Submitted:
04 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
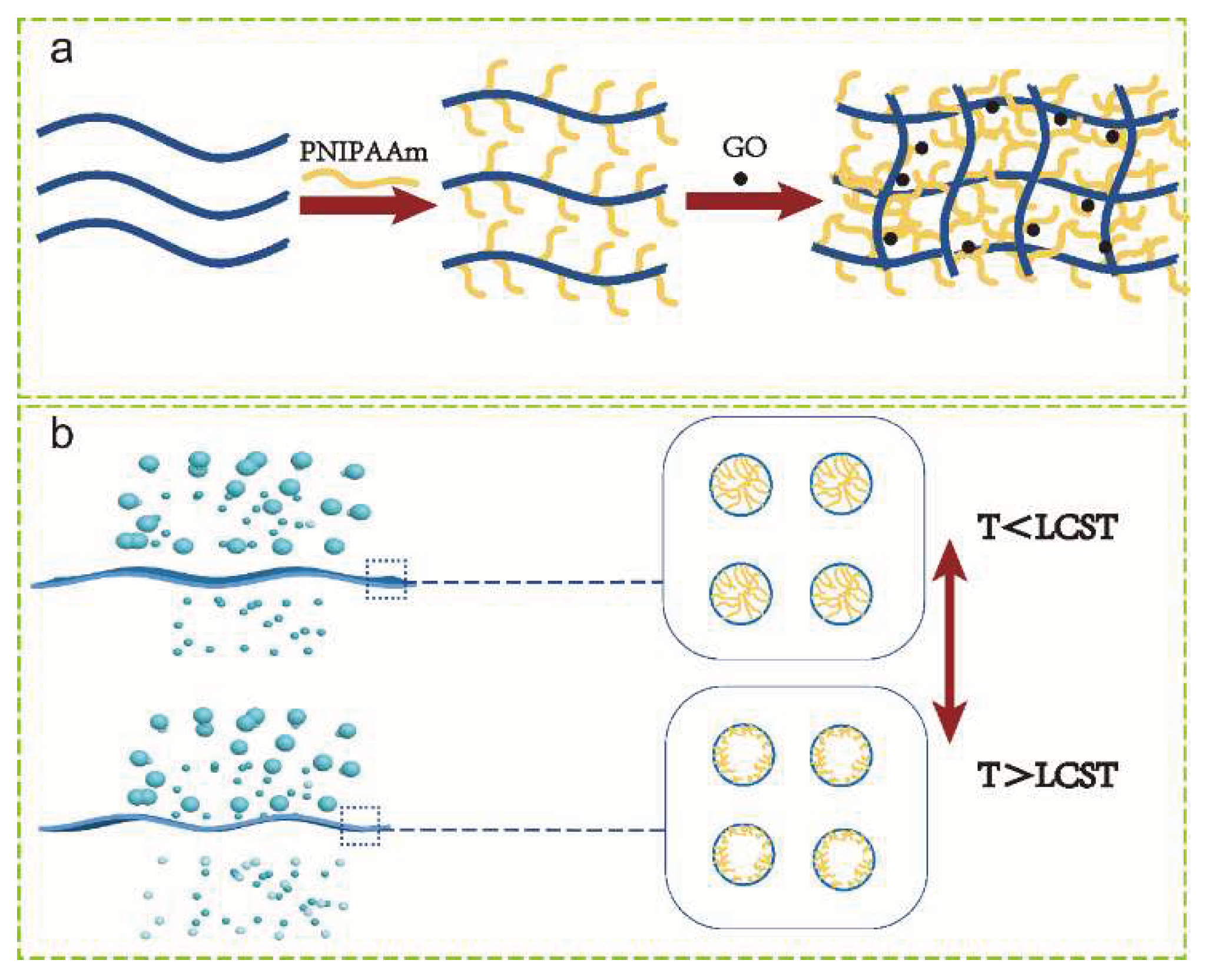
1. Introduction
2. Materials and Methods
2.1. Materials
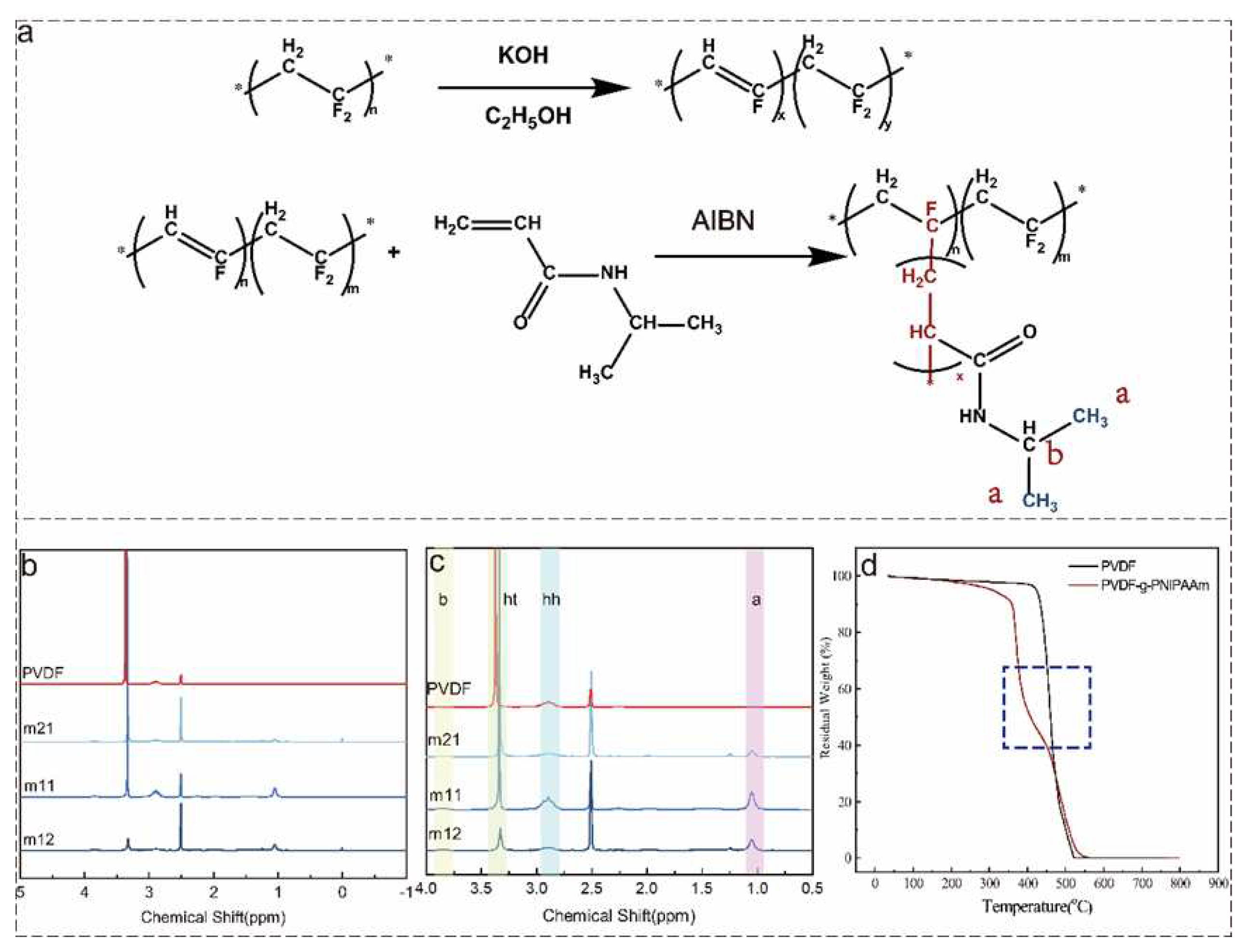
2.2. Synthesis of PVDF-g-PNIPAAm polymer by alkali treatment
2.3. Preparation of temperature-responsive Separation membrane
2.3.1. Preparation of PVDF/PVDF-g-PNIPAAm blending membrane
2.3.2. Preparation of PVDF/PVDF-g-PNIPAAm/GO separation membrane
2.4. PVDF-g-PNIPAAm Characterization
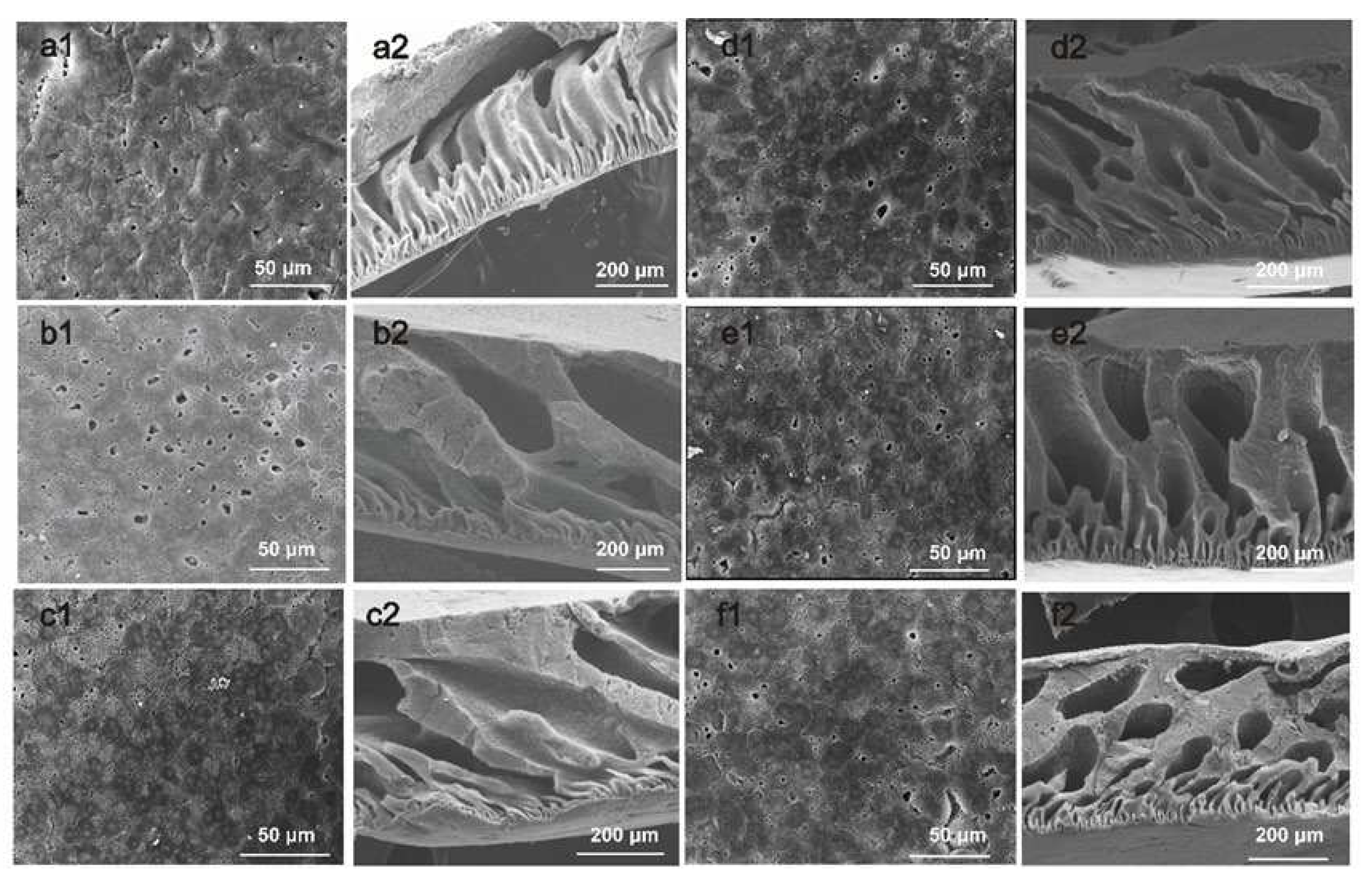
2.5. Membrane Characterization
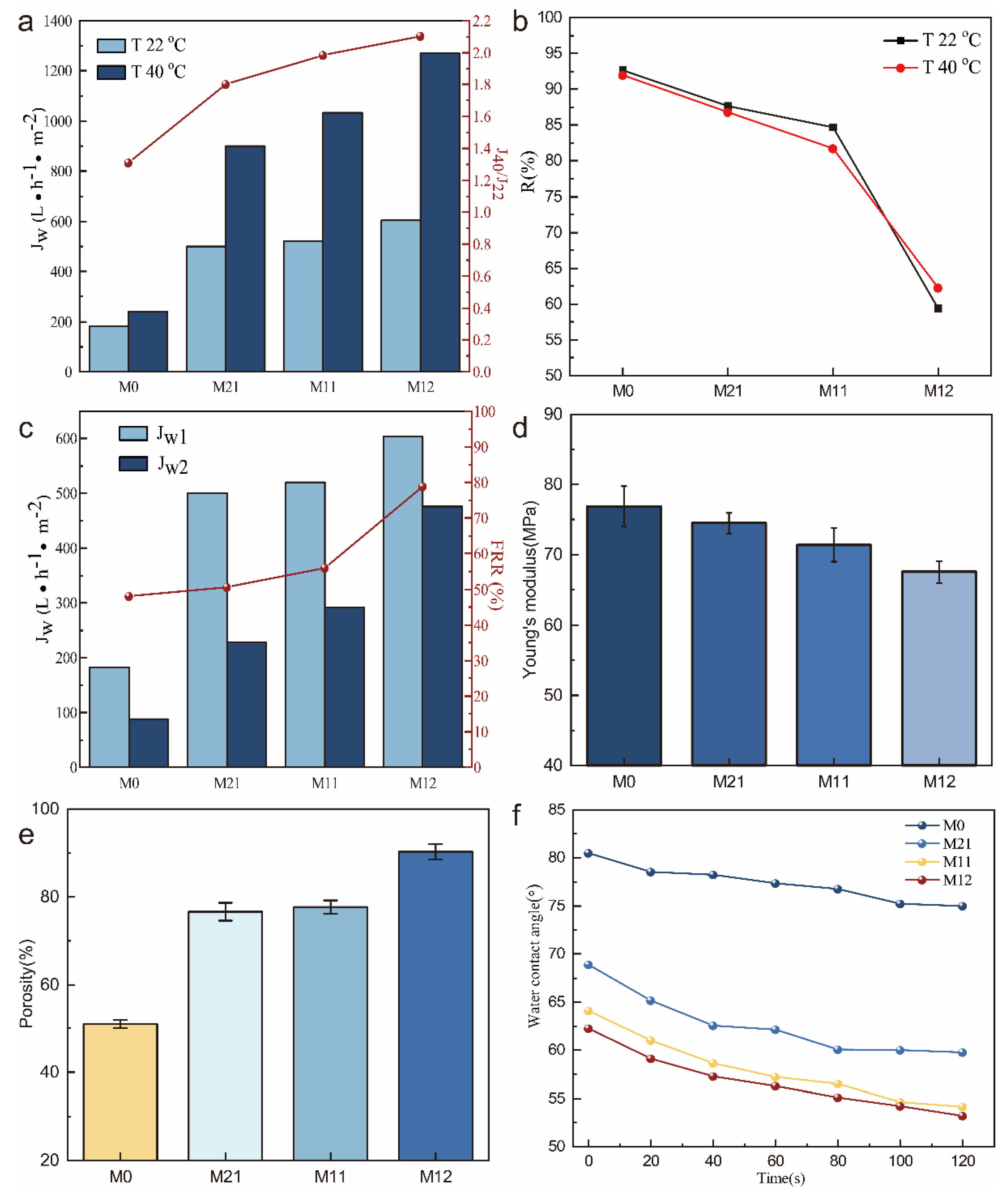
2.6. Water Permeation Experiments
2.7. Rejection property of the membrane
2.8. Antifouling properties of the membrane
2.9. Porosity test of the membrane
3. Results and Discussion
3.1. Characterization of the synthesized PVDF-g-PNIPAAm graft polymers
3.2. Characterizations of PVDF/PVDF-g-PNIPAAm membrane
