase was reduced under moderate and severe water stress if compared with well-watered conditions. However, bacterial inoculation seemed to mitigate the negative impact of water stress on this enzyme, since no significant decreases were observed among the different irrigation regimes in inoculum-supplemented soils. Similar results were observed for alkaline phosphatase and urease, where bacterial inoculation increase by 10% both enzymes’ activity, regardless of water stress conditions.
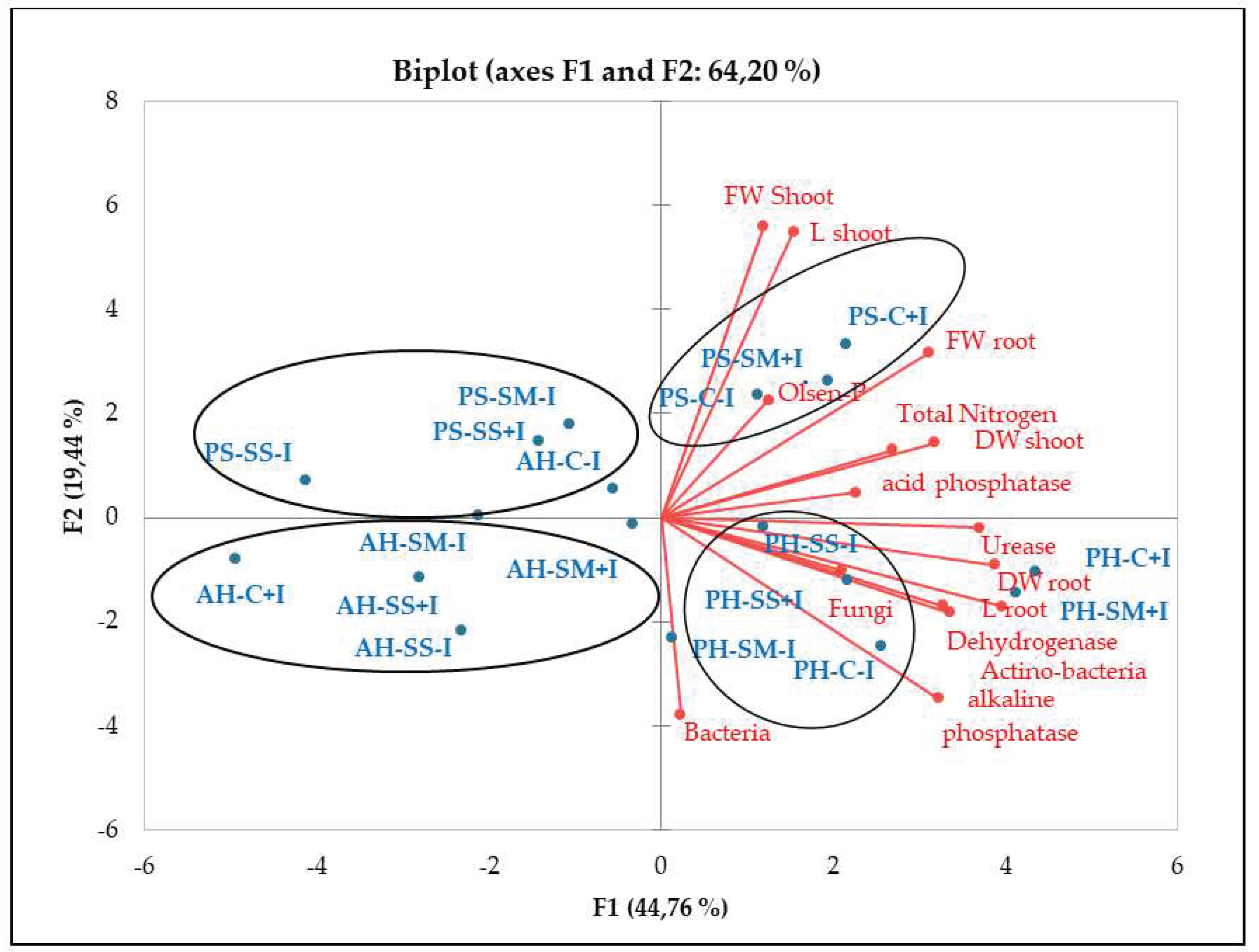
Dehydrogenase activity was also significantly higher (+30%) in inoculated treatments across the three studied plant species. Maximum values of alkaline phosphatase, urease and dehydrogenase were observed in the rhizosphere of P. harmala, particularly when supplemented with the bacterial inoculum as shown by PCA analysis (
Figure 2).
The number of viable bacteria, fungi, and actinomycetes changed remarkably across different treatments and plants species (Table 2). Notably, bacterial and actinomycetes numbers in the rhizosphere of P. harmala were 10-fold higher than those found in the rhizosphere of A. halimus and P. setaceum. Regarding fungi counts no significant differences were observed among plant species. In general, increasing levels of drought stress led to a significant reduction on microbial counts. In the rhizosphere of P. harmala, water deficiency decreased by 17%, 40%, and 30% the number of bacteria, fungi and actinomycetes, respectively, compared to control (well-watered conditions). Furthermore, across the three studied plant species, it was observed a greater abundance of actinomycetes and bacteria in the rhizosphere of inoculated plants if compared to non-inoculated ones. Similar results were obtained for fungi.
Data are shown as means ± standard errors (n= 3). A two-way ANOVA was performed to determine the influence of microbial inoculation (I) and irrigation regimes (R) on enzymatic activity (acid phosphatase, alkaline phosphatase, urease, and dehydrogenase). The results are shown with the test statistic for each case (I: microbial inoculation; R: irrigation regimes; I x R: microbial inoculation x irrigation regimes) and as NS: Non-significant at the level P > 0.05; * significant at the level P < 0.05; ** P < 0.01; *** significant at the level P < 0.001, respectively. One-way ANOVA was performed to determine the influence of irrigation on enzymatic activity for each inoculation treatment at the end of the experiment. Means for the same inoculation treatment showing different letters are significantly different from each other (P < 0.05) according to Duncan test.
Table 2.
Total numbers of bacteria, fungi, and actinomycetes in the rhizosphere of different plant species: Atriplex halimus, Penisetum setaceum and Peganum harmala at the end of the experiment. Plants were grown under 3 water irrigation regimes: Control - watered at 80% of field capacity (FC); Moderate Stress - watered at 45% of FC; Severe Stress - watered at 30% of FC, and under non-inoculated and inoculated conditions.
Table 2.
Total numbers of bacteria, fungi, and actinomycetes in the rhizosphere of different plant species: Atriplex halimus, Penisetum setaceum and Peganum harmala at the end of the experiment. Plants were grown under 3 water irrigation regimes: Control - watered at 80% of field capacity (FC); Moderate Stress - watered at 45% of FC; Severe Stress - watered at 30% of FC, and under non-inoculated and inoculated conditions.
