Submitted:
03 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cattle and Ethics
2.2. Superstimulation
2.3. Transvaginal Ultrasound-Guided Oocyte Aspiration
2.4. In Vitro Maturation, Fertilization, and Culture
2.5. Embryo Transfer
2.6. Statistical Analyses
3. Results
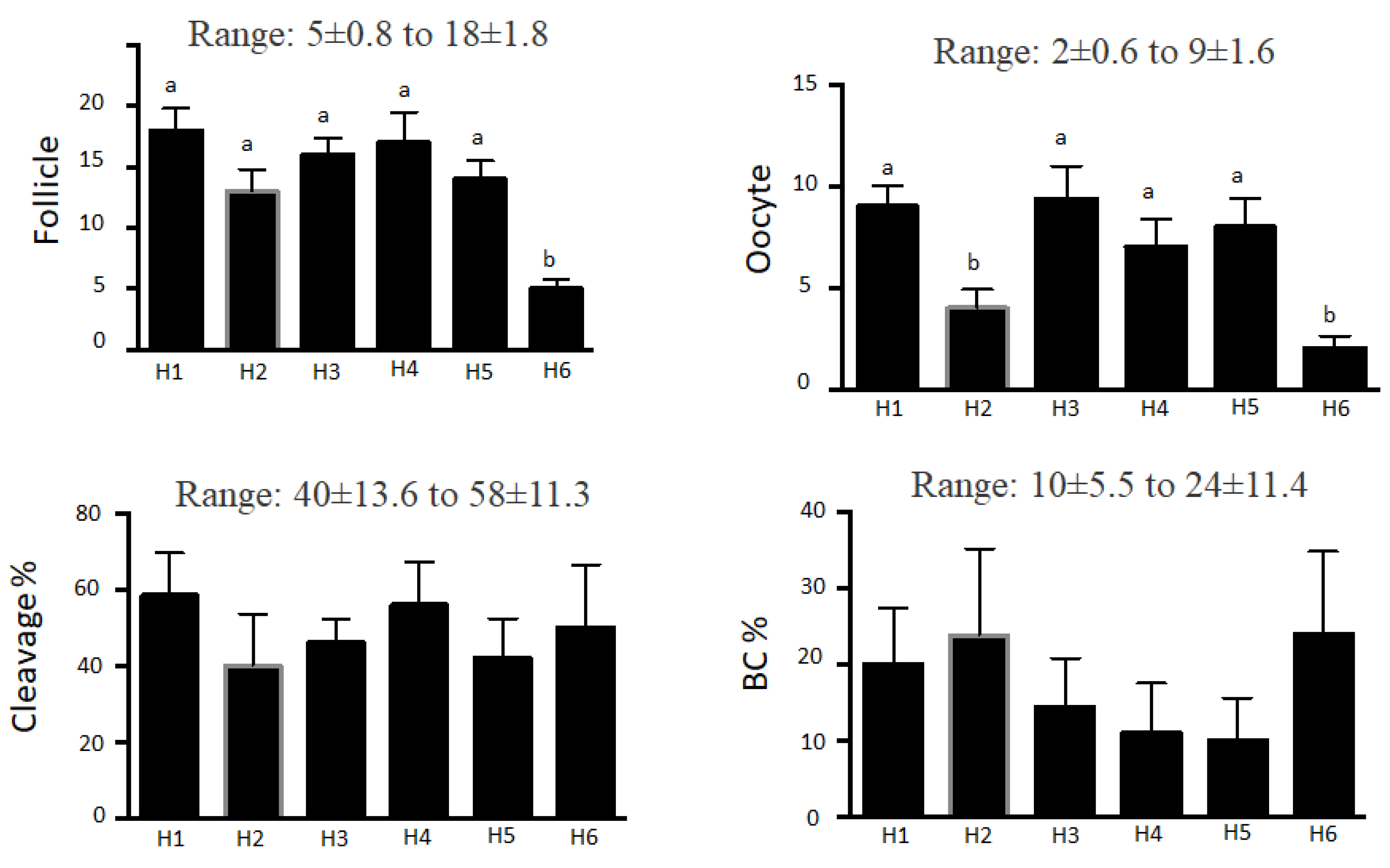
3.1. Evaluation of the Donor Effect on OPU-IVP Outcomes in Crossbred Heifers
3.2. Evaluation of Repeated Superstimulations on OPU-IVP Responses in Crossbred Heifers
3.3. Evaluation of OPU-IVP Outcomes among Purebred Cows
3.4. Developmental Competence of OPU-IVP Embryos
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daly, J.; Smith, H.; McGrice, H.A.; Kind, K.L.; Van Wettere, W.H.E.J. Towards Improving the Outcomes of Assisted Reproductive Technologies of Cattle and Sheep, with Particular Focus on Recipient Management. Animals 2020, 10, 293. [CrossRef]
- Humblot, P.; Le Bourhis, D.; Fritz, S.; Colleau, J.J.; Gonzalez, C.; Guyader Joly, C.; Malafosse, A.; Heyman, Y.; Amigues, Y.; Tissier, M.; et al. Reproductive Technologies and Genomic Selection in Cattle. Vet. Med. Int. 2010, 2010, 1–8. [CrossRef]
- Bó, G.A.; Cedeño, A.; Mapletoft, R.J. Strategies to Increment in Vivo and in Vitro Embryo Production and Transfer in Cattle. Anim. Reprod. 2019, 16, 411–422. [CrossRef]
- Ferré, L.B.; Kjelland, M.E.; Strøbech, L.B.; Hyttel, P.; Mermillod, P.; Ross, P.J. Review: Recent Advances in Bovine in Vitro Embryo Production: Reproductive Biotechnology History and Methods. Animal 2020, 14, 991–1004. [CrossRef]
- Davis, T.C.; White, R.R. Breeding Animals to Feed People: The Many Roles of Animal Reproduction in Ensuring Global Food Security. Theriogenology 2020, 150, 27–33. [CrossRef]
- Viana, J.H. 2021 Statistics of Embryo Production and Transfer in Domestic Farm Animals. N. Am. 2022.
- Greenwood, P.L. Review: An Overview of Beef Production from Pasture and Feedlot Globally, as Demand for Beef and the Need for Sustainable Practices Increase. Animal 2021, 15, 100295. [CrossRef]
- Cordeiro, M.R.C.; Mengistu, G.F.; Pogue, S.J.; Legesse, G.; Gunte, K.E.; Taylor, A.M.; Ominski, K.H.; Beauchemin, K.A.; McGeough, E.J.; Faramarzi, M.; et al. Assessing Feed Security for Beef Production within Livestock-Intensive Regions. Agric. Syst. 2022, 196, 103348. [CrossRef]
- Leroy, J.L.M.R.; Rizos, D.; Sturmey, R.; Bossaert, P.; Gutierrez-Adan, A.; Van Hoeck, V.; Valckx, S.; Bols, P.E.J. Intrafollicular Conditions as a Major Link between Maternal Metabolism and Oocyte Quality: A Focus on Dairy Cow Fertility. Reprod. Fertil. Dev. 2012, 24, 1. [CrossRef]
