Submitted:
04 January 2024
Posted:
05 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Samples Synthesis
2.2. Materials Characterization
2.3. Electrochemical Measurements
2.4. Thermal Stability Test
3. Results and Discussion
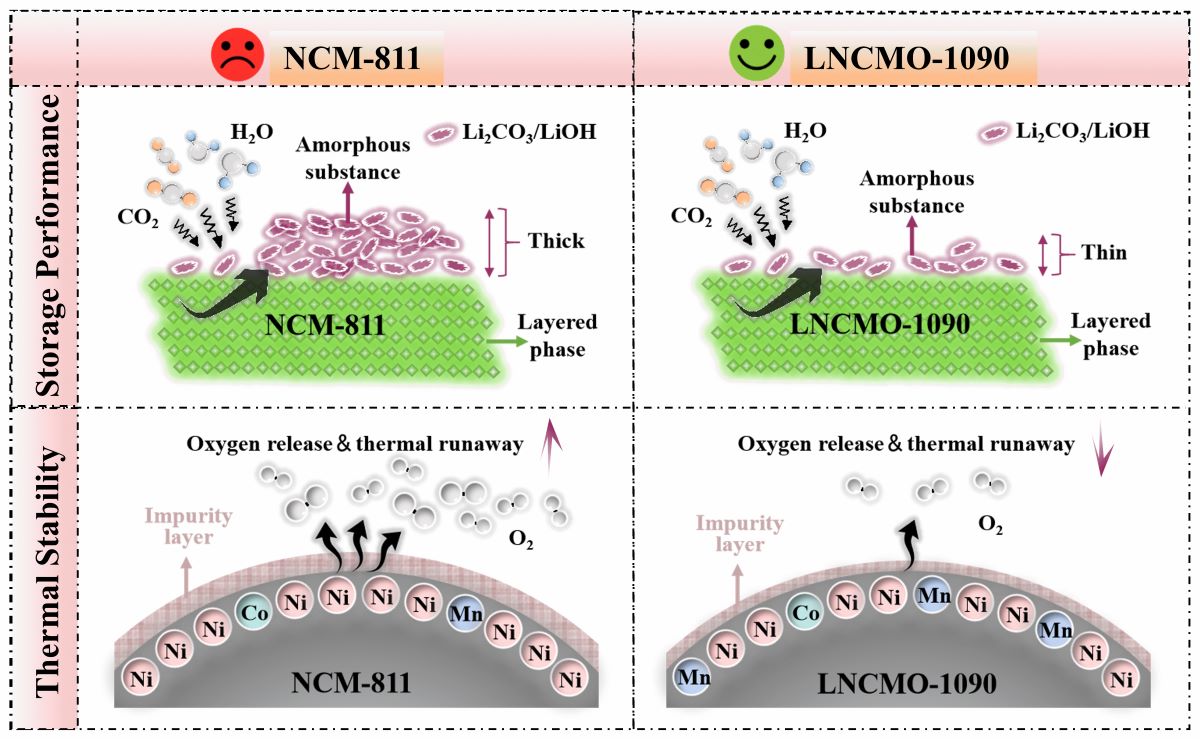
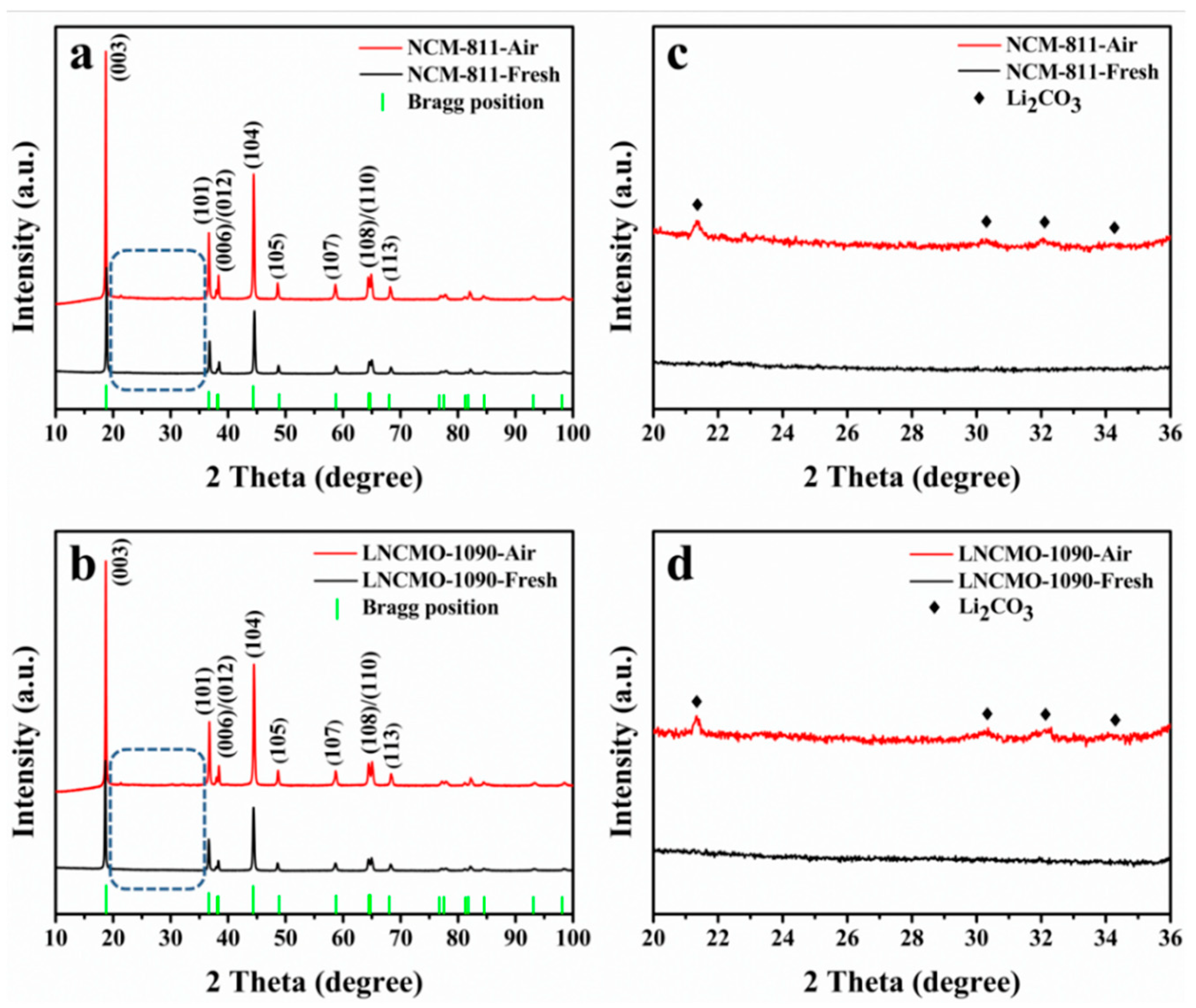
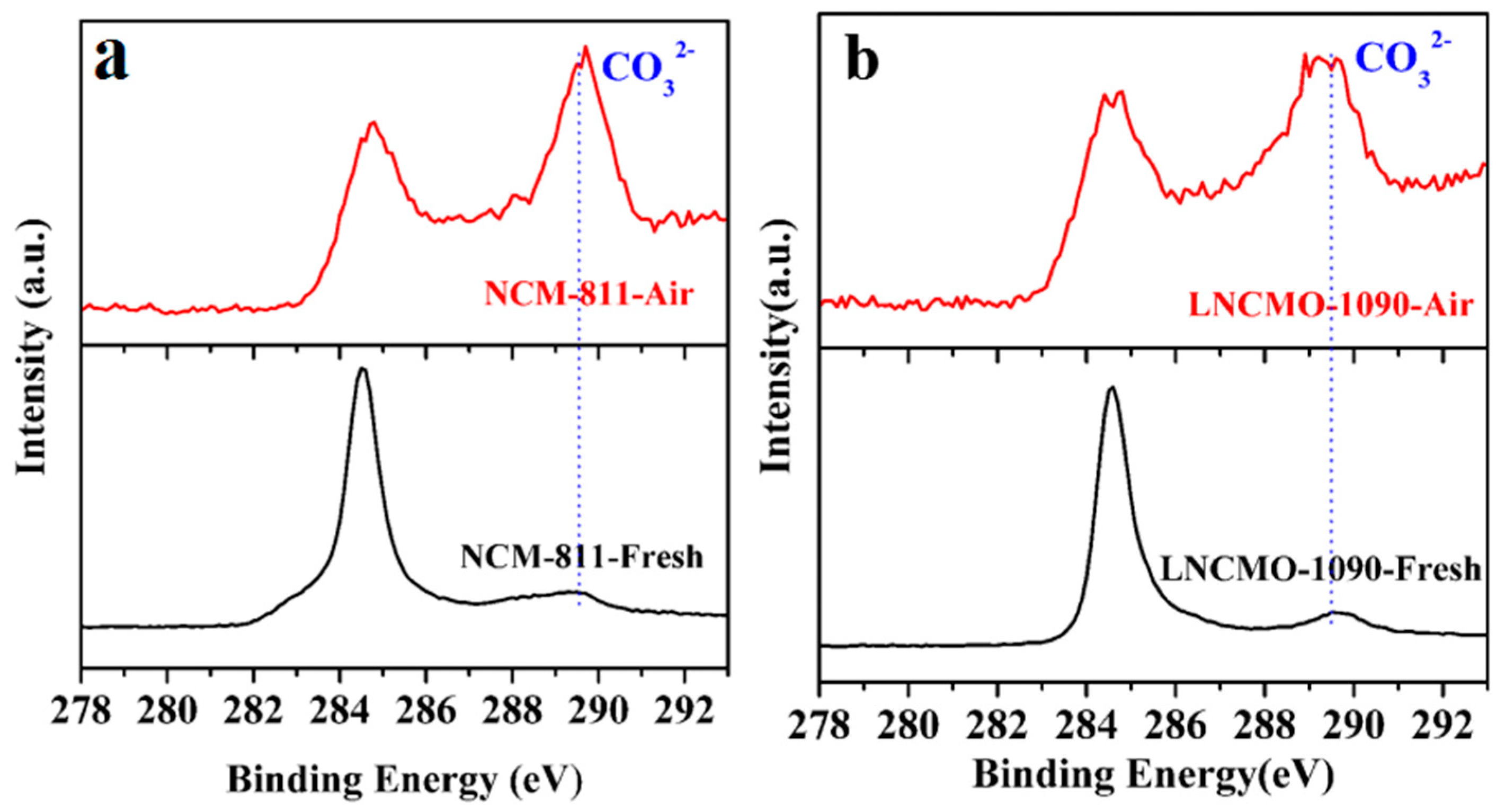
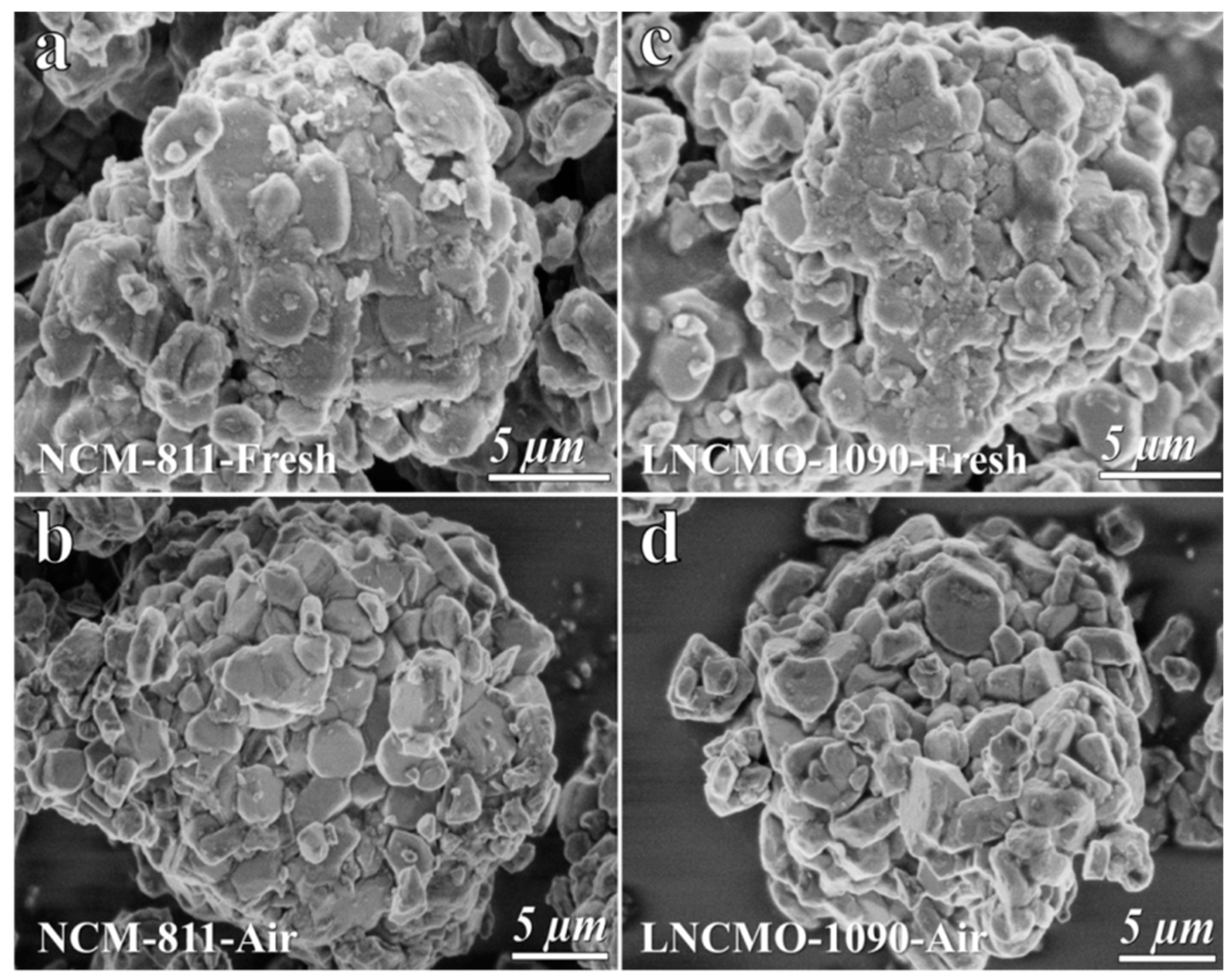
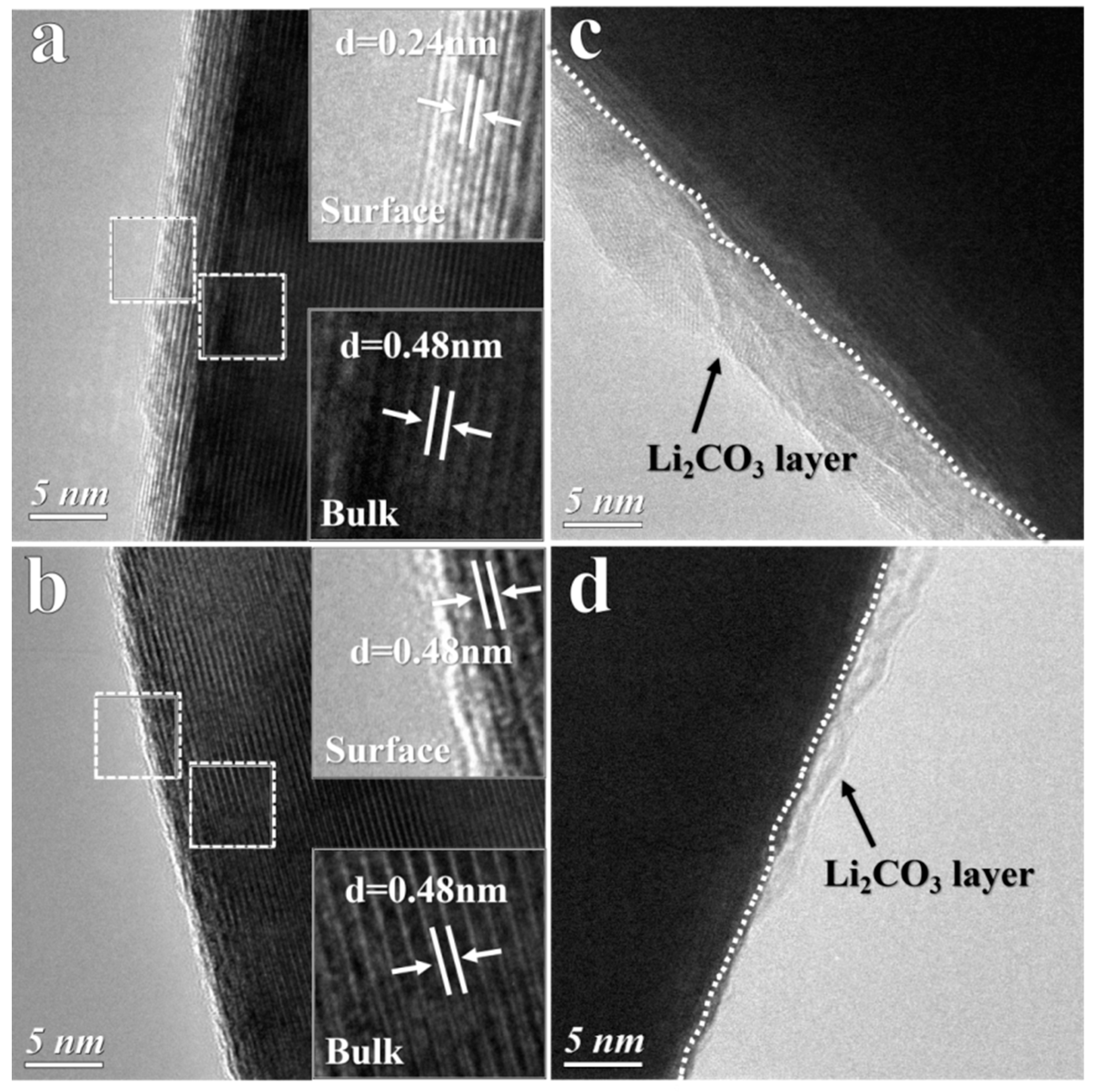
3.1. Structure Change of Ni-rich Cathode During Storage in Air
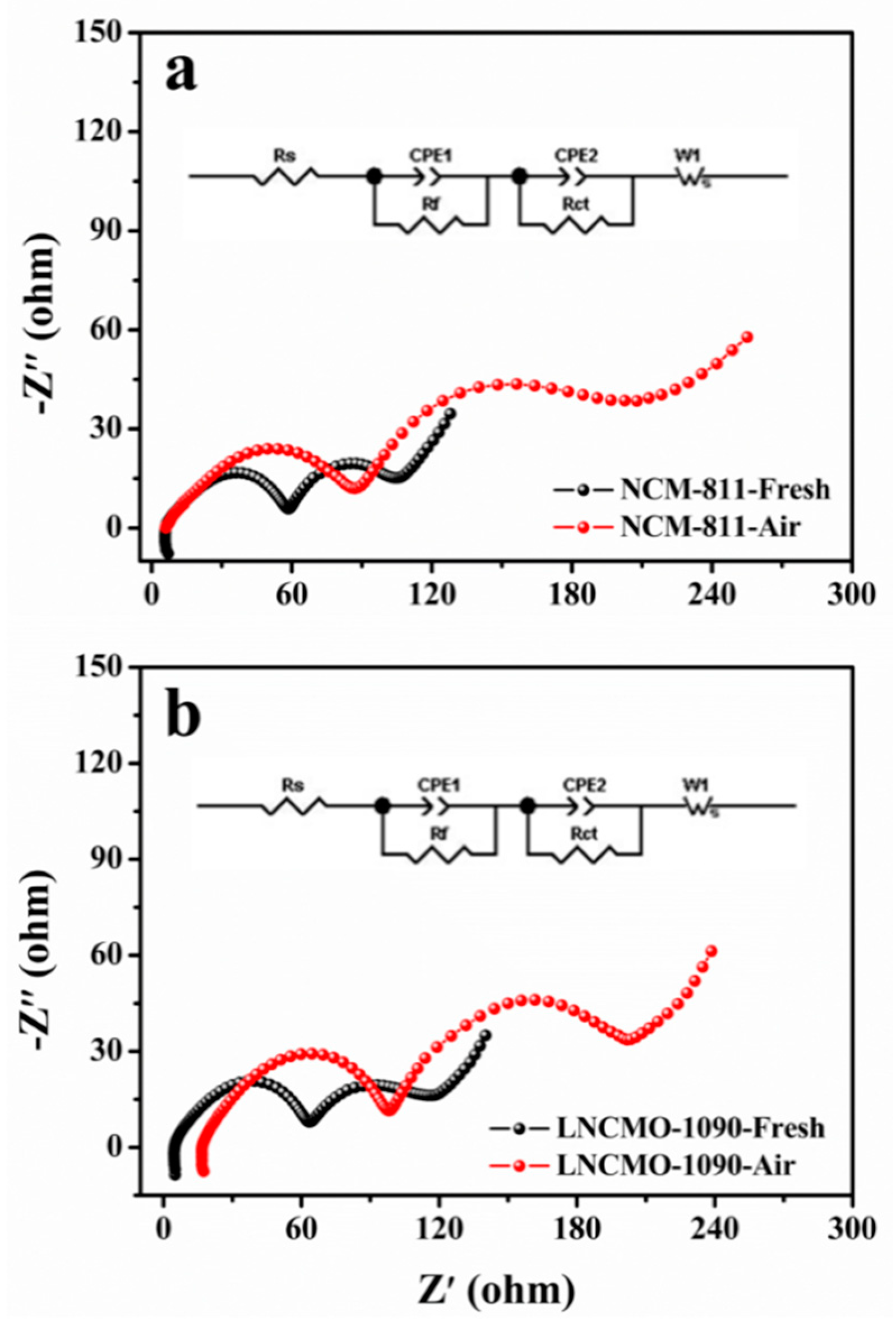
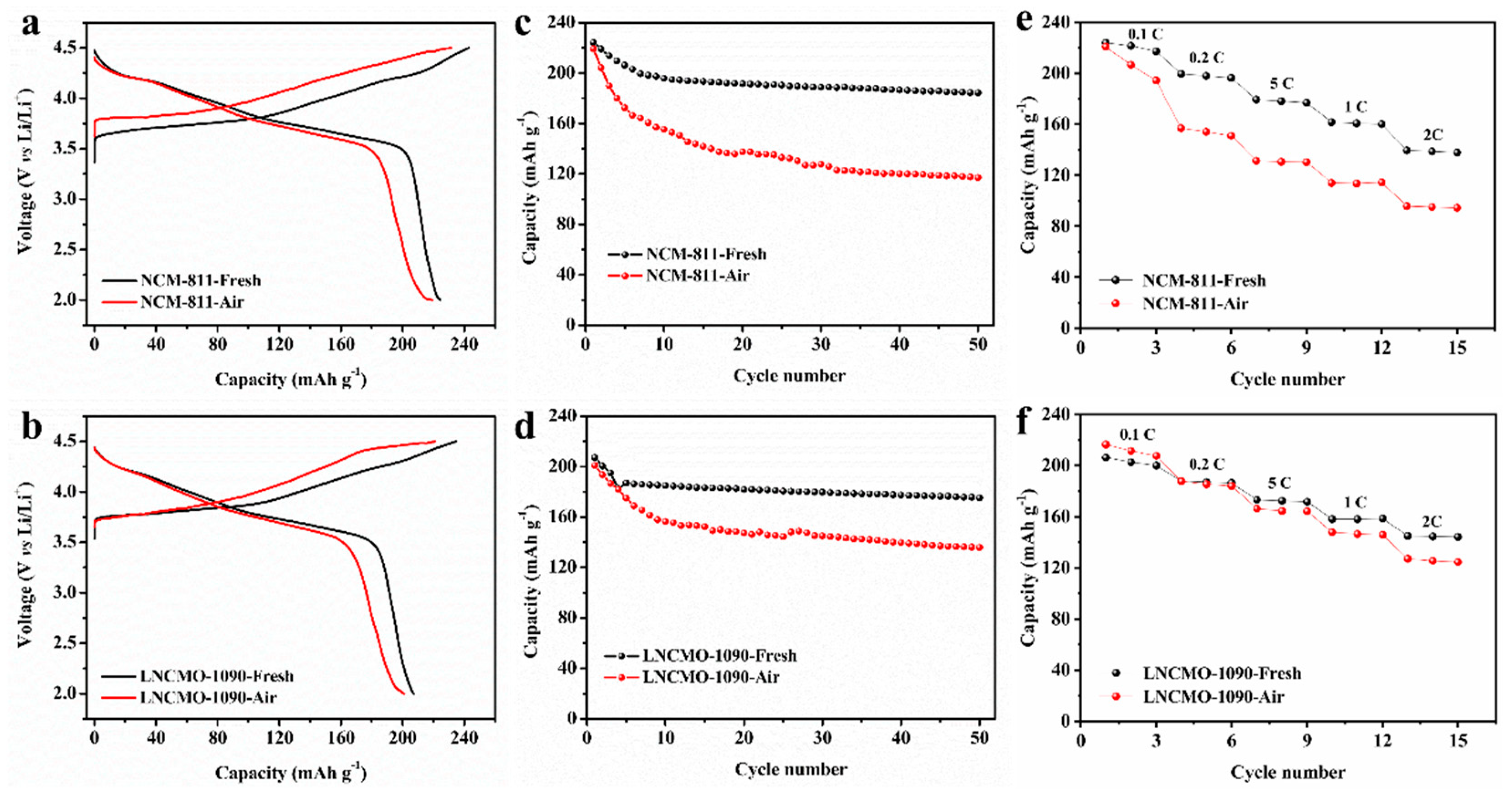
3.2. Changes in Electrochemical Properties of Ni-rich Cathodes During Storage
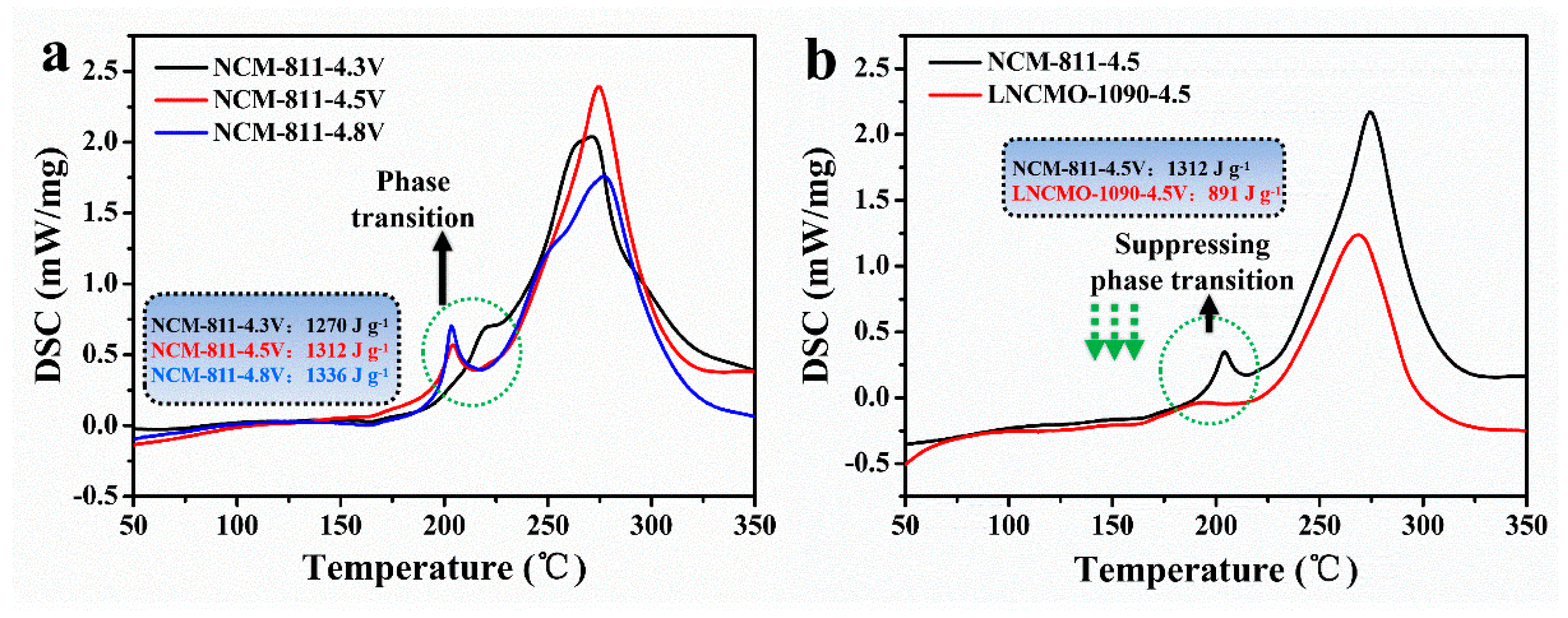
3.3. Thermal Stability of Ni-rich Layered Cathodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, C.-G.; Peng, X.; Dai, P.; Xiao, P.; Zheng, W.-C.; Li, H.-Y.; Li, H.; Indris, S.; Mangold, S.; Hong, Y.-H.; Luo, C.-X.; Shen, C.-H.; Wei, Y.-M.; Huang, L.; Sun, S.-G. Investigation and Suppression of Oxygen Release by LiNi0.8Co0.1Mn0.1O2 Cathode under Overcharge Conditions. Adv. Energy Mater. 2022, 12, 2200569. [Google Scholar] [CrossRef]
- Liu, Y. J.; Zeng, T. Y.; Li, G. T.; Wan, T.; Li, M. Y.; Zhang, X. Y.; Li, M. Q.; Su, M. R.; Dou, A. C.; Zeng, W. S.; Zhou, Y.; Guo, R. Q.; Chu, D. W. The surface double-coupling on single-crystal LiNi0.8Co0.1Mn0.1O2 for inhibiting the formation of intragranular cracks and oxygen vacancies. Energy Storage Mater. 2022, 52, 534–546. [Google Scholar] [CrossRef]
- Freiberg, A. T. S.; Sicklinger, J.; Solchenbach, S.; Gasteiger, H. A. Li2CO3 decomposition in Li-ion batteries induced by the electrochemical oxidation of the electrolyte and of electrolyte impurities. Electrochim. Acta 2020, 346, 136271. [Google Scholar] [CrossRef]
