Submitted:
03 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Regulation of DLK at the transcriptional level
2.2. Regulation of DLK at the posttranscriptional level
2.3. Regulation of DLK at the posttranslational level
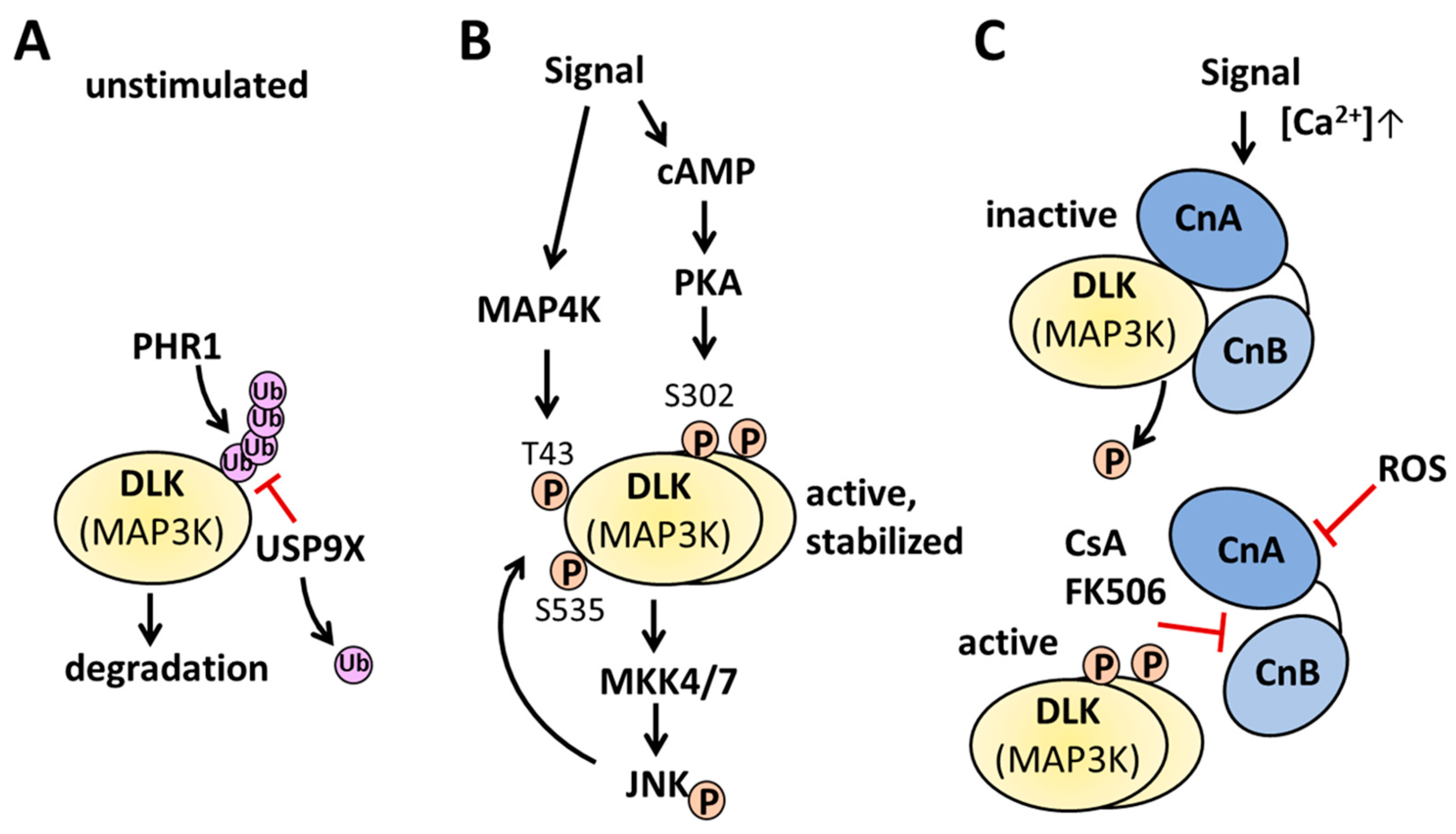
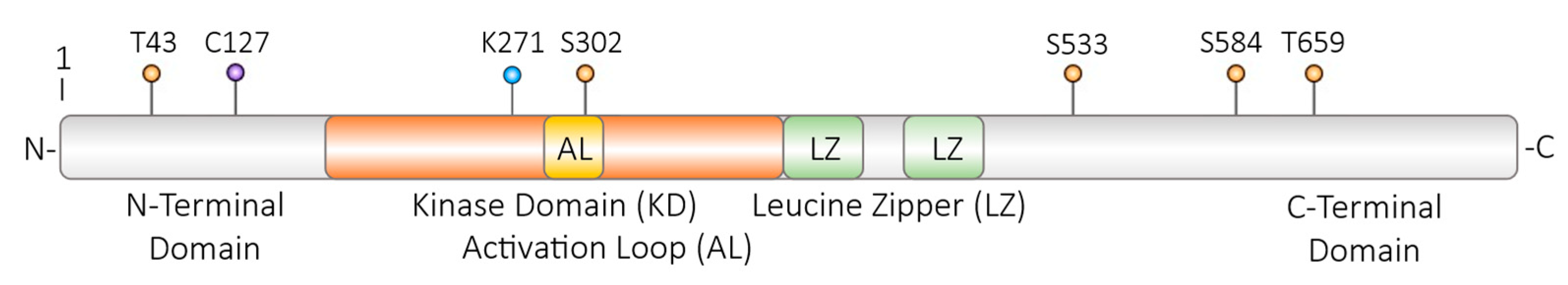
2.3.1. Phosphorylation of DLK
2.3.2. Dephosphorylation of DLK
2.3.3. Palmitoylation of DLK
2.3.4. Regulation by protein-protein interactions
2.3.5. Regulation of DLK by its oligomerization
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Le Pichon, C.E.; Meilandt, W.J.; Dominguez, S.; Solanoy, H.; Lin, H.; Ngu, H.; Gogineni, A.; Sengupta Ghosh, A.; Jiang, Z.; Lee, S.-H.; et al. Loss of dual leucine zipper kinase signaling is protective in animal models of neurodegenerative disease. Science Translational Medicine 2017, 9, eaag0394. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-W.A.; Zhou, B.; Wernig, M.; Südhof, T.C. ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Aβ Secretion. Cell 2017, 168, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Goodwani, S.; Fernandez, C.; Acton, P.J.; Buggia-Prevot, V.; McReynolds, M.L.; Ma, J.; Hu, C.H.; Hamby, M.E.; Jiang, Y.; Le, K.; et al. Dual Leucine Zipper Kinase Is Constitutively Active in the Adult Mouse Brain and Has Both Stress-Induced and Homeostatic Functions. International Journal of Molecular Sciences 2020, 21, 4849. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.S.; Rothstein, J.D.; Cudkowicz, M.E.; Genge, A.; Oskarsson, B.; Hains, A.B.; Chen, C.; Galanter, J.; Burgess, B.L.; Cho, W. A Phase 1 study of GDC-0134, a dual leucine zipper kinase inhibitor, in ALS. Annals of Clinical and Translational Neurology. [CrossRef] [PubMed]
- Hayne, M.; DiAntonio, A. Protein phosphatase 2A restrains DLK signaling to promote proper Drosophila synaptic development and mammalian cortical neuron survival. Neurobiology of Disease 2022, 163, 105586. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Roy, E.R.; Wang, Y.; Watkins, T.; Cao, W. DLK-MAPK Signaling Coupled with DNA Damage Promotes Intrinsic Neurotoxicity Associated with Non-Mutated Tau. Molecular Neurobiology, 2023. [Google Scholar] [CrossRef]
- Welsbie, D.S.; Yang, Z.; Ge, Y.; Mitchell, K.L.; Zhou, X.; Martin, S.E.; Berlinicke, C.A.; Hackler, L., Jr.; Fuller, J.; Fu, J.; et al. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc Natl Acad Sci U S A 2013, 110, 4045–4050. [Google Scholar] [CrossRef] [PubMed]