- Ribeiro, E.S.; Gomes, G.; Greco, L.F.; Cerri, R.L.A.; Vieira-Neto, A.; Monteiro, P.L.J.; Lima, F.S.; Bisinotto, R.S.; Thatcher, W.W.; Santos, J.E.P. Carryover Effect of Postpartum Inflammatory Diseases on Developmental Biology and Fertility in Lactating Dairy Cows. J. Dairy Sci. 2016, 99, 2201–2220. [CrossRef]
- Lopez, H.; Caraviello, D.Z.; Satter, L.D.; Fricke, P.M.; Wiltbank, M.C. Relationship between Level of Milk Production and Multiple Ovulations in Lactating Dairy Cows. J. Dairy Sci. 2005, 88, 2783–2793. [CrossRef]
- Baruselli, P.S.; Souza, A.H.D.; Sá, M.F.D.; Marques, M.O.; Sales, J.N.D.S. Genetic Market in Cattle (Bull, Ai, Ftai, Moet and Ivp): Financial Payback Based on Reproductive Efficiency in Beef and Dairy Herds in Brazil. Anim. Reprod. 2018, 15, 247–255. [CrossRef]
- Demetrio, D.G.B.; Benedetti, E.; Demetrio, C.G.B.; Fonseca, J.; Oliveira, M.; Magalhaes, A.; Santos, R.M.D. How Can We Improve Embryo Production and Pregnancy Outcomes of Holstein Embryos Produced in Vitro? (12 Years of Practical Results at a California Dairy Farm). Anim. Reprod. 2020, 17, e20200053. [CrossRef]
- Soares, J.G.; Martins, C.M.; Carvalho, N.A.T.; Nicacio, A.C.; Abreu-Silva, A.L.; Campos Filho, E.P.; Torres Júnior, J.R.S.; Sá Filho, M.F.; Baruselli, P.S. Timing of Insemination Using Sex-Sorted Sperm in Embryo Production with Bos Indicus and Bos Taurus Superovulated Donors. Anim. Reprod. Sci. 2011, 127, 148–153. [CrossRef]
- Macmillan, K.; Lean, I.; Westwood, C. The Effects of Lactation on the Fertility of Dairy Cows. Aust. Vet. J. 1996, 73, 141–147. [CrossRef]
- Snijders, S.E.M.; Dillon, P.; O’Callaghan, D.; Boland, M.P. Effect of Genetic Merit, Milk Yield, Body Condition and Lactation Number on in Vitro Oocyte Development in Dairy Cows. Theriogenology 2000, 53, 981–989. [CrossRef]
- Ferré, L.B.; Alvarez-Gallardo, H.; Romo, S.; Fresno, C.; Stroud, T.; Stroud, B.; Lindsey, B.; Kjelland, M.E. Transvaginal Ultrasound-guided Oocyte Retrieval in Cattle: State-of-the-art and Its Impact on the in Vitro Fertilization Embryo Production Outcome. Reprod. Domest. Anim. 2023, 58, 363–378. [CrossRef]
- Zangirolamo, A.F.; Morotti, F.; Silva, N.C.D.; Sanches, T.K.; Seneda, M.M. Ovarian Antral Follicle Populations and Embryo Production in Cattle. Anim. Reprod. 2018, 15, 310–315. [CrossRef]
- Chaubal, S.A.; Molina, J.A.; Ohlrichs, C.L.; Ferre, L.B.; Faber, D.C.; Bols, P.E.J.; Riesen, J.W.; Tian, X.; Yang, X. Comparison of Different Transvaginal Ovum Pick-up Protocols to Optimise Oocyte Retrieval and Embryo Production over a 10-Week Period in Cows. Theriogenology 2006, 65, 1631–1648. [CrossRef]