- Kang, H. S.; Santhoshkumar, P.; Park, J. W.; Sim, G. S.; Nanthagopal, M.; Lee, C. W. Glass ceramic coating on LiNi0.8Co0.1Mn0.1O2 cathode for Li-ion batteries. Korean J. Chem. Eng. 2020, 37, 1331–1339. [Google Scholar] [CrossRef]
- Wood, M.; Li, J. L.; Ruther, R. E.; Du, Z. J.; Self, E. C.; Meyer, H. M.; Daniel, C.; Belharouak, I.; Wood, D. L. Chemical stability and long-term cell performance of low-cobalt, Ni-rich cathodes prepared by aqueous processing for high-energy Li-ion batteries. Energy Storage Mater. 2020, 24, 188–197. [Google Scholar] [CrossRef]
- Zheng, W.-C.; Shi, C.-G.; Dai, P.; Huang, Z.; Lin, J.-X.; Chen, H.; Sun, M.-L.; Shen, C.-H.; Luo, C.-X.; Wang, Q.; Feng, X.; Wei, Y.-M.; Huang, L.; Sun, S.-G. A functional electrolyte additive enabling robust interphases in high-voltage Li||LiNi0.8Co0.1Mn0.1O2 batteries at elevated temperatures. J. Mater. Chem. A 2022, 10, 21912–21922. [Google Scholar] [CrossRef]
- Liu, J. X.; Wang, J. Q.; Ni, Y. X.; Zhang, K.; Cheng, F. Y.; Chen, J. Recent breakthroughs and perspectives of high-energy layered oxide cathode materials for lithium ion batteries. Mater. Today 2021, 43, 132–165. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wang, L.; Feng, X. N.; Ren, D. S.; Wu, Y.; Xu, G. L.; Lu, L. G.; Hou, J. X.; Zhang, W. F.; Wang, Y. L.; Xu, W. Q.; Ren, Y.; Wang, Z. F.; Huang, J. Y.; Meng, X. F.; Han, X. B.; Wang, H. W.; He, X. M.; Chen, Z. H.; Amine, K.; Ouyang, M. Thermal runaway mechanism of lithium-ion battery with LiNi0.8Co0.1Mn0.1O2 cathode materials. Nano Energy 2021, 85, 105878. [Google Scholar] [CrossRef]
- Xue, W. J.; Huang, M. J.; Li, Y. T.; Zhu, Y. G.; Gao, R.; Xiao, X. H.; Zhang, W. X.; Li, S. P.; Xu, G. Y.; Yu, Y.; Li, P.; Lopez, J.; Yu, D. W.; Dong, Y. H.; Fan, W. W.; Shi, Z.; Xiong, R.; Sun, C.-J.; Hwang, I.; Lee, W.-K.; Shao-Horn, Y.; Johnson, J. A.; Li, J. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat. Energy 2021, 6, 495–505. [Google Scholar] [CrossRef]