| |
|
Bacteria
(x107 CFU g-1 dry soil) |
Fungi (x104 CFU g-1 dry soil) |
Actinobacteria (x103 CFU g-1 dry soil) |
| Atriplex halimus |
Non-inoculated |
Control |
10.79 ± 0.01a
|
2.33 ± 0.51b
|
28.72 ± 0.01a
|
| Moderate stress |
10.95 ± 0.01a
|
5.00 ± 0.60a
|
24.05 ± 0.02b
|
| Severe stress |
9.053 ± 0.02b
|
4.53 ± 0.11a
|
20.08 ± 0.04c
|
| |
|
***F=119.961 |
***F=39.114 |
*F=9.769 |
| Inoculated |
Control |
11.32 ± 0.01a
|
5.40 ± 0.30a
|
34.72 ± 0.01a
|
| Moderate stress |
11.34 ± 0.03a
|
4.93 ± 0.57a
|
33.33 ± 0.03a
|
| Severe stress |
10.33 ± 0.02b
|
3.20 ± 0.40b
|
22.72 ± 0.02b
|
| |
|
***F=62.214 |
*F=18.510 |
***F=33.444 |
| |
|
***F(I)=105.585 |
*F(I)=7.440 |
***F(I)=33.136 |
| |
|
***F(R)=182.036 |
***F(R)=12.964 |
***F(R)=34.682 |
| |
|
***F(IxR)=16.730 |
***F(IxR)=41.226 |
NSF(IxR)=3.409 |
| |
|
|
Bacteria
(x107 CFU g-1 dry soil)
|
Fungi (x104 CFU g-1 dry soil) |
Actinobacteria (x103 CFU g-1 dry soil) |
| Penisetum setaceum |
Non-inoculated |
Control |
11.48 ± 0.48a
|
3.47 ± 0.23a
|
22.05 ± 0.20a
|
| Moderate stress |
10.68 ± 0.40b
|
2.40 ± 0.01b
|
25.34 ± 0.41b
|
| Severe stress |
9.413 ± 0.12c
|
1.80 ± 0.20c
|
14.72 ± 0.20c
|
| |
***F=36.628 |
***F=68.714 |
***F=25.125 |
| Inoculated |
Control |
12.63 ± 0.18a
|
4.13 ± 0.46a
|
25.32 ± 0.23a
|
| Moderate stress |
12.29 ± 0.13b
|
4.73 ± 0.23a
|
27.36 ± 0.23a
|
| Severe stress |
10.67 ± 0.23c
|
2.67 ± 0.42b
|
18.08 ± 0.11b
|
| |
***F=98.443 |
*F=21.194 |
*F=8.150 |
| |
|
***F(I)=113.483 |
***F(I)=78.233 |
*F(I)=6.036 |
| |
|
***F(R)=105.897 |
***F(R)=44.860 |
***F(R)=25.857 |
| |
|
NSF(IxR)=1.303 |
***F(IxR)=13.000 |
NSF(IxR)=0.143 |
| |
|
|
Bacteria
(x107 CFU g-1 dry soil)
|
Fungi (x104 CFU g-1 dry soil) |
Actinobacteria (x103 CFU g-1 dry soil) |
| Peganum harmala |
Non-inoculated |
Control |
99.10 ± 0.32a
|
4.80 ± 0.69a
|
31.33 ± 0.11a
|
| Moderate stress |
94.51 ± 0.61b
|
3.73 ± 0.23b
|
32.02 ± 0.20a
|
| Severe stress |
88.57 ± 0.14c
|
2.40 ± 0.40c
|
14.73 ± 0.23b
|
| |
***F=19.804 |
***F=18.769 |
***F=81.375 |
| Inoculated |
Control |
106.21 ± 0.72a
|
5.27 ± 0.30a
|
46.75 ± 0.30a
|
| Moderate stress |
105.03 ± 0.11b
|
4.47 ± 0.11b
|
40.08 ± 0.34b
|
| Severe stress |
103.24 ± 0.30c
|
3.67 ± 0.11c
|
32.03 ± 0.20c
|
| |
***F=15.250 |
***F=48.000 |
***F=19.158 |
| |
|
***F(I)=212.253 |
***F(I)=22.443 |
***F(I)=137.815 |
| |
|
***F(R)=29.521 |
***F(R)=44.328 |
***F(R)=69.148 |
| |
|
***F(IxR)=9.23 |
***F(IxR)=1.836 |
*F(IxR)=6.037 |
2.3. Influence of Irrigation Regimes and Microbial Inoculants on Plant Growth
Overall, water stress significantly affected plant growth, by reducing shoot and root length, as well as fresh and dry biomass (
Figure 3,
Figure 4, and
Figure 5). For A. halimus, shoots and roots length decreased by 52% and 50% compared to well-watered plants under severe drought, respectively (
Figure 3A,B). A similar trend was observed for P. setaceum with a reduction of 47% and 66% for shoots and roots length, respectively (
Figure 4A,B). Nevertheless, P. harmala showed comparatively less sensitivity to water stress, as it was observed a lower decrease on roots and shoots length, 16% and 35%, respectively (
Figure 5A, 5B). Shoot and root fresh weight were also significantly affected by water stress, with remarkable reductions being observed with increasing stress severity. Thus, severe drought stress caused a significant decrease in root dry weight of A. halimus and P. setaceum, nearly 42% and 32%, respectively; while root dry weight of P. harmala plants was not significantly affected (
Figure 5F). However, moderate drought stress did not significantly affect root and shoot dry weights of all plants species. Despite the substantial decrease in shoots’ length and in dry and fresh weight with the increasing drought stress intensity, plants did not visually exhibit symptoms of toxicity such as chlorosis.
Bacterial inoculation led to a slight increase in shoots and roots length under well-watered conditions. However, the impact of PGPR inoculation was even more pronounced under water stress conditions. Indeed, under moderate and severe water stress, A. halimus and P. setaceum plants showed significant increases in shoot and root lengths upon PGPR inoculation (
Figure 3A,B,
Figure 4A,B). The higher increases were observed for A. halimus and P. setaceum, which corresponded to 35% and 39%, respectively. Likewise, the application of PGPR led to an increase in the dry weight of both shoots and roots of all three plants species. P. harmala showed the most significant enhancement, with an increase of 59% in roots and of 56% in shoots, respectively under severe stress conditions (
Figure 5E,F).
Data are shown as means ± standard errors (n= 3). A two-way ANOVA was performed to determine the influence of microbial inoculation (I) and irrigation regimes (R) on plant growth parameters (shoot length, root length, shoot fresh biomass, root fresh biomass, shoot dry biomass, and root dry biomass). The results are shown with the test statistic for each case (I: microbial inoculation; R: irrigation regimes; I x R: microbial inoculation x irrigation regimes) and as NS: Non-significant at the level P > 0.05; * significant at the level P < 0.05; ** P < 0.01; *** significant at the level P < 0.001, respectively. One-way ANOVA was performed to determine the influence of irrigation regimes on plant growth parameters for each inoculation treatment at the end of the experiment. Means for the same inoculation treatment showing different letters are significantly different from each other (P < 0.05) according to Duncan test.
3. Discussion
The addition of marble sludge and sheep manure, together with uncontaminated topsoil contributed to increase nutrient content and microbial activity of mine tailings, enabling the successful seed germination and the subsequent plant establishment.
3.1. Improvement of Mine Tailings’ Chemical and Microbiological Properties by the Combined Use of Organo-Mineral Amendments and Bacterial Inoculants
At the end of experiment, it was observed a decrease in pH, which is align with previous research, as plants are known to cause the acidification of the rhizosphere due to the secretion of exudates by the roots, including protons, amino-acids, and sugars [29–31]. These exudates may influence not only soil pH but also metal solubility and its speciation in the rhizosphere [32,33]. The decrease in pH can also be related to the significant build-up of CO2 in the rhizosphere resulting from the respiratory activities of both roots and microorganisms [29,34]. Besides, numerous studies have reported that soil acidification is due to the release of organic acids during organic matter decomposition by microbes [29].
Soil organic matter is essential to soil functions and quality. Our results revealed a significant increase in soil organic carbon and total nitrogen following plant growth and bacterial inoculation. This finding may be related to the slough of root cells, presence of delicate root hairs and the release of organic compounds by the roots. These processes create a unique environment that promotes microbial activity, which, in turn, contributes to the mobilization of nitrogen and phosphorus within the soil [29–31,35]. Furthermore, previous studies have emphasized the crucial role of free-living N2-fixing bacteria in both nitrogen fixation and enhancement of nutrient availability to plants [23,36,37]. The inoculated consortium comprises strains with the capability to produce IAA, which may also have contributed to increase total nitrogen content in inoculated rhizospheres [38]. Similarly, plant establishment and PGPR inoculation enhanced the concentrations of available phosphorus. These results may be attributed to the ability of inoculated strains to convert insoluble P present in marble sludge and sheep manure into soluble forms, such as H2PO4-, by releasing organic acids and phosphatases. Indeed, several authors highlighted the notable role of PGPR in the solubilization of inorganic phosphate and mineralization of organic one [39–43].
Soil microbial communities and their associated enzymatic activity are considered fundamental to soil biochemical cycles. Indeed, soil enzymes mediate many nutrient transformations and organic matter decomposition processes being closely related to soil fertility. In this study, results clearly demonstrated the negative impact of water stress on enzymatic activity, through the decrease in the activities of the enzymes acid and alkaline phosphatase, urease, and dehydrogenase. The impact of water deficiency on soil enzymatic activities has been well-documented [44–49]. Microorganisms play a crucial role in soil fertility by producing and releasing enzymes to obtain nutrients, thereby highlighting the importance of soil enzymes as indicators of soil microbial viability [77]. The decrease in soil moisture due to drought can restrict microbial physiological processes and the availability of substrates essential for enzymes’ production, leading to the decline of their activity [47,49].