- Oetjen, E. Regulation of Beta-Cell Function and Mass by the Dual Leucine Zipper Kinase. Archiv der Pharmazie 2016, 349, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Wallbach, M.; Duque Escobar, J.; Babaeikelishomi, R.; Stahnke, M.-J.; Blume, R.; Schröder, S.; Kruegel, J.; Maedler, K.; Kluth, O.; Kehlenbach, R.H.; et al. Distinct functions of the dual leucine zipper kinase depending on its subcellular localization. Cellular Signalling 2016, 28, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Duque Escobar, J.; Kutschenko, A.; Schröder, S.; Blume, R.; Köster, K.-A.; Painer, C.; Lemcke, T.; Maison, W.; Oetjen, E. Regulation of dual leucine zipper kinase activity through its interaction with calcineurin. Cellular Signalling 2021, 82, 109953. [Google Scholar] [CrossRef]
- Holzman, L.B.; Merritt, S.E.; Fan, G. Identification, molecular cloning, and characterization of dual leucine zipper bearing kinase. A novel serine/threonine protein kinase that defines a second subfamily of mixed lineage kinases. Journal of Biological Chemistry 1994, 269, 30808–30817. [Google Scholar] [CrossRef]
- Gallo, K.A.; Johnson, G.L. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 2002, 3, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Gallo, K.A.; Ellsworth, E.; Stoub, H.; Conrad, S.E. Therapeutic potential of targeting mixed lineage kinases in cancer and inflammation. Pharmacology & Therapeutics 2020, 207, 107457. [Google Scholar] [CrossRef]
- Bisson, N.; Tremblay, M.; Robinson, F.; Kaplan, D.R.; Trusko, S.P.; Moss, T. Mice lacking both mixed-lineage kinase genes Mlk1 and Mlk2 retain a wild type phenotype. Cell Cycle 2008, 7, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Geoffroy, C.G.; Wong, H.N.; Tress, O.; Nguyen, M.T.; Holzman, L.B.; Jin, Y.; Zheng, B. Leucine Zipper-bearing Kinase promotes axon growth in mammalian central nervous system neurons. Scientific Reports 2016, 6, 31482. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.-i.; Feng Cui, D.; Miyata, T.; Ogawa, M.; Kiyonari, H.; Suda, Y.; Aizawa, S.; Banba, Y.; Ohno, S. The c-Jun N-Terminal Kinase Activator Dual Leucine Zipper Kinase Regulates Axon Growth and Neuronal Migration in the Developing Cerebral Cortex. The Journal of Neuroscience 2006, 26, 11992–12002. [Google Scholar] [CrossRef]
- Pozniak, C.D.; Sengupta Ghosh, A.; Gogineni, A.; Hanson, J.E.; Lee, S.H.; Larson, J.L.; Solanoy, H.; Bustos, D.; Li, H.; Ngu, H.; et al. Dual leucine zipper kinase is required for excitotoxicity-induced neuronal degeneration. J Exp Med 2013, 210, 2553–2567. [Google Scholar] [CrossRef] [PubMed]
- Asghari Adib, E.; Smithson, L.J.; Collins, C.A. An axonal stress response pathway: degenerative and regenerative signaling by DLK. Current Opinion in Neurobiology 2018, 53, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Ha, H.; Kim, Y.K.; Cho, Y.; DiAntonio, A. DLK regulates a distinctive transcriptional regeneration program after peripheral nerve injury. Neurobiology of Disease 2019, 127, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Bradke, F. The DLK signalling pathway--a double-edged sword in neural development and regeneration. EMBO Rep 2013, 14, 605–614. [Google Scholar] [CrossRef]
