1. Introduction
Armenia is one of the regions that is exceptionally rich in a variety of natural resources in a relatively small area. The country's climate varies due to factors such as its location, atmospheric circulation and mountainous terrain, as well as the height and position of the mountains. Under conditions of increasing anthropogenic impact on nature, potential changes in the environment are manifested primarily in the biogeochemical processes of land cover [
1]. Changes in climatic conditions have a profound effect on soil formation processes [
2]. This leads to changes in soil biochemistry in terms of composition and quantity [
3], erosion and weathering [
4,
5], and irreversible processes for living organisms [
6]. A noticeable redistribution of elements in soil composition due to their migration is associated with human industrial and agricultural activities [
7,
8].
Different surface soil horizons, formed by the intravital secretions of living organisms, plant root systems, and groundwater, provide for the physicochemical sorption of nitrogen, phosphorus, potassium, and calcium, as well as the biogenic accumulation of humic organic compounds from chemical elements [
9]. In general, these elements are conventionally classified as macro- and trace elements (ME and TE). Their importance for plant growth is generally recognized in the scientific literature [
10,
11]. As participants in specific biochemical and physiological processes responsible for the synthesis of certain substances, both ME and TE have concentration limits when taken up by plants from the soil [
12].
As a result, soils are irreversibly degraded, losing their beneficial properties and agricultural functionality, making it difficult to leave the environment unpolluted, which has adverse effects on the food chain [
13,
14,
15]. That is why, before assessing the degree of soil contamination, it is important to distinguish between the causes of its occurrence: anthropogenic and abiotic [
16,
17].
The Clark-Vernadsky law emphasizes the ubiquitous presence of chemical elements in nature [
18,
19]. This situation can be clarified by introducing Clark's concentration (CC) of chemical elements in the continental crust, which serves as a standard method for comparing geochemical systems [
20,
21]. According to this concept, regional geochemical background landscapes are identified by analyzing the technogenic-geochemical transformation of the chemical composition of natural environments [
22,
23].
The proportion of ME present ranges from hundredths to whole percentages. They are evenly distributed in the various tissues of the body. These elements include chlorine (Cl), calcium (Ca), sulfur (S), phosphorus (P), magnesium (Mg), sodium (Na), and potassium (K). Most ME are an essential component of living organisms [
24]. Their absence has serious consequences, such as disrupted metabolism, impaired cell division, and impaired transfer of genetic material [
25,
26,
27].
TE groups are distributed unevenly and accumulate in living organisms in negligible quantities, typically in concentrations of thousandths and hundred-thousandths of a percent [
28,
29]. TE are essential for plant growth and development [
30,
31]. Among these are strontium (Sr), rubidium (Rb), iron (Fe), copper (Cu), selenium (Se), iodine (I), chromium (Cr), zinc (Zn), fluorine (F), manganese (Mn), cobalt (Co), molybdenum (Mo), cesium (Cs), silicon (Si), bromine (Br), vanadium (V), and boron (B).
For the differentiation of pollution sources, monitoring of the transformed soil cover with sensitive plant test objects is necessary [
32,
33,
34]. Plants are preferred bioindicators over animals because they produce energy and provide food for many organisms at higher trophic levels [
35,
36]. Among the model plants for bio-testing,
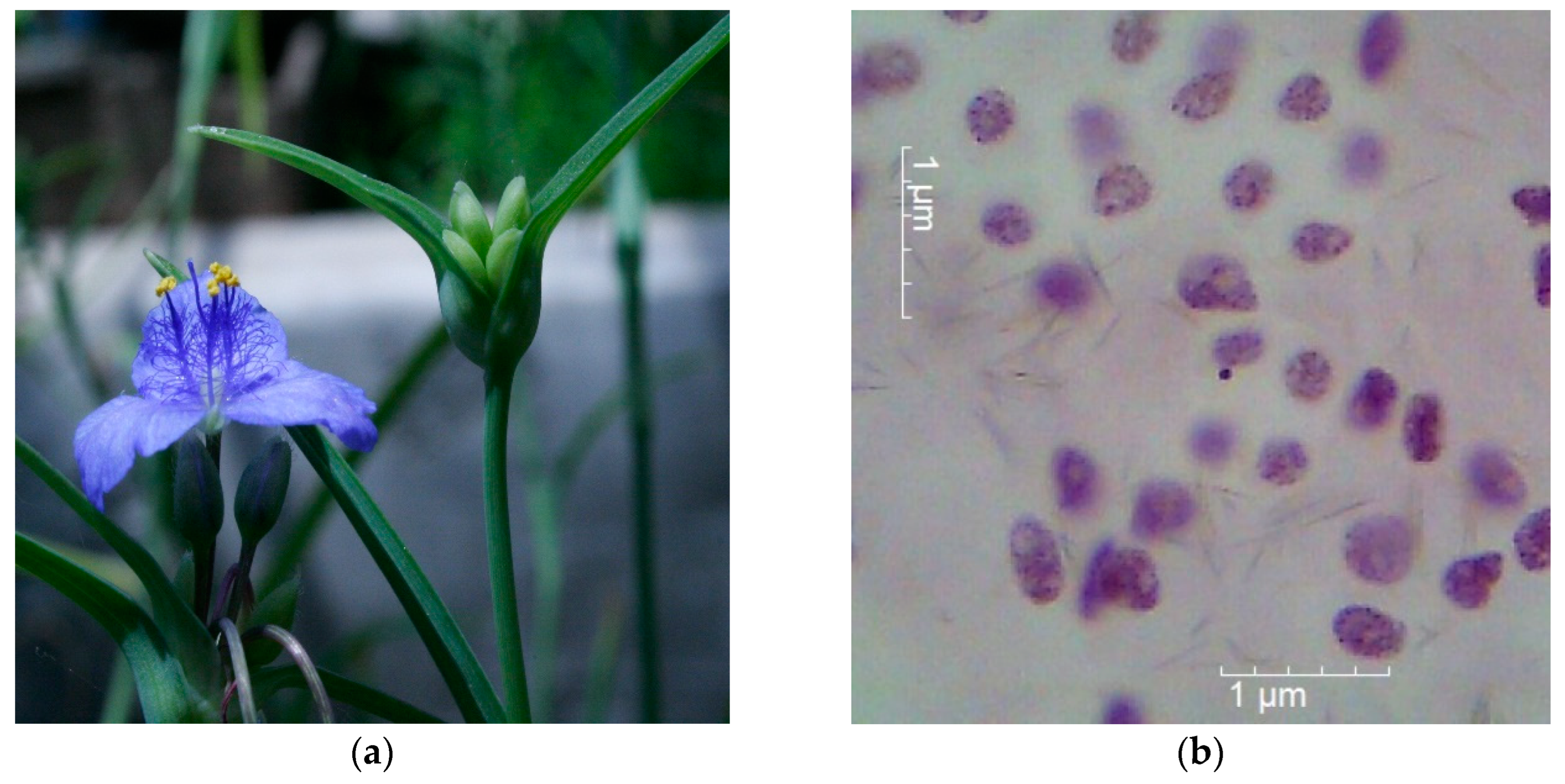
Tradescantia (clone 02) deserves special mention. Its use makes it possible to assess the induction of genetic disorders, point mutations, and clastogenic effects under the influence of sufficiently low concentrations of xenobiotics [
37,
38]. The main marker tests for
Tradescantia clone 02 in biotesting are the micronucleus test to detect disturbances in the process of microsporogenesis with the formation of micronuclei (MN) in tetrads of microspores (clastogenic effect - Trad-MN test). Using Trad-MN test allows the detection of the appearance of chromosomal aberrations (acentric fragments or lagging chromosomes), recorded in the form of MN at the stage of microspore tetrads when microsporogenesis is disturbed [
39].