- Wang, C. H.; Shao, L.; Guo, X.; Xi, X. M.; Yang, L. S.; Huang, C. H.; Zhou, C. X.; Zhao, H. H.; Yin, D. L.; Wang, Z. C. Air-Induced Degradation and Electrochemical Regeneration for the Performance of Layered Ni-Rich Cathodes. ACS Appl. Mater. Inter. 2019, 11, 44036–44045. [Google Scholar] [CrossRef]
- Sun, P. P.; Du, F. H.; Zhou, Q.; Hu, D.; Xu, T.; Mei, C. X.; Hao, Q.; Fan, Z. X.; Zheng, J. W. Efficient preservation of surface state of LiNi0.82Co0.15Al0.03O2 through assembly of hydride terminated polydimethylsiloxane. J. Power Sources 2021, 495, 229761. [Google Scholar] [CrossRef]
- Zeng, L. C.; Shi, K. X.; Qiu, B.; Liang, H. Y.; Li, J. H.; Zhao, W.; Li, S. L.; Zhang, W. G.; Liu, Z. P.; Liu, Q. B. Hydrophobic surface coating against chemical environmental instability for Ni-rich layered oxide cathode materials. Chem. Eng. J. 2022, 437, 135276. [Google Scholar] [CrossRef]
- Xie, Q.; Li, W. D.; Manthiram, A. , A Mg-Doped High-Nickel Layered Oxide Cathode Enabling Safer, High-Energy-Density Li-Ion Batteries. Chem. Mater. 2019, 31, 938–946. [Google Scholar] [CrossRef]
- Han, G.-M.; Kim, Y.-S.; Ryu, H.-H.; Sun, Y.-K.; Yoon, C. S. Structural Stability of Single-Crystalline Ni-Rich Layered Cathode upon Delithiation. ACS Energy Lett. 2022, 7, 2919–2926. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety issues and mechanisms of lithium-ion battery cell upon mechanical abusive loading: A review. Energy Storage Mater. 2020, 24, 85–112. [Google Scholar] [CrossRef]