- Jin, Y.; Zheng, B. Multitasking: Dual Leucine Zipper–Bearing Kinases in Neuronal Development and Stress Management. Annual Review of Cell and Developmental Biology 2019, 35, 501–521. [Google Scholar] [CrossRef]
- Nihalani, D.; Meyer, D.; Pajni, S.; Holzman, L.B. Mixed lineage kinase-dependent JNK activation is governed by interactions of scaffold protein JIP with MAPK module components. The EMBO Journal 2001, 20, 3447–3458. [Google Scholar] [CrossRef] [PubMed]
- Nihalani, D.; Wong, H.N.; Holzman, L.B. Recruitment of JNK to JIP1 and JNK-dependent JIP1 Phosphorylation Regulates JNK Module Dynamics and Activation. Journal of Biological Chemistry 2003, 278, 28694–28702. [Google Scholar] [CrossRef] [PubMed]
- Nihalani, D.; Wong, H.; Verma, R.; Holzman, L.B. Src Family Kinases Directly Regulate JIP1 Module Dynamics and Activation. Molecular and Cellular Biology 2007, 27, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Blouin, R. DLK (Dual Leucine Zipper-Bearing Kinase). In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer International Publishing: Cham, 2018. [Google Scholar]
- Itoh, A.; Wang, Z.; Ito, Y.; Reddy, U.R.; Itoh, T. SP3 acts as a positive regulator on the core promoter of human ZPK gene. Biochemical and Biophysical Research Communications 2004, 313, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Couture, J.P.; Blouin, R. The DLK gene is a transcriptional target of PPARgamma. Biochem J 2011, 438, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ohlstein, J.F.; Strong, A.L.; McLachlan, J.A.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. Journal of Molecular Endocrinology 2014, 53, 345–353. [Google Scholar] [CrossRef]
- Couture, J.P.; Daviau, A.; Fradette, J.; Blouin, R. The mixed-lineage kinase DLK is a key regulator of 3T3-L1 adipocyte differentiation. PLoS One 2009, 4, e4743. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.D.; Anand, D.; Motohashi, H.; Katsuoka, F.; Yamamoto, M.; Lachke, S.A. Deficiency of the bZIP transcription factors Mafg and Mafk causes misexpression of genes in distinct pathways and results in lens embryonic developmental defects. Frontiers in Cell and Developmental Biology 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Jin, Y. Regulation of DLK-1 kinase activity by calcium-mediated dissociation from an inhibitory isoform. Neuron 2012, 76, 534–548. [Google Scholar] [CrossRef]