Based on the concept that comprehensive assessment and monitoring of natural potential are important national priorities of Armenia in the field of environmental safety, the following regions of Armenia were selected Hrazdan, Gavar, and Martuni. In general, the formation of the land cover on the territory of modern Armenia began in the lower Pliocene. Moreover, volcanic eruptions, which dramatically changed the orography of the country, had a great influence on the formation of land cover [
39]. Geographically, Hrazdan is mainly located in a steppe area surrounded by open forests. A short distance from the city, in the basin of the Hrazdan River, there are several deposits. The climate of the region is distinctly continental, with moderately cool, rainy summers and rather cold, snowy winters with an average annual temperature of 4.8°C [
40].
Gavar lies on the banks of the Gavaraget River at an altitude of 1982m. The city is dominated by the Gegham Mountains to the west and Lake Sevan to the east. Gavar has a humid continental climate with an annual rainfall of 450 mm. Gavar has pleasant, dry, and clear summers, but frosty, snowy, and sometimes cloudy winters. Temperatures typically range from -12°C to 24°C throughout the year.
Martuni is situated on the southwestern shore of the high mountain Lake Sevan, at an altitude of 1950 m above sea level. To the north of the town, there is a forest belt around Lake Sevan, and to the south, there is the Vardenis Range. Martuni is pleasant, dry, and clear in summer and frosty, snowy, and sometimes cloudy in winter. Temperatures throughout the year typically range from -12°C to 25°C.
In consideration of the above, the experimental study aimed to investigate the distribution of concentration of some chemical elements from two groups (ME and TE) in soil samples taken from regions of Armenia (Hrazdan, Gavar, and Martuni). These regions have almost similar soil structures, but they differ from each other in terms of climatic conditions. For this reason, we aimed to determine the concentration changes of ME and TE uptake during the four seasons (autumn 2021, winter 2021/22, spring 2022, and summer 2022). As a bioassay of our study, we first used a two-criteria genotoxicity test on soil samples to identify the correlation with the concentrations of ME and TE in soil samples.
2. Materials and Methods
2.1. Soil sampling
The experimental study determined the concentrations of ME such as Ca, S, and K; and TE consisting of Sr, Rb, and Cs in soil samples collected during the agricultural year: autumn 2021, winter 2021/22, spring 2022, and summer 2022. Soil samples were collected from several regions of Armenia, including Hrazdan (two locations), Gavar (two locations), and Martuni (two locations). Geographical coordinates for each sampling site are documented in
Table 1. Sampling areas were identified on private agricultural land with moderate traffic, away from the industrial zone [
6]. Only the Hrazdan soil was located in the south-western direction of the complex industrial comprising the Hrazdan Thermal Power Station and the Hrazdan Cement Plan. To account for the anthropogenic load and the regional wind rose, the sampling process was carried out meticulously [
41]. Soil samples were collected from the control points under dry weather conditions by employing the envelope method at a depth of up to 20 cm. Non-metallic tools were used for point sampling. To prepare the soil sample, at least five incremental samples obtained from the same site were mixed. The samples were subsequently placed in dark glass containers and transported at +4°C for 24 hours for instrumental measurements in the laboratory [
42]. They are mountain semi-desert grey soils formed at altitudes of 850-1350 m above sea level. These soils were formed on alluvial, alluvial-diluvial-pebble, or clastic-pebble carbonates, often gypsum loams. Most of the parent rocks are saline because the area is in a very arid zone. The absence of waterlogging and high porosity characterizes the loose sediments and lava deposits in this region. The density in the upper humus horizons of the non-moor semi-desert grey soils is high, reaching 1.42-1.34 g/cm
3. Mountain semi-desert grey soils are very poor in humus because poor plant residues mineralize rapidly at high temperatures.
2.2. Elemental analysis
After removing any debris from the root system, insects, and other solid components, the soil was ground using a mortar and pestle, and sifted through a 1 mm diameter sieve. An air-dried sample of the tested soil was weighed first using a calibrated analytical balance (OHAUS EP 6102). The sample was then spread thinly onto a clean, flat glass, situated beneath a fume hood where it was stirred continuously for roughly an hour using either a glass or polymer rod. After each 1-2 agitations, the sample was reweighed, and the weight difference was recorded. This process was repeated until a stable dry mass of the samples was established. If the last three weightings' results did not differ by more than the balance's error, we determined the average dry mass of the sample. Subsequently, we ground the obtained soil sample into a powdery mass using a ceramic mortar. The sample was transferred under specific pressure into a sealed container with a 32mm diameter and XRF sample cups made of polypropylene film. Direct X-ray exposure was used to analyze all soil samples for elemental analysis using a portable XRF analyzer by Termo Scientific™ Niton™ [
43].
2.3. Chemical analyses
A 30 g soil sample (within a precision of 0.1 g) was added to a conical volumetric flask. Distilled water was then poured into the flask until it reached the 150 cm mark. The solution was stirred with a propeller stirrer for 3 minutes and left to settle for 5 minutes. Subsequently, a 25 cm
3 volume of the filtrate was poured into a porcelain cup that had been dried and weighed (with a tolerance of 0.001 g). The filtrate was finally evaporated in a water bath. After the evaporation process, the cup was set in a thermostat at 105°C for three hours, cooled in a desiccator, and weighed with a tolerance of 0.001 g. To determine the pH and electrical conductivity of the soil extract, a HACH LANGE HQ 14d water analyzer was utilized. The dense residue's mass fraction in the analyzed soil aqueous extract was calculated employing a formula.
where m is the mass of the beaker containing the residue, g; m
1 is the mass of an empty beaker, g; 500 is the conversion factor in percent; 25 is the volume of the extracted sample, cm
3. These analyses, and the total relative error of these analyses, are provided by standard [
44]. This standard establishes methods for determining the specific electrical conductivity, pH, and residual density of water extracts from saline soils to assess the total concentration of salts when conducting soil, agrochemical, and land reclamation surveys, monitoring the status of the salinity regime of soils. Details of soil chemical characteristics are shown in
Table 2.