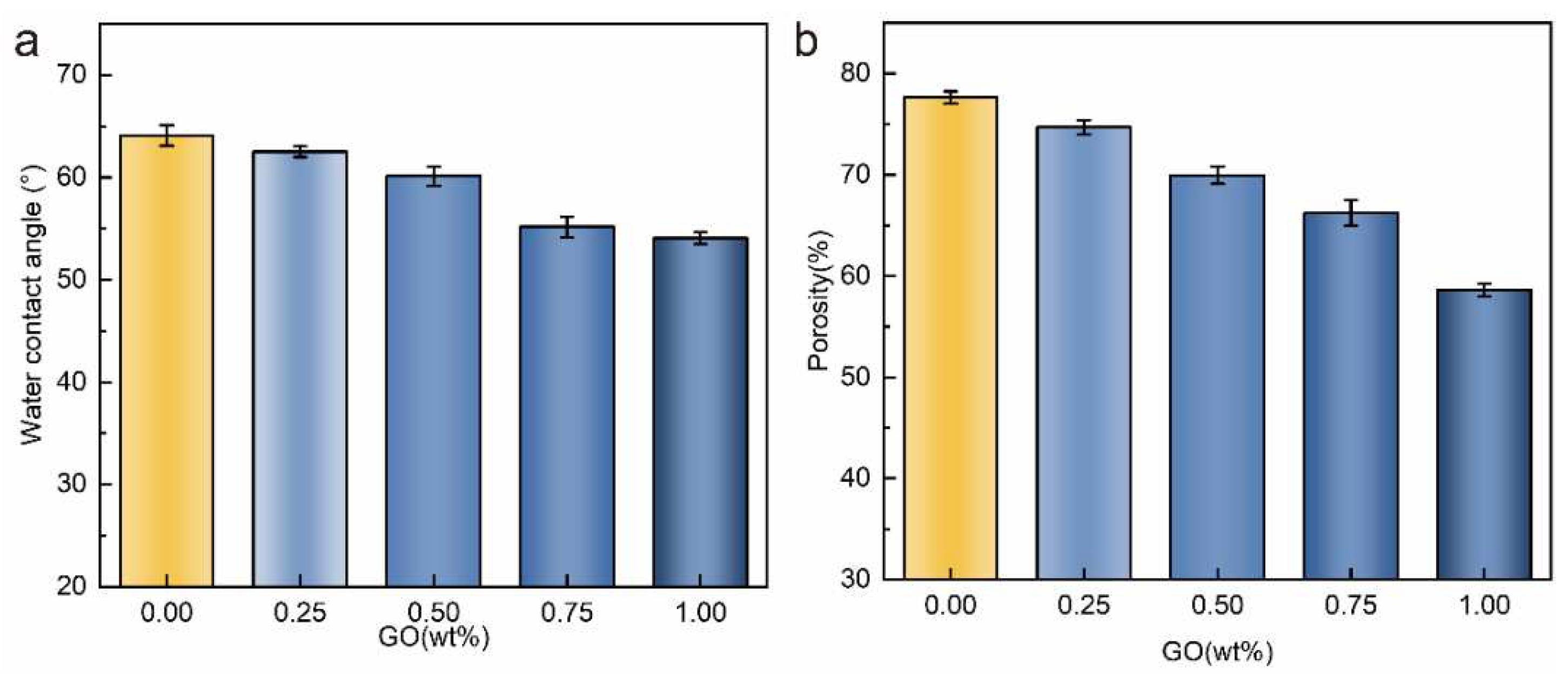
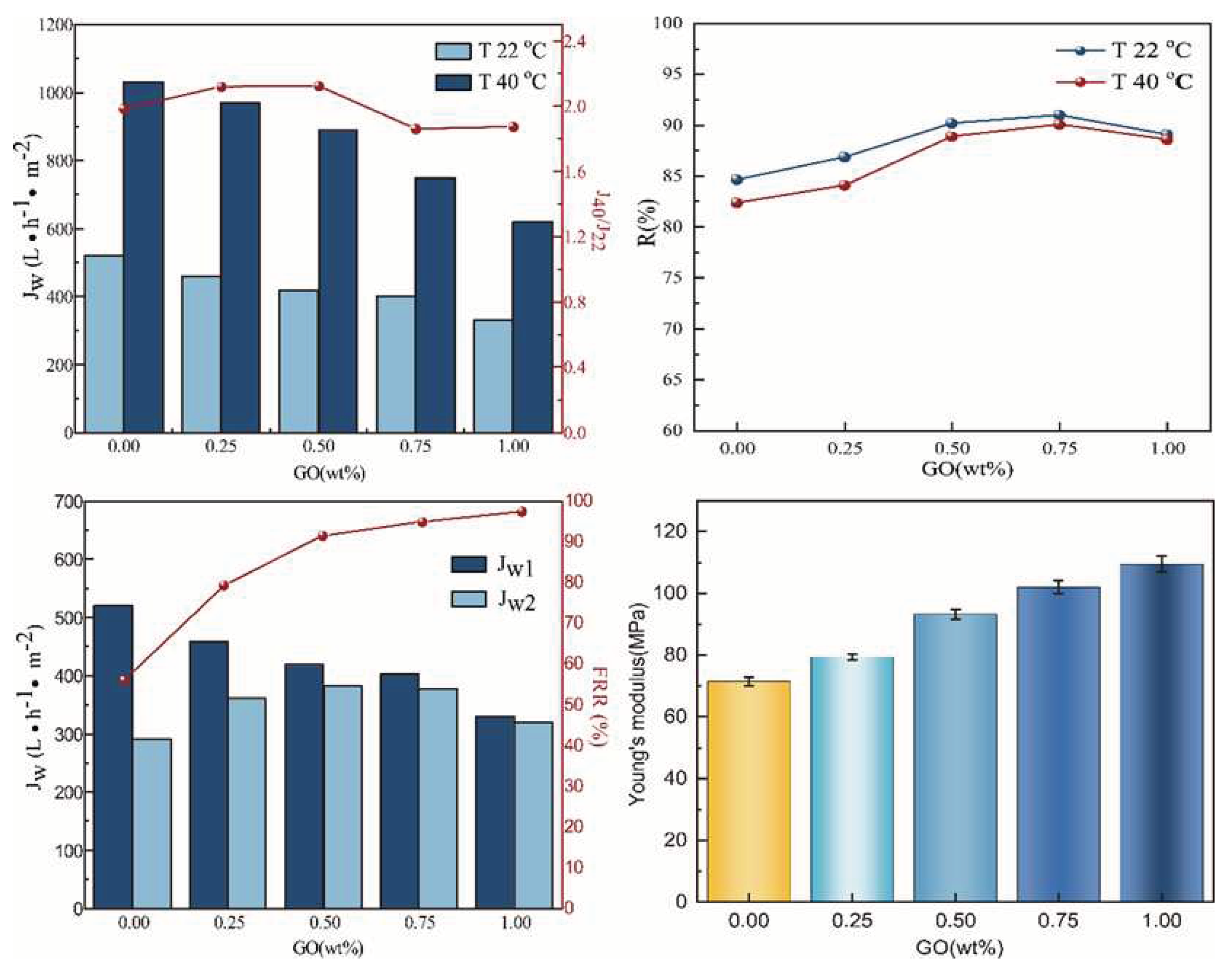
3.1. Characterizations of PVDF/PVDF-g-PNIPAAm/GO membrane
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Wang, W.; Xie, R.; Ju, X.J.; Chu, L.Y. Stimuli-responsive smart gating membranes. Chem Soc Rev 2016, 45, 460-474. [CrossRef]
- Suwal, S.; Doyen, A.; Bazinet, L. Characterization of protein, peptide and amino acid fouling on ion-exchange and filtration membranes: Review of current and recently developed methods. J Membrane Sci 2015, 496, 267-283. [CrossRef]
- Yusuf, A.; Sodiq, A.; Giwa, A.; Eke, J.; Pikuda, O.; De Luca, G.; Di Salvo, J.L.; Chakraborty, S. A review of emerging trends in membrane science and technology for sustainable water treatment. J Clean Prod 2020, 266. [CrossRef]