- Heeyoung, S.; Juyoung, H.; Eun-Sook, J.; Sung Wook, C. MicroRNA Target Recognition: Insights from Transcriptome-Wide Non-Canonical Interactions. Mol. Cells 2016, 39, 375–381. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Lin, Y.-C.-D.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. miRTarBase update 2022: an informative resource for experimentally validated miRNA–target interactions. Nucleic Acids Research 2021, 50, D222–D230. [Google Scholar] [CrossRef]
- Beveridge, N.J.; Tooney, P.A.; Carroll, A.P.; Tran, N.; Cairns, M.J. Down-regulation of miR-17 family expression in response to retinoic acid induced neuronal differentiation. Cellular Signalling 2009, 21, 1837–1845. [Google Scholar] [CrossRef]
- Ye, M.; Li, D.; Yang, J.; Xie, J.; Yu, F.; Ma, Y.; Zhu, X.; Zhao, J.; Lv, Z. MicroRNA-130a Targets MAP3K12 to Modulate Diabetic Endothelial Progenitor Cell Function. Cell Physiol Biochem 2015, 36, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Liu, G.; Li, X.; Liu, Y.; Wang, Y.; Pan, H.; Hu, J. MiR-191-5p alleviates microglial cell injury by targeting Map3k12 (mitogen-activated protein kinase kinase kinase 12) to inhibit the MAPK (mitogen-activated protein kinase) signaling pathway in Alzheimer’s disease. Bioengineered 2021, 12, 12678–12690. [Google Scholar] [CrossRef]
- Yu, J.; Feng, Y.; Wang, Y.; An, R. Aryl hydrocarbon receptor enhances the expression of miR-150-5p to suppress in prostate cancer progression by regulating MAP3K12. Archives of Biochemistry and Biophysics 2018, 654, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.; Merritt, S.E.; Fan, G.; Yu, G.G.; Holzman, L.B. Characterization of Dual Leucine Zipper-bearing Kinase, a Mixed Lineage Kinase Present in Synaptic Terminals Whose Phosphorylation State Is Regulated by Membrane Depolarization via Calcineurin. Journal of Biological Chemistry 1996, 271, 16888–16896. [Google Scholar] [CrossRef] [PubMed]
- Huntwork-Rodriguez, S.; Wang, B.; Watkins, T.; Ghosh, A.S.; Pozniak, C.D.; Bustos, D.; Newton, K.; Kirkpatrick, D.S.; Lewcock, J.W. JNK-mediated phosphorylation of DLK suppresses its ubiquitination to promote neuronal apoptosis. J Cell Biol 2013, 202, 747–763. [Google Scholar] [CrossRef]
- Börchers, S.; Babaei, R.; Klimpel, C.; Duque Escobar, J.; Schröder, S.; Blume, R.; Malik, M.N.H.; Oetjen, E. TNFα-induced DLK activation contributes to apoptosis in the beta-cell line HIT. Naunyn-Schmiedeberg’s Archives of Pharmacology 2017, 390, 813–825. [Google Scholar] [CrossRef]
- Hao, Y.; Frey, E.; Yoon, C.; Wong, H.; Nestorovski, D.; Holzman, L.B.; Giger, R.J.; DiAntonio, A.; Collins, C. An evolutionarily conserved mechanism for cAMP elicited axonal regeneration involves direct activation of the dual leucine zipper kinase DLK. eLife 2016, 5, e14048. [Google Scholar] [CrossRef]
- Ghosh-Roy, A.; Wu, Z.; Goncharov, A.; Jin, Y.; Chisholm, A.D. Calcium and Cyclic AMP Promote Axonal Regeneration in <span class="named-content project" id="named-content-1">Caenorhabditis elegans</span> and Require DLK-1 Kinase. The Journal of Neuroscience 2010, 30, 3175–3183. [Google Scholar] [CrossRef] [PubMed]