PCA analysis underlines the positive relationship between soil enzymatic activity and available phosphorus, organic carbon, and total nitrogen (
Figure 2). Results also revealed significant variations in enzymatic activity across plant species. Notably, higher values of alkaline phosphatase, urease and dehydrogenase were observed in the rhizosphere of P. harmala. Likewise, a higher abundance of bacteria and actinomycetes was observed in P. harmala’s rhizosphere than in A. halimus and P. setaceum. These differences can be attributed to the distinctive root morphology of each plant species, coupled with variations in the quantity and quality of their respective rhizodeposits. All together, these factors are able to significantly influence the composition and functioning of the rhizosphere microbiome, potentially leading to an increase in soil enzymatic activities. Our results are consistent with previous studies conducted by [50] and [30]. These studies corroborate the role of plant species in shaping soil microbial biomass and enzymatic activity by providing substrates that stimulate enzyme synthesis. In line with this, [51] reported that successive plantations of Eucalyptus urophylla resulted in a decrease in soil bacterial community diversity, microbial biomass, and enzyme activity. Conversely, [52] reported that growing Trifolium repens in metal-contaminated soil mitigated the decline in enzymes activities caused by contamination. This was achieved through an increase in soil’s organic matter content resulting from the release of roots exudates and accumulation of litter.
In this study, dehydrogenase activity was significantly increased by the inoculation with PGPR, which may be related to the production of enzymes by the bacterial strains present in the inoculum or through their interactions with native microorganisms [42,51,53]. Furthermore, actinomycetes and bacterial counts were higher in the rhizosphere of inoculated plants, suggesting that the selected bacteria possess the ability to foster native microbial communities, a characteristic that could also explain their positive impact on soil enzymatic activity. Furthermore, bacterial inoculum seems to enhance nutrient content in rhizosphere, improving plant-root system and supporting microbes’ diversity and activity [42,50,53]. PGPR are able to produce exopolysaccharides, which are extracellular polymeric substances that enhance water retention and thus regulate the diffusion of organic carbon sources, facilitating nutrient and water uptake by root cells [54,55], being of utmost importance to cope with metal exposure and water deficiency. This further demonstrates the multifaceted ways in which PGPR may enhance soil conditions and ultimately influence enzymatic activities.
3.2. Plant Growth in Amended Mine Tailings under Water Stress Conditions
Results highlight the potential of the three plant species to be cultivated in amended mine tailings. The improvements in soil fertility attributed to the incorporation of marble sludge and sheep manure had profound effects on plant growth. Notably, the pH adjustments provided by marble sludge and the nutrient enrichment provided by sheep manure acted synergistically to thrive the growth of both rhizospheric microorganisms and plants.
Plant growth was negatively influenced by drought stress, being P. setaceum the most affected species. In contrast, P. harmala and A. halimus demonstrated a higher resilience to drought stress, suggesting their evolutionary adaptations to endure harsh environmental conditions. Besides, further analysis (
Figure 2) showed that P. harmala exhibited higher shoot biomass production and root elongation in comparison to A. halimus. [56] also reported reductions in root and shoot biomass of Atriplex sp. under water stress conditions. Indeed, drought stress is known to influence key aspects of plant growth such as root diameter, root volume, and the process of root differentiation [57]. Moreover, it can also negatively impact the balance of endogenous hormones in roots, leading to a decrease in IAA and a simultaneous increase in abscisic acid content [57]. The complex dynamics between soil moisture and metal toxicity add another layer of complexity to this phenomenon. Indeed, it has been reported that a decrease in water availability may increase metal toxicity in soil solution, which subsequently hinder roots growth [58,59].
In this study, P. harmala arise as a suitable plant to withstand the challenges posed by both drought and metal toxicity. These results are in line with previous works which show its capacity to thrive over a wide range of metals while developing a resilient and deeply-rooted system [60]. This adaptability might be attributed to its ability to release high and low molecular weight root exudates, which shield the root apex from metal toxicity during severe environmental stresses [61–63]. These exudates, coupled with modifications in cell wall and plasma-membranes properties provide mechanical strength to the root system, further enhancing its ability to withstand harsh abiotic stressors. Furthermore, the preservation of root elongation, through osmotic adjustment and abscisic acid accumulation, has also been highlighted as a key factor for sustaining root growth under adverse conditions [64]. These strategies corroborate the potential of P. harmala plants to cope with environmental challenges, demonstrating their adaptability and resilience.
The outcomes of the present study showed that bacterial inoculation had a pronounced and positive impact on plant growth, which was particularly evident under water stress conditions. The improvement of plant growth by PGPR inoculation in metal-contaminated soils and under drought stress conditions could be attributed to its capacity to produce siderophores, phytohormones (e.g., IAA), solubilize inorganic phosphate, and the activity of the enzyme ACC-deaminase. Moreover, the rhizobacteria used as inoculum showed high ability to produce exopolysaccharides and ammonia under different abiotic stresses, including drought, metal, and salt. Exopolysaccharide-producing bacteria are acknowledged for their role in preserving soil moisture and enhancing plant growth, even in arid conditions. Furthermore, the produced ammonia by bacteria acts as an extra nitrogen source to the host plant, leading to an increase in overall plant biomass [65–67]. Moreover, the synthesis of IAA by plant-associated rhizobacteria appears to be an additional factor contributing to plant growth in metal-polluted soils, through the stimulation of plant root elongation, which enhance plant nutrient uptake [8,68]. Moreover, the concurrent application of sheep manure and PGPR inoculation plays a pivotal role in stimulating soil microbial activity, thereby enriching plant nutrition. The introduction of organic amendments appears to be a carbon source as well as an energy reservoir for the inoculated bacteria, fostering beneficial microbial activity and consequently enhancing their functional traits that contribute to improved plant nutrition.
4. Materials and Methods
4.1. Plant Species
Three plant species were used: Atriplex halimus (Chenopodiaceae), Pennisetum setaceum (Poaceae) and Peganum harmala (Zygophyllaceae). These species were selected as they are native shrubs, thriving within the geographical area of the study. In addition, they show high tolerance to metallic stress and are well adapted to arid conditions especially to hot summers and low annual rainfall. Moreover, members of Chenopodiaceae family, specifically Atriplex spp., are known to exhibit exceptional tolerance to both salinity and drought, while P. setaceum is a salt-resistant perennial herb that can provide an extensive and dense canopy cover, coupled with a well-established deeper root network, effectively preventing erosion [69]. On the other hand, P. harmala, is a perennial herbaceous that thrives naturally in degraded and metalliferous lands within arid and semiarid regions. Notably, its root system can reach impressive depth, ranging from of 5 to 6 m. Given these remarkable attributes, these plants species are highly effective in stabilizing contaminants in soils and may act as pioneers for the revegetation of these areas.
The seeds of P. harmala and P. setaceum were collected in the vicinity of Kettara mine tailings, while plants of A. halimus were obtained through transplantation to ensure their establishment and growth for experimental purposes.
4.2. PGPR Consortium, Seed Sterilization and Seedling Growth
In this assay, a consortium consisting of four bacterial strains, namely Mesorhizobium tamadayense BKM04, Enterobacter xiangfangensis BKM30, Pseudomonas azotifigens BKM07 and Streptomyces caelestis BKM05 was used. These strains were chosen based on their proven ability to withstand environmental stressors, including drought, salt stress and high levels of metals, as well as their capacity to promote plant growth [27,70]. To prepare the inoculum, single colonies of each strain were transferred from tryptic soy agar (TSA) plates to a nutrient broth and incubated with agitation (200 rpm) at 28°C for 48 h. Subsequently, the cultures were centrifuged at 10000 rpm for 10 min, and the pellet washed twice with a sterile saline solution (0.9% (w/v) NaCl). Bacterial cells were then resuspended in 1% (w/v) of methylcellulose solution prepared in 10 mM of MgSO4. Equal number of cells of each rhizobacteria were mixed to form the inoculum (≈ 2 x 108 colony forming units (CFU) mL-1).
