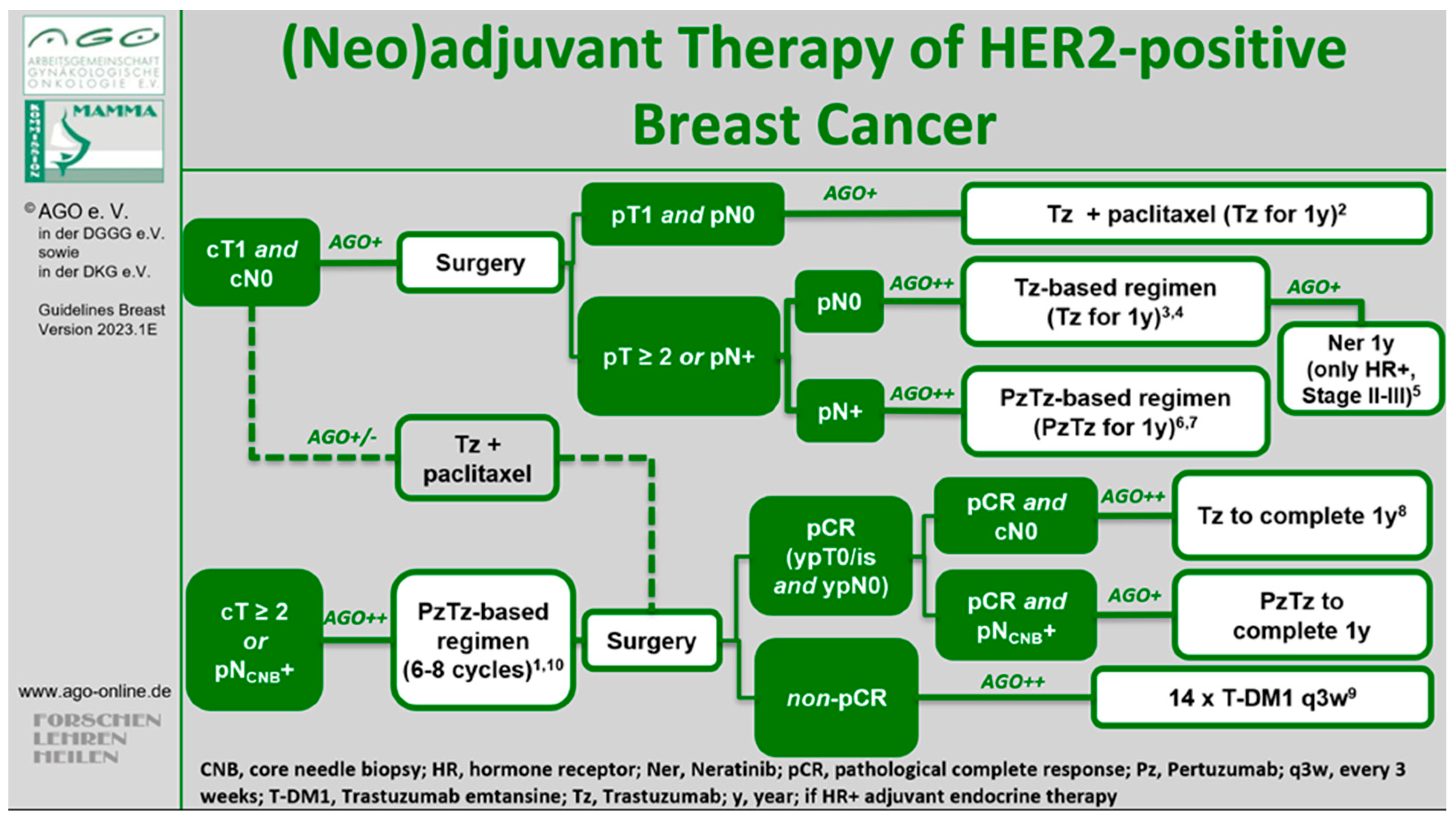
1. Introduction
Breast cancer (BC) is the most common cancer in women worldwide and every 8th woman in the western world is affected by it during their lifetime (1). The identification of the nuclear estrogen receptor (ER) and the differentiation between ER-positive and ER-negative breast carcinomas in the 1970s by the American scientist Elwood Jensen marked a milestone and the beginning of a tumor biological approach in oncology, from which the still-existing anti-estrogen therapy originated. This development continued in the 1980s and 1990s when another important cellular factor, the human epidermal growth factor receptor (HER), was first described by Shih et al. (2), leading to the development of targeted antibody-based therapy, primarily initiated by the German biochemist Axel Ullrich and the U.S. American oncologist Dennis Slamon (3).
The HER2 receptor is a 1255 amino acid glycoprotein belonging to a family of transmembrane tyrosine kinases, with its coding proto-oncogene located on the long arm of chromosome 17 (q21) (4). Ligand binding is only possible after the receptor forms heterodimers with other HER receptors and is regulated by autophosphorylation of the HER2 tyrosine kinase, subsequently regulating the Ras-MAPK pathway (5). HER2 overexpression, due to an increased rate of heterodimer formation and reduced endocytosis, leads to upregulation of the downstream Ras-MAPK pathway, resulting in enhanced cell growth and the inhibition of pro-apoptotic factors in the tumor cell, making it more biologically aggressive. HER2 gene amplification, first described in BC cells in 1985 (6), results in an up to a 45-fold increase in receptor density on the cell membrane (7, 8). Since then, activating point mutations, which intensify the signalling cascade without the presence of gene amplification and receptor overexpression, have also been described (9).
In 1987 Slamon et al. reported that the amplification of the Her-2/neu oncogene is correlated with increased relapse risk and reduced survival after BC in humans (10). They stated that Her-2/neu amplification had greater prognostic value than other prognostic factors used at that time, such as hormone receptor status and lymph node disease (10). An aggressive disease biology such as enhanced cell proliferation and reduced progression-free survival (PFS) and overall survival (OS) was associated with amplification of HER2 gene (10, 11, 12, 13). Back then, types of BC considered “aggressive” were generally treated with chemotherapy. However, this strategy did not lead to marked improvements in patient survival as median PFS after diagnosis of distant disease was as low as 4.6 months and OS 20 months in patients treated with chemotherapy alone (14). In this context, identification of the HER2 receptor as a potential target for therapy was a gamechanger.
