Submitted:
04 January 2024
Posted:
05 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
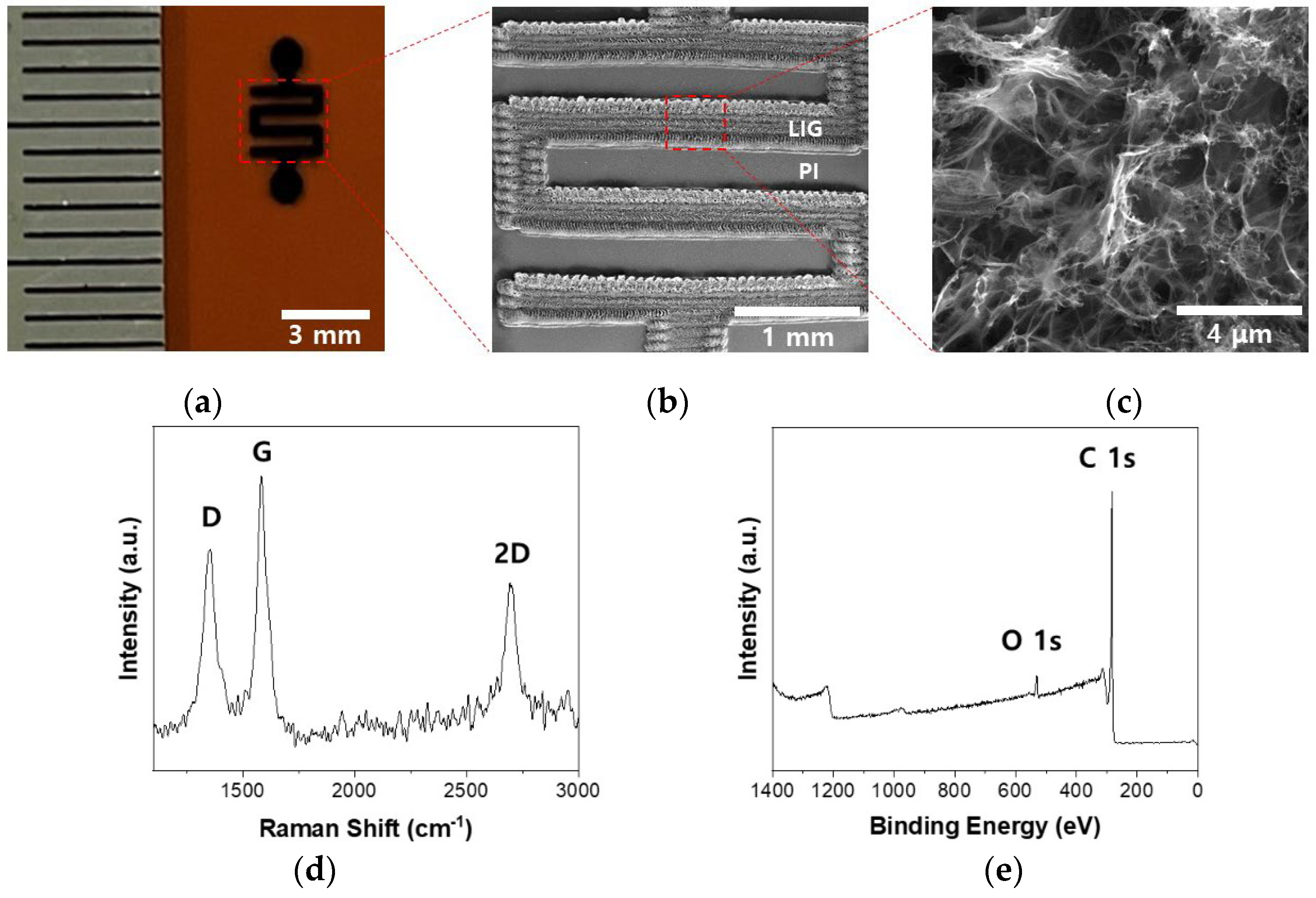
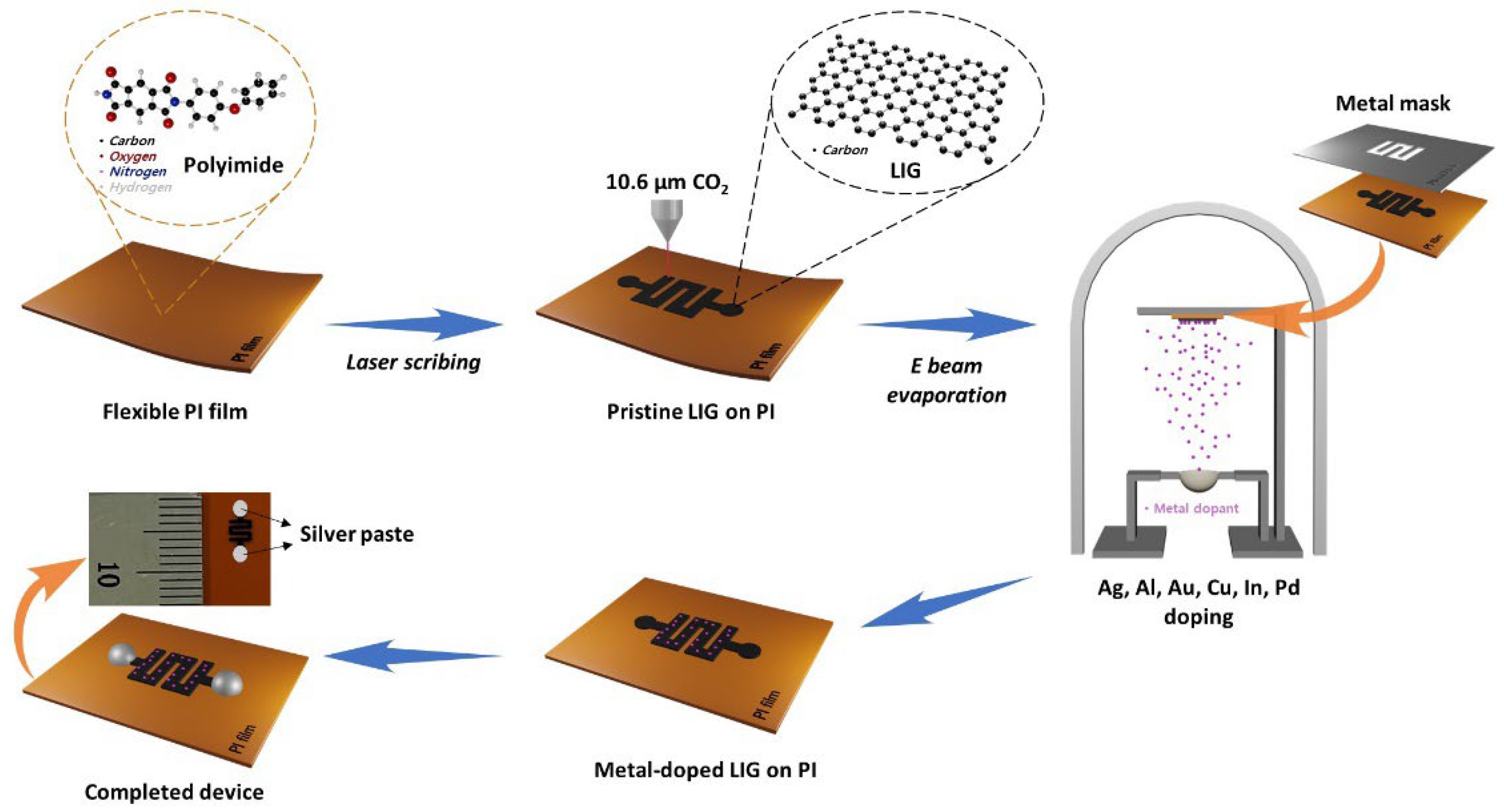
2.1. Fabrication and Characterization of the P-LIG sensor device.
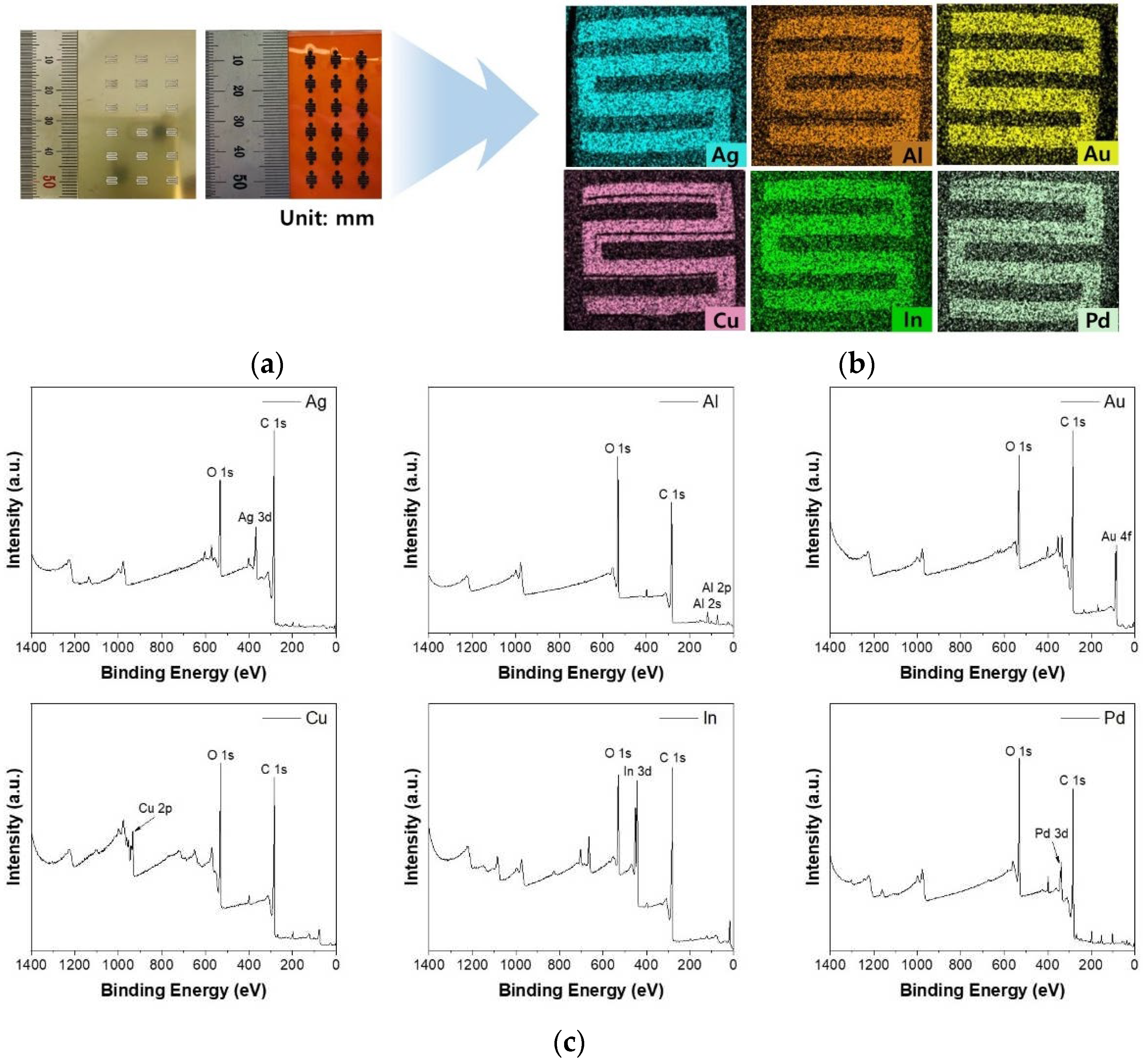
2.2. Metal-doping in P-LIG and characterization of the M-LIG sensor devices
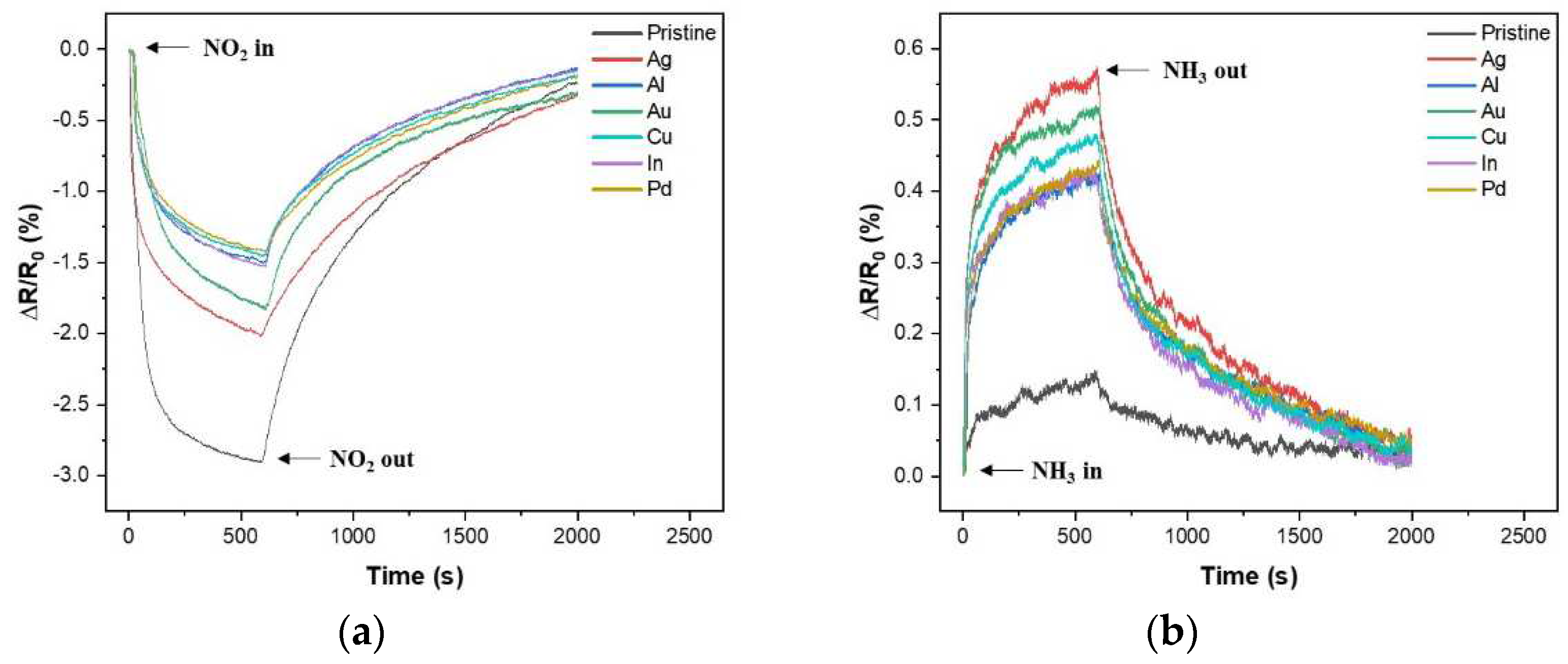
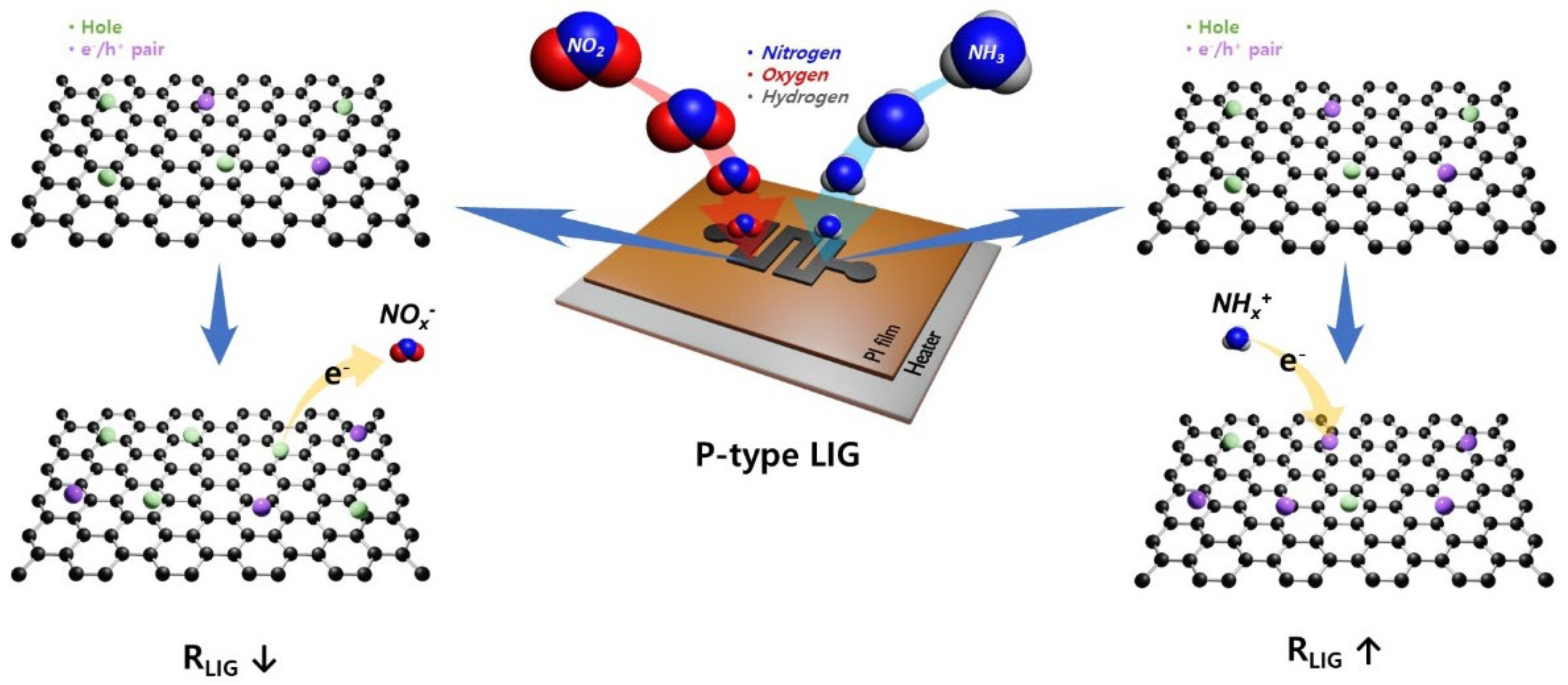
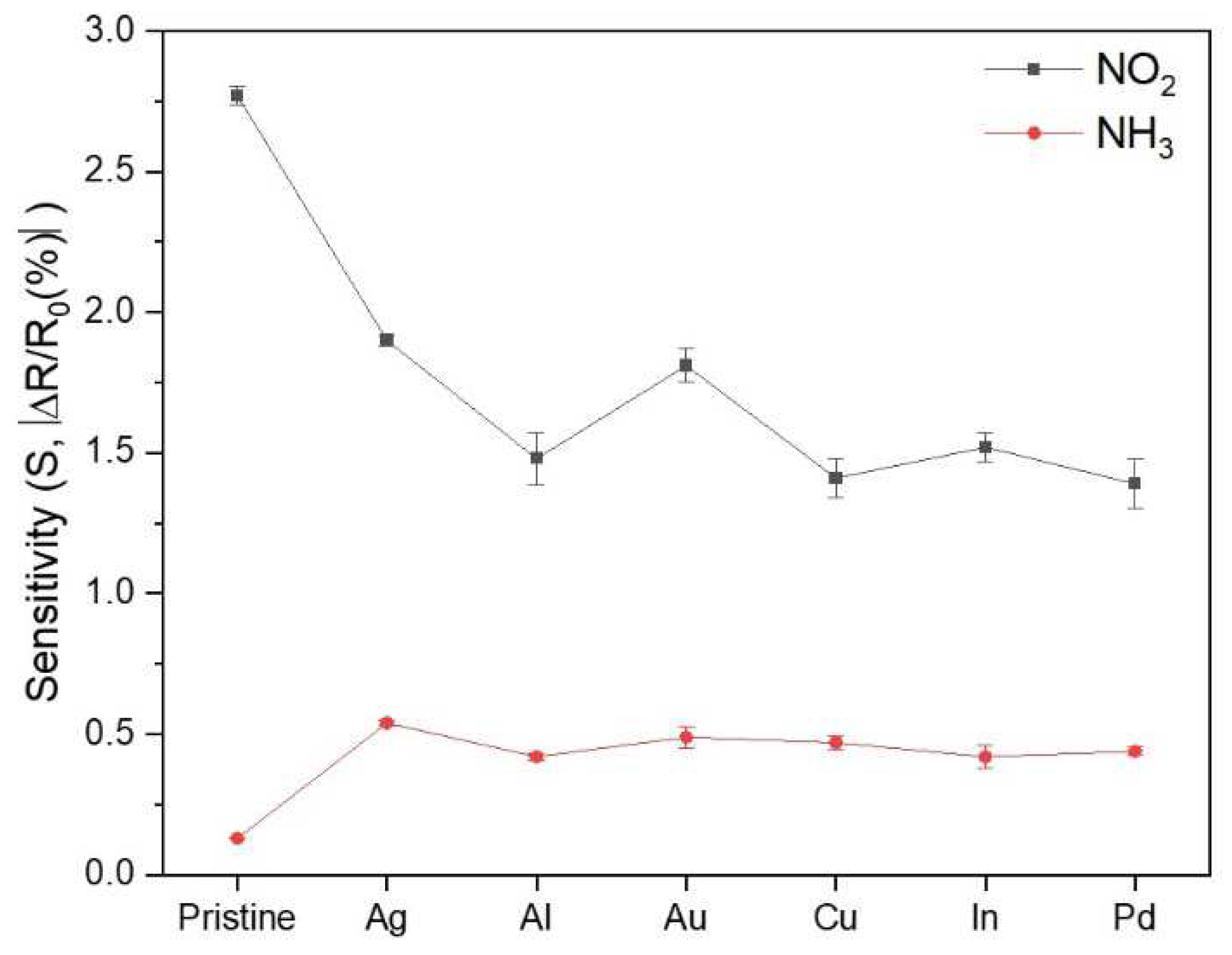
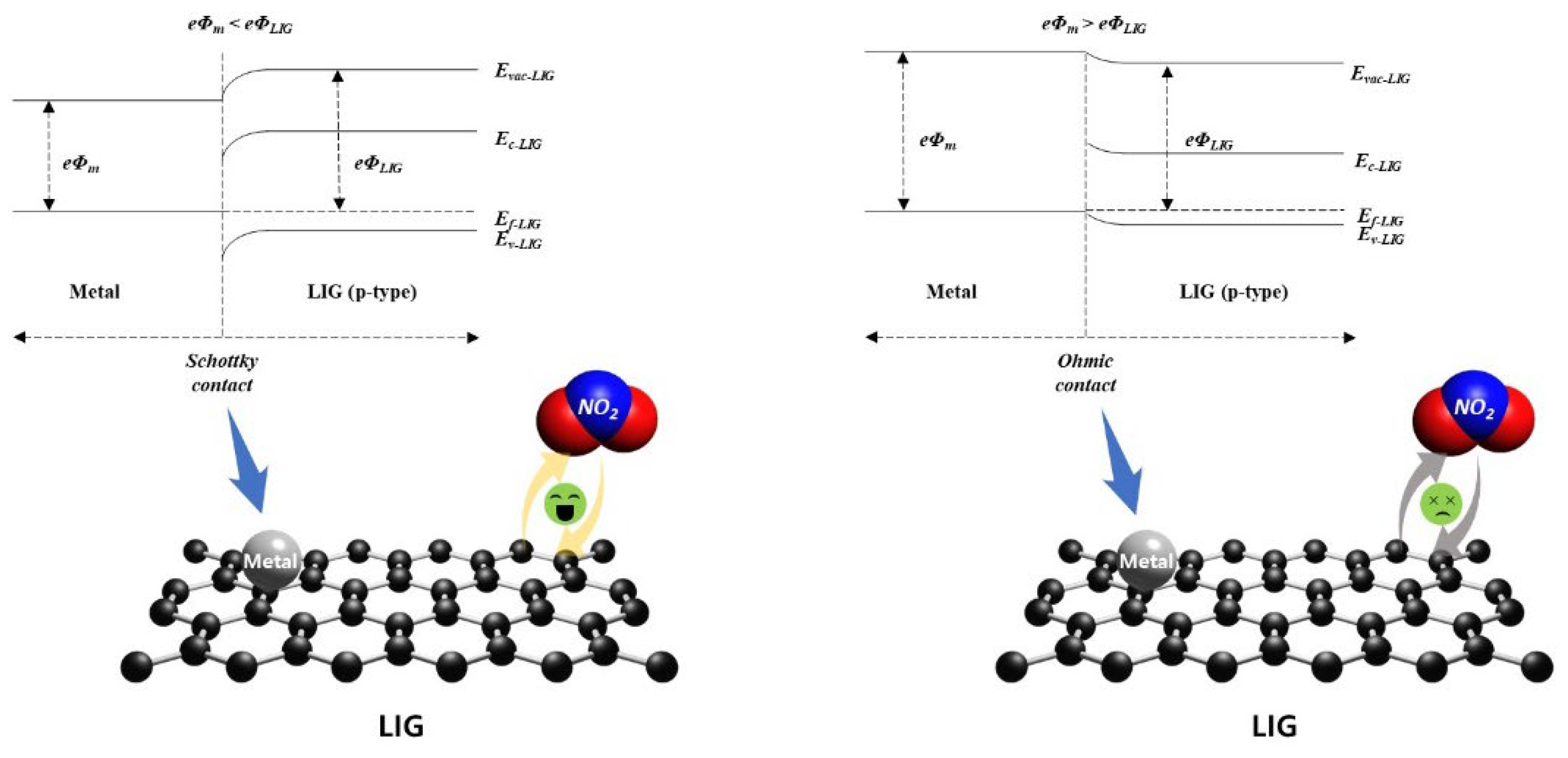
2.3. Gas-sensing performance characterizations
3. Conclusions
4. Materials and Methods
4.1. Synthesis of LIG on PI
4.2. Preparation of P-LIG and M-LIG Devices
4.3. Bare LIG and Metal-doped LIG Characterizations
4.4. Sensor Testing Equipment
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, Z.; Tao, L.-Q.; Zou, S.; Zhu, C.; Wang, G.; Sun, H.; Ren, T.-L. A Multi-functional NO2 gas monitor and Self-Alarm based on Laser-Induced graphene. Chemical Engineering Journal 2022, 428. [Google Scholar] [CrossRef]
- Yan, W.; Yan, W.; Chen, T.; Xu, J.; Tian, Q.; Ho, D. Size-Tunable Flowerlike MoS2 Nanospheres Combined with Laser-Induced Graphene Electrodes for NO2 Sensing. ACS Applied Nano Materials 2020, 3, 2545–2553. [Google Scholar] [CrossRef]
- Kwon, B.; Bae, H.; Lee, H.; Kim, S.; Hwang, J.; Lim, H.; Lee, J.H.; Cho, K.; Ye, J.; Lee, S.; et al. Ultrasensitive N-Channel Graphene Gas Sensors by Nondestructive Molecular Doping. ACS Nano 2022, 16, 2176–2187. [Google Scholar] [CrossRef]