Seeds of P. harmala and P. setaceum were sterilized using 70% of ethanol followed by 3% sodium hypochlorite for 5 min. Subsequently, seeds were washed several times with deionized sterilized water. Sterilized seeds were then soaked in the inoculum for 30 min. For A. halimus, young shoots were collected from plants growing in their natural habitat. The collected plant material was first thoroughly washed in running tap water for 15 min to remove any dust and other particles. Explants of 5-7 cm, containing the nodes and axillary bud were excised from the shoots. These explants were subjected to surface-sterilization with 70% ethanol for 1 min and subsequently rinsed several times with sterile distilled water. Finally, they were carefully placed in plastic cups containing a mixture of agriculture soil and peat and kept under greenhouse conditions for 2 months. During this period, the plants were irrigated 1–2 times per week until they were ready for their final transplantation into pots for the experiment. Rooted plants were then inoculated with 10 mL of bacterial inoculum after transplantation to the pots.
4.3. Sampling and Characterization of Mine Tailings, Topsoil, and Organo-Mineral Amendments
Mine tailings and topsoil (upper soil layer removed prior to rock mine excavation) were collected from an abandoned pyrrhotite mine (Kettara), located at 30 km of northwest of Marrakech (Morocco). Mine tailings are highly contaminated with various metals, posing serious environmental problems [12,13,70,71]. Topsoil and the organic (sheep manure) and inorganic (crushed marble sludge, MBS) amendments were used to reduce metal toxicity and improve chemical properties of tailings. Sheep manure was collected from a sheep farm in Ourika located 32 km south of Marrakech (Morocco), while MBS was collected from the industrial zone of Marrakech (Sidi Ghanem). The physicochemical properties of mine tailings, topsoil, and amendments are shown in Table 1. Mine tailings and topsoil were sieved (<2 mm) and mixed in a ratio of 40% of topsoil and 60% of tailings. Then, 6% of crushed marble sludge and 10% of sheep manure were added. These proportions were used based on previous results (data not showed) that showed an improvement on mine tailings’ toxicity and nutrient status, as well as a better microbial activity, seed germination and seedling growth.
Table 1.
-Physicochemical properties of mine tailings, topsoil, and organo-mineral amendments (marble sludge and sheep manure).
Table 1.
-Physicochemical properties of mine tailings, topsoil, and organo-mineral amendments (marble sludge and sheep manure).
| Parameters |
Mine Tailings |
Topsoil |
Marble Sludge |
Sheep Manure |
| pH H2O |
2.36 ± 0.01 |
8.02 ± 0.07 |
10.33 ± 0.24 |
8.36 ± 0.11 |
| pH KCl |
2.19 ± 0.12 |
7.88 ± 0.13 |
8.89 ± 0.11 |
7.92 ± 0.21 |
| EC (mS cm-1) |
7.86 |
2.44 |
0.198 |
11.47 |
| Organic Carbon (%) |
Nd* |
1.13 ± 0.08 |
1.2 ± 0.05 |
40 ± 0.11 |
| Total N (%) |
Nd* |
0.17 ± 0.06 |
0.14 ± 0.16 |
1.28 ± 0.05 |
| Olsen-P (mg kg-1) |
Nd* |
43.42 ± 0.04 |
20 ± 1.23 |
1047 ± 11.24 |
| CaCO3 (%) |
- |
16.89 ± 0.14 |
57.42 ± 0.25 |
- |
| Texture |
Loam |
Sandy Loam |
- |
- |
| Clay (%) |
7.47 |
5.58 |
- |
- |
| Silt (%) |
37.37 |
39.08 |
- |
- |
| Sand (%) |
50.47 |
52.89 |
- |
- |
| Moisture content (%) |
41.92 |
40.72 |
40.62 |
40.49 |
4.4. Greenhouse Pot Experiment
Pots containing 1 kg of the prepared mixture (section 2.3) were irrigated with deionized water to reach 70% of field capacity. Then, they were transferred to the greenhouse and left to stabilize for 30 days. Following this period, 0.3 g of P. harmala and 0.5 g of P. setaceum non-inoculated and inoculated seeds were sown in pots. Three rooted plants of A. halimus with similar size (2 months old) were carefully transplanted to the pots containing the mixture. Subsequently, in the treatments with inoculation, 10 mL of the bacterial consortium was added.
All pots were watered to reach 70% of field capacity with distilled water. After emergence (≈30 days after sowing), the P. harmala plants were selectively thinned to 3, while A. halimus were thinned to one. A re-inoculation was performed four weeks after the emergence of P. harmala and P. setaceum seedlings, as well as after the transplantation of A. halimus cuttings. To simulate real environmental conditions 3 irrigation regimes corresponding to 3 levels of water deficit were applied one week after reinoculation: Control - plants watered at 80% of field capacity (FC); Moderate Stress - plants watered at 45% of FC; and Severe Stress - plants watered at 30% of FC, for both microbial treatments (non-inoculated and inoculated).
The experiment was conducted in in semi-controlled greenhouse conditions under natural light, with temperature adjusted to 30 °C. Each treatment was replicated 4 times.
After 4 months, soil and plants were harvest and analyzed. Soil samples were sieved through a 2 mm mesh to eliminate particulate matter and plant debris. The samples were then divided into 2 fractions: the first fraction was stored at 4°C for biological analysis, while the second one was air-dried for subsequent physicochemical analysis.
4.4.1. Soil Physicochemical Analysis
Soil pH and electrical conductivity (EC) were determined in a soil distilled water suspension with a ratio of 1:2.5 (w/v) and 1:5 (w/v), respectively. Total nitrogen amount was determined using the Kjeldahl method based on soil mineralization with sulfuric acid at high temperature. The molybdenum blue method was employed to determine the available phosphorus [72], while organic carbon was estimated using the oxidation of organic matter by potassium dichromate as outlined by [73].
4.4.2. Soil Enzymatic Activity
Acid and alkaline phosphomonoesterase activities were measured by incubating soil with a substrate containing a p-nitrophenyl group according to [74]. Briefly, a mixture of 1 g of soil, 4 ml of modified universal buffer, 0.25 ml of toluene, and 1 ml of 15 mM p-nitrophenyl phosphate (pNPP) was incubated for 1 h at 37°C. After stopping the reaction with 1 ml of calcium chloride (0.5M) and 4 ml of sodium hydroxide (0.5 M), the color intensity was measured at 400 nm to quantify phosphatase activity as μg of p-nitrophenol released per g of dry soil per hour (µg p-nitrophenol g-1 h-1). Dehydrogenase activity was quantified following [75] method, based on the reduction of 2,3,5-triphenyltetrazolium chloride (TTC) to 1,3,5-triphenyltetrazolium formazan (TPF). Each soil sample (5 g) was incubated with 5 mL of 0.8% TTC for 24 h in the dark at 37 °C. The formed TPF was subsequently extracted using acetone and analyzed spectrophotometrically at 546 nm. Dehydrogenase activity was expressed as µg TPF released per gram of dry soil per hour (µg-1 dry soil h-1). The urease activity was evaluated using [76] method, involving the determination of the ammonium concentration resulting from soil and urea incubation. Four replicates were done for all assays.
4.4.3. Enumeration of Culturable Microorganisms
The plate count technique was used to quantify the viable microbial population (CFU g-1 dry soil) in each sample. Serial dilutions were prepared by suspending 10 g of soil in 90 ml sterile saline solution (0.9% NaCl) and shaken for 30 min. After 15 min standing, serial dilutions (from 10-1 to 10-9) were prepared. From each dilution, 100 μL were spread onto TSA medium supplemented with 50 mg L-1 of actidione and incubated at 28°C for 48 h for bacteria. Fungi were cultivated and incubated at 20º for 7 days on Potato Dextrose Agar (PDA) medium supplemented with Rose Bengal, while actinomycetes were grown on International Streptomyces Project 2 (ISP2).
4.4.4. Plant Biometric Parameters
After four months of the experiment, plants were harvested and thoroughly washed with tap water to remove any attached particles and then rinsed with deionized water. Shoot height was measured, as well as dry biomass of shoots and roots after drying samples at 70 °C for 48h.
4.4.5. Statistical Analysis
A two-way ANOVA was performed to determine the influence of microbial inoculation (I) and irrigation regimes (R) on different parameters with the SPSS program (IBM, Armonk, NY, USA, version 25.0). One-way ANOVA was carried out to determine the influence of the irrigation regimes on the different parameters for each inoculation treatment at the end of the experiment. A statistical principal component analysis (PCA) based on Pearson’s correlation matrix was performed with XLSTAT to identify possible correlations between the biological and physicochemical parameters taking into account the microbial inoculation and irrigation regimes for each plant species.