- Irfan, M.; Waqas, S.; Arshad, U.; Khan, J.A.; Legutko, S.; Kruszelnicka, I.; Ginter-Kramarczyk, D.; Rahman, S.; Skrzypczak, A. Response Surface Methodology and Artificial Neural Network Modelling of Membrane Rotating Biological Contactors for Wastewater Treatment. Materials 2022, 15. [CrossRef]
- Khorshidi, B.; Thundat, T.; Fleck, B.A.; Sadrzadeh, M. A Novel Approach Toward Fabrication of High Performance Thin Film Composite Polyamide Membranes. Sci Rep-Uk 2016, 6. [CrossRef]
- Kim, H.S.; Park, S.J.; Nguyen, D.Q.; Bae, J.Y.; Bae, H.W.; Lee, H.; Lee, S.D.; Choi, D.K. Multi-functional zwitterionic compounds as new membrane materials for separating oletin-paraffin mixtures. Green Chem 2007, 9, 599-604. [CrossRef]
- Sun, L.W.; Song, L.J.; Luan, S.F.; Yin, J.H. Progress in Photo-initiated Living Graft Polymerization of Biomaterials. Acta Polym Sin 2021, 52, 223-234. [CrossRef]
- Yin, B.B.; Wu, Y.N.; Liu, C.F.; Wang, P.; Wang, L.; Sun, G.X. An effective strategy for the preparation of a wide-temperature-range proton exchange membrane based on polybenzimidazoles and polyacrylamide hydrogels. J Mater Chem A 2021, 9, 3605-3615. [CrossRef]
- Diez-Pascual, A.M.; Shuttleworth, P.S. Layer-by-Layer Assembly of Biopolyelectrolytes onto Thermo/pH-Responsive Micro/Nano-Gels. Materials 2014, 7, 7472-7512. [CrossRef]
- Liu, H.W.; Yang, S.S.; Liu, Y.W.; Miao, M.J.; Zhao, Y.; Sotto, A.; Gao, C.J.; Shen, J.N. Fabricating a pH-responsive membrane through interfacial in-situ assembly of microgels for water gating and self-cleaning. J Membrane Sci 2019, 579, 230-239. [CrossRef]
- Ni, X.Q.; Xing, X.; Deng, Y.F.; Li, Z. Applications of Stimuli-Responsive Hydrogels in Bone and Cartilage Regeneration. Pharmaceutics 2023, 15. [CrossRef]