- Singh, A.; Shiby, S.; Sahu, A.; Pachori, P.; Tanwar, M.; Kumar, R.; Palani, A.I. Parametric investigation on laser interaction with polyimide for graphene synthesis towards flexible devices. Journal of Physics D: Applied Physics 2022, 56. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-based gas sensors. Journal of Materials Chemistry A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Eriksson, J.; Puglisi, D.; Kang, Y.H.; Yakimova, R.; Spetz, A.L. Adjusting the electronic properties and gas reactivity of epitaxial graphene by thin surface metallization. Physica B: Condensed Matter 2014, 439, 105–108. [Google Scholar] [CrossRef]
- Cho, B.; Yoon, J.; Hahm, M.G.; Kim, D.-H.; Kim, A.R.; Kahng, Y.H.; Park, S.-W.; Lee, Y.-J.; Park, S.-G.; Kwon, J.-D. Graphene-based gas sensor: metal decoration effect and application to a flexible device. Journal of Materials Chemistry C 2014, 2, 5280–5285. [Google Scholar] [CrossRef]
- Javey, A.; Guo, J.; Wang, Q.; Lundstrom, M.; Dai, H. Ballistic carbon nanotube field-effect transistors. nature 2003, 424, 654–657. [Google Scholar] [CrossRef]
- Chen, Z.; Appenzeller, J.; Knoch, J.; Lin, Y.-m.; Avouris, P. The role of metal− nanotube contact in the performance of carbon nanotube field-effect transistors. Nano letters 2005, 5, 1497–1502. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F. Adsorption of H 2 O, N H 3, CO, N O 2, and NO on graphene: A first-principles study. Physical Review B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Singhal, A.V.; Charaya, H.; Lahiri, I. Noble metal decorated graphene-based gas sensors and their fabrication: a review. Critical Reviews in Solid State and Materials Sciences 2017, 42, 499–526. [Google Scholar] [CrossRef]
- Giovannetti, G.; Khomyakov, P.A.; Brocks, G.; Karpan, V.v.; van den Brink, J.; Kelly, P.J. Doping graphene with metal contacts. Physical review letters 2008, 101, 026803. [Google Scholar] [CrossRef]
- Chan, K.T.; Neaton, J.; Cohen, M.L. First-principles study of metal adatom adsorption on graphene. Physical Review B 2008, 77, 235430. [Google Scholar] [CrossRef]
- Khomyakov, P.; Giovannetti, G.; Rusu, P.; Brocks, G.v.; Van den Brink, J.; Kelly, P.J. First-principles study of the interaction and charge transfer between graphene and metals. Physical Review B 2009, 79, 195425. [Google Scholar] [CrossRef]
- Liang, X.-Y.; Ding, N.; Ng, S.-P.; Wu, C.-M.L. Adsorption of gas molecules on Ga-doped graphene and effect of applied electric field: A DFT study. Applied Surface Science 2017, 411, 11–17. [Google Scholar] [CrossRef]
- Ni, J.; Quintana, M.; Song, S. Adsorption of small gas molecules on transition metal (Fe, Ni and Co, Cu) doped graphene: A systematic DFT study. Physica E: Low-dimensional Systems and Nanostructures 2020, 116, 113768. [Google Scholar] [CrossRef]
- Yang, L.; Xiao, W.; Wang, J.; Li, X.; Wang, L. Formaldehyde gas sensing properties of transition metal-doped graphene: a first-principles study. Journal of Materials Science 2021, 56, 12256–12269. [Google Scholar] [CrossRef]