The introduction of trastuzumab, the first monoclonal antibody against the HER2 receptor over 20 years ago was a milestone in the treatment of HER2 positive breast carcinomas (14, 15). Trastuzumab binds to the extracellular domain of the HER2 receptor (16). Prevention of receptor dimerization, immune activation, increased endocytotic receptor destruction as well as inhibition of shedding the extra-cellular domain are possible mechanisms of trastuzumab receptor binding (17). Its introduction marked the beginning of a new era in targeted tumor therapy and was followed by approvals of monoclonal antibodies in nearly all oncological entities. The name trastuzumab is derived from the last three syllables, "-tu" "-zu" and "-mab" which, according to the established nomenclature for monoclonal antibodies, indicate both the therapeutic use against tumors ("-tu") and the biochemical structure as a humanized ("-zu") monoclonal antibody ("-mab") (18).
While there have been further developments in anti-HER2 therapy, such as the introduction of dual blockade with trastuzumab/pertuzumab or trastuzumab emtansin (T-DM1) as the first antibody-drug conjugate (ADC) for BC, all of these developments have followed the principle of therapy targeting HER2 overexpression, as the use of trastuzumab in tumors other than those HER2 positive did not show clinical benefit.
HER2 enriched tumor cells are defined by a strong immunohistochemical (IHC) staining reaction (3+). In the case of moderate staining (2+), an additional positive confirmation using in situ hybridization (ISH) is required. According to the current pathological classification, all 2+ tumors with negative ISH or low to absent immunohistochemical staining (1+/0) are considered HER2-negative (19, 20). However, a significant number of tumors exhibits low to moderate expression of HER2 (2+ with negative ISH/1+), which have traditionally been treated according to the recommendations for HR+ HER2 negative or triple-negative breast cancer (TNBC) (21). Recently, the DESTINY-Breast 04 trial has shown a significant clinical benefit of therapy with ADC trastuzumab deruxtecan (T-DXd) in patients with advanced breast cancer (aBC) with HER2-low expression. As a result, T-DXd is currently approved for use in both HER2 low and HER2 enriched tumors (22). It is worth noting that due to existing limitations and uncertainties in the exact definition of the term HER2low, the ASCO–College of American Pathologists (CAP) does not recommend this term for the mentioned tumors. Instead, it is recommended to include a reporting footnote for such tumors that may be eligible for a treatment targeting non-amplified/non-overexpressed levels of HER2 expression for cytotoxic drug delivery (20).
2. HER2 positive early breast cancer: “from no hope to excellent prognosis in 20 years”
For decades, HER2 overexpressing early breast cancer (eBC) was associated with poor outcomes and higher mortality rates compared with other BC subtypes. However, following the discovery of HER2 receptor as a potential target for personalized therapy, the development of a specific antibody immediately improved the therapy options and prognosis of that subtype of BC. The benefit of trastuzumab in the setting of eBC was shown in several large, randomized trials (23, 24, 25) and a new standard in treatment of HER2 positive BC was set. FDA approval for trastuzumab was granted in the U.S. in 1998, making trastuzumab the first approved antibody for treatment of HER2 positive BC (
Table 1).
The second discovered and used monoclonal antibody was pertuzumab, which binds at a different epitope of the extracellular domain (26). Pertuzumab prevents HER2 from dimerization with other receptors from the EGFR/ErbB group, especially the HER3 receptor (27, 28). The most potent signaling heterodimer is considered to be the HER2/HER3 heterodimer, strongly promoting cell proliferation in HER2 positive cancer (28).
The NeoSphere phase II study showed that pertuzumab and trastuzumab administered together in combination with neoadjuvant chemotherapy significantly increased the rate of pathological complete response (pCR) in HER2 positive BC patients (29). Because of the binding to different HER2 epitopes, trastuzumab and pertuzumab have a complementary mechanism of action providing a more comprehensive blockade of HER2 signaling pathway and a greater antitumor activity than each antibody administered alone (30). The APHINITY trial was a phase III study that demonstrated that adjuvant pertuzumab in combination with trastuzumab and chemotherapy significantly improved invasive disease-free survival (iDFS) among patients with operable HER2 positive BC (31, 32).
While prognosis of BC has significantly improved since the introduction of HER2 targeted therapy, the risk of disease recurrence and death remained high in patients with residual invasive disease at surgery, especially when compared to those who achieved pCR through neoadjuvant chemotherapy (29, 33, 34, 35, 36). The ADC T-DM1 consists of trastuzumab and the microtubule inhibitor emtansine as a cytotoxic agent (37). After binding to the HER2 receptor, trastuzumab emtansine is internalized into the BC cell and undergoes proteolytic degradation, releasing the active cytotoxic agent emtansine and resulting in cell death (37). The efficacy of T-DM1 in the post-neoadjuvant setting was examined in the KATHERINE trial (38). 1,486 patients with HER2 positive BC and residual invasive disease in the breast or axilla after standard neoadjuvant treatment were randomly assigned to receive 14 cycles of either T-DM1 or trastuzumab. The final iDFS and updated OS analysis were recently presented at the San Antonio Breast Cancer Symposium (SABCS) 2023 (39). This analysis revealed a significantly improved OS after 7 years (89.1% in the T-DM1 arm vs. 84.4% in trastuzumab arm, hazard ratio [HR] 0.66; 95% CI 0.51, 0.87; p=0.0027). The final iDFS benefit after 7 years of T-DM1 was sustained in the intention-to-treat population (80.8% vs. 67.1%, HR 0.54, 95% CI 0.44, 0.66). While T-DM1 was associated with a higher incidence of adverse events (98.8% vs. 93.3%) and a higher rate of treatment discontinuation (18% vs. 2.1%), compared to trastuzumab, it is worth noting that the regimen is generally well tolerated and has a lower toxicity than conventional monochemotherapies. Based on the KATHERINE trial, T-DM1 was the first evidence-based post-neoadjuvant strategy to show improved clinical outcome in BC patients, leading to increased use of neoadjuvant therapy (39).
A different way to interact with the HER2 pathway is via acting on intracellular tyrosine kinase domains. Neratinib as a small molecule inhibits EGFR, HER2 and HER4 tyrosine kinase. Reduction of EGFR and HER2 autophosphorylation and downstream signaling results in growth reduction of EGFR and HER2 dependent cell lines. The binding of neratinib to the targeted kinase is irreversible (40). Compared to trastuzumab and pertuzumab where extracellular binding to the receptor occurs, neratinib exhibits its intracellular effects in BC cells which became resistant to trastuzumab treatment (41) or are co-activated through EGFR signaling as well (42). The ExteNET trial showed that neratinib administered for 1 year improved iDFS in HR-positive HER2 positive (sometimes referred to as “triple positive”) patient population after trastuzumab treatment (43).
3. Post-neoadjuvant therapy: time for new players?
One of the goals of modern cancer research is to find strategies that would effectively combat tumor cells with as little toxicity as possible to other rapidly dividing cells. Therefore, numerous trials over the past few years have focused on drugs designed to increase the transfer of cytotoxic compounds to cancer cells by using antibodies targeting antigens presented on those cells. In this context, ADCs are still a relatively new group of cancer-targeted drugs, of which only a small fraction have so far been tested in large clinical trials.
