Submitted:
04 January 2024
Posted:
08 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
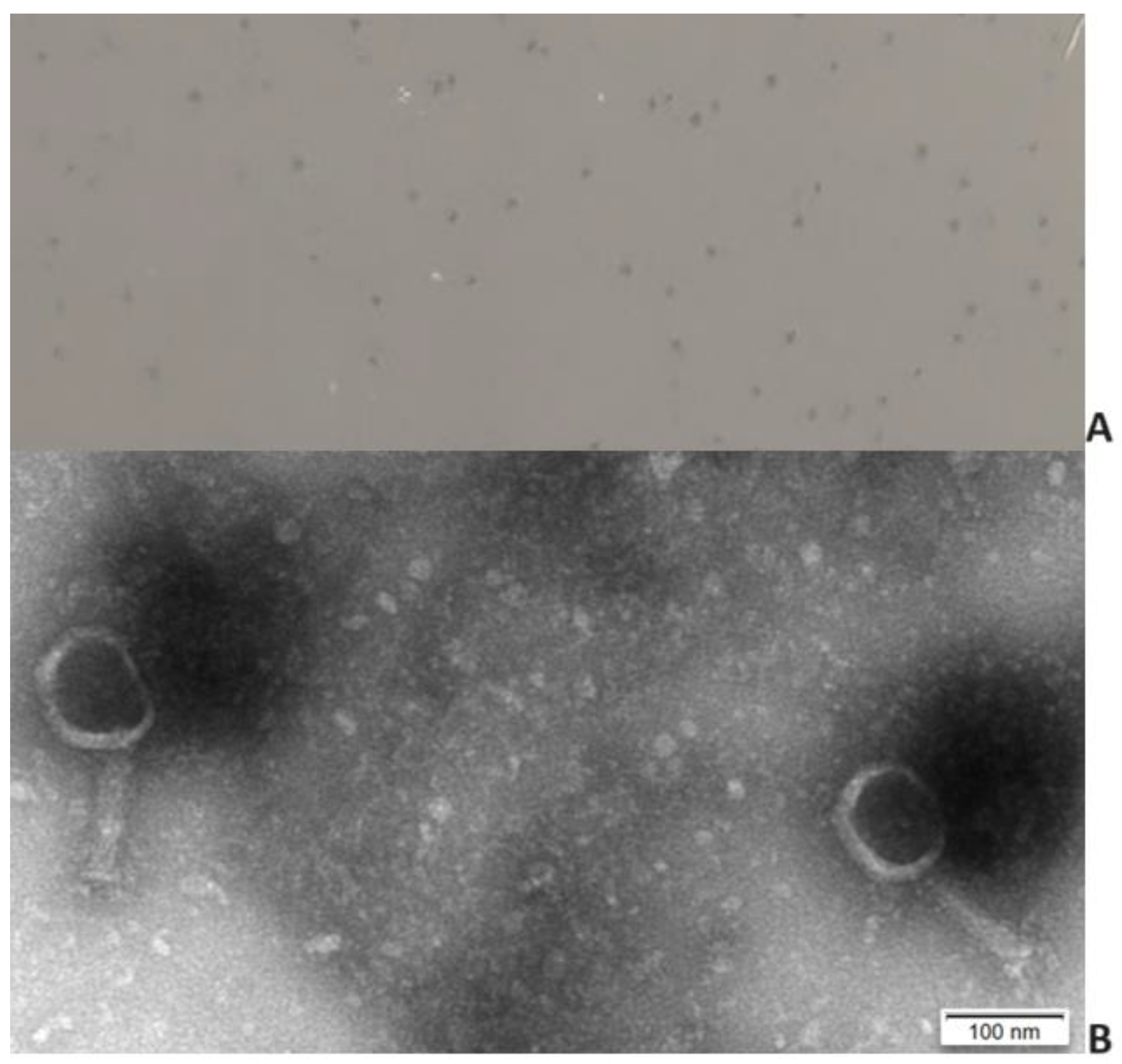
2.1. Phage isolation and virion morphology
2.2. Genome analysis
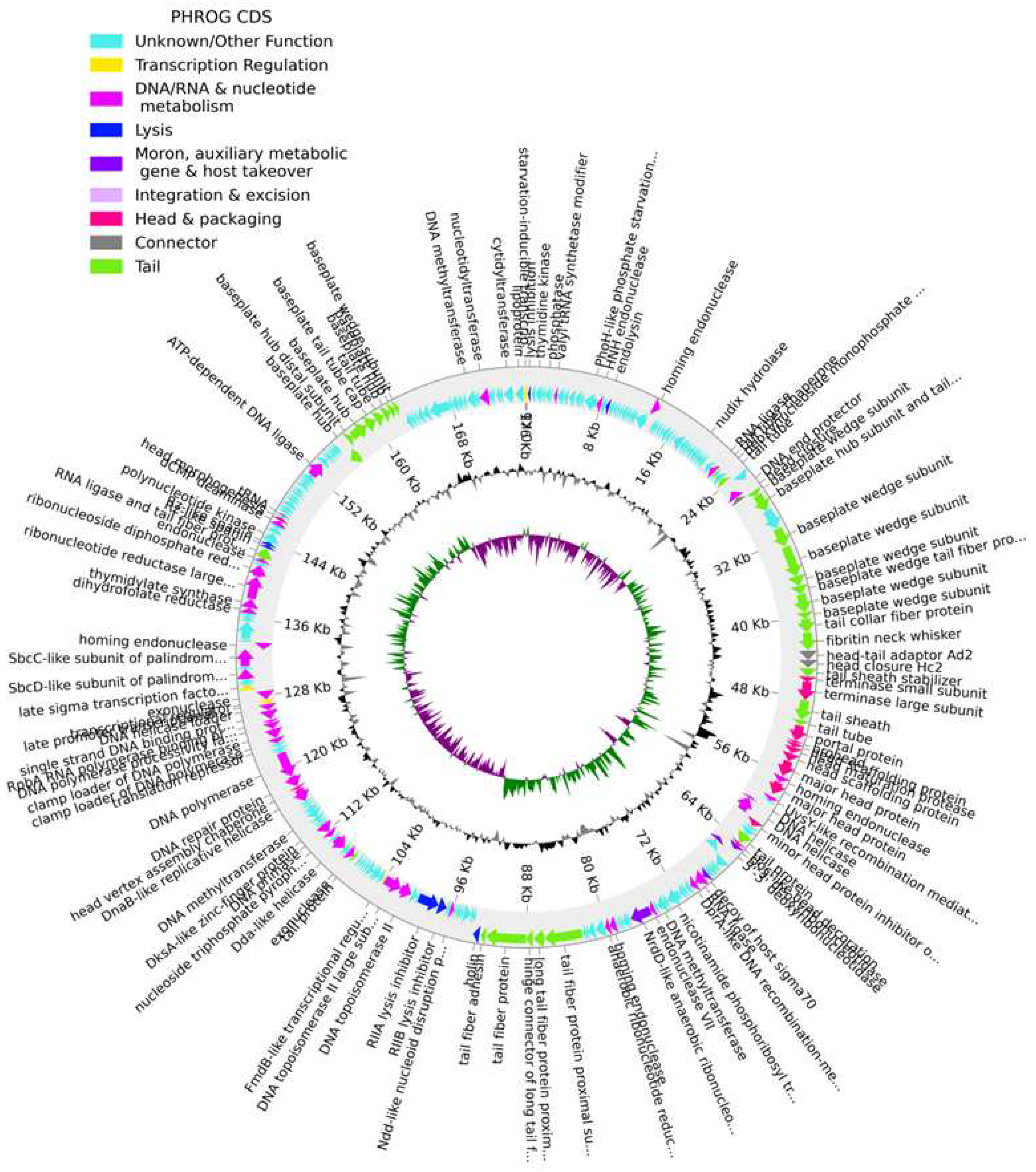
2.3. Phage host range and efficiency of plating
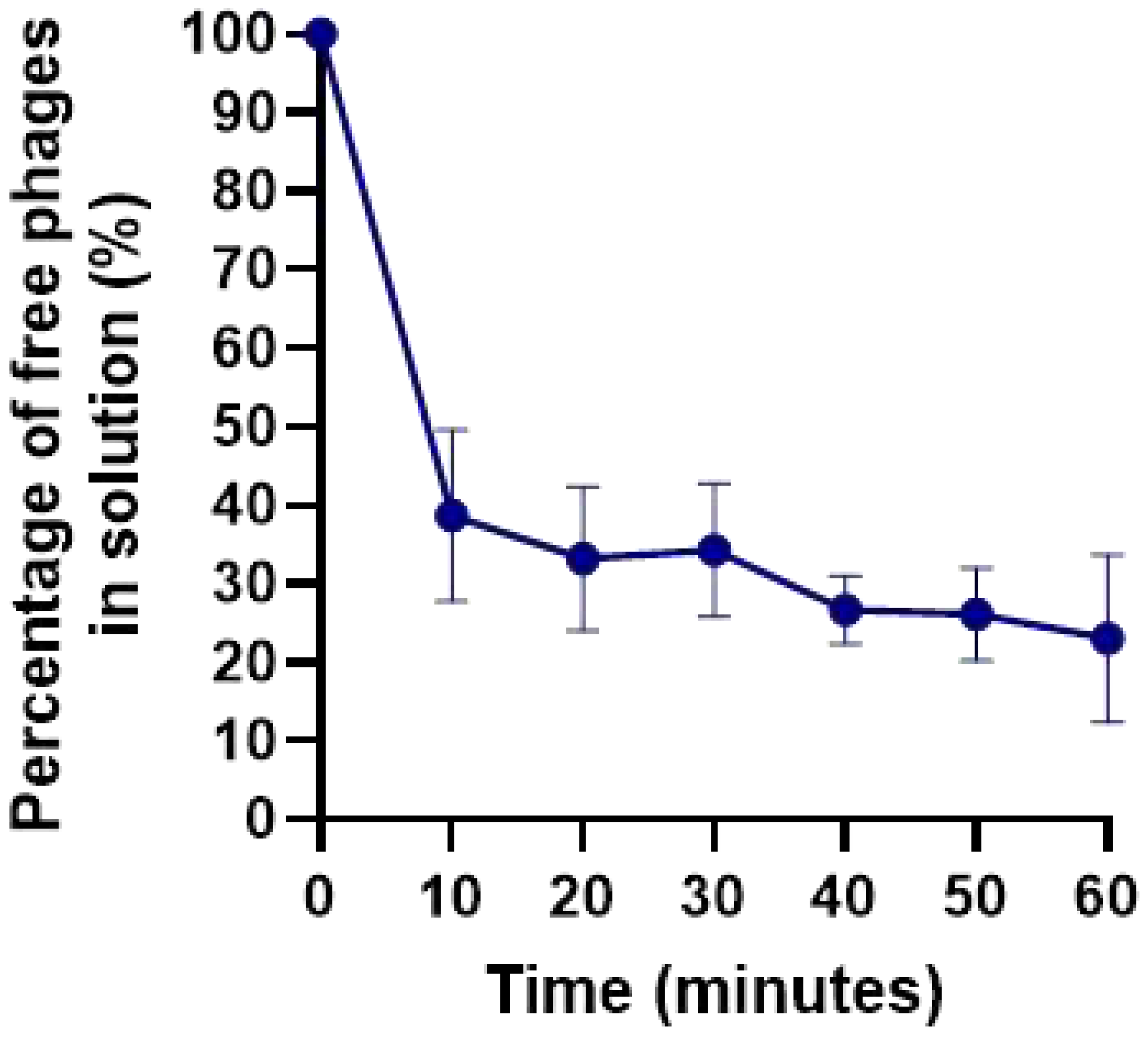
2.4. Adsorption curve
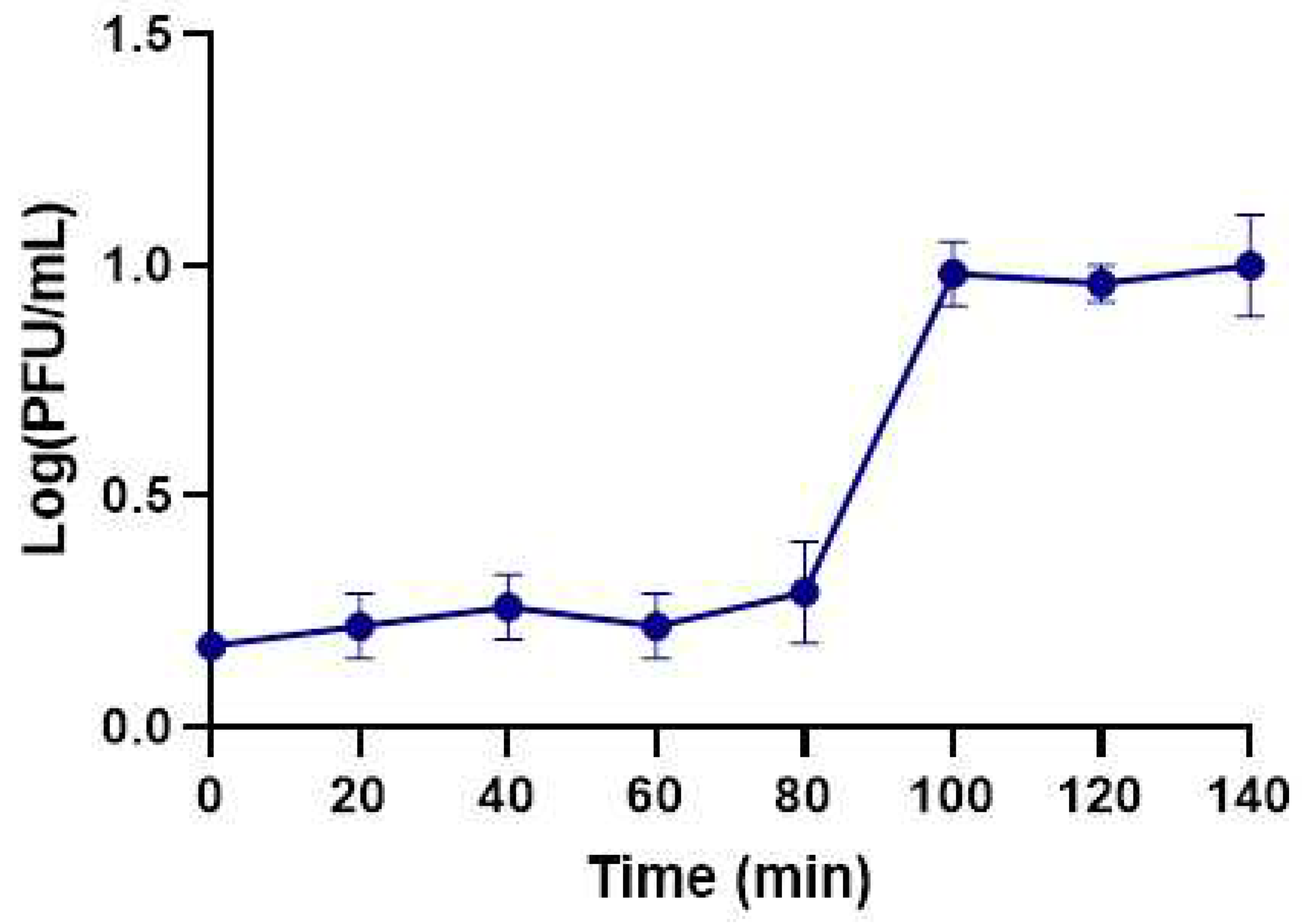
2.5. One-step growth curve
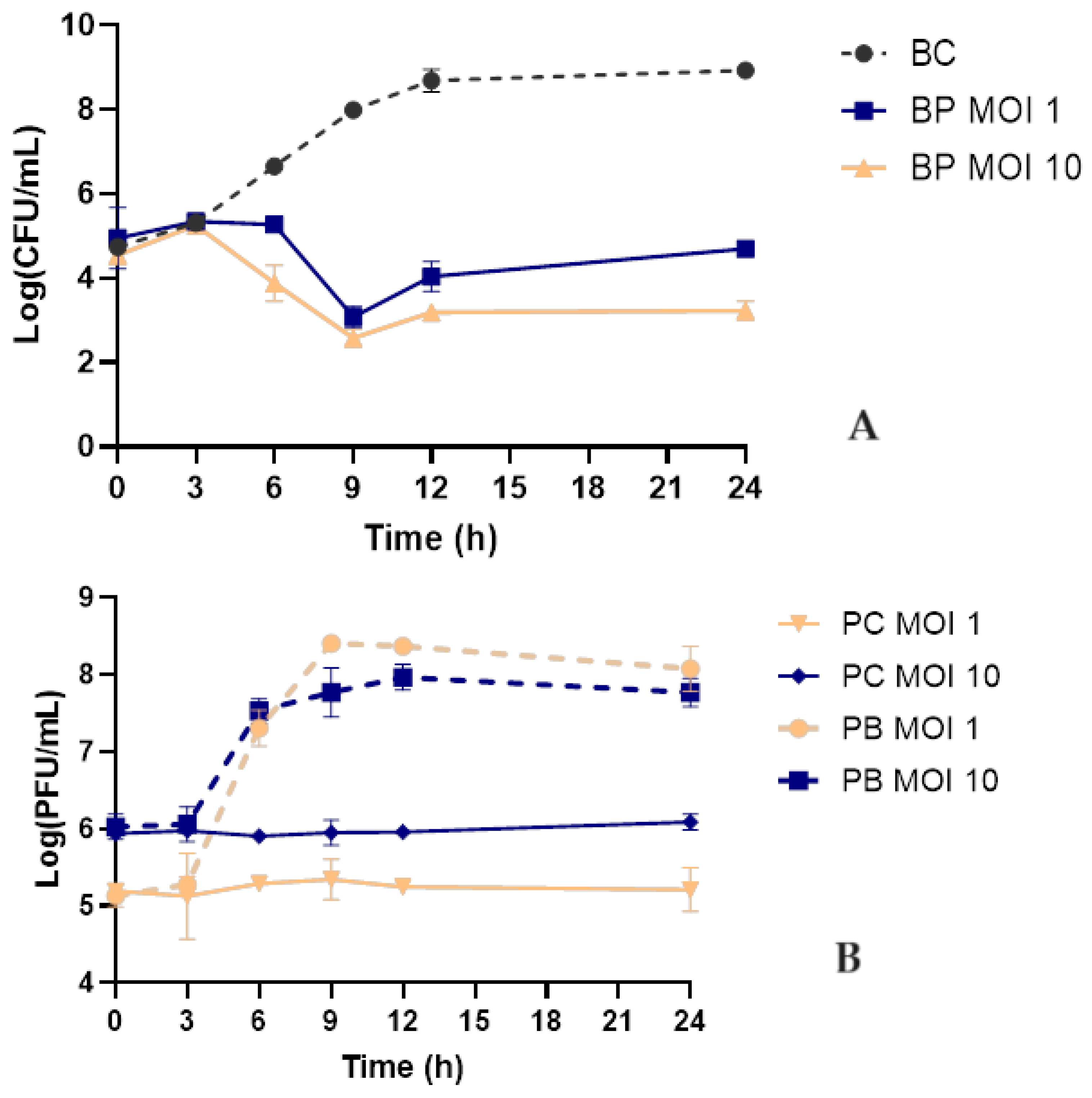
2.6. Bacterial kill curve
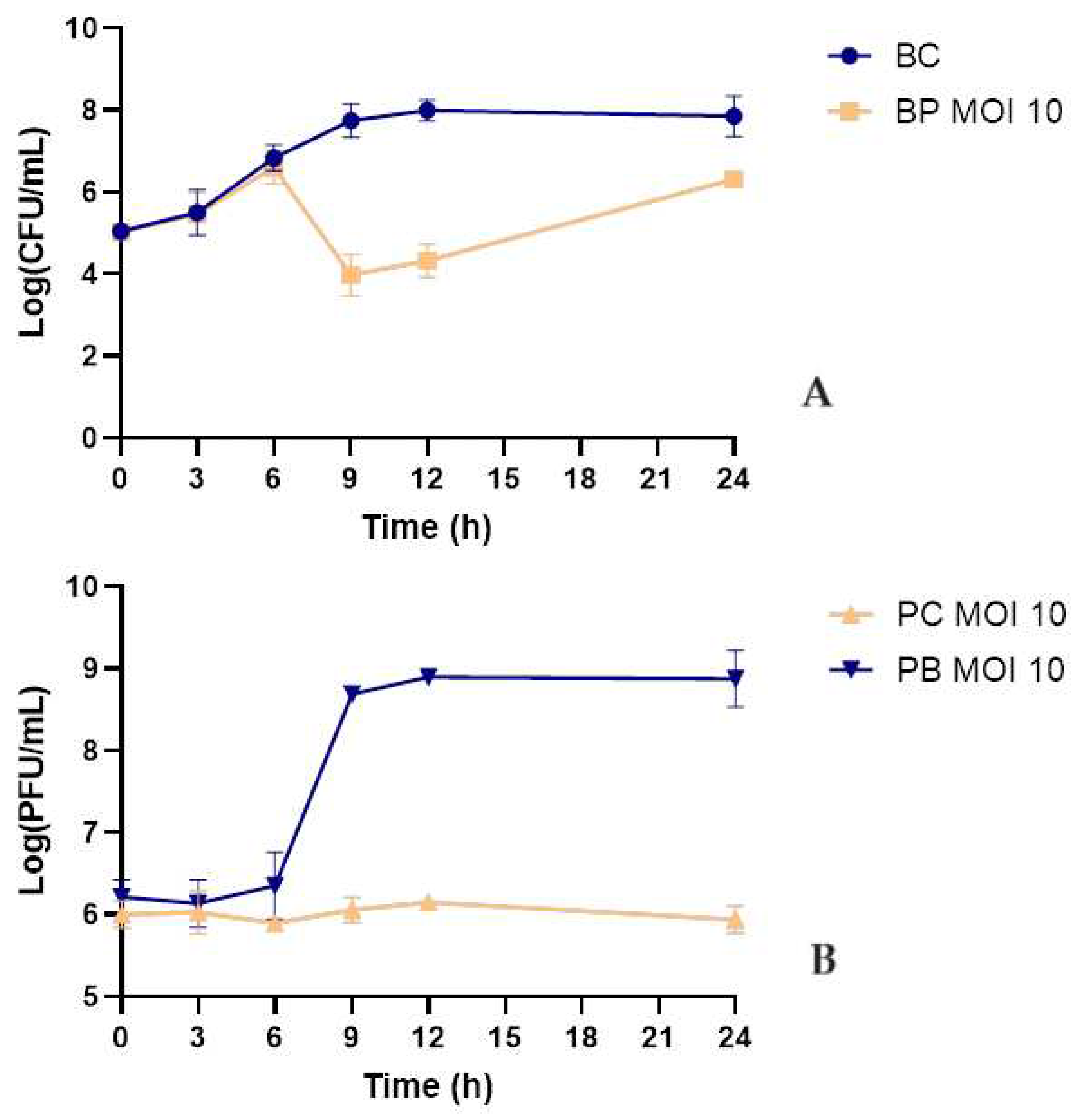
2.7. Bacterial kill curve in urine