5. Conclusions
The present study highlights the beneficial effects of organo-mineral amendments and topsoil on the growth of A. halimus; P. harmala and P. setaceum in mine tailings by improving their chemical and microbiological properties. Significant changes in pH; organic carbon; total nitrogen; and available phosphorus were observed; resulting from both plants’ growth and bacterial inoculation.
Microbial community abundance and enzymatic activities were different across the rhizospheres of the three plant species. Notably, the rhizosphere of P. harmala exhibited higher number of bacteria and actinomycetes, as well as increased enzymatic activities compared to those found in the rhizosphere of A. halimus and P. setaceum. This suggests that root morphology, rhizodeposits quantity and quality play a pivotal role in shaping the composition and activity of the rhizosphere microbiome, subsequently influencing soil enzymatic activities.
Plant growth was significantly influenced by water stress, with decreases in shoot and root lengths, as well as in fresh and dry weights. P. harmala exhibited greater resilience to water stress compared to other plant species. Furthermore, PGPR inoculation exerted a positive influence on plant growth, particularly under water-stressed conditions, driving heightened shoot and root lengths, alongside augmented dry weights.
The present study emphasizes the use of native plant species, like P. harmala, for reclaiming and restoring soil health in challenging environmental settings like metal contaminated mine tailings in arid and semiarid regions. The successful integration of organo-mineral amendments and microbial inoculants further strength these efforts, offering a sustainable way to enhance soil quality and foster plant growth even under stressful conditions.
Author Contributions
Conceptualization, A.M., A.B. and L.B.; methodology, A.M.; formal analysis, A.M.; investigation, A.M.; writing—original draft preparation, A.M.; writing—review and editing, A.B, S.P. and L.B.; supervision, A.B; project administration, A.B; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Convention de coopération CNRST-Morocco/FCT-Portugal, Centre National de Recherche Scientifique et Techniques [grant no. PPR 22/2015] and Ministry of Higher Education, Scientific Research and Innovation (MESRSI)/DRSI), Morocco (PRIMA European program for Research and Innovation solutions in the Mediterranean region) N°1607.
Data Availability Statement
All data generated or analyzed during this study are included in this published article, further information is available from the corresponding authors upon reasonable request.
Acknowledgments
The authors acknowledge the financial support of the "Convention de Coopération CNRST-Morocco/FCT-Portugal" and CNRST, Centre National de Recherche Scientifique et Techniques [grant no. PPR 22/2015] and the scientific collaboration under the Fundação para a Ciência e Tecnologia (FCT) project UID/Multi/50016/2020. The authors are also gratefully acknowledging Ministry of Higher Education, Scientific Research and Innovation (MESRSI)/DRSI), Morocco (PRIMA European program for Research and Innovation solutions in the Mediterranean region) N°1607.
Conflicts of Interest
The authors declare no conflict of interest .
References
- Franzaring, Franzaring, J., S. Ancora, L. Paoli, A. H. Fongoh, P. Büttner, A. Fangmeier, S. Schlosser, and F. Monaci. "Phytotoxicity of polymetallic mine wastes from southern Tuscany and Saxony." Ecotoxicology and Environmental Safety 162, 2018: 505-513. [CrossRef]
- Akcil, Ata, Ceren Erust, Sevda Ozdemiroglu, Viviana Fonti, and Francesca Beolchini. "A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes." Journal of Cleaner Production 86, 2015: 24-36. [CrossRef]
- Shackira, A. M., and Jos T. Puthur. "Phytostabilization of heavy metals: Understanding of principles and practices." Plant-metal interactions (2019): 263-282. [CrossRef]
- Tang, Chunfang, Yonghua Chen, Qianni Zhang, Jianbin Li, Fuyun Zhang, and Zhiming Liu. "Effects of peat on plant growth and lead and zinc phytostabilization from lead-zinc mine tailing in southern China: Screening plant species resisting and accumulating metals." Ecotoxicology and environmental safety 176, 2019: 42-49. [CrossRef]
- Testiati E, Parinet J, Massiani C, Laffont-Schwob I, Rabier J, Pfeifer HR & Prudent P. Trace metal and metalloid contamination levels in soils and in two native plant species of a former industrial site: evaluation of the phytostabilization potential. J. Hazard. Mater. 2013: 248: 131-141. [CrossRef]
- Dary M, Chamber-Pérez MA, Palomares AJ, Pajuelo E. “In situ” phytostabilisation of heavy metal polluted soils using Lupinusluteus inoculated with metal resistant plant growth promoting rhizobacteria. J. Hazard. Mater. 2010, 177: 323-330. [CrossRef]
- Clemente,R., Walker, D.J., Pardo, T., Martínez-Fernández, D. & Bernal, M.P. The use of a halophytic plant species and organic amendments for the remediation of a trace elements-contaminated soil under semi-arid conditions. J. Hazard. Mater. 2012, 223–224, 63– 71. [CrossRef]
- Ma, Ying, Mani Rajkumar, Chang Zhang, and Helena Freitas. "Inoculation of Brassica oxyrrhina with plant growth promoting bacteria for the improvement of heavy metal phytoremediation under drought conditions." Journal of hazardous materials 320 2016: 36-44. [CrossRef]
- Visser, A., Kroes, J., van Vliet, M. T., Blenkinsop, S., Fowler, H. J., & Broers, H. P. Climate change impacts on the leaching of a heavy metal contamination in a small lowland catchment. Journal of contaminant hydrology, 2012, 127(1-4), 47-64. [CrossRef]
- Jha, S., & Srivastava, R. Impact of drought on vegetation carbon storage in arid and semi-arid regions. Remote Sensing Applications: Soci and Envi, 2018, 11, 22-29. [CrossRef]
- Párraga-Aguado, I., González-Alcaraz, M.N., Álvarez-Rogel, J., Conesa, 593 H.M. Assessment of the employment of halophyte plant species for the phytomanagement of mine tailings in semiarid areas. Ecol. Eng. 2014, 71, 598–604. [CrossRef]
- Boularbah A, Schwartz C, Bitton G, Aboudrar W, Ouhammou A & Morel JL. Heavy metal contamination from mining sites in South Morocco: 2. Assessment of metal accumulation and toxicity in plants. Chem. 2006b 63: 811-817. [CrossRef]
- Boularbah A, Schwartz C, Bitton G & Morel JL. Heavy metal contamination from mining sites in South Morocco: 1. Use of a biotest to assess metal toxicity of tailings and soils. Chem. 2006a, 63: 802-810. [CrossRef]
- Mendez MO, Maier RM. Phytostabilization of mine tailings in arid and semiarid environments–an emerging remediation technology. Environ Health Perspect. 2008, 116:278–283.