T-DM1 was the first representative of this group to be approved for treatment of a solid tumor. As an ADC, it consists of a cytotoxic agent (emtansine) and an antibody (trastuzumab) connected through a stable thioether linker. Earlier studies have shown that all three of these components (the antibody, linker, and cytotoxic payload), which make up the structure of ADCs, have a critical impact on therapy outcomes. Changing even just the linker itself results in significant differences in the pharmacokinetics, anticancer activity, and toxicity of the drug (37).
Besides T-DM1, there are two other ADCs currently approved for BC therapy in Europe. Trastuzumab deruxtecan (T-DXd) is an antibody against HER2 (trastuzumab), conjugated with a topoisomerase I inhibitor (deruxtecan, a derivative of exatecan) through tetrapeptide-based linker, approved for the treatment of HER2 positive as well as HER2-low metastatic BC. Sacituzumab govitecan, an antibody against Trop-2, conjugated with the active metabolite of irinotecan, SN-38, is currently approved for the treatment of metastatic triple-negative and HR-positive HER2 negative BC (45).
While T-DM1 remains the only approved post-neoadjuvant strategy for HER2 positive patients with non-pCR following neoadjuvant therapy, studies on the efficacy of new drugs in this setting are currently underway. The multicenter open-label randomized TruDy / DESTINY-Breast05 study aims to compare the efficacy of T-DXd with T-DM1 as post-neoadjuvant treatment in HER2 positive patients with invasive tumor rest (46). Similar to the KATHERINE study, patients included in this trial must have received at least 16 weeks of taxane and trastuzumab before surgery. The recruitment is scheduled to close in 2024. The rationale for this study were superior outcomes of T-DXd, compared to T-DM1, achieved in the metastatic setting. In the DESTINY-Breast03 study patients with HER2 positive metastatic BC were randomized to T-DXd vs. T-DM1 (47). The trial showed significantly longer OS and PFS in T-DXd arm (OS: HR 0,64; P=0,0037; 2-year-OS 77.4% vs. 69.9%; median PFS: 28.8 vs. 6.8 months, HR 0.33; P<0.0001).
While maintaining an optimistic approach to trials on further applications of the antibody-drug conjugates, it is important to keep in mind the potentially increased toxicity of these drugs, which goes hand in hand with enhanced antitumor activity. In case of T-DXd pulmonal toxicity is an adverse event of special interest and treatment-related deaths have been reported in earlier studies. In the DESTINY-Breast03 trial, more patients discontinued treatment in the T-DXd group (20% vs. 7% in the T-DM1 group), and more dose reductions (25 vs. 15%), as well as drug interruptions due to adverse events (42% vs. 17%) were observed in the T-DXd arm. The most frequent reasons for the discontinuation were pneumonitis, interstitial lung disease and pneumonia. The drug-related interstitial lung disease or pneumonitis occurred in 15% patients treated with T-DXd and only 3% patients treated with T-DM1 (47). However, no therapy-related deaths were reported, suggesting an improved toxicity management and the importance of learning curve, compared to previous studies.
4. Treatment de-escalation: who is “low risk”?
De-escalation is currently taking place both in surgical and systemic treatment of BC. In both the surgical and systemic therapy settings, it is essential to strike the right balance between benefits and side effects. This aims to improve the quality of life while maintaining a high level of oncological safety and, at the same time, reducing treatment-related morbidity. The introduction of sentinel lymph node biopsy is considered a milestone in de-escalating surgical interventions for BC patients. Currently, there are varying guideline recommendations regarding the omission of sentinel lymph node biopsy (SLNB) in older patients with low-risk tumors (48, 49). Additionally, one of the most controversially discussed settings in surgical treatment remains the optimal approach for patients who initially had a positive node status but achieved a complete axillary response (cN+ → ycN0) after neoadjuvant chemotherapy (50, 51, 52).
The right balance between appropriate systemic therapy and avoiding unnecessary side effects to enhance the quality of life is particularly important in the case of low-risk tumors. Especially in case of small, node-negative HER2 positive eBC, it remains a challenge for clinicians to establish the safest and most efficient treatment plan while considering the significant potential for toxic side effects associated with chemotherapy and HER2 targeted therapy, and the generally excellent prognosis. While several trials demonstrated improved clinical outcomes in patients treated with trastuzumab and multiagent chemotherapy, it is worth noting that the number of patients with small node-negative tumors was very low (23, 24, 25, 53).
The open-label single-arm, multicenter, phase II Adjuvant Paclitaxel and Trastuzumab (APT) trial aimed at addressing this issue (54). The study enrolled 406 patients with small (≤ 3 cm) HER2 positive eBC between 2007 and 2010. All patients received primary surgery and were either node-negative or had micrometastatic nodal involvement (N1mi). The adjuvant treatment regimen consisted of weekly paclitaxel at 80 mg/m² and trastuzumab at a loading dose of 4 mg/kg followed by 2 mg/kg weekly for 12 weeks, followed by trastuzumab weekly at 2 mg/kg or once every 3 weeks at 6 mg/kg to complete a full year of treatment. Patients who underwent lumpectomy received radiation therapy after completing the 12-week chemotherapy, while those with hormone receptor positive HER2 positive (“triple-positive”) tumors subsequently received endocrine therapy (54). Side effects (e.g., cardiac toxicity) was minimal and notably lower compared to anthracycline-containing regimens in other studies. However, a head-to-head comparison of the de-escalated treatment from the APT study and other regimens is not available. In 2023, the 10-year follow-up analysis indicated an iDFS rate of 91.3% (95% CI 88.3 - 94.4). In patients with HR-positive disease, iDFS rate was 91.6% (95% CI 88.0; 95.4) and in HR negative tumors 90.6% (95% CI 85.1; 96.4). 10-year recurrence-free survival (RFS) was 96.3% (95% CI 94.3; 98.3; in HR positive tumors 96.2% [95% CI 93.8; 98.7] and in HR negative disease 96.4% [95% CI 93.0;99.9]). 10-year-OS rate was 94.3% (95% CI 91.8;96.8), and 10-year BC-specific survival rate was 98.8% (95% CI 97.6;100) (55). These longer follow-up results showing a favorable prognosis indicate that adjuvant paclitaxel and trastuzumab represent a reasonable treatment standard and it has been widely adopted as the recommended therapeutic approach for patients with node-negative HER2 positive eBC and incorporated into national and international guidelines, such as the NCCN (56), ESMO (57), St. Gallen International Consensus (58) and AGO Breast Committee (LoE 2b/B/AGO+) (59). Since only a small percentage of patients (8.9%) had a tumor size between 2 and 3 cm and/or N1mi disease (1.5%), some guidelines considered the APT regimen as suitable only for node-negative patients with a tumor size not exceeding 2 cm (
Figure 1).