- Larhammar, M.; Huntwork-Rodriguez, S.; Rudhard, Y.; Sengupta-Ghosh, A.; Lewcock, J.W. The Ste20 Family Kinases MAP4K4, MINK1, and TNIK Converge to Regulate Stress-Induced JNK Signaling in Neurons. The Journal of Neuroscience 2017, 37, 11074–11084. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Broyer, R.M.; Lee, C.D.; Lu, T.; Louie, M.J.; La Torre, A.; Al-Ali, H.; Vu, M.T.; Mitchell, K.L.; Wahlin, K.J.; et al. Inhibition of GCK-IV kinases dissociates cell death and axon regeneration in CNS neurons. Proceedings of the National Academy of Sciences 2020, 117, 33597–33607. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Qiu, W.R.; Wang, X.; Meyer, C.F.; Tan, T.H. Human HPK1, a novel human hematopoietic progenitor kinase that activates the JNK/SAPK kinase cascade. Genes & Development 1996, 10, 2251–2264. [Google Scholar] [CrossRef]
- Leung, I.W.-L.; Lassam, N. The Kinase Activation Loop Is the Key to Mixed Lineage Kinase-3 Activation via Both Autophosphorylation and Hematopoetic Progenitor Kinase 1 Phosphorylation*. Journal of Biological Chemistry 2001, 276, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Karney-Grobe, S.; Russo, A.; Frey, E.; Milbrandt, J.; DiAntonio, A. HSP90 is a chaperone for DLK and is required for axon injury signaling. Proceedings of the National Academy of Sciences 2018, 115, E9899–E9908. [Google Scholar] [CrossRef] [PubMed]
- Daviau, A.; Proulx, R.; Robitaille, K.; Di Fruscio, M.; Tanguay, R.M.; Landry, J.; Patterson, C.; Durocher, Y.; Blouin, R. Down-regulation of the mixed-lineage dual leucine zipper-bearing kinase by heat shock protein 70 and its co-chaperone CHIP. J Biol Chem 2006, 281, 31467–31477. [Google Scholar] [CrossRef]
- Lee, B.; Oh, Y.; Cho, E.; DiAntonio, A.; Cavalli, V.; Shin, J.E.; Choi, H.W.; Cho, Y. FK506-binding protein-like and FK506-binding protein 8 regulate dual leucine zipper kinase degradation and neuronal responses to axon injury. Journal of Biological Chemistry 2022, 298, 101647. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.M.; Collura, K.M.; Ketschek, A.; Noma, K.; Ferguson, T.A.; Jin, Y.; Gallo, G.; Thomas, G.M. Palmitoylation controls DLK localization, interactions and activity to ensure effective axonal injury signaling. Proc Natl Acad Sci U S A 2016, 113, 763–768. [Google Scholar] [CrossRef]
- Daviau, A.; Di Fruscio, M.; Blouin, R. The mixed-lineage kinase DLK undergoes Src-dependent tyrosine phosphorylation and activation in cells exposed to vanadate or platelet-derived growth factor (PDGF). Cellular Signalling 2009, 21, 577–587. [Google Scholar] [CrossRef]