- Roover, R.D.; Genicot, G.; Leonard, S.; Bols, P.; Dessy, F. Ovum Pick up and in Vitro Embryo Production in Cows Superstimulated with an Individually Adapted Superstimulation Protocol. Anim. Reprod. Sci. 2005, 86, 13–25. [CrossRef]
- Chastant-Maillard, S.; Quinton, H.; Lauffenburger, J.; Cordonnier-Lefort, N.; Richard, C.; Marchal, J.; Mormede, P.; Renard, J. Consequences of Transvaginal Follicular Puncture on Well-Being in Cows. Reproduction 2003, 555–563. [CrossRef]
- Van Wagtendonk-de Leeuw, A.M. Ovum Pick Up and In Vitro Production in the Bovine after Use in Several Generations: A 2005 Status. Theriogenology 2006, 65, 914–925. [CrossRef]
- Blondin, P.; Bousquet, D.; Twagiramungu, H.; Barnes, F.; Sirard, M.-A. Manipulation of Follicular Development to Produce Developmentally Competent Bovine Oocytes1. Biol. Reprod. 2002, 66, 38–43. [CrossRef]
- Bó, G.A.; Mapletoft, R.J. Historical Perspectives and Recent Research on Superovulation in Cattle. Theriogenology 2014, 81, 38–48. [CrossRef]
- Landry, D.A.; Bellefleur, A.-M.; Labrecque, R.; Grand, F.-X.; Vigneault, C.; Blondin, P.; Sirard, M.-A. Effect of Cow Age on the in Vitro Developmental Competence of Oocytes Obtained after FSH Stimulation and Coasting Treatments. Theriogenology 2016, 86, 1240–1246. [CrossRef]
- Vieira, L.M.; Rodrigues, C.A.; Castro Netto, A.; Guerreiro, B.M.; Silveira, C.R.A.; Moreira, R.J.C.; Sá Filho, M.F.; Bó, G.A.; Mapletoft, R.J.; Baruselli, P.S. Superstimulation Prior to the Ovum Pick-up to Improve in Vitro Embryo Production in Lactating and Non-Lactating Holstein Cows. Theriogenology 2014, 82, 318–324. [CrossRef]
- Sirard, M.-A. 40 Years of Bovine IVF in the New Genomic Selection Context. Reproduction 2018, 156, R1–R7. [CrossRef]
- Petyim, S.; Båge, R.; Madej, A.; Larsson, B. Ovum Pick-up in Dairy Heifers: Does It Affect Animal Well-being? Reprod. Domest. Anim. 2007, 42, 623–632. [CrossRef]
- Pieterse, M.C.; Kappen, K.A.; Kruip, Th.A.M.; Taverne, M.A.M. Aspiration of Bovine Oocytes during Transvaginal Ultrasound Scanning of the Ovaries. Theriogenology 1988, 30, 751–762. [CrossRef]
- Kruip, Th.A.M.; Den Daas, J.H.G. In Vitro Produced and Cloned Embryos: Effects on Pregnancy, Parturition and Offspring. Theriogenology 1997, 47, 43–52. [CrossRef]
- Thompson, J.G.; Peterson, A.J. Bovine Embryo Culture in Vitro: New Developments and Post-Transfer Consequences. Hum. Reprod. 2000, 15, 59–67. [CrossRef]
- Gardner, D.K.; Lane, M. Development of Viable Mammalian Embryos in Vitro. In Principles of Cloning; Elsevier, 2002; pp. 187–213 ISBN 978-0-12-174597-4.