2.8. Frequency of emergence of phage-resistant mutants
3. Discussion
4. Materials and Methods
4.1. Bacterial strains and growth conditions
4.2. Phage isolation and purification
4.3. Electron Microscope Examination
4.4. Phage DNA Extraction
4.5. Phage Genome Assembly and Annotation
4.6. Phage host range and efficiency of plating
4.7. Adsorption curve
4.8. One-step growth assay
4.9. Bacterial kill curve in TSB culture media
4.10. Bacterial kill curve in urine samples.
4.10.1. Urine sample collection and handling.
4.10.2. Bacterial kill curves in urine
4.11. Rate of emergence of phage-resistant bacterial mutant
4.12. Statistical analysis.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- World Health Organization (WHO) Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Heal.; World Health Organization, 2017; Vol. 13;
- Lee, Y.-L.; Ko, W.-C.; Hsueh, P.-R. Geographic patterns of global isolates of carbapenem-resistant Klebsiella pneumoniae and the activity of ceftazidime/avibactam, meropenem/vaborbactam, and comparators against these isolates: Results from the Antimicrobial Testing Leadership and Surveillan. Int. J. Antimicrob. Agents 2022, 60, 106679. [Google Scholar] [CrossRef]
- Gorrie, C.L.; Mirčeta, M.; Wick, R.R.; Judd, L.M.; Lam, M.M.C.; Gomi, R.; Abbott, I.J.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F.; et al. Genomic dissection of Klebsiella pneumoniae infections in hospital patients reveals insights into an opportunistic pathogen. Nat. Commun. 2022, 13, 3017. [Google Scholar] [CrossRef]
- European Center for Disease Prevention and Control and World Health Organization (WHO) Annual epidemiological report for 2019: Healthcare - associated infections acquired in intensive care units; Stockholm, 2023.
- Karampatakis, T.; Tsergouli, K.; Behzadi, P. Carbapenem-Resistant Klebsiella pneumoniae: Virulence Factors, Molecular Epidemiology and Latest Updates in Treatment Options. Antibiotics 2023, 12, 234. [Google Scholar] [CrossRef]
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Hakansson, A.P.; Darrieux, M.; Converso, T.R. Klebsiella pneumoniae Biofilms and Their Role in Disease Pathogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- European Center for Disease Prevention and Control and World Health Organization (WHO) Antimicrobial resistance surveillance in Europe; 2023.