- Wu, C.-C.; Wu, H.-J.; Wang, C.-H.; Lin, C.-H.; Hsu, S.-C.; Chen, Y.-R.; Hsiao, M.; Schuyler, S.C.; Lu, F.L.; Ma, N.; et al. Akt suppresses DLK for maintaining self-renewal of mouse embryonic stem cells. Cell Cycle 2015, 14, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Tulgren, E.D.; Baker, S.T.; Rapp, L.; Gurney, A.M.; Grill, B. PPM-1, a PP2Cα/β phosphatase, Regulates Axon Termination and Synapse Formation in Caenorhabditis elegans. Genetics 2011, 189, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.T.; Opperman, K.J.; Tulgren, E.D.; Turgeon, S.M.; Bienvenut, W.; Grill, B. RPM-1 Uses Both Ubiquitin Ligase and Phosphatase-Based Mechanisms to Regulate DLK-1 during Neuronal Development. PLOS Genetics 2014, 10, e1004297. [Google Scholar] [CrossRef] [PubMed]
- Oetjen, E.; Lechleiter, A.; Blume, R.; Nihalani, D.; Holzman, L.; Knepel, W. Inhibition of membrane depolarisation-induced transcriptional activity of cyclic AMP response element binding protein (CREB) by the dual-leucine-zipper-bearing kinase in a pancreatic islet beta cell line. Diabetologia 2006, 49, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Plaumann, S.; Blume, R.; Börchers, S.; Steinfelder, H.J.; Knepel, W.; Oetjen, E. Activation of the Dual-Leucine-Zipper-Bearing Kinase and Induction of β-Cell Apoptosis by the Immunosuppressive Drug Cyclosporin A. Molecular Pharmacology 2008, 73, 652–659. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, J.; Hendricks, M.; Le Guyader, S.; Subburaju, S.; Grunewald, B.; Scholich, K.; Jesuthasan, S. Formation of the retinotectal projection requires Esrom, an ortholog of PAM (protein associated with Myc). Development 2005, 132, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Lewcock, J.W.; Genoud, N.; Lettieri, K.; Pfaff, S.L. The Ubiquitin Ligase Phr1 Regulates Axon Outgrowth through Modulation of Microtubule Dynamics. Neuron 2007, 56, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund, M.; Nix, P.; Hauth, L.; Jorgensen, E.M.; Bastiani, M. Axon Regeneration Requires a Conserved MAP Kinase Pathway. Science 2009, 323, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Liao, E.H.; Hung, W.; Abrams, B.; Zhen, M. An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature 2004, 430, 345–350. [Google Scholar] [CrossRef]
- Nakata, K.; Abrams, B.; Grill, B.; Goncharov, A.; Huang, X.; Chisholm, A.D.; Jin, Y. Regulation of a DLK-1 and p38 MAP Kinase Pathway by the Ubiquitin Ligase RPM-1 Is Required for Presynaptic Development. Cell 2005, 120, 407–420. [Google Scholar] [CrossRef]
- Takekawa, M.; Saito, H. A Family of Stress-Inducible GADD45-like Proteins Mediate Activation of the Stress-Responsive MTK1/MEKK4 MAPKKK. Cell 1998, 95, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Valakh, V.; Walker, L.J.; Skeath, J.B.; DiAntonio, A. Loss of the Spectraplakin Short Stop Activates the DLK Injury Response Pathway in <em>Drosophila</em>. The Journal of Neuroscience 2013, 33, 17863–17873. [Google Scholar] [CrossRef]