- Marsico, T.V.; Camargo, J.D.; Valente, R.S.; Sudano, M.J. Embryo Competence and Cryosurvival: Molecular and Cellular Features. Anim. Reprod. 2019, 16, 423–439. [CrossRef]
- Lonergan, P.; Rizos, D.; Gutierrez-Adan, A.; Fair, T.; Boland, M. Oocyte and Embryo Quality: Effect of Origin, Culture Conditions and Gene Expression Patterns. Reprod. Domest. Anim. 2003, 38, 259–267. [CrossRef]
- Tharasanit, T.; Thuwanut, P. Oocyte Cryopreservation in Domestic Animals and Humans: Principles, Techniques and Updated Outcomes. Animals 2021, 11, 2949. [CrossRef]
- Magdanz, V.; Boryshpolets, S.; Ridzewski, C.; Eckel, B.; Reinhardt, K. The Motility-Based Swim-up Technique Separates Bull Sperm Based on Differences in Metabolic Rates and Tail Length. PLOS ONE 2019, 14, e0223576. [CrossRef]
- Stringfellow, D.A.; Givens, M. Manual of the International Embryo Transfer Society (IETS): A Procedural Guide and General Information for the Use of Embryo Transfer Technology Emphasizing Sanitary Procedures; 4th ed.; International Embryo Transfer Society, 2010; Vol. Savory, Ill Champaign, IL: IETS;
- Youngs, C.R. Cryopreservation of Preimplantation Embryos of Cattle, Sheep, and Goats. J. Vis. Exp. 2011, 2764. [CrossRef]
- De Roover, R.; Bols, P.E.J.; Genicot, G.; Hanzen, Ch. Characterisation of Low, Medium and High Responders Following FSH Stimulation Prior to Ultrasound-Guided Transvaginal Oocyte Retrieval in Cows. Theriogenology 2005, 63, 1902–1913. [CrossRef]
- Hasler, J.F.; Henderson, W.B.; Hurtgen, P.J.; Jin, Z.Q.; McCauley, A.D.; Mower, S.A.; Neely, B.; Shuey, L.S.; Stokes, J.E.; Trimmer, S.A. Production, Freezing and Transfer of Bovine IVF Embryos and Subsequent Calving Results. Theriogenology 1995, 43, 141–152. [CrossRef]
- Jaton, C.; Koeck, A.; Sargolzaei, M.; Price, C.A.; Baes, C.; Schenkel, F.S.; Miglior, F. Short Communication: Genetic Correlations between Number of Embryos Produced Using in Vivo and in Vitro Techniques in Heifer and Cow Donors. J. Dairy Sci. 2016, 99, 8222–8226. [CrossRef]
- Abraham, M.C.; Ruete, A.; Brandt, Y.C.B. 260 Breed Influences Outcome of in Vitro Production of Embryos in Cattle. Reprod. Fertil. Dev. 2010, 22, 287. [CrossRef]
- Pontes, J.H.F.; Melo Sterza, F.A.; Basso, A.C.; Ferreira, C.R.; Sanches, B.V.; Rubin, K.C.P.; Seneda, M.M. Ovum Pick up, in Vitro Embryo Production, and Pregnancy Rates from a Large-Scale Commercial Program Using Nelore Cattle (Bos Indicus) Donors. Theriogenology 2011, 75, 1640–1646. [CrossRef]