- Maestri E, Marmiroli M, Visioli G, Marmiroli N. Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ Exp Biol. 2010, 68:1–1. [CrossRef]
- Zou T, Li T, Zhang X, Yu H & Huang H. Lead accumulation and phytostabilization potential of dominant plant species growing in a lead–zinc mine tailing. Environ. Earth Sci.2012, 65: 621-630. [CrossRef]
- Ali H, Khan E & Sajad MA. Phytoremediation of heavy metals: concepts and applications.c).Chemo. 2013, 91:869–881. [CrossRef]
- Meeinkuirt, W., Pokethitiyook, P., Kruatrachue, M., Tanhan, P., Chaiyarat, R. Phytostabilization of a Pb-contaminated mine tailing by various tree species in potand field trial experiments. Int. J. Phytoremediation. 2012, 14, 925–938. 2012; 14, 925–938. [CrossRef]
- Wasilkowski, D., Nowak, A., Płaza, G., & Mrozik, A. Effects of pulp and Na bentonite amendments on the mobility of trace elements, soil enzymes activity and microbial parameters under ex situ aided phytostabilization. Plos one. 2017, 12(1), e0169688. [CrossRef]
- Zhou, Hu, Chong Chen, Daozhong Wang, Emmanuel Arthur, Zhongbin Zhang, Zichun Guo, Xinhua Peng, and Sacha J. Mooney. "Effect of long-term organic amendments on the full-range soil water retention characteristics of a Vertisol." Soil and Tillage Research 202, 2020: 104663. [CrossRef]
- Kang, Sung-Won, and Kwang-Ho Ahn. "The Influence of Organic Matter Origin on the Chlorine Bulk Decay Coefficient in Reclaimed Water." Water 14, no. 5. 2022: 765. [CrossRef]
- Taban, M., and S. A. R. Movahedi Naeini. "Effect of aquasorb and organic compost amendments on soil water retention and evaporation with different evaporation potentials and soil textures." Communications in soil science and plant analysis 37, no. 13-14. 2006: 2031-2055. [CrossRef]
- Ju, W., Liu, L., Fang, L., Cui, Y., Duan, C., Wu, H. Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol. Environ. Saf. 2019. 167, 218–226. [CrossRef]
- Zainab, N., Din, B. U., Javed, M. T., Afridi, M. S., Mukhtar, T., Kamran, M. A. and Chaudhary, H. J. Deciphering metal toxicity responses of flax (Linum usitatissimum L.) with exopolysaccharide and ACC-deaminase producing bacteria in industrially contaminated soils. Plant physiology and biochemistry. 2020. 152, 90-99. [CrossRef]
- Benidire L, Pereira S, Aboudrar W, Hafidi M, Castro P, Boularbah A. Remediation of metal-contaminated mine tailings by the application of organic and mineral amendments. J Soils Sediments. 2022. 1-14. [CrossRef]
- Rani R, Kumar V, Gupta P, Chandra A. Potential use of Solanum lycopersicum and plant growth promoting rhizobacterial (PGPR) strains for the phytoremediation of endosulfan stressed soil. Chemosphere. 2021. 279: 130589. [CrossRef]
- Madline, A., Benidire, L., & Boularbah, A. Alleviation of salinity and metal stress using plant growth-promoting rhizobacteria isolated from semiarid Moroccan copper-mine soils. Environmental Science and Pollution Research. 2021. 28(47), 67185-67202. [CrossRef]
- Sandhya VS KZ, Ali SZ, Grover M, Reddy G & Venkateswarlu B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 62: 21- 30. [CrossRef]
- Wang, X., Tang, C., Severi, J., Butterly, C. R., and Baldock, J. A. Rhizosphere priming effect on soil organic carbon decomposition under plant species differing in soil acidification and root exudation. New Phytologist. 2016. 211(3), 864-873. [CrossRef]
- Gispert, M., Emran, M., Pardini, G., Doni, S., Ceccanti, B. The impact of land management and abandonment on soil enzymatic activity, glomalin content and aggregate stability. Geoderma. 2013. 202–203, 51–61. [CrossRef]
- Yadav, B. K., Akhtar, M., and Panwar, J. Rhizospheric plant-microbe interactions: key factors to soil fertility and plant nutrition. In Plant microbes symbiosis: applied facets. 2015. (pp. 127-145). Springer, New Delhi. [CrossRef]
- Bowsher, A. W., Ali, R., Harding, S. A., Tsai, C. J., and Donovan, L. A. Evolutionary divergences in root exudate composition among ecologically-contrasting Helianthus species. Plos one. 2016. 11(1), e0148280. [CrossRef]
- Gamage, S. S. W., Masakorala, K., Brown, M. T., and Gamage, S. M. K. W. Comparative phytoremediation potentials of Impatiens balsamina L. and Crotalaria retusa L. for soil contaminated with used lubricating oil. Environmental Advances. 2021. 5, 100095. [CrossRef]
- Chaparro, J. M., Sheflin, A. M., Manter, D. K., and Vivanco, J. M. Manipulating the soil microbiome to increase soil health and plant fertility. Biology and Fertility of Soils. 2012. 48(5), 489-499. [CrossRef]
- Kim, Jae Hwan, So-Jeong Kim, and In-Hyun Nam. "Effect of treating acid sulfate soils with phosphate solubilizing bacteria on germination and growth of tomato (Lycopersicon esculentum L.)." International Journal of Environmental Research and Public Health 18, no. 17. 2021. 8919. [CrossRef]
- Altaf, M. Functional diversity of nitrogen-fixing plant growth-promoting Rhizobacteria: The story so far. In Soil Nitrogen Ecology. 2021. (pp. 327-348). Springer, Cham. [CrossRef]
- Zeng, Q., Ding, X., Wang, J., Han, X., Iqbal, H., and Bilal, M. Insight into soil nitrogen and phosphorus availability and agricultural sustainability by plant growth-promoting rhizobacteria. Environmental Science and Pollution Research. 2022. 1-18. [CrossRef]
- Pramanik, K., Mitra, S., Sarkar, A., and Maiti, T. K. Alleviation of phytotoxic effects of cadmium on rice seedlings by cadmium resistant PGPR strain Enterobacter aerogenes MCC 3092. Journal of hazardous materials. 2018. 351, 317-329. [CrossRef]
- Benidire L, Madline A, Pereira, SIA, Castro, PML, Boularbah A. Synergistic effect of organo-mineral amendments and plant growth-promoting rhizobacteria (PGPR) on the establishment of vegetation cover and amelioration of mine tailings. Chemosphere. 2020. 262: 127803. [CrossRef]
- Daraz, U., Li, Y., Sun, Q., Zhang, M., and Ahmad, I. Inoculation of Bacillus spp. modulate the soil bacterial communities and available nutrients in the rhizosphere of vetiver plant irrigated with acid mine drainage. Chemosphere. 2021. 263, 128345. [CrossRef]
- Madejón, P., Navarro-Fernández, C. M., Madejón, E., López-García, Á., and Marañón, T. Plant response to mycorrhizal inoculation and amendments on a contaminated soil. Science of the Total Environment. 2021. 789, 147943. [CrossRef]
- Tirry, N., Kouchou, A., Laghmari, G., Lemjereb, M., Hnadi, H., Amrani, K. and El Ghachtouli, N. (2021). Improved salinity tolerance of Medicago sativa and soil enzyme activities by PGPR. Biocatalysis and Agricultural Biotechnology. 2021. 31, 101914. [CrossRef]
- Dey, G., Banerjee, P., Maity, J. P., Sharma, R. K., Gnanachandrasamy, G., Huang, Y. H., and Chen, C. Y. Heavy metals distribution and ecological risk assessment including arsenic resistant PGPR in tidal mangrove ecosystem. Marine Pollution Bulletin. 2022. 181, 113905. [CrossRef]
- Sardans, J.; Peñuelas, J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Pohlon, E.; Fandino, A.O. & Marxsen, J. Bacterial community composition and extracellular enzyme activity in temperate streambed sediment during drying and rewetting. PLoS ONE. 2013. 8, e83365. [CrossRef]
- Siebielec, S., Siebielec, G.; Klimkowicz-Pawlas, A.; Gał ˛azka, A.; Grz ˛adziel, J.; Stuczy ´nski, T. Impact of water stress on microbial community and activity in sandy and loamy soils. Agronomy. 2020, 10, 1429. [CrossRef]
- Schreckinger, J.; Mutz, M.; Mendoza-Lera, C.; Frossard, A. Attributes of drying define the structure and functioning of microbial communities in temperate riverbed sediment. Front. Microbiol. 2021, 12, 676615. [Google Scholar] [CrossRef]
- Sardans, J., and Peñuelas, J. Soil enzyme activity in a Mediterranean forest after six years of drought. Soil Science Society of America Journal. 2010. 74(3), 838-851. [CrossRef]
- Gao, W., Reed, S. C., Munson, S. M., Rui, Y., Fan, W., Zheng, Z., and Hao, Y. Responses of soil extracellular enzyme activities and bacterial community composition to seasonal stages of drought in a semiarid grassland. Geoderma. 2021. 401, 115327. [CrossRef]
- Chaudhary, D. R., Rathore, A. P., and Sharma, S. Effect of halotolerant plant growth promoting rhizobacteria inoculation on soil microbial community structure and nutrients. Applied Soil Ecology. 2022. 150, 103461. [CrossRef]
- Zhu, L., Wang, X., Chen, F., Li, C., and Wu, L. Effects of the successive planting of Eucalyptus urophylla on soil bacterial and fungal community structure, diversity, microbial biomass, and enzyme activity. Land degradation and development. 2019. 30(6), 636-646. [CrossRef]
- Lin, H., Liu, C., Li, B., and Dong, Y. Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. Journal of Hazardous Materials. 2021. 402, 123829. [CrossRef]
- Piromyou, P., Noisangiam, R., Uchiyama, H., Tittabutr, P., Boonkerd, N., and Teaumroong, N. Indigenous microbial community structure in rhizosphere of Chinese kale as affected by plant growth-promoting rhizobacteria inoculation. Pedosphere, 2013. 23(5), 577-592. [CrossRef]
- Ilyas, N., Mumtaz, K., Akhtar, N., Yasmin, H., Sayyed, R. Z., Khan, W., and Ali, Z. Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability, 2020. 12(21), 8876. [CrossRef]
- Kumar, A., Singh, S., Gaurav, A. K., Srivastava, S., and Verma, J. P. Plant growth-promoting bacteria: biological tools for the mitigation of salinity stress in plants. Frontiers in Microbiology, 2020. 11, 1216. [CrossRef]
- Shi, G., Xia, S., Ye, J., Huang, Y., Liu, C., and Zhang, Z. PEG-simulated drought stress decreases cadmium accumulation in castor bean by altering root morphology. Environmental and Experimental Botany, 2015. 111, 127-134. [CrossRef]
- Wang, J. Q., Li, H., Liu, Q., and Xiang, D. (2019). Effects of drought stress on root development and physiological characteristics of sweet potato at seedling stage. Ying Yong Sheng tai xue bao= The Journal of Applied Ecology, 2019. 30(9), 3155-3163. [CrossRef]
- Xiao, X. X. "The physiological and biochemical response of longan (Dimocarpus longan Lour.) to aluminum stress and rectification of aluminum toxicity." Fujian Journal of Agricultural Science 17, 2002: 182-185.