- Li, L. J.; Fu, L. Z.; Li, M.; Wang, C.; Zhao, Z. X.; Xie, S. C.; Lin, H. C.; Wu, X. W.; Liu, H. D.; Zhang, L.; Zhang, Q. B.; Tan, L. B-doped and La4NiLiO8-coated Ni-rich cathode with enhanced structural and interfacial stability for lithium-ion batteries. J. Energy Chem. 2022, 71, 588–594. [Google Scholar] [CrossRef]
- Fan, X. M.; Ou, X.; Zhao, W. G.; Liu, Y.; Zhang, B.; Zhang, J. F.; Zou, L. F.; Seidl, L.; Li, Y. Z.; Hu, G. R.; Battaglia, C.; Yang, Y. In situ inorganic conductive network formation in high-voltage single-crystal Ni-rich cathodes. Nat. Commun. 2021, 12, 5320. [Google Scholar] [CrossRef] [PubMed]
- Yin, S. Y.; Deng, W. T.; Chen, J.; Gao, X.; Zou, G. Q.; Hou, H. S.; Ji, X. B. Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries. Nano Energy 2021, 83, 105854. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y. Enhancement on the Cycling Stability of the Layered Ni-Rich Oxide Cathode by In-Situ Fabricating Nano-Thickness Cation-Mixing Layers. J. Electrochem. Soc. 2016, 163, A2665–A2672. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C. S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources, 2013; 233, 121–130. [Google Scholar]
- Yang, J.; Hou, M.; Haller, S.; Wang, Y.; Wang, C.; Xia, Y. Improving the Cycling Performance of the Layered Ni-Rich Oxide Cathode by Introducing Low-Content Li2MnO3. Electrochim. Acta 2016, 189, 101–110. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y. Suppressing the Phase Transition of the Layered Ni-Rich Oxide Cathode during High-Voltage Cycling by Introducing Low-Content Li2MnO3. ACS Appl. Mater. Inter. 2016, 8, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Kim, H.; Lee, W.; Ahn, S.-J.; Lee, E.; Yoon, W.-S. Stabilizing effects of Al-doping on Ni-rich LiNi0.80Co0.15Mn0.05O2 cathode for Li rechargeable batteries. J. Power Sources 2020, 474, 228592. [Google Scholar] [CrossRef]