2.4. Bioassays
Tradescantia (clone 02) was used as a model plant to determine the extent of clastogenicity in the contaminated soil samples. This bioassay is included in the International Programme for Monitoring and Testing of Environmental Contaminants [
45]. During the experiment, 5-10 plants per option underwent cytogenetic monitoring. The plants were planted directly in the pot with soil from the study sites. A few weeks after the formation of inflorescences containing 16-20 flower buds (the 7th or 8th pair of buds from the top of the inflorescence pyramid), tetrads in early prophase I (pachytene and diplotene) show sensitivity to mutagens. The buds were then fixed in acetoacetic acid fixative consisting of three parts 96% alcohol and one-part glacial acetic acid. Temporary acetocarmine preparations were then made according to standard methods [
46]. Early tetrads have complete membranes around the four nuclei. As a result, the four cells of the tetrads do not separate during preparation and the resulting micronuclei, which are indicators of contamination, are close together. One to five micronuclei can form in each tetrad. The number of tetrads with micronuclei was determined and the number of micronuclei per 100 tetrads was recalculated according to the standard method [
47]. Cells were analyzed at 10x20 or 10x40 magnification using a Motic Images Swift M10L series microscope.
2.5. Calculation of geochemical coefficients
The total pollution index (Z
c) by characterizing the accumulation of chemical elements in soil samples, was calculated using the formulas
where n is the number of chemical elements that are measured in a sample of soil, K
c is the ratio of the concentration of a chemical element in the investigated soil samples to the value (C
s, mg/kg) of its background (i.e. CC) concentration (B
f, mg/kg) [
48,
49].
2.6. Statistical analysis
All results were averaged and statistically processed using the computer program Stastgraphics Centurion 16. 2. - (STATGRAPHICS Centurion XVI Version 16.2.04). The calculation of mean concentrations of elements and analysis of variances to estimate statistically significant differences between groups of the samples was performed.
3. Results and discussion
3.1. Distribution of elements in soils
The qualitative and quantitative composition of soil depends on the environment in which it was formed [
50,
51]. Special attention is given to soil formation processes that are directly related to climatic conditions [
52]. The process of soil chemistry formation can be influenced by abiotic and anthropogenic factors, depending on the specific natural conditions of the location. However, the direct influence of various pollutants on the qualitative composition of the soil is a determining factor [
53]. Therefore, it is important to study the changes in the concentration of ME and TE present in the soil, as well as to determine the factors that control the concentration of these elements, and their toxicity [
54,
55,
56]. These spatial-climatic investigations can help solve many environmental problems.
3.1.1. Macro-elements
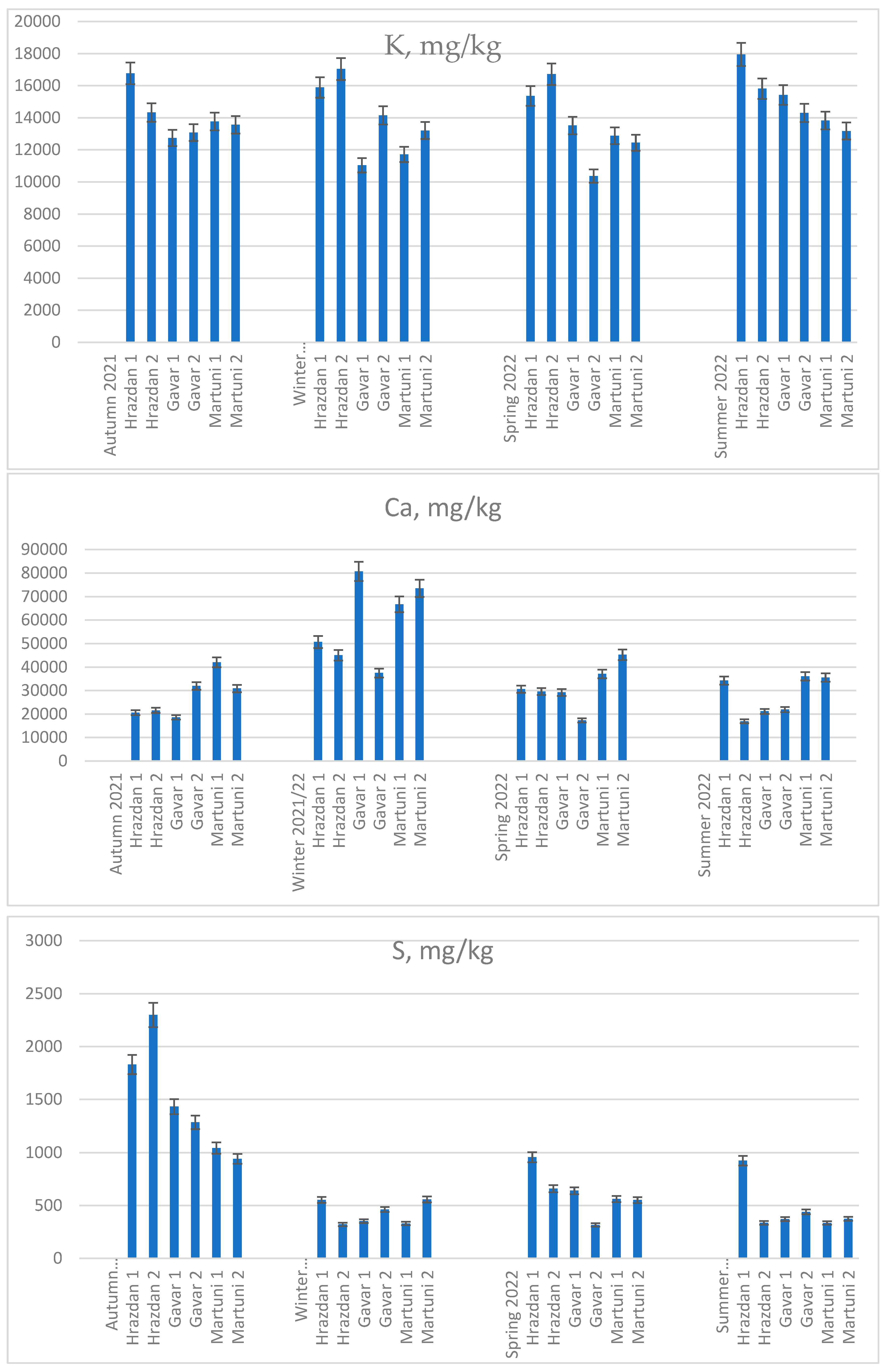
The concentration of K, Ca and S in soil samples from different locations is shown in
Figure 1. In the ME group, the concentration of K, Ca and S was determined in soil samples from private agricultural land during the whole sowing period from autumn 2021 to summer 2022. The concentration of K showed statistically significant differences, which slightly increased during the summer. At the same time, the highest accumulation of K was found in G1 soil samples in autumn, with an average of 17368 mg/kg. In winter and spring, the highest accumulation was found in the G2 soil samples.