- Orrego, F., Ortíz-Calderón, C., Lutts, S., and Ginocchio, R. Effect of single and combined Cu, NaCl and water stresses on three Atriplex species with phytostabilization potential. South African Journal of Botany, 2020. 131, 161-168. [CrossRef]
- Nedjimi B, Beladel B, Guit B. Biodiversity of halophytic vegetation in chott Zehrez lake of Djelfa (Algeria). Am J Plant Sci3. 2012. 1513–1660. [CrossRef]
- Geng, M., Xu, M., Xiao, H., Wang, H., He, L., Zhao, Z., and Yu, M. Protective role of mucilage against Al toxicity to root apex of pea (Pisum sativum). Acta physiologiae plantarum. 2012. 34(4), 1261-1266. https://link.springer.com/article/10.1007/s11738-011-0919-1.
- Liao, M., and Xie, X. M. Cadmium release in contaminated soils due to organic acids. Pedosphere, 2004. 14(2), 223-228.
- Bali, A. S., Sidhu, G. P. S., & Kumar, V. Root exudates ameliorate cadmium tolerance in plants: a review. Environmental Chemistry Letters, 2020. 18(4), 1243-1275. [CrossRef]
- Yamaguchi, M., &Sharp, R. E. Complexity and coordination of root growth at low water potentials: recent advances from transcriptomic and proteomic analyses. Plant, Cell and Environment, 2010. 33(4), 590-603. [CrossRef]
- Naseem, H., Ahsan, M., Shahid, M. A., and Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. Journal of basic microbiology, 2018. 58(12), 1009-1022. [CrossRef]
- Abdelkrim, S., Jebara, S. H., Saadani, O., Abid, G., Taamalli, W., Zemni, H., and Jebara, M. In situ effects of Lathyrus sativus-PGPR to remediate and restore quality and fertility of Pb and Cd polluted soils. Ecotoxicology and environmental safety. 2020. 192, 110260. [CrossRef]
- Morcillo, R. J., and Manzanera, M. The effects of plant-associated bacterial exopolysaccharides on plant abiotic stress tolerance. Metabolites, 2021. 11(6), 337. [CrossRef]
- Ahmed, B., Shahid, M., Syed, A., Rajput, V. D., Elgorban, A. M., Minkina, T., and Lee, J. Drought tolerant Enterobacter sp./Leclercia adecarboxylata secretes indole-3-acetic acid and other biomolecules and enhances the biological attributes of Vigna radiata (L.) R. Wilczek in water deficit conditions. Biology, 2021. 10(11), 1149. [CrossRef]
- Hou, X., Teng, W., Hu, Y., Yang, Z., Li, C., Scullion, J., and Zheng, R. Potential phytoremediation of soil cadmium and zinc by diverse ornamental and energy grasses. BioResources, 2020. 15(1), 616-640. [CrossRef]
- Benidire L, Pereira, SIA, Castro, PML, Boularbah, A. Assessment of plant growth-promoting bacterial populations in the rhizosphere of metallophytes from the Kettara mine, Marrakech. Environ Sci Pollut Res, 2016. 23: 21751-21765. [CrossRef]
- El Hamiani O, El Khalil H, Sirguey C, Ouhammou A, Bitton G, Schwartz C, Boularbah A. Metal concentrations in plants from mining areas in South Morocco: health risks assessment of consumption of edible and aromatic plants. CLEAN–Soil Air Water, 2015. 43: 399-407. [CrossRef]
- Olsen SR, Sommers LE. Phosphorus. In: Page AL, Miller RH, Keeney DR (eds.), Methods of Soil Analysis Part 2, Second Edition. Agron Soc Am, 1982. Madison, pp. 403e430.
- Aubert G (ed). Methodes d’analyses des sols. C.R.D.P., 1978. Marseille, p. 360.
- Tabatabai MA, Bremner JM. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem, 1969. 1: 301e307. [CrossRef]
- Alef K, Nannipieri P. Methods in applied soil microbiology and biochemistry. Inst Ecol Chem Geochem, 1995. Univ Bayreuth, Germany, 569p.
- Kandeler E, Gerber H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils, 1988. 6: 68-72. [CrossRef]
- Zhu, L., Wang, X., Chen, F., Li, C., and Wu, L. Effects of the successive planting of Eucalyptus urophylla on soil bacterial and fungal community structure, diversity, microbial biomass, and enzyme activity. Land degradation and development, 2019. 30(6), 636-646. [CrossRef]
Figure 1.
-Activities of acid phosphatase (A, E, I), alkaline phosphatase (B, F, J), urease (C, G, K) and dehydrogenase (D, H, L) in the rhizosphere of different plant species: Atriplex halimus, Pennisetum setaceum and Peganum harmala. Plants were grown under 3 water irrigation regimes: Control - watered at 80% of field capacity (FC); Moderate Stress - watered at 45% of FC; Severe Stress - watered at 30% of FC, and under non-inoculated and inoculated conditions.
Figure 1.
-Activities of acid phosphatase (A, E, I), alkaline phosphatase (B, F, J), urease (C, G, K) and dehydrogenase (D, H, L) in the rhizosphere of different plant species: Atriplex halimus, Pennisetum setaceum and Peganum harmala. Plants were grown under 3 water irrigation regimes: Control - watered at 80% of field capacity (FC); Moderate Stress - watered at 45% of FC; Severe Stress - watered at 30% of FC, and under non-inoculated and inoculated conditions.
Figure 2.
PCA diagram of correlations between chemical and biological parameters for different plants studied inoculated or not under different water regime. PH: P. harmala, AH: A. halimus, PS: P. setaceum, C: control (well-watered), SM: moderated stress, SS: severe stress, +I: inoculated, -I: non inoculated, FW: Fresh weight, DW: dry weight.
Figure 2.
PCA diagram of correlations between chemical and biological parameters for different plants studied inoculated or not under different water regime. PH: P. harmala, AH: A. halimus, PS: P. setaceum, C: control (well-watered), SM: moderated stress, SS: severe stress, +I: inoculated, -I: non inoculated, FW: Fresh weight, DW: dry weight.
Figure 3.
Shoot length (A), root length (B), shoot fresh biomass (C), root fresh biomass (D), shoot dry biomass (E) and root dry biomass (F) of Atriplex halimus growing under different water regimes (Control - watered at 80% of field capacity (FC); Moderate Stress - watered at 45% of FC; Severe Stress - watered at 30% of FC) and under non-inoculated and inoculated conditions.
Figure 3.
Shoot length (A), root length (B), shoot fresh biomass (C), root fresh biomass (D), shoot dry biomass (E) and root dry biomass (F) of Atriplex halimus growing under different water regimes (Control - watered at 80% of field capacity (FC); Moderate Stress - watered at 45% of FC; Severe Stress - watered at 30% of FC) and under non-inoculated and inoculated conditions.
Figure 4.