- Abbo, L.; Hooton, T. Antimicrobial Stewardship and Urinary Tract Infections. Antibiotics 2014, 3, 174–192. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hong, Q.; Chang, R.Y.K.; Kwok, P.C.L.; Chan, H.-K. Phage–Antibiotic Therapy as a Promising Strategy to Combat Multidrug-Resistant Infections and to Enhance Antimicrobial Efficiency. Antibiotics 2022, 11, 570. [Google Scholar] [CrossRef]
- Rehman, S.; Ali, Z.; Khan, M.; Bostan, N.; Naseem, S. The dawn of phage therapy. Rev. Med. Virol. 2019, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tandogdu, Z.; Cai, T.; Koves, B.; Wagenlehner, F.; Bjerklund-Johansen, T.E. Urinary Tract Infections in Immunocompromised Patients with Diabetes, Chronic Kidney Disease, and Kidney Transplant. Eur. Urol. Focus 2016, 2, 394–399. [Google Scholar] [CrossRef]
- European Center for Disease Prevention and Control Annual Epidemiological Report for 2018: Healthcare- associated infections acquired in intensive care unitsHealthcare-associated infections acquired in intensive care units; 2018.
- Al-Anany, A.M.; Hooey, P.B.; Cook, J.D.; Burrows, L.L.; Martyniuk, J.; Hynes, A.P.; German, G.J. Phage Therapy in the Management of Urinary Tract Infections: A Comprehensive Systematic Review. PHAGE 2023, 4, 112–127. [Google Scholar] [CrossRef]
- Sybesma, W.; Zbinden, R.; Chanishvili, N.; Kutateladze, M.; Chkhotua, A.; Ujmajuridze, A.; Mehnert, U.; Kessler, T.M. Bacteriophages as Potential Treatment for Urinary Tract Infections. Front. Microbiol. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Leitner, L.; Mehnert, U.; Chkhotua, A.; Kessler, T.M.; Sybesma, W. Adapted Bacteriophages for Treating Urinary Tract Infections. Front. Microbiol. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Le, T.; Nang, S.C.; Zhao, J.; Yu, H.H.; Li, J.; Gill, J.J.; Liu, M.; Aslam, S. Therapeutic Potential of Intravenous Phage as Standalone Therapy for Recurrent Drug-Resistant Urinary Tract Infections. Antimicrob. Agents Chemother. 2023, 67. [Google Scholar] [CrossRef] [PubMed]
- Laforêt, F.; Antoine, C.; Blasdel Reuter, B.; Detilleux, J.; Pirnay, J.-P.; Brisse, S.; Fall, A.; Duprez, J.-N.; Delcenserie, V.; Thiry, D. In Vitro and In Vivo Assessments of Two Newly Isolated Bacteriophages against an ST13 Urinary Tract Infection Klebsiella pneumoniae. Viruses 2022, 14, 1079. [Google Scholar] [CrossRef] [PubMed]
- Zurabov, F.; Glazunov, E.; Kochetova, T.; Uskevich, V.; Popova, V. Bacteriophages with depolymerase activity in the control of antibiotic resistant Klebsiella pneumoniae biofilms. Sci. Rep. 2023, 13, 15188. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Du, F.; Long, M.; Li, P. Limitations of Phage Therapy and Corresponding Optimization Strategies: A Review. Molecules 2022, 27, 1857. [Google Scholar] [CrossRef] [PubMed]
- Patient, S.; Zhang, B. Comparison of two distinct subpopulations of Klebsiella pneumoniae ST16 co-occurring in a single patient. Microbiol. Spectr. 2022, 10, e0262421. [Google Scholar]
- Cai, R.; Wang, Z.; Wang, G.; Zhang, H.; Cheng, M.; Guo, Z.; Ji, Y.; Xi, H.; Wang, X.; Xue, Y.; et al. Biological properties and genomics analysis of vB_KpnS_GH-K3, a Klebsiella phage with a putative depolymerase-like protein. Virus Genes 2019, 55, 696–706. [Google Scholar] [CrossRef]
- Majkowska-Skrobek, G.; Latka, A.; Berisio, R.; Squeglia, F.; Maciejewska, B.; Briers, Y.; Drulis-Kawa, Z. Phage-borne depolymerases decrease Klebsiella pneumoniae resistance to innate defense mechanisms. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Obradović, M.; Malešević, M.; Di Luca, M.; Kekić, D.; Gajić, I.; McAuliffe, O.; Neve, H.; Stanisavljević, N.; Vukotić, G.; Kojić, M. Isolation, characterization, genome analysis and host resistance development of two novel lastavirus phages active against pandrug-resistant Klebsiella pneumoniae. Viruses 2023, 15, 628. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef]
- Abedon, S.T.; Danis-Wlodarczyk, K.M.; Alves, D.R. Phage therapy in the 21st century: Is there modern, clinical evidence of phage-mediated efficacy? Pharmaceuticals 2021, 14, 1–25. [Google Scholar] [CrossRef]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e0207121. [Google Scholar] [CrossRef]
- Peng, Q.; Fang, M.; Liu, X.; Zhang, C.; Liu, Y.; Yuan, Y. Isolation and Characterization of a Novel Phage for Controlling Multidrug-Resistant Klebsiella pneumoniae. Microorganisms 2020, 8, 542. [Google Scholar] [CrossRef]