High concentrations of Ca were found at all soil sampling sites in winter. In soil samples from H1, the average concentration in autumn, spring and summer was 28514 mg/kg, which was 44% lower than in samples from the same region in winter. Soils from G1 and G2 differed in relatively low Ca concentrations in autumn, spring and summer. According to the obtained results, high Ca concentrations were found in all investigated sites of soil sampling in winter in the sites of Hrazdan H1 > H2. In the sites of Gavar, the situation was not as simple as it seemed. The highest Ca concentration in G1 was 80685 mg/kg and the lowest in G2 was 37500 mg/kg. A similar picture is observed both in autumn and in spring, but with a fourfold lower concentration.
The situation was completely different for the accumulation of S in the soil samples. This element had a high concentration in all soil samples only in autumn: at the Hrazdan site (H1<H2), then decreasing in order at the Gavar (G1>G2) and Martuni (M1>M2) sites. In the other sampling seasons, 4-5 times decrease in the concentration of the element in soil samples is observed in winter, spring and summer. The comparative series was maintained in the sampling sites for S concentration.
3.1.2. Trace elements (TE)
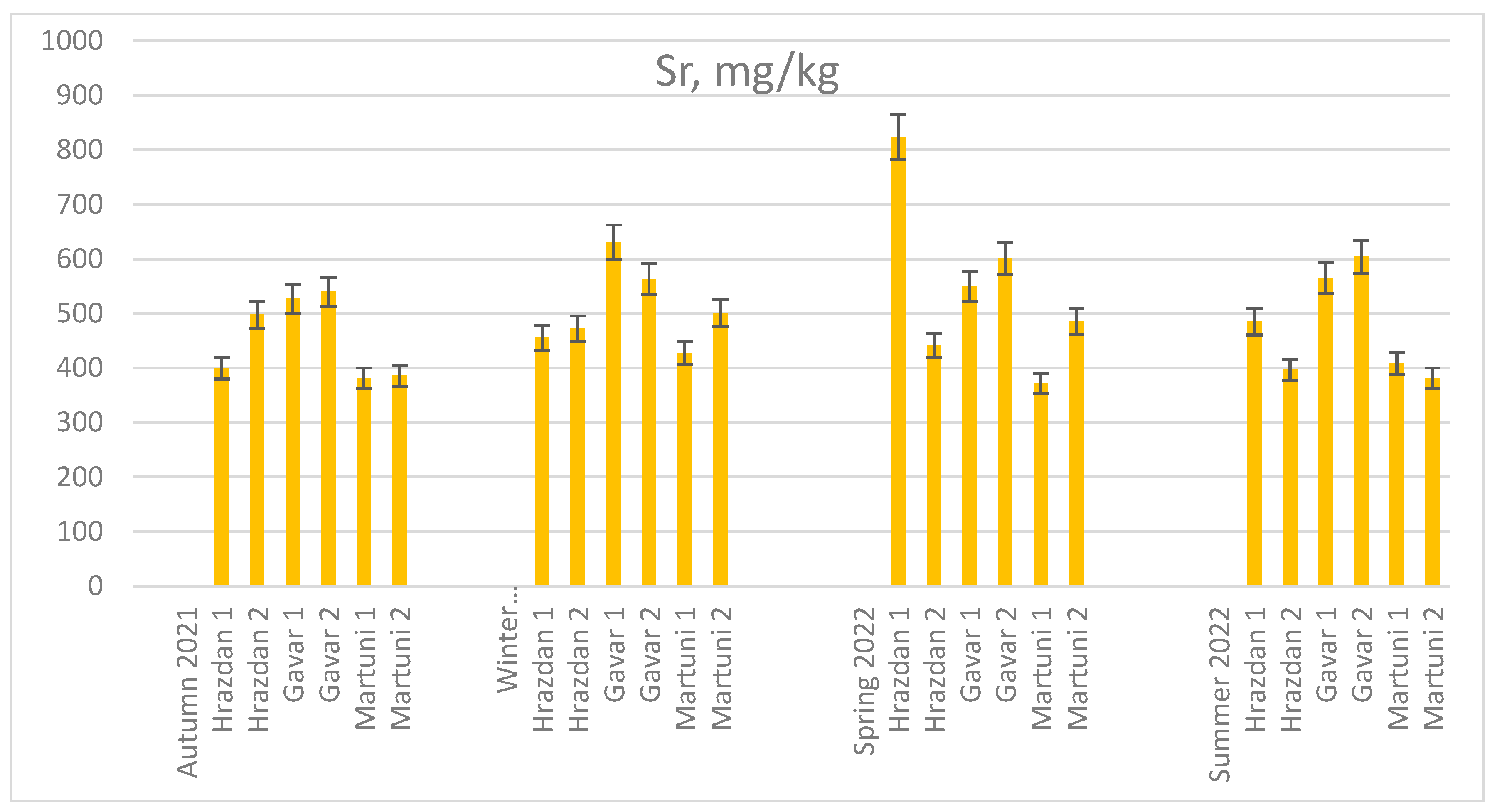
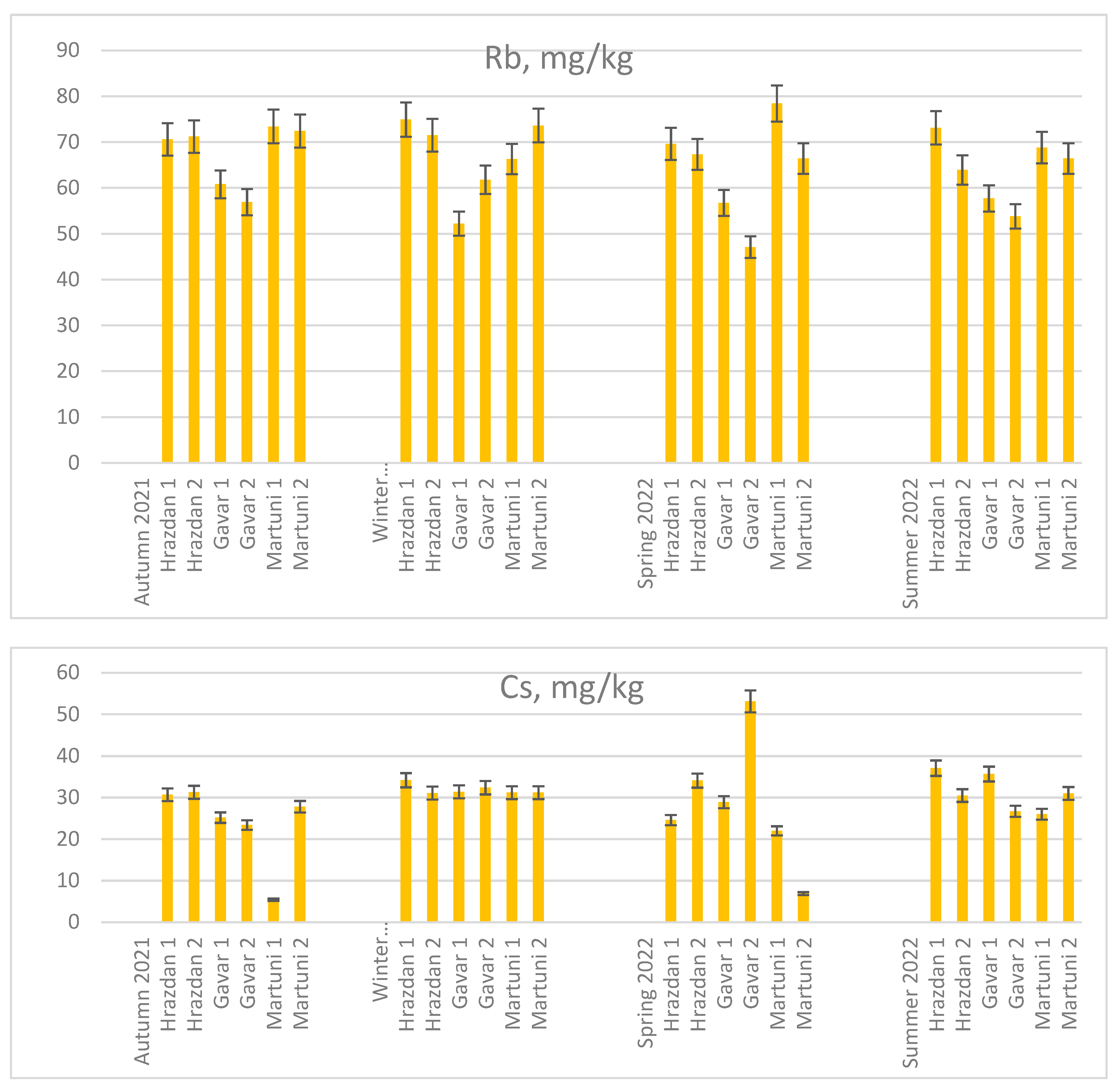
The concentration of Sr, Rb, and Cs in soil samples from different sites is shown in
Figure 2. In most cases, except soil taken from H1 in spring 2022, the soil samples from Gavar sites contained the highest Sr. The seasonal changes in the Sr accumulation were the following: winter` 21/22≥ summer` 22> autumn` 21. However, relatively low levels of this element data were obtained in the soil samples from the Martuni sites, with the highest levels of accumulation occurring in the spring at M2.
Compared the Rb concentration in soil sampling sites was found in winter G1<G2. In spring` 22 the Rb concentration is less than 50 mg/kg in the soil collected from site G2.
The Cs concentration in the general context of the studies was maintained at an average level of 29 mg/kg in the soil samples from all sites. Furthermore, on the one hand, the lowest Cs concentrations were found in the Martuni soil samples in autumn (M1- 5.4 mg/kg) and spring (M2 - 6.9 mg/kg). On the other hand, the highest Cs concentrations were found in soil samples from the Gavar sites (G2 - 53.1 mg/kg).
3.2. Geo-ecological coefficient of ME and TE assessment of soil pollution
In order to assess the degree of soil contamination, an unconditional constant value was introduced as no maximum allowable concentration values were established for all chemical elements [
19,
57]. CC of chemical elements has often been used to assess pollution levels in available environmental studies to identify the type of chemical element accumulation: anthropogenic or abiotic [
58].This indicator characterizes the relative concentration of chemical elements in nature. The pollution index (
Zc) was calculated considering the CC to interpret the results obtained on the chemical element concentration in soil samples [
21,
59]. This methodology also aimed to differentiate between anthropogenic and absolute impacts.
Table 3 shows the comparative pollution index for each group of ME and TE elements in soil samples collected from all sample sites during the study period. Regardless of the season, the meaning of the pollution index for TE is higher than the value of the other groups of elements. This could be attributed to the fact that TE typically comprise heavy metals that had demonstrated harmful effects on the environment in various literature [
60,
61]. In the autumn, the superiority of the ME in the total pollution index was observed in all soil samples.