- Valakh, V.; Frey, E.; Babetto, E.; Walker, L.J.; DiAntonio, A. Cytoskeletal disruption activates the DLK/JNK pathway, which promotes axonal regeneration and mimics a preconditioning injury. Neurobiol Dis 2015, 77, 13–25. [Google Scholar] [CrossRef]
- JANSSENS, V.; GORIS, J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochemical Journal 2001, 353, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Roy, J.; Martinez-Martinez, S.; Lopez-Maderuelo, M.D.; Nino-Moreno, P.; Orti, L.; Pantoja-Uceda, D.; Pineda-Lucena, A.; Cyert, M.S.; Redondo, J.M. A conserved docking surface on calcineurin mediates interaction with substrates and immunosuppressants. Mol Cell 2009, 33, 616–626. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Biddle, K.; Augustine, T.; Azmi, S. Post-Transplantation Diabetes Mellitus. Diabetes Therapy 2020, 11, 779–801. [Google Scholar] [CrossRef] [PubMed]
- Dennis, K.M.J.H.; Heather, L.C. Post-translational palmitoylation of metabolic proteins. Frontiers in Physiology 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Holland, S.M.; Ketschek, A.; Collura, K.M.; Hesketh, N.L.; Hayashi, T.; Gallo, G.; Thomas, G.M. Palmitoylation couples the kinases DLK and JNK3 to facilitate prodegenerative axon-to-soma signaling. Science Signaling 2022, 15, eabh2674. [Google Scholar] [CrossRef]
- Tortosa, E.; Sengupta Ghosh, A.; Li, Q.; Wong, W.R.; Hinkle, T.; Sandoval, W.; Rose, C.M.; Hoogenraad, C.C. Stress-induced vesicular assemblies of dual leucine zipper kinase are signaling hubs involved in kinase activation and neurodegeneration. The EMBO Journal 2022, 41, e110155. [Google Scholar] [CrossRef]
- Martin, D.D.O.; Kanuparthi, P.S.; Holland, S.M.; Sanders, S.S.; Jeong, H.-K.; Einarson, M.B.; Jacobson, M.A.; Thomas, G.M. Identification of Novel Inhibitors of DLK Palmitoylation and Signaling by High Content Screening. Scientific Reports 2019, 9, 3632. [Google Scholar] [CrossRef]
- Niu, J.; Sanders, S.S.; Jeong, H.-K.; Holland, S.M.; Sun, Y.; Collura, K.M.; Hernandez, L.M.; Huang, H.; Hayden, M.R.; Smith, G.M.; et al. Coupled Control of Distal Axon Integrity and Somal Responses to Axonal Damage by the Palmitoyl Acyltransferase ZDHHC17. Cell Reports 2020, 33, 108365. [Google Scholar] [CrossRef] [PubMed]
- Grill, B.; Murphey, R.K.; Borgen, M.A. The PHR proteins: intracellular signaling hubs in neuronal development and axon degeneration. Neural Development 2016, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Mooney, L.M.; Whitmarsh, A.J. Docking Interactions in the c-Jun N-terminal Kinase Pathway*. Journal of Biological Chemistry 2004, 279, 11843–11852. [Google Scholar] [CrossRef]