- Zhao, M.; Dong, F.; Yan, L.; Xu, L.; Zhang, X.; Chen, P.; Song, Z.; Chu, W. High efficiency room temperature detection of NO 2 gas based on ultrathin metal/graphene devices. RSC advances 2016, 6, 84082–84089. [Google Scholar] [CrossRef]
- Zhu, J.; Cho, M.; Li, Y.; Cho, I.; Suh, J.-H.; Del Orbe, D.; Jeong, Y.; Ren, T.-L.; Park, I. Biomimetic Turbinate-like Artificial Nose for Hydrogen Detection Based on 3D Porous Laser-Induced Graphene. ACS Applied Materials & Interfaces 2019, 11, 24386–24394. [Google Scholar]
- Tseng, S.-F.; Chen, P.-S.; Hsu, S.-H.; Hsiao, W.-T.; Peng, W.-J. Investigation of fiber laser-induced porous graphene electrodes in controlled atmospheres for ZnO nanorod-based NO2 gas sensors. Applied Surface Science 2023, 620. [Google Scholar] [CrossRef]
- Wang, F.; Wang, K.; Zheng, B.; Dong, X.; Mei, X.; Lv, J.; Duan, W.; Wang, W. Laser-induced graphene: preparation, functionalization and applications. Materials technology 2018, 33, 340–356. [Google Scholar] [CrossRef]
- Stanford, M.G.; Yang, K.; Chyan, Y.; Kittrell, C.; Tour, J.M. Laser-Induced Graphene for Flexible and Embeddable Gas Sensors. ACS Nano 2019, 13, 3474–3482. [Google Scholar] [CrossRef]
- Dosi, M.; Lau, I.; Zhuang, Y.; Simakov, D.S.A.; Fowler, M.W.; Pope, M.A. Ultrasensitive Electrochemical Methane Sensors Based on Solid Polymer Electrolyte-Infused Laser-Induced Graphene. ACS Appl Mater Interfaces 2019, 11, 6166–6173. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene: From Discovery to Translation. Adv Mater 2019, 31, e1803621. [Google Scholar] [CrossRef]