- Becker, D.; Boerner, M.; Noelle, R.; Diehl, M.; Klein, S.; Rodehorst, U.; Schmuch, R.; Winter, M.; Placke, T. Surface Modification of Ni-Rich LiNi0.8Co0.1Mn0.1O2 Cathode Material by Tungsten Oxide Coating for Improved Electrochemical Performance in Lithium-Ion Batteries. ACS Appl. Mater. Inter. 2019, 11, 18404–18414. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H. L.; Cheung, E. A.; Avdeev, M.; Maynard-Casely, H. E.; Abraham, D. P.; Sharma, N. Consequences of long-term water exposure for bulk crystal structure and surface composition/chemistry of nickel-rich layered oxide materials for Li-ion batteries. J. Power Sources 2020, 470, 228370. [Google Scholar] [CrossRef]
- Xu, C. L.; Xiang, W.; Wu, Z. G.; Qiu, L.; Ming, Y.; Yang, W.; Yue, L. C.; Zhang, J.; Zhong, B. H.; Guo, X. D.; Wang, G. K.; Liu, Y. X. Dual-site lattice modification regulated cationic ordering for Ni-rich cathode towards boosted structural integrity and cycle stability. Chem. Eng. J. 2021, 403, 126314. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, Q.; Li, B. H.; Manthiram, A. An in-depth understanding of the effect of aluminum doping in high-nickel cathodes for lithium-ion batteries. Energy Storage Mater. 2021, 34, 229–240. [Google Scholar] [CrossRef]
- Tang, M.; Yang, J.; Chen, N.; Zhu, S.; Wang, X.; Wang, T.; Zhang, C.; Xia, Y. Overall structural modification of a layered Ni-rich cathode for enhanced cycling stability and rate capability at high voltage. J. Mater. Chem. A 2019, 7, 6080–6089. [Google Scholar] [CrossRef]
- Zhang, F.; Lou, S. F.; Li, S.; Yu, Z. J.; Liu, Q. S.; Dai, A.; Cao, C. T.; Toney, M. F.; Ge, M. Y.; Xiao, X. H.; Lee, W. K.; Yao, Y. D.; Deng, J. J.; Liu, T. C.; Tang, Y. P.; Yin, G. P.; Lu, J.; Su, D.; Wang, J. J. Surface regulation enables high stability of single-crystal lithium-ion cathodes at high voltage. Nat. Commun. 