3.3. Biotesting of soil samples using the Trad-MN testing system
The Trad-MN test of the model plant
Tradescantia (clone 02) was used in the plant-soil system to biotest the degree of clastogenicity of the investigated soil samples (
Figure 4). The percentage of tetrads with MN and MN in microspore tetrads were used as marker test criteria. The use of the test makes it possible to detect the appearance of chromosomal aberrations (acentric fragments or lagging chromosomes), which was recorded in the form of MN at the stage of microspore tetrads when the process of microsporogenesis is disrupted.
This test takes into account two criteria: the percentage of tetrads with micronuclei and the percentage of MN in tetrads (
Table 4). A considerable rise in the occurrence of both testing criteria was noted in all the studied variants during the examination of clastogenic outcomes in sporogenic cells of a model plant, based on Trad-MN results, compared to the experimental conditioned average background.
In soil samples collected in autumn, the lowest frequency of occurrence of tetrads in MN in the test -system compared to the conditional background was in the Hrazdan sites with H1<H2 (p<0.01), and the highest with an almost twofold increase was in the Gavar sites with the value G1 >G2 (p<0.01). Significant changes were within the average control range (p<0.05) in the Martuni sites.
In all the soil samples collected in the winter, the Trad-MN test showed an increase in the frequency of tetrads in MN, which is a sign of contamination of the samples. In the Hrazdan sites, the average increase in the frequency of tetrads in MN was up to 58%, in the Gavar and Martuni sites it was 2.8 times (but only in G2) and 3.1 times, respectively.
The highest percentage increase in the frequency of tetrads was observed in the spring samples from MN. Compared with the conditional background for soil samples from the Hrazdan sites was increased 2.1 times (with H1<H2, p<0.001), from the Gavar sites it was 2.7 times (with G1>G2, p<0.001) and from Martuni it was 2.0 times (with M1<M2, p<0.001).
When analyzing the results for the summer, a decrease in the frequency of occurrence of tetrads in the MN of the model plant was observed. The change in the frequency of their occurrence was 2.1 times greater in soil samples from the Hrazdan and Gavar sites compared to the conditional control (with H1<H2 and G1<G2). For soil samples from the Martuni sites, the test system showed an increase in the frequency of occurrence of tetrads in MN to 87.5% (with M1=M2, p<0.001). The comparisons for soil samples, according to the test criterion for the occurrence of tetrads in the MN of our model plant, are as follows
| Autumn, 2021 |
Hrazdan region |
< |
Martuni region |
< |
Gavar region |
| H1<H2 |
|
M1>M2 |
|
G1>G2 |
| Winter, 2021/22 |
Hrazdan region |
< |
Gavar region |
< |
Martuni region |
| H1<H2 |
|
only by G2 |
|
M1<M2 |
| Spring, 2022 |
Martuni region |
< |
Hrazdan region |
< |
Gavar region |
| M1<M2 |
|
H1<H2 |
|
G1>G2 |
| Summer, 2022 |
Hrazdan region |
< |
Martuni region |
< |
Gavar region |
| H1<H2 |
|
M1=M2 |
|
G1<G2 |
Another test, the frequency of occurrence of MN in tetrads of the model plant, was characterized by taking into account the sign of mutagenicity of the material tested [
62]. Furthermore, in the seasonal soil sampling, the comparative ranges of biotests in the case of the tetrads in the MN were almost similar across sites (
Table 4). The frequency of occurrence of MN in the tetrads of the model plant test system in the autumn soil samples in the Hrazdan sites is within the statistical mean of the conditional background (with H1<H2, p<0.001). Soil samples from the Gavar and Martuni sites showed a 57% and 17% increase in the frequency of MN in the tetrads of the test plant respectively.