However, the single-arm study design should be subject to critical discussion. In principle, randomized controlled trials are considered the gold standard to assess long-term outcomes or treatment effects. On the other hand, due to favorable prognosis of patients with low-risk HER2 positive BC, conducting a randomized trial in this setting may be challenging. It has been estimated that it would probably require a non-inferiority trial involving a cohort of more than 1000 patients to reliably compare de-escalated therapy with standard treatment (61). On the other hand, adjuvant clinical trials involving much higher patient numbers such as TAILORx (62), RxPONDER (63), monarchE (64), NATALEE (65) and OlympiA (66) have been successfully conducted in the last decade. It is important to consider and to discuss further limitations: approx. 20% of the recruited patients hat a tumor size < 0.5 cm, making them eligible to omit any systemic therapy whatsoever (60). Additionally, two-thirds of the recruited patients had HR positive disease, which is already associated with a favorable prognosis, and received endocrine therapy as part of their systemic therapy. Indeed, the iDFS rate in patients with HR negative tumors was lower compared to HR positive tumors, with a total of 10 iDFS events observed in the HR negative cohort (vs. 19 events in the HR positive group)
Without doubt, the main critical issue regarding the APT trial is its adjuvant design. Due to the necessity of primary surgical therapy to adequately assess the nodal status and tumor size, patients are not eligible for neoadjuvant therapy, and it remains unknown, whether they might have benefited from a post-neoadjuvant strategy in case of non-pCR. On the other hand, those diagnosed with either sentinel lymph node metastasis (cN0 / pN1) or a tumor larger than 2 cm (cT1 / pT2) are usually recommended a polychemotherapy with one or two antibodies. Therefore, optimal pre-therapeutic diagnostic workup including high-quality ultrasound of the axilla seems crucial to identify patients who are at risk of pathological upstaging. In this context, a potential surgical de-escalation e.g., with omittance of sentinel node biopsy in low-risk patients requires a critical multidisciplinary discussion (67). Otherwise, a metastatic sentinel node in a patient deemed cN0 who is in fact pN+ could remain undiagnosed with the patient proceeding to receive de-escalated systemic therapy (APT regimen). Such simultaneous de-escalation of both surgical and systemic therapy might potentially lead to reduced prognosis. Further, if the initially low-risk HER2 positive tumor becomes high-risk postoperatively, the treatment concept of post-neoadjuvant therapy becomes unfeasible. In patients with HER2 positive early-stage BC who retain residual invasive disease following standard neoadjuvant treatment, the prognosis is notably less favorable compared to those who achieved a pCR, and those receiving post-neoadjuvant T-DM1 in this setting show significantly improved iDFS and OS (39).
One of the ongoing trials on de-escalation of therapy is DECRESCENDO (68). In this single-arm phase II study 1065 patients with HR negative HER2 positive eBC will receive neoadjuvant taxane with trastuzumab plus pertuzumab for 12 weeks. After surgery, the patients who achieved pCR will receive 14 additional cycles of dual HER2 blockade and patients with non-pCR will be treated with T-DM1 +/- anthracycline-based post-neoadjuvant chemotherapy. Another study with a similar design is the CompassHER2 phase II trial conducted by the ECOG-ACRIN Cancer Research Group (NCT04266249).
While the APT, DECRESCENDO and CompassHER2 studies explore a de-escalation of chemotherapy, other trials focused on the duration of HER2 targeted treatment. The ShortHER phase III trial was designed to assess whether a shorter trastuzumab course is non-inferior to a conventional one-year course (69). 1254 patients were randomized to a 9-week weekly administration of adjuvant trastuzumab in combination with 3 cycles of docetaxel, followed by 3 cycles of FEC chemotherapy vs. 4 cycles of doxorubicin and cyclophosphamide, followed by 4 cycles of a docetaxel, along with trastuzumab, continued for a total duration of one year. 672 (54%) patients were node-negative, 383 (30%) had 1-3 positive nodes and 198 (16%) had 4 or more metastatic nodes. The recent follow-up analysis reported a 10-year-DFS of 77% in the long arm and 78% in the short arm (HR 1.06; 90%CI 0.86-1.31) and 10-year-OS of 89% in the long and 88% in the short arm (HR 1.15; 90% CI 0.85-1.56) (70). Despite these comparable long-term outcomes, ShortHER failed to demonstrate non-inferiority of shorter treatment duration. Therefore, one-year course of trastuzumab remains standard treatment. However, it is worth noting that survival differences in patients node-negative(10-year-DFS: 81% in the long arm vs. 85% in the short arm, HR 0.74, 90% CI 0.54; 1.04; 10y-OS long arm 89%; short arm 95% HR 0.57 90% CI 0.33; 0.99) and with 1-3 positive nodes (10-year-DFS: 77% in the long arm vs. 79% in the short arm, HR 1.11, 90% CI 0.76; 1.64; 10y-OS long arm 92%; short arm 89% HR 1.37 90% CI 0.77; 2.44) were minimalwhile patients with four or more metastatic nodes clearly benefitted from a one-year trastuzumab treatment (10y-DFS long arm: 63%; short arm: 53% HR 1.84 90%CI 1.24; 2.75; 10y-OS long arm: 84%; short arm 64% HR1.87 90%CI 1.11; 3.14) (69).
Several trials explored a 6-month duration of trastuzumab treatment. At the ESMO Congress 2021, a meta-analysis was presented that included data from five randomized non-inferiority studies (71). PHARE (72), HORG (73) und PERSEPHONE (74) examined the outcomes of 6 vs. 12 months of adjuvant trastuzumab (n = 7950), while SOLD and ShortHER compared 9 weeks with 12 months (n = 3428). In the trials comparing 12 vs. 6 months, the 5-year-iDFS was 89.3% (12 months) and 88.6% (6 months; HR 1.07 90% CI 0.98-1.17; p = 0.002). The non-inferiority limit (HR 1.2) was formally met, leading the authors to conclude a non-inferiority of a 6-month trastuzumab regimen (71).
While these studies did not change our treatment standard, they showed that patients are likely to derive most benefit from trastuzumab in the first weeks / months of therapy. Therefore, when low- or intermediate-risk patients need to discontinue treatment due to toxicity, the results of these trials may be used to provide reassurance. More importantly, they may open the door to a more affordable treatment option for the many patients worldwide who cannot afford the high cost of a full year of trastuzumab.