- Shoot length (A), root length (B), shoot fresh biomass (C), root fresh biomass (D), shoot dry biomass (E) and root dry biomass (F) of Pennisetum setaceum growing under different water regimes (Control-watered at 80% of field capacity (FC); Moderate Stress-watered at 45% of FC; Severe Stress-watered at 30% of FC) and under non-inoculated and inoculated conditions. Data are shown as means ± standard errors (n= 3). A two-way ANOVA was performed to determine the influence of microbial inoculation (I) and irrigation regimes (R) on plant growth parameters (shoot length, root length, shoot fresh biomass, root fresh biomass, shoot dry biomass, and root dry biomass). The results are shown with the test statistic for each case (I: microbial inoculation; R: irrigation regimes; I x R: microbial inoculation x irrigation regimes) and as NS: Non-significant at the level P > 0.05; * significant at the level P < 0.05; ** P < 0.01; *** significant at the level P < 0.001, respectively. One-way ANOVA was performed to determine the influence of irrigation regimes on plant growth parameters for each inoculation treatment at the end of the experiment. Means for the same inoculation treatment showing different letters are significantly different from each other (P < 0.05) according to Duncan test.
Figure 4.
- Shoot length (A), root length (B), shoot fresh biomass (C), root fresh biomass (D), shoot dry biomass (E) and root dry biomass (F) of Pennisetum setaceum growing under different water regimes (Control-watered at 80% of field capacity (FC); Moderate Stress-watered at 45% of FC; Severe Stress-watered at 30% of FC) and under non-inoculated and inoculated conditions. Data are shown as means ± standard errors (n= 3). A two-way ANOVA was performed to determine the influence of microbial inoculation (I) and irrigation regimes (R) on plant growth parameters (shoot length, root length, shoot fresh biomass, root fresh biomass, shoot dry biomass, and root dry biomass). The results are shown with the test statistic for each case (I: microbial inoculation; R: irrigation regimes; I x R: microbial inoculation x irrigation regimes) and as NS: Non-significant at the level P > 0.05; * significant at the level P < 0.05; ** P < 0.01; *** significant at the level P < 0.001, respectively. One-way ANOVA was performed to determine the influence of irrigation regimes on plant growth parameters for each inoculation treatment at the end of the experiment. Means for the same inoculation treatment showing different letters are significantly different from each other (P < 0.05) according to Duncan test.
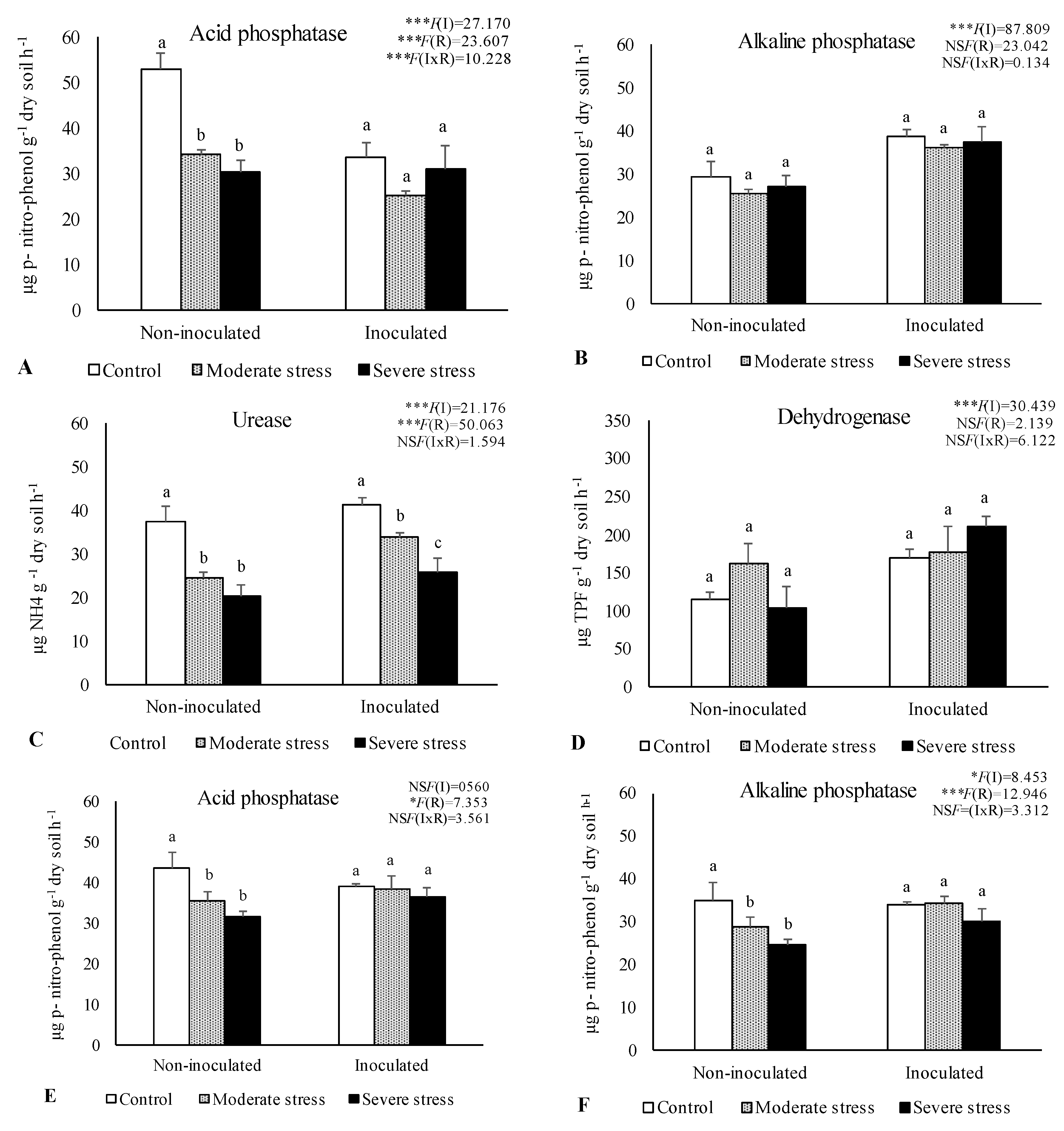
Figure 5.
- Shoot length (A), root length (B), shoot fresh biomass (C), root fresh biomass (D), shoot dry biomass (E) and root dry biomass (F) of Peganum harmala growing under different water regimes (Control-watered at 80% of field capacity (FC); Moderate Stress-watered at 45% of FC; Severe Stress-watered at 30% of FC) and under non-inoculated and inoculated conditions. Data are shown as means ± standard errors (n= 3). A two-way ANOVA was performed to determine the influence of microbial inoculation (I) and irrigation regimes (R) on plant growth parameters (shoot length, root length, shoot fresh biomass, root fresh biomass, shoot dry biomass, and root dry biomass). The results are shown with the test statistic for each case (I: microbial inoculation; R: irrigation regimes; I x R: microbial inoculation x irrigation regimes) and as NS: Non-significant at the level P > 0.05; * significant at the level P < 0.05; ** P < 0.01; *** significant at the level P < 0.001, respectively. One-way ANOVA was performed to determine the influence of irrigation regimes on plant growth parameters for each inoculation treatment at the end of the experiment. Means for the same inoculation treatment showing different letters are significantly different from each other (P < 0.05) according to Duncan test.
Figure 5.
- Shoot length (A), root length (B), shoot fresh biomass (C), root fresh biomass (D), shoot dry biomass (E) and root dry biomass (F) of Peganum harmala growing under different water regimes (Control-watered at 80% of field capacity (FC); Moderate Stress-watered at 45% of FC; Severe Stress-watered at 30% of FC) and under non-inoculated and inoculated conditions. Data are shown as means ± standard errors (n= 3). A two-way ANOVA was performed to determine the influence of microbial inoculation (I) and irrigation regimes (R) on plant growth parameters (shoot length, root length, shoot fresh biomass, root fresh biomass, shoot dry biomass, and root dry biomass). The results are shown with the test statistic for each case (I: microbial inoculation; R: irrigation regimes; I x R: microbial inoculation x irrigation regimes) and as NS: Non-significant at the level P > 0.05; * significant at the level P < 0.05; ** P < 0.01; *** significant at the level P < 0.001, respectively. One-way ANOVA was performed to determine the influence of irrigation regimes on plant growth parameters for each inoculation treatment at the end of the experiment. Means for the same inoculation treatment showing different letters are significantly different from each other (P < 0.05) according to Duncan test.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).