- Mahdavi, H.; Rezaei, M.; Ahmadian-Alam, L.; Amini, M.M. A novel ternary Pd-GO/N-doped TiO2 hierarchical visible-light sensitive photocatalyst for nanocomposite membrane. Korean J Chem Eng 2020, 37, 946-954. [CrossRef]
- Wang, X.; Feng, M.; Liu, Y.; Deng, H.N.; Lu, J. Fabrication of graphene oxide blended polyethersulfone membranes via phase inversion assisted by electric field for improved separation and antifouling performance. J Membrane Sci 2019, 577, 41-50. [CrossRef]
- Rybak, A.; Rybak, A.; Kaszuwara, W.; Nyc, M.; Auguscik, M. Metal substituted sulfonated poly(2,6-dimethyl-1,4-phenylene oxide) hybrid membranes with magnetic fillers for gas separation. Sep Purif Technol 2019, 210, 479-490. [CrossRef]
- Chen, Q.M.; Yu, X.W.; Pei, Z.Q.; Yang, Y.; Wei, Y.; Ji, Y. Multi-stimuli responsive and multi-functional oligoaniline-modified vitrimers. Chem Sci 2017, 8, 724-733. [CrossRef]
- Okada, K.; Miura, Y.; Chiya, T.; Tokudome, Y.; Takahashi, M. Thermo-responsive wettability via surface roughness change on polymer-coated titanate nanorod brushes toward fast and multi-directional droplet transport. Rsc Adv 2020, 10, 28032-28036. [CrossRef]
- Ye, Q.S.; Wang, R.; Chen, C.; Chen, B.L.; Zhu, X.Y. High-Flux pH-Responsive Ultrafiltration Membrane for Efficient Nanoparticle Fractionation. Acs Appl Mater Inter 2021, 13, 56575-56583. [CrossRef]
- Li, J.J.; Zhu, L.T.; Luo, Z.H. Electrospun fibrous membrane with enhanced swithchable oil/water wettability for oily water separation. Chem Eng J 2016, 287, 474-481. [CrossRef]
- Wu, L.; Li, C.R.; Tao, Z.; Wang, H.; Ran, J.; Bakangura, E.; Zhang, Z.H.; Xu, T.W. Anionic quaternary ammonium fluorous copolymers bearing thermo-responsive grafts for fuel cells. Int J Hydrogen Energ 2014, 39, 9387-9396. [CrossRef]