A significant increase in the frequency of MN in tetrads occurring in Tradescantia tetrads (clone 02) compared to the spring was observed in all soil samples collected in winter. For this characteristic, the soil samples from the Hrazdan sites were 57% more mutagenic than the conditional background. Analysis of the results for soil samples from Martuni showed an average increase in the frequency of occurrence of MN in tetrads of 2 times and for samples from Gavar - 3.6 times (but only for G2).
The frequency of MN in tetrads was significantly highest in spring soil samples, ranging from 1.7 times (Martuni) to 2.3 times (Gavar) on average. At the same time, the highest percentage of occurrence of MN in tetrads was detected in soil samples from site G1 (7.5±0.5, p<0.001) and the lowest from site M1 (4.0±0.4, p<0.01).
The genotoxicity of soils collected during the summer was also assessed by mutagenicity. A decrease in the frequency of occurrence of MN in tetrads of sporogenic Tradescantia cells was observed. Moreover, in soils collected from the Hrazdan sites, their frequency was on average 1.4 times higher compared to the conditional background (with H1<H2, p<0.01). Analysis of the results of soil samples from the Gavar sites showed a 77% increase in clastogenicity. The highest percentage of MN occurrence in the test tetrads (84%) was found using soil samples from Martuni. It was noteworthy that M1≤M2 (p<0.001).
3.4. Correlation analysis
Table 5 shows the correlations between the ME and TE groups in soil samples across all sites during the experimental cycle. Both positive and negative correlations with varying degrees of significance were found. It should be noted that the results of the correlation analysis may be influenced by the similarity of the soil type. In the autumn, a strong negative relationship was observed between Rb and Sr, Cs and Ca. A direct strong correlation was observed in the concentration in the soil between such ME and TE, which are Rb and K, Cs and S. In the winter, a strong negative correlation occurred between K and Ca, Rb and Sr. A strong positive correlation was observed between ME and TE, such as Rb and K, Cs and S. In the soil samples collected in spring, there was only a positive correlation between Rb and Ca, S and K, and correspondingly a negative correlation between Cs and Ca (as in autumn), Cs and Rb. In the soil samples collected in summer, there was a strong positive correlation between Rb and Ca (as in spring), S and K (as in spring), Cs and S. In all soil samples a negative correlation between was between Rb and Sr.
In addition, the comparative correlation of the soil samples according to the location of their collection is shown in
Table 6. Subsequent elemental analyses throughout the experimental cycle showed a significant positive correlation, indicating a homogeneous structure of the soils selected during the research.
4. Conclusions
A combined assessment system is needed to facilitate efficient soil remediation, taking into account the current soil conditions and the factors influencing the biogeochemical processes there. Therefore, the study of several elements in soil samples requires special attention to their main parameters, distribution patterns, and assessment of correlations between groups, as they have not been extensively studied.
The analysis showed significant differences depending on the sampling sites and the season. In the ME group, the concentration of K showed statistically significant differences, slightly increasing during the summer season, with the highest accumulation in the Gavar sites (for G1 soil samples in autumn and for G2 soil samples in winter and spring). High concentrations of Ca were found at all soil sampling sites in winter. However, soil samples from G1 and G2 differed in having relatively low Ca concentrations in autumn, spring, and summer. As for the accumulation of S in the soil samples, a high concentration was only observed in autumn. During the winter, spring, and summer sampling periods, a 4-5 times lower concentration of this element was observed in the soil samples. In most of the cases considered, seasonal changes in Sr, Rb and Cs accumulation concentrations in soil samples were as follows: winter'21/22≥ summer'22> autumn'21. However, relatively low levels of TE were found in the soil samples from the Martuni sites. In comparison, the Rb concentration in the soil sampling sites was found in winter G1<G2. The Cs concentration in the general context of the studies was maintained at an average level of 29 mg/kg in the soil samples from all sites.
Irrespective of the season, TE group elements showed a higher pollution index than other element groups. But in autumn, ME showed a higher total pollution index in all soil samples. Similarly, in the spring soil sampling, ME was a higher value Zc at the following sites: H1, G1, M1 and M2.
Summarizing the results of genotoxic analysis of soils from the study sites, it can be stated that there was a considerable increase in soil clastogenicity, according to both test criteria starting from autumn. During the spring of soil sampling, there was a peak in the percentage of occurrence of both tetrads criteria in MN and MN in tetrads of Tradescantia (clone 02). In the subsequent summer sampling, a significant decrease was seen in all genotoxicity assessment criteria for the materials under study.
Thus, the observed characteristic trends in the seasonal distribution of the studied series of elements from the group of both ME and TE in soil samples from regions of Armenia with different climatic indicators indicate the presence of common factors influencing the chemical composition of soils, to understand which it is necessary to know the features of the spatial-temporary distribution of elements in the studied areas. The study conducted a comparative analysis of the concentration of some chemical elements of the ME and TE groups in soil samples from three regions of Armenia, which differ from each other in their agro-geographical indicators - Hrazdan, Gavar and Martuni was carried out.
Author Contributions
Conceptualization, A.S., and A.K.; methodology, A.S., R.H.; software, A.K; validation, A.K.; formal analysis, M.K., and A.A.; investigation, A.S., M.K., A.A., and A.K.; resources, A.K.; data curation, A.K., M.K., and A.A; writing—original draft preparation, A.S., M.K., R.H., and A.K.; writing—review and editing, A.S., A.K. and R.H; visualization, A.K.; supervision, A.S.; project administration, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of MESCS RA, in the frames of the research project № 21T-2H216.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available on request from the authors.