- Valente, R.S.; Marsico, T.V.; Sudano, M.J. Basic and Applied Features in the Cryopreservation Progress of Bovine Embryos. Anim. Reprod. Sci. 2022, 239, 106970. [CrossRef]
- Valente, R.S.; Almeida, T.G.D.; Alves, M.F.; Paschoal, D.M.; Basso, A.C.; Sudano, M.J. Cellular and Apoptotic Status Monitoring According to the Ability and Speed to Resume Post-Cryopreservation Embryonic Development. Theriogenology 2020, 158, 290–296. [CrossRef]
- Lonergan, P.; Rizos, D.; Ward, F.; Boland, M.P. Factors Influencing Oocyte and Embryo Quality in Cattle. Reprod. Nutr. Dev. 2001, 41, 427–437. [CrossRef]
- Wrenzycki, C. Gene Expression Analysis and in Vitro Production Procedures for Bovine Preimplantation Embryos: Past Highlights, Present Concepts and Future Prospects. Reprod. Domest. Anim. 2018, 53, 14–19. [CrossRef]
- De Andrade Melo-Sterza, F.; Poehland, R. Lipid Metabolism in Bovine Oocytes and Early Embryos under in Vivo, in Vitro, and Stress Conditions. Int. J. Mol. Sci. 2021, 22, 3421. [CrossRef]
- Edidin, M. Lipids on the Frontier: A Century of Cell-Membrane Bilayers. Nat. Rev. Mol. Cell Biol. 2003, 4, 414–418. [CrossRef]
- Lin, T.; Lee, J.E.; Kang, J.W.; Oqani, R.K.; Cho, E.S.; Kim, S.B.; Il Jin, D. Melatonin Supplementation during Prolonged in Vitro Maturation Improves the Quality and Development of Poor-quality Porcine Oocytes via Anti-oxidative and Anti-apoptotic Effects. Mol. Reprod. Dev. 2018, 85, 665–681. [CrossRef]
- Pero, M.E.; Zullo, G.; Esposito, L.; Iannuzzi, A.; Lombardi, P.; De Canditiis, C.; Neglia, G.; Gasparrini, B. Inhibition of Apoptosis by Caspase Inhibitor Z-VAD-FMK Improves Cryotolerance of in Vitro Derived Bovine Embryos. Theriogenology 2018, 108, 127–135. [CrossRef]
- Dias, L.R.O.; Leme, L.O.; Sprícigo, J.F.W.; Pivato, I.; Dode, M.A.N. Effect of Delipidant Agents during in Vitro Culture on the Development, Lipid Content, Gene Expression and Cryotolerance of Bovine Embryos. Reprod. Domest. Anim. 2020, 55, 11–20. [CrossRef]
- Mortimer, D.; Cohen, J.; Mortimer, S.T.; Fawzy, M.; McCulloh, D.H.; Morbeck, D.E.; Pollet-Villard, X.; Mansour, R.T.; Brison, D.R.; Doshi, A.; et al. Cairo Consensus on the IVF Laboratory Environment and Air Quality: Report of an Expert Meeting. Reprod. Biomed. Online 2018, 36, 658–674. [CrossRef]
- Gardner, D.K.; Kelley, R.L. Impact of the IVF Laboratory Environment on Human Preimplantation Embryo Phenotype. J. Dev. Orig. Health Dis. 2017, 8, 418–435. [CrossRef]
- Consensus Group, C. ‘There Is Only One Thing That Is Truly Important in an IVF Laboratory: Everything’ Cairo Consensus Guidelines on IVF Culture Conditions. Reprod. Biomed. Online 2020, 40, 33–60. [CrossRef]