- Xu, Z.; Kukekov, N.V.; Greene, L.A. POSH acts as a scaffold for a multiprotein complex that mediates JNK activation in apoptosis. The EMBO Journal 2003, 22, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nature Reviews Molecular Cell Biology 2010, 11, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Gallardo, L.; Martín-Benito, J.; Marcilla, M.; Espadas, G.; Sabidó, E.; Valpuesta, J.M. The cochaperone CHIP marks Hsp70- and Hsp90-bound substrates for degradation through a very flexible mechanism. Scientific Reports 2019, 9, 5102. [Google Scholar] [CrossRef] [PubMed]
- Hébert, S.S.; Daviau, A.; Grondin, G.; Latreille, M.; Aubin, R.A.; Blouin, R. The Mixed Lineage Kinase DLK Is Oligomerized by Tissue Transglutaminase during Apoptosis. Journal of Biological Chemistry 2000, 275, 32482–32490. [Google Scholar] [CrossRef]
- Robitaille, K.; Daviau, A.; Tucholski, J.; Johnson, G.V.W.; Rancourt, C.; Blouin, R. Tissue transglutaminase triggers oligomerization and activation of dual leucine zipper-bearing kinase in calphostin C-treated cells to facilitate apoptosis. Cell Death & Differentiation 2004, 11, 542–549. [Google Scholar] [CrossRef]
- Robitaille, K.; Daviau, A.; Lachance, G.; Couture, J.P.; Blouin, R. Calphostin C-induced apoptosis is mediated by a tissue transglutaminase-dependent mechanism involving the DLK/JNK signaling pathway. Cell Death & Differentiation 2008, 15, 1522–1531. [Google Scholar] [CrossRef]
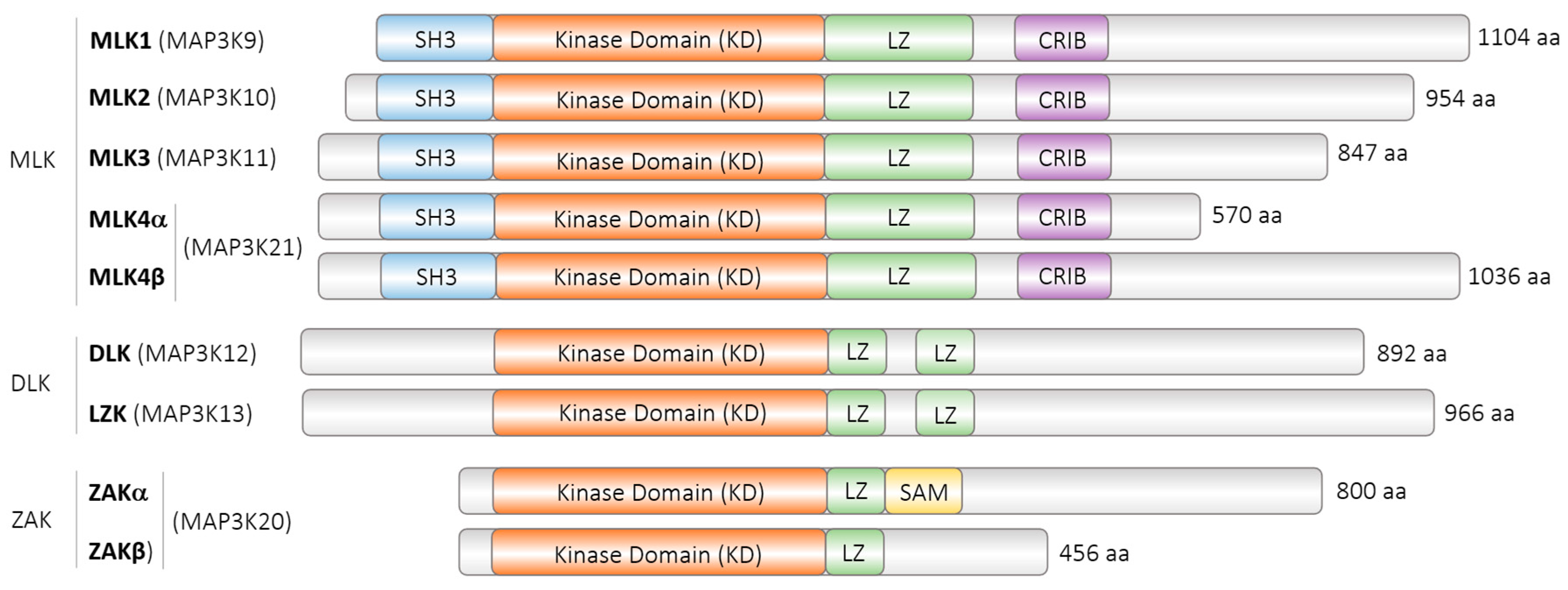
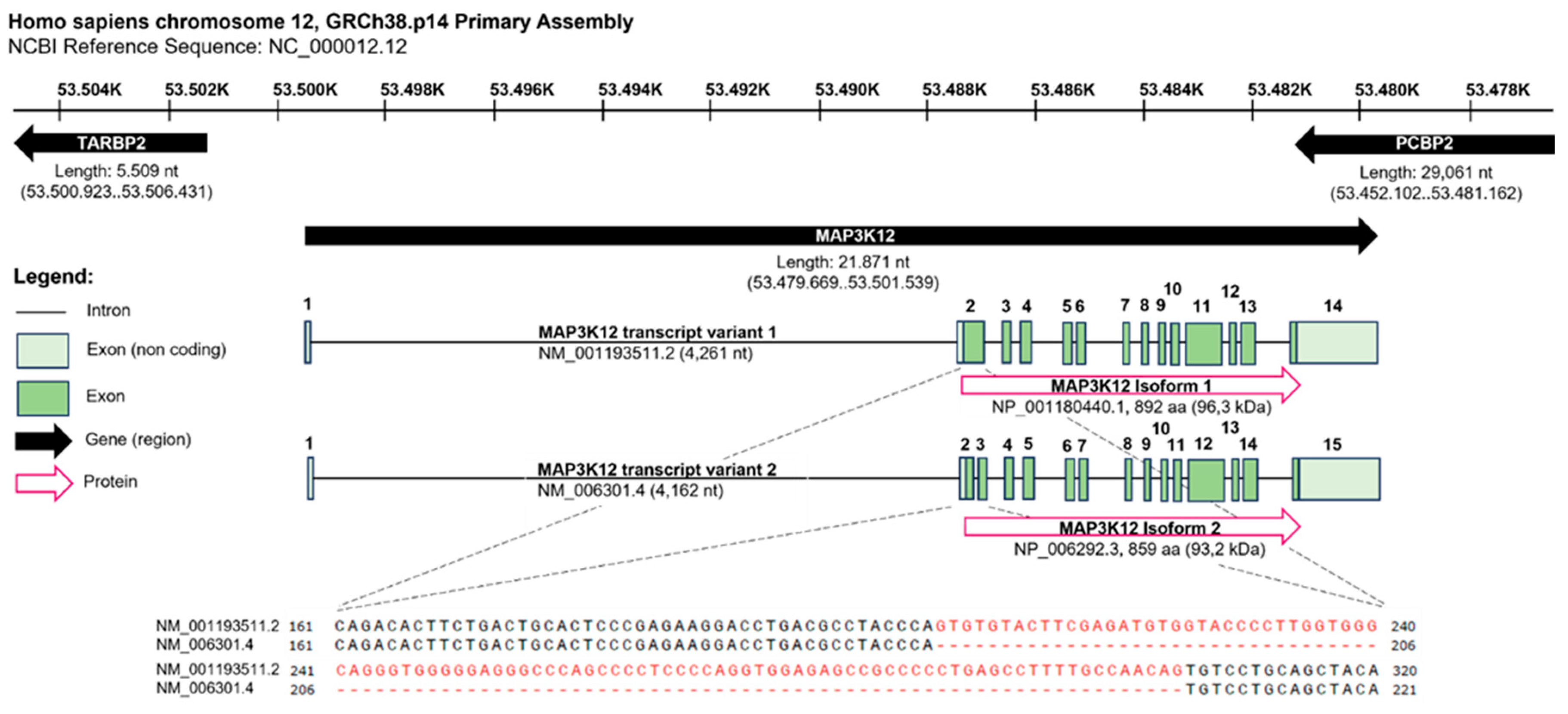
| Model Organism | Protein | Accession Number | Isoforms | Length |
|---|---|---|---|---|
| Homo sapiens | MAP3K12, ZPK | NP_001180440.1 | Isoform 1 | 892 aa |
| Homo sapiens | MAP3K12, ZPK | NP_006292.3 | Isoform 2 | 859 aa |
| Mus musculus | MAP3K12, DLK | NP_001157115.1 | (not described) | 888 aa |
| Rattus norvegicus | MAP3K12, MUK | NP_037187.1 | (not described) | 888 aa |
| Mesocricetus auratus | MAP3K12, DLK | XP_040609646.1 | Isoform X1 | 920 aa |
| Mesocricetus auratus | MAP3K12, DLK | XP_012966775.1 | Isoform X2 | 862 aa |
| Mesocricetus auratus | MAP3K12, DLK | XP_005067383.1 | Isoform X3 | 892 aa |
| Caenorhabditis elegans | DLK-1 | NP_001021443.1 | long | 928 aa |
| Caenorhabditis elegans | DLK-1 | NP_001021445.1 | short | 577 aa |
| Drosophila melanogaster | Wallenda, wnd | NP_649137.3 | Isoform (A) | 977 aa |
| Drosophila melanogaster | Wallenda, wnd | NP_788540.1 | Isoform (B) | 977 aa |
| Drosophila melanogaster | Wallenda, wnd | NP_788541.1 | Isoform (C) | 977 aa |
| Drosophila melanogaster | Wallenda, wnd | NP_001189132.1 | Isoform (D) | 950 aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