Acknowledgments
The authors would like to express their gratitude to PhD Avalyan R. and PhD Agajhanyan E. for their valuable assistance during the genotoxicity analysis at the RI "Biology" of YSU. Special thanks to Mr. Aslikyan and Mr. Galstyan from the RA NAS "National Bureau of Expertises" SNPO for their technical assistance regarding the use of the Termo Scientific™ Niton™ X-ray fluorescence analyzer.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ito, A.; Hajima, T. Biogeophysical and biogeochemical impacts of land-use change simulated by MIROC-ES2L. Prog Earth Planet Sci 2020, 7, 54. [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ Sci Pollut Res 2022, 29, 42539–42559 . [CrossRef]
- Piaszczyk, W.; Błońska, E.; Lasota, J. Soil biochemical properties and stabilisation of soil organic matter in relation to deadwood of different species. FEMS Microbiology Ecology 2019, 95, fiz011. [CrossRef]
- Gulahmadov, N., Chen, Y.; Gulakhmadov, M.; Satti, Z.; Naveed, M.; Davlyatov, R.; Ali, S.; Gulakhmadov, A. Assessment of temperature, precipitation, and snow cover at different altitudes of the Varzob River basin in Tajikistan. App. Sci. 2023, 13, 5583. [CrossRef]
- Larsen, I.; Eger, A.; Almond, P. C.; Thaler, E.; Rhodes, J. M.; Prasicek, G. The influence of erosion and vegetation on soil production and chemical weathering rates in the Southern Alps, New Zealand. Data and Datasets 2022, 161. [CrossRef]
- Sinclair, A.R.E; Byrom, A.E. Understanding ecosystem dynamics for conservation of biota. Journal of Animal Ecology 2006, 75, 64–79. [CrossRef]
- Tóth, G.; Hermann, T.; Szatmári, G.; Pásztor, L. Maps of heavy metals in the soils of the European Union and proposed priority areas for detailed assessment. Science of the total environment 2016, 565, 1054–1062. [CrossRef]
- Sukiasyan, A.; Kalantaryan, M.; Ledashcheva, T.; Kirakosyan, A. Seasonal comparative geochemistry of ultra trace elements concentration in agricultural soils near Lake Sevan. E3S Web of Conferences 2023, 407. [CrossRef]
- Osman, K.T. Plant Nutrients and Soil Fertility Management. In: Soils. Springer, Dordrecht, 2013; [CrossRef]
- Uchida, R. Еssential nutrients for plant growth: nutrient functions and deficiency symptoms. In Plant nutrient management in Hawaii’s soils, approaches for tropical and subtropical agriculture; Silva, J. A.; Uchida, R. Eds.; College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa, 2000; Chapter 3, pp. 31–55.
- Dhaliwal, S.S.; Naresh, R.K.; Mandal, A.; Singh, R.; Dhaliwal, M.K. Dynamics and transformations of micronutrients in agricultural soils as influenced by organic matter build-up: A review. Environmental and Sustainability Indicators 2019, 1–2, 100007. [CrossRef]
- Sukiasyan, A.R.; Jhangiryan, T.A.; Hunanyan, S.A.; Kirakosyan A.A. Translocation of heavy metals into plants from the soil near the Alaverdi copper smelting enterprise. Theoretical and applied ecology 2023, 3, 120–128. [CrossRef]
- Samec, P.; Kučera, A.; Tomášová, G. Soil Degradation Processes Linked to Long-Term Forest-Type Damage. IntechOpen. 2023. [CrossRef]
- Chaurasia, M.; Patel, K.; Rao, K.S. Impact of anthropogenic land uses on soil microbiological activity in a peri-urban landscap. Environmental Monitoring and Assessment 2023,195, 10. [CrossRef]
- FAO and UNEP. 2021. Global assessment of soil pollution: Report. Rome. [CrossRef]
- Kaown, D.; Koh, E.-H.; Mayer, B.; Ju, Y.J.; Kim, J.; Lee, H.-L.; Lee, S.-S.; Park, D. K.; Lee, K.-K. Differentiation of natural and anthropogenic contaminant sources using isotopic and microbial signatures in a heavily cultivated coastal area Environmental Pollution 2021, 273, 116493. [CrossRef]
- Xin, X.; Shentu, J.; Zhang, T.; Yang, X.; Baligar, V.C.; He, Z. Sources, indicators, and assessment of soil contamination by potentially toxic metals. Sustainability 2022, 14, 15878. [CrossRef]
- Perelman, A.I. Geochemistry: Accounting for geological specialties of universities. Moscow: High School, Russia, 1989; 423 p.
- Bitimbayev, M.; Rysbekov, K.; Akhmetkanov, D.; Kunayev, M.; Lozynskyi, V.; Elemesov, К. The role and importance of chemical elements clarks in the practical expanded reproduction of mineral resources. Engineering Journal of Satbayev University 2022, 144, 48–56. [CrossRef]
- Taylor, S.R. Abundance of chemical elements in the continental crust: a new table. Geochimica et Cosmochimica Acta, 1964, 28, 1273–1285. [CrossRef]
- Kasimov, N.S.; Vlasov, D.V. Global and regional geochemical indexes of production of chemical elements. Geography, Environment, Sustainability 2014, 7, 52–65. [CrossRef]
- Bychinsky, V.; Charykova, M.; Omara, R. Geochemical modeling of soils and technogenic sediments interactions with natural waters using SELECTOR software (Chaabet-el-Hamra mine, Algeria). Geochemistry 2021, 81, 125799. [CrossRef]
- Sukiasyan, A.R. New approach to determining the environmental risk factor by the biogeochemical coefficients of heavy metals. South of Russia: ecology, development 2018,13,108–118. [CrossRef]
- Chellan, P.; Sadler, P.J. The elements of life and medicines. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2015, 13. [CrossRef]
- Engwa, G. A.; Ferdinand, P. U.; Nwalo, F. N.; Unachukwu, M. N. Mechanism and health effects of heavy metal toxicity in humans. IntechOpen 2019. [CrossRef]
- Chibuike, G. U.; Obiora, S. C. Heavy metal polluted soils: effect on plants and bioremediation methods. Applied and environmental soil science 2014, 12 pages. [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [CrossRef]
- Gumienna-Kontecka, E.; Rowińska-Żyrek, M.; Łuczkowski M. Тhe role of trace elements in living organisms. In Book Recent advances in trace elements, Chojnacka, K., Saeid, A., Eds.; First Published: 23 February 2018 . [CrossRef]
- Nielsen, F.H.; Trace and ultratrace elements. Reference Module in Biomedical Sciences, Elsevier, 2014. [CrossRef]
- Kaur, H.; Kaur, H.; Kaur, H.; Srivastava S. . The beneficial roles of trace and ultratrace elements in plants. Plant Growth Regul 2023, 100, 219–236. [CrossRef]
- Shallari, S. Heavy metals in soils and plants of serpentine and industrial sites of Albania. Sci. Total Environ 1998, 209, 133–142. [CrossRef]
- Ichikawa, S. Tradescantia stamen-hair system as an excellent botanical tester of mutagenicity: its responses to ionizing radiations and chemical mutagens, and some synergistic effects found. Mutat. Res. 1992, 270, 3–22. [CrossRef]
- Nannas, N.J.; Dawe, R.K, Genetic and genomic toolbox of Zea mays. Genetics 2015, 199, 655–69. [CrossRef]
- Notten, M.J.; Oosthoek, A.J.; Rozema, J.; Aerts, R. Heavy metal concentrations in a soil-plant-snail food chain along a terrestrial soil pollution gradient. Environ. Pollut 2005, 138, 178–190. [CrossRef]
- Gautam, M.; Mishra, S.; Agrawal, M.; Bioindicators of soil contaminated with organic and inorganic pollutants. In New paradigms in environmental biomonitoring using plants, S. Tiwari, Sh. Agrawal, Eds.; Elsevier, 2022, pp. 271–298. [CrossRef]
- Osipov, R.G.; Shevchenko, V.A. The use of Tradescantia (clones 02 and 4430) in studies on radiation and chemical mutagenesis. J. Gen. Biol 1984, ХLV, 226–232.