- Sartori, R.; Spies, C.; Wiltbank, M.C. Effects of Dry Matter and Energy Intake on Quality of Oocytes and Embryos in Ruminants. Reprod. Fertil. Dev. 2017, 29, 58. [CrossRef]
- Baruselli, P.S.; Ferreira, R.M.; Vieira, L.M.; Souza, A.H.; Bó, G.A.; Rodrigues, C.A. Use of Embryo Transfer to Alleviate Infertility Caused by Heat Stress. Theriogenology 2020, 155, 1–11. [CrossRef]
- Pontes, J.H.F.; Silva, K.C.F.; Basso, A.C.; Rigo, A.G.; Ferreira, C.R.; Santos, G.M.G.; Sanches, B.V.; Porcionato, J.P.F.; Vieira, P.H.S.; Faifer, F.S.; et al. Large-Scale in Vitro Embryo Production and Pregnancy Rates from Bos Taurus, Bos Indicus, and Indicus-Taurus Dairy Cows Using Sexed Sperm. Theriogenology 2010, 74, 1349–1355. [CrossRef]
- Watanabe, Y.F.; Souza, H.A.; Mingoti, R.D.; Ferreira, R.M.; Batista, E.O.S.; Dayan, A.; Watanabe, O.Y.; Meirelles, F.V.; Nogueira, M.F.G.; Ferraz, J.B.S.; et al. Number of Oocytes Retrieved per Donor during OPU and Its Relationship with in Vitro Embryo Production and Field Fertility Following Embryo Transfer. Anim. Reprod. 2017, 14, 635–644. [CrossRef]
- Batista, E.; Macedo, G.; Sala, R.; Ortolan, M.; Sá Filho, M.; Del Valle, T.; Jesus, E.; Lopes, R.; Rennó, F.; Baruselli, P. Plasma Antimullerian Hormone as a Predictor of Ovarian Antral Follicular Population in Bos Indicus (Nelore) and Bos Taurus (Holstein) Heifers. Reprod. Domest. Anim. 2014, 49, 448–452. [CrossRef]
- Guerreiro, B.M.; Batista, E.O.S.; Vieira, L.M.; Sá Filho, M.F.; Rodrigues, C.A.; Castro Netto, A.; Silveira, C.R.A.; Bayeux, B.M.; Dias, E.A.R.; Monteiro, F.M.; et al. Plasma Anti-Mullerian Hormone: An Endocrine Marker for in Vitro Embryo Production from Bos Taurus and Bos Indicus Donors. Domest. Anim. Endocrinol. 2014, 49, 96–104. [CrossRef]
| Trial | No. follicles | No. oocytes | Cleavage (%) | Blastocyst rate (%) |
|---|---|---|---|---|
| 1 | 14±2.8 | 6±1.7 | 66±15 | 31±14.2 |
| 2 | 14±3.5 | 6±1.1 | 67±5.5 | 20±13.3 |
| 3 | 19±2.4 | 11±2.1 | 68±7.9 | 20±7.1 |
| 4 | 16±2.0 | 9±1.1 | 56±8.2 | 23±11.5 |
| 5 | 14±2.9 | 5±2.1 | 42±17 | 39±13.1 |
| 6 | 11±2.1 | 5±1.1 | 34±16.2 | 18±10.2 |
| 7 | 13±2.1 | 7±1.8 | 27±13 | 5.5±3.7 |
| 8 | 12±2.4 | 6±2.5 | 44±12.1 | 15±7.1 |
| 9 | 12±2.4 | 6±1.2 | 33±17.1 | 0 |
| Cow (No. trials) | No. follicles | No. oocytes | Cleavage (%) | Blastocyst rate (%) |
|---|---|---|---|---|
| A (2) | 56.0±1.0a | 17.5±3.5a | 54.5±16.5 | 20.0±1a |
| B (4) | 24.7±5.6b | 9.5±2.3ab | 70±15.9 | 52.5±18.3ab |
| C (4) | 9.0±1.2c | 4±0.8b | 89±6.2 | 78±7.7b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