- Wang, Y.-n.; Gan, J.; Wang, D.-m.; Xin, H.-h.; Zhu, Y.-f. Characteristics and electrical properties of polyimide films fluorinated for different durations. Materials Today Communications 2021, 26, 102098. [Google Scholar] [CrossRef]
- Robertson, A.C.F.a.J. Interpretation of Raman spectra of disordered and amorphous carbon. Physical review B 2000, 61, 14095. [Google Scholar]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Physical review B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Yao, J.; Liu, L.; Zhang, S.; Wu, L.; Tang, J.; Qiu, Y.; Huang, S.; Wu, H.; Fan, L. Metal-incorporated laser-induced graphene for high performance supercapacitors. Electrochimica Acta 2023, 441, 141719. [Google Scholar] [CrossRef]
- Zhang, Y.; Franklin, N.W.; Chen, R.J.; Dai, H. Metal coating on suspended carbon nanotubes and its implication to metal–tube interaction. Chemical Physics Letters 2000, 331, 35–41. [Google Scholar] [CrossRef]
- Kim, T.; Eom, T.H.; Jang, H.W. Self-activated Graphene Gas Sensors: A Mini Review. Journal of Sensor Science and Technology 2020, 29, 220–226. [Google Scholar] [CrossRef]
- Eranna, G.; Joshi, B.; Runthala, D.; Gupta, R. Oxide materials for development of integrated gas sensors—a comprehensive review. Critical Reviews in Solid State and Materials Sciences 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Kim, T.Y.; Park, C.H.; Marzari, N. The Electronic Thermal Conductivity of Graphene. Nano Lett 2016, 16, 2439–2443. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.L.; Liu, Z.; Zhou, G.; Hao, S.; Wu, J.; Gu, B.-L.; Duan, W. Adsorption of gas molecules on graphene nanoribbons and its implication for nanoscale molecule sensor. The Journal of Physcial Chemistry C 2008, 112, 13442–13446. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sensors and Actuators B: Chemical 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Jung, I.; Dikin, D.A.; Piner, R.D.; Ruoff, R.S. Tunable electrical conductivity of individual graphene oxide sheets reduced at “low” temperatures. Nano letters 2008, 8, 4283–4287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lu, Y.-H.; Cai, Y.-Q.; Zhang, C.; Feng, Y.-P. Adsorption of gas molecules on transition metal embedded graphene: a search for high-performance graphene-based catalysts and gas sensors. Nanotechnology 2011, 22, 385502. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.S.; Foukolaei, V.P. Density functional study of Al-doped graphene nanostructure towards adsorption of CO, CO2 and H2O. Synthetic Metals 2015, 210, 171–178. [Google Scholar]
- Subrahmanyam, K.; Manna, A.K.; Pati, S.K.; Rao, C. A study of graphene decorated with metal nanoparticles. Chemical Physics Letters 2010, 497, 70–75. [Google Scholar] [CrossRef]
- Jia, Z.; Yan, B.; Niu, J.; Han, Q.; Zhu, R.; Yu, D.; Wu, X. Transport study of graphene adsorbed with indium adatoms. Physical Review B 2015, 91, 085411. [Google Scholar] [CrossRef]