5. Chemo-free therapy: ready for prime time?
In the sense of de-escalation, omitting non-targeted cytotoxic chemotherapy altogether is often hailed as the ultimate goal of oncological research. In this context, chemotherapy-free regimens have recently been evaluated in clinical trials such as WSG ADAPT, PherGAIN, ATEMT or WSG ADAPT HER2-IV (
Table 2). The introduction of immunotherapy, ADCs and tyrosine kinase inhibitors have considerably broadened the spectrum of potential therapeutic strategies.
While different clinical settings are currently under investigation, the majority of trials focused on de-escalated neoadjuvant treatment and used pCR as a surrogate endpoint. Some of these studies have been conducted within the ADAPT umbrella trial designed by the German WSG Group. The non-inferiority study WSG-ADAPT-HER2+/HR- investigated the efficacy of chemo-free neoadjuvant regimen trastuzumab/pertuzumab, compared to paclitaxel plus trastuzumab/pertuzumab in patients with HR negative HER2 positive eBC (75). The duration of treatment was 12 weeks in both arms. 36% of patients in the chemotherapy-free cohort achieved a pCR, compared to 91% in the paclitaxel plus trastuzumab/pertuzumab group. After a median follow-up of 60 months, there were numerical but not significant differences between the treatment groups regarding survival endpoints (5-year-iDFS: 98% [95% CI 84–100] in the chemotherapy group vs. 87% [95% CI 78–93] in the chemo-free group, HR 0.32, 95% CI 0.07–1.49; p = 0.15; relapse-free survival: 98% [95% CI 84–100] vs. 89% [95% CI 79–94], HR 0.41, 95% CI 0.09–1.91; p = 0.25); distant DFS: 98% [95% CI 84–100] vs. 92% [95% CI 83–96], HR 0.35, 95% CI 0.04–3.12; p = 0.36], OS: 98% [95% CI 84–100] vs. 94% [95% CI 86–97], HR 0.41, 95% CI 0.05–3.63; p = 0.43) (75). Interestingly, only two iDFS events occurred in patients achieving pCR (one in each arm), demonstrating that omission of further chemotherapy did not affect iDFS in patients with a pCR after de-escalated chemo-free neoadjuvant regimen. Since clinical outcomes were better after chemotherapy-containing treatment, identifying patients most likely to achieve pCR after chemo-free therapy remains challenging. Parameters that might be helpful in this context are among others the molecular subtype and immunohistochemical HER2 expression (75). Indeed, Graeser et al. were able to show that distinct gene signatures were associated with pCR versus iDFS and that patients with upregulated immune response signatures could be suitable candidates for de-escalation concepts in HR negative HER2 positive eBC (76).
The combination of neoadjuvant HER2 targeted therapy with endocrine treatment in patients with HR positive HER2 positive tumors has been explored in the WSG-TP-II study (77). In this trial, 207 patients were randomized to 12 weeks of dual HER2 blockade (trastuzumab / pertuzumab) in combination with either paclitaxel or endocrine therapy. pCR rate was significantly lower in the chemo-free group (23.7% vs. 56.4%). Interestingly, HER2 messenger RNA levels were associated with tumor response and in patients with the highest quartile by HER2 messenger RNA pCR rates were comparable in both arms, suggesting that this assay may help to identify patients more likely to respond well to combined endocrine and anti-HER2 therapy.
Another approach to optimized patient selection was tested in the PHERGain trial. Here, imaging tools were used to identify patients who are likely to benefit from de-escalated neoadjuvant treatment (78, 79). 356 patients with HER2 positive tumors were randomly allocated to 2 cycles of conventional TCHP regimen (docetaxel / carboplatin / trastuzumab / pertuzumab) vs. chemo-free trastuzumab / pertuzumab, combined with endocrine treatment in triple-positive patients. Early metabolic response was evaluated by [¹⁸F]FDG-PET at baseline and after 2 cycles. Patients in the standard arm continued to receive TCHP for further 4 cycles. In the experimental arm, responders received further 6 cycles of chemo-free treatment while non-responders (approx. 20% of patients) were switched to 6 courses of TCHP. 38% of early responders achieved pCR after 8 courses of chemo-free neoadjuvant therapy and these patients had excellent clinical outcomes with 3-year-iDFS of 98.8%. However, when all patients in the experimental arm were considered, the iDFS was lower than in the standard arm (95.4% vs. 98.3%), despite the fact that all patients with non-pCR after chemo-free therapy were recommended 6 courses of TCHP after surgery. The study is ongoing and OS data pending but nevertheless PET imaging seems to be a promising tool to distinguish responders from non-responders (78, 79).
Potential limitations of chemotherapy-free regimes must be considered, especially in the setting of advanced stages of disease. The KRISTINE study randomized patients with stage II-III HER2 positive tumors to a combination of T-DM1 plus pertuzumab vs. docetaxel, carboplatin, trastuzumab plus pertuzumab (80, 81). Patients allocated to T-DM1 plus pertuzumab continued the same treatment after surgery, and patients who received TCHP received adjuvant trastuzumab / pertuzumab. pCR rates were lower in the experimental arm (44.4%), compared to TCHP (55.7%, P = 0.016). After a median follow-up of 37 months, the risk of an EFS event was higher with T-DM1 plus pertuzumab (HR 2.61, 95% CI 1.36-4.98) and more locoregional progressions were observed before surgery (6.7% vs. 0%). iDFS rates after surgery were similar between arms (HR 1.11, 95% CI 0.52-2.40). While grade ≥ 3 adverse events were less common with T-DM1 plus pertuzumab (31.8% vs. 67.7%), toxicity leading to treatment discontinuation after surgery occurred more frequently in the T-DM1 plus pertuzumab arm (18.4% vs. 3.8%).
Current ongoing studies investigate further questions such as the comparison of neoadjuvant T-DXd vs. chemotherapy plus dual HER2 blockade (trastuzumab / pertuzumab). The ADAPT-HER2-IV will randomized patients with low- to intermediate-risk HER2 positive tumors to 12 weeks of neoadjuvant T-DXd vs. paclitaxel plus trastuzumab / pertuzumab) and those with intermediate- to high-risk tumors to 18 weeks of T-DXd vs. conventional neoadjuvant chemotherapy with dual HER2 blockade. The trial is designed as superiority trial to demonstrate higher pCR rates in both clinically relevant subgroups treated by T-DXd. Recruitment began in 2023.
Even though the concept of chemotherapy-free regimens seems likely to become an option at least for some carefully selected patients, survival data from most trials are still pending. Especially ADCs appear to be promising candidates for a de-escalated treatment due to their favorable safety and enhanced efficacy (82). However, optimal duration, dosage and combination partners for an ADC-based neoadjuvant therapy remain to be cleared.