2020, 11, 3050. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xu, H.; Wang, B.; Tuo, K.; Wang, P.; Wang, S.; Liang, W.; Lu, H.; Li, S. Structural evolution of nickel-rich layered cathode material LiNi0.8Co0.1Mn0.1O2 at different current rates. Ionics 2021, 27, 517–526. [Google Scholar] [CrossRef]
- Yin, E.; Grimaud, A.; Rousse, G.; Abakumov, A. M.; Senyshyn, A.; Zhang, L.; Trabesinger, S.; Iadecola, A.; Foix, D.; Giaume, D.; Tarascon, J.-M. Structural evolution at the oxidative and reductive limits in the first electrochemical cycle of Li1.2Ni0.13Mn0.54Co0.13O2. Nat. Commun. 2020, 11, 1252. [Google Scholar] [CrossRef]
- Xiong, C.; Fu, H. K.; Wu, L. J.; Yuan, G. Q. Enhanced Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Cathode Material for lithium ion batteries by WO3 surface coating. Int. J. Electrochem. Sc. 2020, 15, 8990–9002. [Google Scholar] [CrossRef]
- Liu, X. S.; Zheng, B. Z.; Zhao, J.; Zhao, W. M.; Liang, Z. T.; Su, Y.; Xie, C. P.; Zhou, K.; Xiang, Y. X.; Zhu, J. P.; Wang, H. C.; Zhong, G. M.; Gong, Z. L.; Huang, J. Y.; Yang, Y. Electrochemo-Mechanical Effects on Structural Integrity of Ni-Rich Cathodes with Different Microstructures in All Solid-State Batteries. Adv. Energy Mater. 2021, 11, 2003583. [Google Scholar] [CrossRef]
- Li, X. L.; Jin, L. B.; Song, D. W.; Zhang, H. Z.; Shi, X. X.; Wang, Z. Y.; Zhang, L. Q.; Zhu, L. Y. LiNbO3-coated LiNi0.8Co0.1Mn0.1O2 cathode with high discharge capacity and rate performance for all-solid-state lithium battery. J. Energy Chem. 2020; 40, 39–45. [Google Scholar]
- Lee, S. H.; Park, G. J.; Sim, S. J.; Jin, B. S.; Kim, H. S. Improved electrochemical performances of LiNi0.8Co0.1Mn0.1O2 cathode via SiO2 coating. J. Alloys Compd. 2019, 791, 193–199. [Google Scholar] [CrossRef]