- Chandni, U.; Henriksen, E.A.; Eisenstein, J. Transport in indium-decorated graphene. Physical Review B 2015, 91, 245402. [Google Scholar] [CrossRef]
- Mirzaei, A.; Bharath, S.P.; Kim, J.-Y.; Pawar, K.K.; Kim, H.W.; Kim, S.S. N-Doped Graphene and Its Derivatives as Resistive Gas Sensors: An Overview. Chemosensors 2023, 11, 334. [Google Scholar] [CrossRef]
- Chakraborty, N.; Mondal, S. Dopant-mediated surface charge imbalance for enhancing the performance of metal oxide chemiresistive gas sensors. Journal of Materials Chemistry C 2022, 10, 1968–1976. [Google Scholar] [CrossRef]
- Kropp, T.; Mavrikakis, M. Transition metal atoms embedded in graphene: how nitrogen doping increases CO oxidation activity. ACS Catalysis 2019, 9, 6864–6868. [Google Scholar] [CrossRef]
- Wang, S.; Huang, D.; Xu, S.; Jiang, W.; Wang, T.; Hu, J.; Hu, N.; Su, Y.; Zhang, Y.; Yang, Z. Two-dimensional NiO nanosheets with enhanced room temperature NO 2 sensing performance via Al doping. Physical Chemistry Chemical Physics 2017, 19, 19043–19049. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cui, X.; Liu, J.; Zhou, X.; Cheng, X.; Sun, P.; Hu, X.; Li, X.; Zheng, J.; Lu, G. Design of superior ethanol gas sensor based on Al-doped NiO nanorod-flowers. Acs Sensors 2016, 1, 131–136. [Google Scholar] [CrossRef]