- Shah, M.; Bukhari, S.; Redaina; Adnan, M.; Imran, M.; Jamal, M. Isolation and characterization of lytic bacteriophage from waste water to control clinical multidrug resistant Klebsiella pneumoniae. Kuwait J. Sci. 2023, 50, 681–689. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, F.; Liang, S.; Chen, Q.; Wang, W.; Wang, Y.; Mart, A.J. Isolation and characterization of vB _ kpnM _ 17-11, a novel pnhage ffficcient against carbapenem- resistant Klebsiella pneumoniae. 2022, 12, 1–13. [CrossRef]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, H.; McKean, K.; Wang, I. Phage fitness may help predict phage therapy efficacy. Bacteriophage 2014, 4, e964081. [Google Scholar] [CrossRef] [PubMed]
- Baqer, A.; Fang, K.; Mohd-Assaad, N.; Adnan, S.; Nor, N. In Vitro Activity, Stability and Molecular Characterization of Eight Potent Bacteriophages Infecting Carbapenem-Resistant Klebsiella pneumoniae. Viruse 2023, 15, 117. [Google Scholar] [CrossRef]
- Chen, C.; Li, T.; Chen, H. Isolation and characterization of novel bacteriophage vB _ KpP _ HS106 for Klebsiella pneumonia K2 and applications in foods. Front. Microbiol. 2023, 14, 1227147. [Google Scholar] [CrossRef]
- Choi, C.; Kuatsjah, E.; Wu, E.; Yuan, S. The Effect of Cell Size on the Burst Size of T4 Bacteriophage Infections of Escherichia coli B23. J. Exp. Microbiol. Immunol. (JEMI 2010, 14, 85–91. [Google Scholar]
- Droubogiannis, S.; Katharios, P. Genomic and Biological Profile of a Novel Bacteriophage, Vibrio phage Virtus, Which Improves Survival of Sparus aurata Larvae Challenged with Vibrio harveyi. Pathogens 2022, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.J.; Costa, L.; Pereira, C.; Cunha, Â.; Calado, R.; Gomes, N.C.M.; Almeida, A. Influence of environmental variables in the efficiency of phage therapy in aquaculture. Microb. Biotechnol. 2014, 7, 401–413. [Google Scholar] [CrossRef]
- Nakai, T.; Sugimoto, R.; Park, K.H.; Matsuoka, S.; Mori, K.; Nishioka, T.; Maruyama, K. Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis. Aquat. Organ. 1999, 37, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Duarte, J.; Costa, P.; Braz, M.; Almeida, A. Bacteriophages in the control of Aeromonas sp. in aquaculture systems: An integrative view. Antibiotics 2022, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Pereira, C.; Santos, L.; Klumpp, J.; Almeida, A. Potential of phage cocktails in the inactivation of Enterobacter cloacae — An in vitro study in a buffer solution and in urine samples. Virus Res. 2016, 211, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.; Moutinho, C.; Pinto, F.; Del Fiol, F.; Jozala, A.; Chaud, M.; Vila, M.; Teixeira, J.; Balcão, V. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol. Res. 2016, 191, 51–80. [Google Scholar] [CrossRef]
- Lima, R.; Del Fiol, F.S.; Balcão, V.M. Prospects for the use of new technologies to combat multidrug-resistant bacteria. Front. Pharmacol. 2019, 10, 692. [Google Scholar] [CrossRef] [PubMed]
- Filippov, A.; Sergueev, K. V.; He, Y.; Huang, X.Z.; Gnade, B.T.; Mueller, A.J.; Fernandez-Prada, C.; Nikolich, M.P. Bacteriophage-resistant mutants in Yersinia pestis: Identification of phage receptors and attenuation for mice. PLoS One 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Pereira, C.; Moreirinha, C.; Lewicka, M.; Almeidab, P.; Clemente, C.; Romalde, J.L.; Nunes, M.L.; Almeida, A. Characterization and in vitro evaluation of new bacteriophages for the biocontrol of Escherichia coli. Virus Res. 2016, 227, 171–182. [Google Scholar] [CrossRef]
- Wright, R.C. .; Friman, V.-P.; Smith, M.C.M.; Brockhurst, M.A.; Cross-resistance is modular in bacteria–phage interactions. PLoS Biol. 2018, 16, e2006057. [Google Scholar] [CrossRef]
- Pinheiro, L.; Pereira, C.; Frazão, C.; Balcão, V.; Almeida, A. Efficiency of phage φ6 for biocontrol of Pseudomonas syringae pv. syringae: An in vitro preliminary study. Microorganisms 2019, 7, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Pereira, C.; Costa, P.; Almeida, A. Bacteriophages with potential to inactivate Aeromonas hydrophila in cockles : In vitro and in vivo preliminary studies. Antibiotics 2021, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Culot, A.; Grosset, N.; Gautier, M. Overcoming the challenges of phage therapy for industrial aquaculture: A review. Aquaculture 2019, 513, 734423. [Google Scholar] [CrossRef]
- Silva, I.; Tacão, M.; Tavares, R.D.S.; Miranda, R.; Araújo, S.; Manaia, C.M.; Henriques, I. Fate of cefotaxime-resistant Enterobacteriaceae and ESBL-producers over a full-scale wastewater treatment process with UV disinfection. Sci. Total Environ. 2018, 639, 1028–1037. [Google Scholar] [CrossRef]
- Pereira, S.R. do S. Viruses as therapeutic and infectious agents.Thesis MsC, University of Aveiro, Portugal, 2014.