- Montvydienė, D.; Marčiulionienė, D.; Karlavičienė, V.; Hogland, W. Phytotoxicity assessment of effluent waters, surface water and sediments. In: Dangerous Pollutants (Xenobiotics) in Urban Water Cycle. NATO Science for Peace and Security Series.; Hlavinek, P., Bonacci, O., Marsalek, J., Mahrikova, I. Eds.; Springer, Dordrecht. 2008. [CrossRef]
- Aghajanyan, E.A.; Avalyan, R.E.; Simonyan, A.E.; Atoyants, A.L.; Gabrielyan, B.K.; Aroutiounian, R.M.; Khosrovyan, A. Clastogenecity evaluation of water of Lake Sevan (Armenia) using Tradescantia micronucleus assay. Chemosphere 2018, 209, 1–6. [CrossRef]
- Fayvush, G.M.; Aleksanyan, A.S. Habitats of Armenia. Yerevan, Institute of Botany NAS RA, 2016. 360 pp.
- https://en.climate-data.org/asia/armenia/kotayk/hrazdan-2023/.
- Sukiasyan, A.; Kalantaryan, M.; Ledashcheva, T.; Kirakosyan, A. Seasonal comparative geochemistry of ultra trace elements concentration in agricultural soils near Lake Sevan. E3S Web of Conferences 2023, 407. [CrossRef]
- Elliott, D.; Schwartz, M.; Scott, G.; Haymes, S.; Heimiller, D.; George R. Wind energy resource: Atlas of Armenia. Technical report National Renewable Energy Laboratory (U.S. Department of Energy Office of Scientific and Technical Information. July. 2003).
- Thermo scientific sample collection and preparation tools for exploration and mining [https://www.malvernpanalytical.com/en/assets/pn12871_le_sample_prep_of_geological_or_mining_and_other_inorganic_samples_tcm50-95064.pdf].
- GOST 26423-85. Soils. Methods for determining specific electricity conductivity, pH and density residue water extract Edition May 2011 with Amendment IUS 8-86, 8 pages.
- Grant, W.F. The present status of higher plant bioassay for the detection of environmental mutagens. Mutation Research 1999, 310, 175–185. [CrossRef]
- Mišík, M.; Pichler, C.; Rainer, B.; Nersesyan, A.; Mišíková, K.; Knasmueller, S. Micronucleus assay with tetrad cells of Tradescantia. Methods Mol Biol 2019, 2031, 325–335. [CrossRef]
- Ma, T.H.; Cabrera, G.L.; Chen, R.; Gill, B.S.; Sandhu, S. S.; Vandenberg, A.L.; Salomone, M.F. Tradescantia micronucleus bioassay. Mutat. Res.1994, 310, 221–230. [CrossRef]
- Muller, G. Die Schwermetallbelstung der Sedimente des Neckars und seiner Nebenflusse: eine estandsaufnahme. Chem. Zeitung 1981, 105, 157–164.
- Sukiasyan, A. New approach to determining the environmental risk factor by the biogeochemical coefficients of heavy metals. South of Russia: ecology, development 2018, 13, 108 – 118. [CrossRef]
- Blagodatsky, S.; Smith, P. Soil physics meets soil biology: Towards better mechanistic prediction of greenhouse gas emissions from soil. Soil Biol. Biochem 2012, 47, 78–92. [CrossRef]
- Shtangeeva, I. Accumulation of scandium, cerium, europium, hafnium, and tantalum in oats and barley grown in soils that differ in their characteristics and level of contamination. Environmental Science and Pollution Research 2022, 29(27), 40839–40853. [CrossRef]
- Sukiasyan, A.; Kirakosyan, A. Ecological evaluation of heavy metal pollution of different soil-climatic regions of Armenia by biogeochemical coefficients. DRC Sustainable Future: Journal of Environment, Agriculture, and Energy 2020, 1, 94–102. [CrossRef]
- Sukiasyan, A.; Hovhannisyan, A.; Aslikyan, M.; Galstyan, A.; Simonyan, A.; Kroyan. S.; Kirakosyan, A. Assessment of ultra-trace elements pollution in the arable soils near the Lake Sevan correcting for its toxicity. In Processing of the XXIV International Conference “Actual Problems of Ecology and Environmental Management”, Russia, 20-22 April, Moscow.
- Benhamdoun, A.; Achtak, H.; Vinti, G., Dahbi, A. Soil contamination by trace metals and assessment of the risks associated: the dumping site of Safi city (Northwest Morocco). Environ Monit Assess 2023,195(8), 941. [CrossRef]
- Amanuel, Y.A.W.; Kassegne, A.B. Assessment of trace metals in soil and vegetables samples irrigated from Borkena river, South Wollo Zone, Amhara Region, Ethiopia. Sustain. Environ. 2022, 8, 2035045. [CrossRef]
- Temesgen, E.; Seyoum, L. Trace metals bio concentration from soil to vegetables and appraisal of health risk in Koka and Wonji farms. Environmental Science and Pollution Research 2017, 24(12), 11807–11815. [CrossRef]
- Vodyanitskii, Y.N. Heavy Metals and Metalloids in Soils; GNU Soil Institute. V.V. Dokuchaev Russian Academy of Agricultural Sciences: Moscow, Russia, 2008; 86p.
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp Suppl. 2012, 101,133–64. [CrossRef]
- Antoniadis, V.; Shaheen, S.M.; Levizou, E.; Shahid, M.; Niazi, N. Kh.; Vithanage, M.; Ok, Y.S.; Bolan, N.; Rinklebe, J. A critical prospective analysis of the potential toxicity of trace element regulation limits in soils worldwide: Are they protective concerning health risk assessment? - A review, Environment International 2019, 127, 819–847. [CrossRef]
- Yadav, M.; George, N.; Dwibedi, V. Emergence of toxic trace elements in plant environment: Insights into potential of silica nanoparticles for mitigation of metal toxicity in plants. Environmental Pollution 2023, 333, 122112. [CrossRef]
- Vodyanitskiy, Yu.; Ladonin, D.; Savichev A. Soil contamination with heavy metals. Moskva: Soil Institute after. V.V. Dokuchaeva RAAS, 2012.
- Guimarães, E.T.; Domingos, M.; Alves, E.S.; Caldini, N.; Lobo, D.J.A.; Lichtenfels, A.J.F.C.; Saldiva, P.H.N. Detection of the genotoxicity of air pollutants in and around the city of São Paulo (Brazil) with the Tradescantia-micronucleus (Trad-MCN) assay. Environmental and Experimental Botany 2000, 44, 1–8. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).