- Moafi, A.; Heidari, O.; Soltannia, B.; Wlodarski, W.; Shahi, F.; Parvin, P. Reduction of metal nanoparticle decorated flexible graphene oxide by laser at various temperatures and under selected atmospheres. Carbon Trends 2022, 6, 100140. [Google Scholar] [CrossRef]
- Eom, W.; Jang, J.-S.; Lee, S.H.; Lee, E.; Jeong, W.; Kim, I.-D.; Choi, S.-J.; Han, T.H. Effect of metal/metal oxide catalysts on graphene fiber for improved NO2 sensing. Sensors and Actuators B: Chemical 2021, 344, 130231. [Google Scholar] [CrossRef]
- Karim, W.; Spreafico, C.; Kleibert, A.; Gobrecht, J.; VandeVondele, J.; Ekinci, Y.; van Bokhoven, J.A. Catalyst support effects on hydrogen spillover. Nature 2017, 541, 68–71. [Google Scholar] [CrossRef]
- Kim, B.-K.; Park, N.; Na, P.S.; So, H.-M.; Kim, J.-J.; Kim, H.; Kong, K.-J.; Chang, H.; Ryu, B.-H.; Choi, Y. The effect of metal cluster coatings on carbon nanotubes. Nanotechnology 2005, 17, 496. [Google Scholar] [CrossRef]
- Rut’kov, E.V.; Afanas’eva, E.Y.; Gall, N.R. Graphene and graphite work function depending on layer number on Re. Diamond and Related Materials 2020, 101. [Google Scholar] [CrossRef]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and metal oxide nanoparticles in chemiresistors: does the nanoscale matter? small 2006, 2, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sensors and actuators B: Chemical 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Kohl, D. The role of noble metals in the chemistry of solid-state gas sensors. Sensors and Actuators B: Chemical 1990, 1, 158–165. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