- Pereira, C.; Moreirinha, C.; Lewicka, M.; Almeida, P.; Clemente, C.; Cunha, Â.; Delgadillo, I.; Romalde, J.; Nunes, M.L.; Almeida, A. Bacteriophages with potential to inactivate Salmonella Typhimurium: Use of single phage suspensions and phage cocktails. Virus Res. 2016, 220, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.H. Bacteriophages; John Wiley and Sons Inc: New York, 1959. [Google Scholar]
- Jakočiūnė, D.; Moodley, A. A Rapid Bacteriophage DNA Extraction Method. Methods Protoc. 2018, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001; ISBN 9780879695774. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Schultz, M.B.; Zobel, J.; Holt, K.E. Bandage: Interactive visualization of de novo genome assemblies. Bioinformatics 2015, 31, 3350–3352. [Google Scholar] [CrossRef]
- Bushnell, B.; Rood, J.; Singer, E. BBMerge – Accurate paired shotgun read merging via overlap. PLoS One 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Garneau, J.R.; Depardieu, F.; Fortier, L.C.; Bikard, D.; Monot, M. PhageTerm: A tool for fast and accurate determination of phage termini and packaging mechanism using next-generation sequencing data. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Millard, A. Phage genome annotation: Where to begin and end. PHAGE Ther. Appl. Res. 2021, 2, 183–193. [Google Scholar] [CrossRef]
- Nayfach, S.; Camargo, A.P.; Schulz, F.; Eloe-Fadrosh, E.; Roux, S.; Kyrpides, N.C. CheckV assesses the quality and completeness of metagenome-assembled viral genomes. Nat. Biotechnol. 2021, 39, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Bouras, G.; Nepal, R.; Houtak, G.; Psaltis, A.J.; Wormald, P.J.; Vreugde, S. Pharokka: a fast scalable bacteriophage annotation tool. Bioinformatics 2023, 39, 1–4. [Google Scholar] [CrossRef]
- Terzian, P.; Ndela, E.O.; Galiez, C.; Lossouarn, J.; Bucio, R.E.P.; Mom, R.; Toussaint, A.; Petit, M.-A.; Enault, F. PHROG: families of prokaryotic virus proteins clustered using remote homology. NAR Genomics Bioinforma. 2021, 3, lqab067. [Google Scholar] [CrossRef]
- Hyman, P.; Abedon, S.T. Practical methods for determining phage growth parameters. In Bacteriophages: methods and protocols, volume 1: isolation, characterization, and interactions; 2009; Vol. 501 ISBN 9781603271646. [CrossRef]
| Bacterial strains | Phage KP-1 | |
|---|---|---|
| Spot test | EOP (%) | |
| Klebsiella pneumoniae Scc 24 | + | 100 |
| Klebsiella pneumoniae Scc 1 | - | 0 |
| Klebsiella pneumoniae Scc 5 | - | 0 |
| Klebsiella pneumoniae Scc 11 | - | 0 |
| Klebsiella pneumoniae Scc 15 | - | 0 |
| Klebsiella pneumoniae Scc 17 | - | 0 |
| Escherichia coli ATCC 13706 | + | 32.98 ± 2.85 |
| Escherichia coli ATCC 25922 | - | 0 |
| Escherichia coli Scc 9 | - | 0 |
| Escherichia coli Scc 33 | - | 0 |
| Escherichia coli Scc 34 | - | 0 |
| Escherichia coli Scc 35 | - | 0 |
| Escherichia coli Scc 36 | - | 0 |
| Escherichia coli Scc 37 | - | 0 |
| Escherichia coli Scc 39 | - | 0 |
| Escherichia coli Scc 40 | - | 0 |
| Escherichia coli Scc 41 | - | 0 |
| Escherichia coli Scc 43 | - | 0 |
| Escherichia coli Scc 45 | - | 0 |
| Escherichia coli Scc 47 | - | 0 |
| Escherichia coli Scc 48 | - | 0 |
| Escherichia coli Scc 49 | - | 0 |
| Escherichia coli Scc 51 | - | 0 |
| Escherichia coli Scc 52 | - | 0 |
| Escherichia coli Scc 53 | - | 0 |
| Escherichia coli Scc 55 | - | 0 |
| Escherichia coli Scc 56 | - | 0 |
| Escherichia coli Scc 58 | - | 0 |
| Escherichia coli Scc 69 | - | 0 |
| Escherichia coli Scc 77 | - | 0 |
| Escherichia coli Scc 78 | - | 0 |
| Escherichia coli Scc 91 | - | 0 |
| Escherichia coli Scc 98 | - | 0 |
| Escherichia coli BC30 | - | 0 |
| Escherichia coli AE11 | - | 0 |
| Escherichia coli AD6 | - | 0 |
| Escherichia coli AF15 | - | 0 |
| Escherichia coli AN19 | - | 0 |
| Escherichia coli AC5 | - | 0 |
| Escherichia coli AJ23 | - | 0 |
| Escherichia coli BN65 | - | 0 |
| Escherichia coli BM62 | - | 0 |
| Citrobacter freundii 6F | - | 0 |
| Enterobacter cloacae | - | 0 |
| Providencia sp. | - | 0 |
| Salmonella enterica serovar Enteriditis CVA | - | 0 |
| Salmonella enterica serovar Enteriditis CVB | - | 0 |
| Salmonella enterica serovar Enteriditis CVC | - | 0 |
| Salmonella enterica serovar Enteriditis CVD | - | 0 |
| Salmonella enterica serovar Enteriditis CVE | - | 0 |
| Salmonella enterica serovar Typhimurium ATCC 14028 | - | 0 |
| Salmonella enterica serovar Typhimurium ATCC 13311 | - | 0 |
| Shigella flexneri DSM 4782 | - | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