- Pan, Y.Q.; He, L.; Ren, Y.S.; Wang, W.; Wang, T.H. Analysis of Influencing Factors on the Gas Separation Performance of Carbon Molecular Sieve Membrane Using Machine Learning Technique. Membranes-Basel 2022, 12. [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Hliavitskaya, T.A.; Penkova, A.V.; Ermakov, S.S.; Ulbricht, M. Modification of Polysulfone Ultrafiltration Membranes via Addition of Anionic Polyelectrolyte Based on Acrylamide and Sodium Acrylate to the Coagulation Bath to Improve Antifouling Performance in Water Treatment. Membranes-Basel 2020, 10. [CrossRef]
| Membranes | PVDF (wt%) | PVDF-g-PNIPAAm (wt%) | GO(wt%) |
|---|---|---|---|
| 1 | 19.8 | 0.20 | 0.00 |
| 2 | 19.8 | 0.20 | 0.25 |
| 3 | 19.8 | 0.20 | 0.50 |
| 4 | 19.8 | 0.20 | 0.75 |
| 5 | 19.8 | 0.20 | 1.00 |
| number | The mass ratio of PVDF to NIPAAm | XPNIPAAm(%) |
|---|---|---|
| m21 | 2:1 | 4.9 |
| m11 | 1:1 | 8.3 |
| m12 | 1:2 | 16.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