6. Conclusions
In recent decades, an increasing number of targeted oncological therapeutics has been approved for use based on molecular parameters such as receptor expression, amplification, and mutation status. Due to the introduction of numerous targeted anti-HER2 strategies, HER2 positive breast carcinoma, previously considered an entity with a particularly poor prognosis, became a well-treatable disease and one of the subtypes with best clinical outcomes. This groundbreaking medical development began with approval of trastuzumab following the pivotal study by Slamon et al. in the year 2000 and marked the beginning of a new era in targeted tumor therapy. The gold standard for HER2 positive BC with residual invasive disease following neoadjuvant therapy is T-DM1 administered in the post-neoadjuvant setting. The most recent survival data presented at the SABCS 2023 showed that T-DM1 improves OS in these high-risk patients (39).
It remains to be seen whether the data from the TruDy (Destiny Breast 05) study, a head-to-head comparison of T-DM1 and another ADC T-DXd in case of non-pCR will challenge this standard of care, potentially establishing a role for T-DXd in the post-neoadjuvant setting in the future (46). Currently, T-DXd is only approved for HER2 positive and HER2 low metastatic BC (22, 47). However, medical history demonstrates that new therapeutic options usually prove their efficacy in advanced stages before being introduced into the (neo-)adjuvant treatment. Early clinical management in dealing with new side effects is considered essential, as potentially life-threatening ADC-mediated complications must be approached with particular caution in the curative setting.
Therapeutic de-escalation is an important and forward-looking approach in oncology, with an increasing significance in the field of both surgical senology and systemic therapy for patients with BC. The right balance between appropriate systemic therapy and avoiding morbidity to enhance the quality of life is particularly important in the case of low-risk tumors. In this context, the “APT regimen”, i.e., 12 weeks of paclitaxel in combination with a 1-year course of trastuzumab, has been widely adopted in the global guidelines as standard of care for low-risk patients, even if the main critical issue remains the absence of the option for post-neoadjuvant therapy, if the initially low-risk HER2 positive tumor becomes a high-risk tumor postoperatively (54, 55, 85) (56, 57, 58, 59).
One of the most exciting opportunities created by the introduction of targeted therapy is the potential of limiting chemotherapy-related toxicity. Several ongoing studies, including WSG ADAPT umbrella trials (75), PherGAIN (78), ATEMPT (84), and WSG ADAPT HER2-IV (NCT05704829) are currently assessing chemo-free regimens as part of strategies aimed at de-escalating therapy in the field of oncology. Despite promising early results from these studies, combination of anti-HER2 treatment with chemotherapy backbone remains standard of care.
The evolution of HER2 directed therapy in BC is a success story and achieving a curative treatment even for HER2 metastatic BC is no longer unthinkable. The advent of innovative new therapeutics highlights the importance of effectively managing novel spectrums of side effects. Additionally, the question of the combination and sequence of new therapeutics in various treatment lines remains yet to be clarified.
Funding
This research received no external funding.
Conflicts of Interest
Maggie Banys-Paluchowski received honoraria for lectures and participation in advisory boards: Roche, Novartis, Pfizer, pfm, Eli Lilly, Onkowissen, Seagen, AstraZeneca, Eisai, Amgen, Samsung, Canon, MSD, GSK, Daiichi Sankyo, Gilead, Sirius Medical, Syantra, resitu, Pierre Fabre, ExactSciences; Study support from: EndoMag, Mammotome, MeritMedical, Sirius Medical, Gilead, Hologic, ExactSciences; Travel reimbursement: Eli Lilly, ExactSciences, Pierre Fabre, Pfizer, Daiichi Sankyo, Roche. She is a member of AGO Breast Committee and S3 guideline expert panel. Achim Rody has received lecture and consulting honoraria from Roche, Pfizer, Novartis, Celgen, Novartis, ExactSciences, Pierre Fabre, Lilly, Seagen, Astra Zeneca, Eisai, MSD, Hexal, Amgen. He is a member of AGO Breast Committee and S3 guideline expert panel. The other authors declare no conflicts of interest.
References
- Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022;66:15-23. [CrossRef]
- Shih C, Padhy LC, Murray M, Weinberg RA. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature. 1981;290(5803):261-4. [CrossRef]
- Williams CL., H. Michael Shepard, Dennis J. Slamon, and Axel Ullrich honored with the 2019 Lasker~DeBakey Clinical Medical Research Award. J Clin Invest. 2019;129(10):3963-5. [CrossRef] [PubMed]
- Yarden, Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61 Suppl 2:1-13. [CrossRef] [PubMed]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127-37. [CrossRef] [PubMed]
- King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985;229(4717):974-6. [CrossRef] [PubMed]
- Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24(2):85-95. [CrossRef] [PubMed]
- Reese DM, Slamon DJ. HER-2/neu signal transduction in human breast and ovarian cancer. Stem Cells. 1997;15(1):1-8. [CrossRef] [PubMed]
- Weigelt B, Reis-Filho JS. Activating mutations in HER2: neu opportunities and neu challenges. Cancer Discov. 2013;3(2):145-7. [CrossRef]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177-82. [CrossRef]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707-12. [CrossRef]
- Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, et al. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117(8):2051-8. [CrossRef]
- Badache A, Gonçalves A. The ErbB2 signaling network as a target for breast cancer therapy. J Mammary Gland Biol Neoplasia. 2006;11(1):13-25. [CrossRef]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783-92. [CrossRef]
- Marczyk VR, Rosa DD, Maia AL, Goemann IM. Overall Survival for HER2-Positive Breast Cancer Patients in the HER2-Targeted Era: Evidence From a Population-Based Study. Clin Breast Cancer. 2022;22(5):418-23. [CrossRef] [PubMed]
- Goldenberg, MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther. 1999;21(2):309-18. [CrossRef] [PubMed]
- Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18(6):977-84. [CrossRef] [PubMed]
- Guimaraes Koch SS, Thorpe R, Kawasaki N, Lefranc MP, Malan S, Martin ACR, et al. International nonproprietary names for monoclonal antibodies: an evolving nomenclature system. MAbs. 2022;14(1):2075078. [CrossRef] [PubMed]
- Wolff AC, Hammond MEH, Allison KH, Harvey BE, McShane LM, Dowsett M. HER2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update Summary. J Oncol Pract. 2018;14(7):437-41. [CrossRef]
- Wolff AC, Somerfield MR, Dowsett M, Hammond MEH, Hayes DF, McShane LM, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO–College of American Pathologists Guideline Update. Journal of Clinical Oncology. 2023;41(22):3867-72. [CrossRef]
- Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J Clin Oncol. 2020;38(17):1951-62. [CrossRef]
- Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med. 2022;387(1):9-20. [CrossRef]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr., Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673-84. [CrossRef] [PubMed]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659-72. [CrossRef] [PubMed]
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273-83. [CrossRef]
- Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109-19. [CrossRef] [PubMed]
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9(7):463-75. [CrossRef]
- Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127-37. [CrossRef] [PubMed]
- Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25-32. [CrossRef]
- Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68(14):5878-87. [CrossRef]
- von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017;377(2):122-31. [CrossRef]
- Piccart M, Procter M, Fumagalli D, de Azambuja E, Clark E, Ewer MS, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer in the APHINITY Trial: 6 Years’ Follow-Up. J Clin Oncol. 2021;39(13):1448-57. [CrossRef]
- Untch M, Fasching PA, Konecny GE, Hasmüller S, Lebeau A, Kreienberg R, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29(25):3351-7. [CrossRef]
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-72. [CrossRef]
- de Azambuja E, Holmes AP, Piccart-Gebhart M, Holmes E, Di Cosimo S, Swaby RF, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15(10):1137-46. [CrossRef]
- Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Waldron-Lynch M, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer. 2018;89:27-35. [CrossRef] [PubMed]
- Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280-90. [CrossRef] [PubMed]
- von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380(7):617-28. [CrossRef] [PubMed]
- Sibylle Loibl MM, Michael Untch, Chiun-Sheng Huang, Eleftherios Mamounas, Norman Wolmark, Adam Knott, Asna Siddiqui, Thomas Boulet, Beatrice Nyawira, Eleonora Restuccia, Charles Geyer Jr. Phase III study of adjuvant ado-trastuzumab emtansine vs trastuzumab for residual invasive HER2-positive early breast cancer after neoadjuvant chemotherapy and HER2-targeted therapy: KATHERINE final IDFS and updated OS analysis. San Antonio Breast Cancer Symposium; December 5-9, 2023; Texas2023. 5 December.
- Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64(11):3958-65. [CrossRef]
- Xia W, Liu LH, Ho P, Spector NL. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene. 2004;23(3):646-53. [CrossRef]
- Rampaul RS, Pinder SE, Nicholson RI, Gullick WJ, Robertson JF, Ellis IO. Clinical value of epidermal growth factor receptor expression in primary breast cancer. Adv Anat Pathol. 2005;12(5):271-3. [CrossRef]
- Chan A, Moy B, Mansi J, Ejlertsen B, Holmes FA, Chia S, et al. Final Efficacy Results of Neratinib in HER2-positive Hormone Receptor-positive Early-stage Breast Cancer From the Phase III ExteNET Trial. Clin Breast Cancer. 2021;21(1):80-91.e7. [CrossRef]
- Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195-205. [CrossRef]
- Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. New England Journal of Medicine. 2021;384(16):1529-41. [CrossRef]
-
Geyer CE, Jr., Untch M, Prat A, Rastogi P, Niikura N, Mathias E, et al. Abstract OT1-02-03: Trastuzumab deruxtecan (T-DXd; DS-8201) vs trastuzumab emtansine (T-DM1) in high-risk patients with HER2-positive, residual invasive early breast cancer after neoadjuvant therapy: A randomized, phase 3 trial (DESTINY-Breast05). Cancer Research. 2022;82(4_Supplement):OT1-02-3-OT1--3.
- Hurvitz SA, Hegg R, Chung WP, Im SA, Jacot W, Ganju V, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401(10371):105-17. [CrossRef] [PubMed]
- Brackstone M, Baldassarre FG, Perera FE, Cil T, Chavez Mac Gregor M, Dayes IS, et al. Management of the Axilla in Early-Stage Breast Cancer: Ontario Health (Cancer Care Ontario) and ASCO Guideline. J Clin Oncol. 2021;39(27):3056-82. [CrossRef] [PubMed]
- Banys-Paluchowski M, Thill M, Kuhn T, Ditsch N, Heil J, Wockel A, et al. AGO Recommendations for the Surgical Therapy of Breast Cancer: Update 2022. Geburtshilfe Frauenheilkd. 2022;82(10):1031-43. [CrossRef]
- Tauber N, Bjelic-Radisic V, Thill M, Banys-Paluchowski M. Controversies in axillary management of patients with breast cancer - updates for 2024. Curr Opin Obstet Gynecol. 2023. [CrossRef]
- Banys-Paluchowski M, Untch M, Krawczyk N, Thurmann M, Kühn T, Sehouli J, et al. Current trends in diagnostic and therapeutic management of the axilla in breast cancer patients receiving neoadjuvant therapy: results of the German-wide NOGGO MONITOR 24 survey. Arch Gynecol Obstet. 2023;307(5):1547-56. [CrossRef] [PubMed]
- Alcaide SM, Diana CAF, Herrero JC, Vegue LB, Perez AV, Arce ES, et al. Can axillary lymphadenectomy be avoided in breast cancer with positive sentinel lymph node biopsy? Predictors of non-sentinel lymph node metastasis. Arch Gynecol Obstet. 2022;306(6):2123-31. [CrossRef] [PubMed]
- Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, de Azambuja E, Procter M, Suter TM, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382(9897):1021-8. [CrossRef]
- Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134-41. [CrossRef]
- Tolaney SM, Tarantino P, Graham N, Tayob N, Parè L, Villacampa G, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24(3):273-85. [CrossRef]
- Giordano SH, Elias AD, Gradishar WJ. NCCN Guidelines Updates: Breast Cancer. J Natl Compr Canc Netw. 2018;16(5s):605-10. [CrossRef]
- Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(10):1674. [CrossRef]
- Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32(10):1216-35. [CrossRef]
- Park-Simon T-W, Müller V, Jackisch C, Albert U-S, Banys-Paluchowski M, Bauerfeind I, et al. Arbeitsgemeinschaft Gynäkologische Onkologie Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2023. Breast Care. 2023;18(4):289-305. [CrossRef]
- Park-Simon TW, Müller V, Jackisch C, Albert US, Banys-Paluchowski M, Bauerfeind I, et al. Arbeitsgemeinschaft Gynäkologische Onkologie Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2023. Breast Care (Basel). 2023;18(4):289-305. [CrossRef]
- Amiri-Kordestani L, Xie D, Tolaney SM, Bloomquist E, Tang S, Ibrahim A, et al. A Food and Drug Administration analysis of survival outcomes comparing the Adjuvant Paclitaxel and Trastuzumab trial with an external control from historical clinical trials. Annals of Oncology. 2020;31(12):1704-8. [CrossRef]
- Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. New England Journal of Medicine. 2018;379(2):111-21. [CrossRef] [PubMed]
- Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, et al. 21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. New England Journal of Medicine. 2021;385(25):2336-47. [CrossRef] [PubMed]
- Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol. 2020;38(34):3987-98. [CrossRef] [PubMed]
- Slamon DJ, Fasching PA, Hurvitz S, Chia S, Crown J, Martín M, et al. Rationale and trial design of NATALEE: a Phase III trial of adjuvant ribociclib + endocrine therapy versus endocrine therapy alone in patients with HR+/HER2- early breast cancer. Ther Adv Med Oncol. 2023;15:17588359231178125.
- Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. New England Journal of Medicine. 2021;384(25):2394-405. [CrossRef] [PubMed]
- Gentilini OD, Botteri E, Sangalli C, Galimberti V, Porpiglia M, Agresti R, et al. Sentinel Lymph Node Biopsy vs No Axillary Surgery in Patients With Small Breast Cancer and Negative Results on Ultrasonography of Axillary Lymph Nodes: The SOUND Randomized Clinical Trial. JAMA Oncol. 2023;9(11):1557-64. [CrossRef]
- Debien V, Adam V, Caparica R, Fumagalli D, Velghe C, Gaye J, et al. DECRESCENDO: De-escalation of adjuvant chemotherapy in patients with HER2+/HR-/node-negative early breast cancer who achieve pCR after neoadjuvant taxane and subcutaneous dual anti-HER2 blockade. Journal of Clinical Oncology. 2022;40(16_suppl):TPS621-TPS.
- Conte P, Bisagni G, Piacentini F, Sarti S, Minichillo S, Anselmi E, et al. Nine-Week Versus One-Year Trastuzumab for Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: 10-Year Update of the ShortHER Phase III Randomized Trial. J Clin Oncol. 2023;41(32):4976-81. [CrossRef]
- Conte PF, Bisagni G, Piacentini F, Sarti S, Minichillo S, Anselmi E, et al. Nine-weeks versus one-year trastuzumab for early-stage HER2+ breast cancer: 10-year update of the Short-HER phase III randomized trial. Journal of Clinical Oncology. 2023;41(17_suppl):LBA637-LBA. [CrossRef]
- Earl HM, Hiller L, Dunn JA, Conte PF, D’Amico R, Guarneri V, et al. LBA11 Individual patient data meta-analysis of 5 non-inferiority RCTs of reduced duration single agent adjuvant trastuzumab in the treatment of HER2 positive early breast cancer. Annals of Oncology. 2021;32:S1283. [CrossRef]
- Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet. 2019;393(10191):2591-8. [CrossRef] [PubMed]
- Mavroudis D, Saloustros E, Malamos N, Kakolyris S, Boukovinas I, Papakotoulas P, et al. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol. 2015;26(7):1333-40. [CrossRef] [PubMed]
- Earl HM, Hiller L, Vallier A-L, Loi S, McAdam K, Hughes-Davies L, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. The Lancet. 2019;393(10191):2599-612. [CrossRef]
- Nitz U, Gluz O, Graeser M, Christgen M, Kuemmel S, Grischke EM, et al. De-escalated neoadjuvant pertuzumab plus trastuzumab therapy with or without weekly paclitaxel in HER2-positive, hormone receptor-negative, early breast cancer (WSG-ADAPT-HER2+/HR-): survival outcomes from a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2022;23(5):625-35. [CrossRef]
- Graeser M, Gluz O, Biehl C, Ulbrich-Gebauer D, Christgen M, Palatty J, et al. Impact of RNA Signatures on pCR and Survival after 12-Week Neoadjuvant Pertuzumab plus Trastuzumab with or without Paclitaxel in the WSG-ADAPT HER2+/HR- Trial. Clin Cancer Res. 2023;29(4):805-14. [CrossRef]
- Gluz O, Nitz UA, Christgen M, Kuemmel S, Holtschmidt J, Schumacher J, et al. Efficacy of Endocrine Therapy Plus Trastuzumab and Pertuzumab vs De-escalated Chemotherapy in Patients with Hormone Receptor-Positive/ERBB2-Positive Early Breast Cancer: The Neoadjuvant WSG-TP-II Randomized Clinical Trial. JAMA Oncol. 2023;9(7):946-54. [CrossRef]
- Pérez-García JM, Gebhart G, Ruiz Borrego M, Stradella A, Bermejo B, Schmid P, et al. Chemotherapy de-escalation using an (18)F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): a multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021;22(6):858-71. [CrossRef]
- Cortes J, Pérez-García JM, Ruiz-Borrego M, Stradella A, Bermejo B, Escrivá-de-Romaní S, et al. 3-year invasive disease-free survival (iDFS) of the strategy-based, randomized phase II PHERGain trial evaluating chemotherapy (CT) de-escalation in human epidermal growth factor receptor 2-positive (HER2[+]) early breast cancer (EBC). Journal of Clinical Oncology. 2023;41(17_suppl):LBA506-LBA. [CrossRef]
- Hurvitz SA, Martin M, Symmans WF, Jung KH, Huang CS, Thompson AM, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19(1):115-26. [CrossRef] [PubMed]
- Hurvitz SA, Martin M, Jung KH, Huang CS, Harbeck N, Valero V, et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes From the Phase III KRISTINE Study. J Clin Oncol. 2019;37(25):2206-16. [CrossRef] [PubMed]
- Matikas A, Johansson H, Grybäck P, Bjöhle J, Acs B, Boyaci C, et al. Survival Outcomes, Digital TILs, and On-treatment PET/CT During Neoadjuvant Therapy for HER2-positive Breast Cancer: Results from the Randomized PREDIX HER2 Trial. Clin Cancer Res. 2023;29(3):532-40. [CrossRef] [PubMed]
- Nitz UA, Gluz O, Christgen M, Grischke EM, Augustin D, Kuemmel S, et al. De-escalation strategies in HER2-positive early breast cancer (EBC): final analysis of the WSG-ADAPT HER2+/HR- phase II trial: efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab ± weekly paclitaxel. Ann Oncol. 2017;28(11):2768-72.
- Tolaney SM, Tayob N, Dang C, Yardley DA, Isakoff SJ, Valero V, et al. Adjuvant Trastuzumab Emtansine Versus Paclitaxel in Combination With Trastuzumab for Stage I HER2-Positive Breast Cancer (ATEMPT): A Randomized Clinical Trial. Journal of Clinical Oncology. 2021;39(21):2375-85. [CrossRef]
- Tolaney SM, Guo H, Pernas S, Barry WT, Dillon DA, Ritterhouse L, et al. Seven-Year Follow-Up Analysis of Adjuvant Paclitaxel and Trastuzumab Trial for Node-Negative, Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer. J Clin Oncol. 2019;37(22):1868-75. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).