- Li, Y. J.; Deng, S. Y.; Chen, Y. X.; Gao, J.; Zhu, J.; Xue, L. L.; Lei, T. X.; Cao, G. L.; Guo, J.; Wang, S. L. Dual functions of residue Li-reactive coating with C4H6CoO4 on high-performance LiNiO2 cathode material. Electrochim. Acta 2019, 300, 26–35. [Google Scholar] [CrossRef]
- Zhang, Y. R.; Katayama, Y.; Tatara, R.; Giordano, L.; Yu, Y.; Fraggedakis, D.; Sun, J. G. W.; Maglia, F.; Jung, R.; Bazant, M. Z.; Shao-Horn, Y. Revealing electrolyte oxidation via carbonate dehydrogenation on Ni-based oxides in Li-ion batteries by in situ Fourier transform infrared spectroscopy. Energ. Environ. Sci. 2020, 13, 183–199. [Google Scholar] [CrossRef]
- Cheng, W. D.; Hao, S.; Ji, Y. Y.; Li, L.; Liu, L.; Xiao, Y.; Wu, Y. X.; Huo, J. S.; Tang, F.; Liu, X. Q. Optimizing surface residual alkali and enhancing electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode by LiH2PO4. Nanotechnology 2022, 33, 045404. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.-T.; Ruess, R.; Pan, R.; Walther, F.; Rohnke, M.; Hori, S.; Kanno, R.; Schroeder, D.; Janek, J. A mechanistic investigation of the Li10GeP2S12|LiNi1-x-yCoxMnyO2 interface stability in all-solid-state lithium batteries. Nat. Commun. 2021, 12, 6669. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. F.; Ji, X.; Yue, J.; Hou, S.; Wang, P. F.; Cui, C. Y.; Chen, J.; Shao, B. W.; Li, J. R.; Han, F. D.; Tu, J. P.; Wang, C. S. High Interfacial-Energy Interphase Promoting Safe Lithium Metal Batteries. J. Am. Chem. Soc. 2020, 142, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liang X., H. Ryu H. H.; Yoon, C. S.; Sun Y. K., Ni-Rich Layered Cathodes for Lithium-Ion Batteries: From Challenges to the Future. Energy Storage Mater. 2023, 63, 102969. [Google Scholar] [CrossRef]
- Jung, S. H.; Kim, U. H.; Kim, J. H.; Jun, S. G.; Yoon, C. S.; Jung, Y. S.; Sun, Y. K. Ni-Rich Layered Cathode Materials with Electrochemo-Mechanically Compliant Microstructures for All-Solid-State Li Batteries. Adv. Energy Mater. 2020, 10, 1903360. [Google Scholar] [CrossRef]
- Xu, G. L.; Liu, X.; Daali, A.; Amine, R.; Chen, Z. H.; Amine, K. Challenges and Strategies to Advance High-Energy Nickel-Rich Layered Lithium Transition Metal Oxide Cathodes for Harsh Operation. Adv. Funct. Mater. 2020, 30, 2004748. [Google Scholar] [CrossRef]
- Wang, Q. D.; Yao, Z. P.; Zhao, C. L.; Verhallen, T.; Tabor, D. P.; Liu, M.; Ooms, F.; Kang, F. Y.; Aspuru-Guzik, A.; Hu, Y. S.; Wagemaker, M.; Li, B. H. Interface chemistry of an amide electrolyte for highly reversible lithium metal batteries. Nat. Commun. 2020, 11, 4188. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Guo, W. B.; Wu, H. L.; Lin, L.; Liu, Q.; Han, X.; Xie, Q. S.; Liu, P. F.; Zheng, H. F.; Wang, L. S.; Yu, X. Q.; Peng, D. L. Challenges and Recent Advances in High Capacity Li-Rich Cathode Materials for High Energy Density Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2005937. [Google Scholar] [CrossRef] [PubMed]
- Ren, D. S.; Feng, X. N.; Liu, L. S.; Hsu, H. J.; Lu, L. G.; Wang, L.; He, X. M.; Ouyang, M. G. Investigating the relationship between internal short circuit and thermal runaway of lithium-ion batteries under thermal abuse condition. Energy Storage Mater. 2021, 34, 563–573. [Google Scholar] [CrossRef]
- Csernica, P. M.; Kalirai, S. S.; Gent, W. E.; Lim, K.; Yu, Y. S.; Liu, Y. Z.; Ahn, S. J.; Kaeli, E.; Xu, X.; Stone, K. H.; Marshall, A. F.; Sinclair, R.; Shapiro, D. A.; Toney, M. F.; Chueh, W. C. Persistent and partially mobile oxygen vacancies in Li-rich layered oxides. Nat. Energy 2021, 6, 642–652. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




