Submitted:
04 January 2024
Posted:
08 January 2024
You are already at the latest version
Abstract
Keywords:
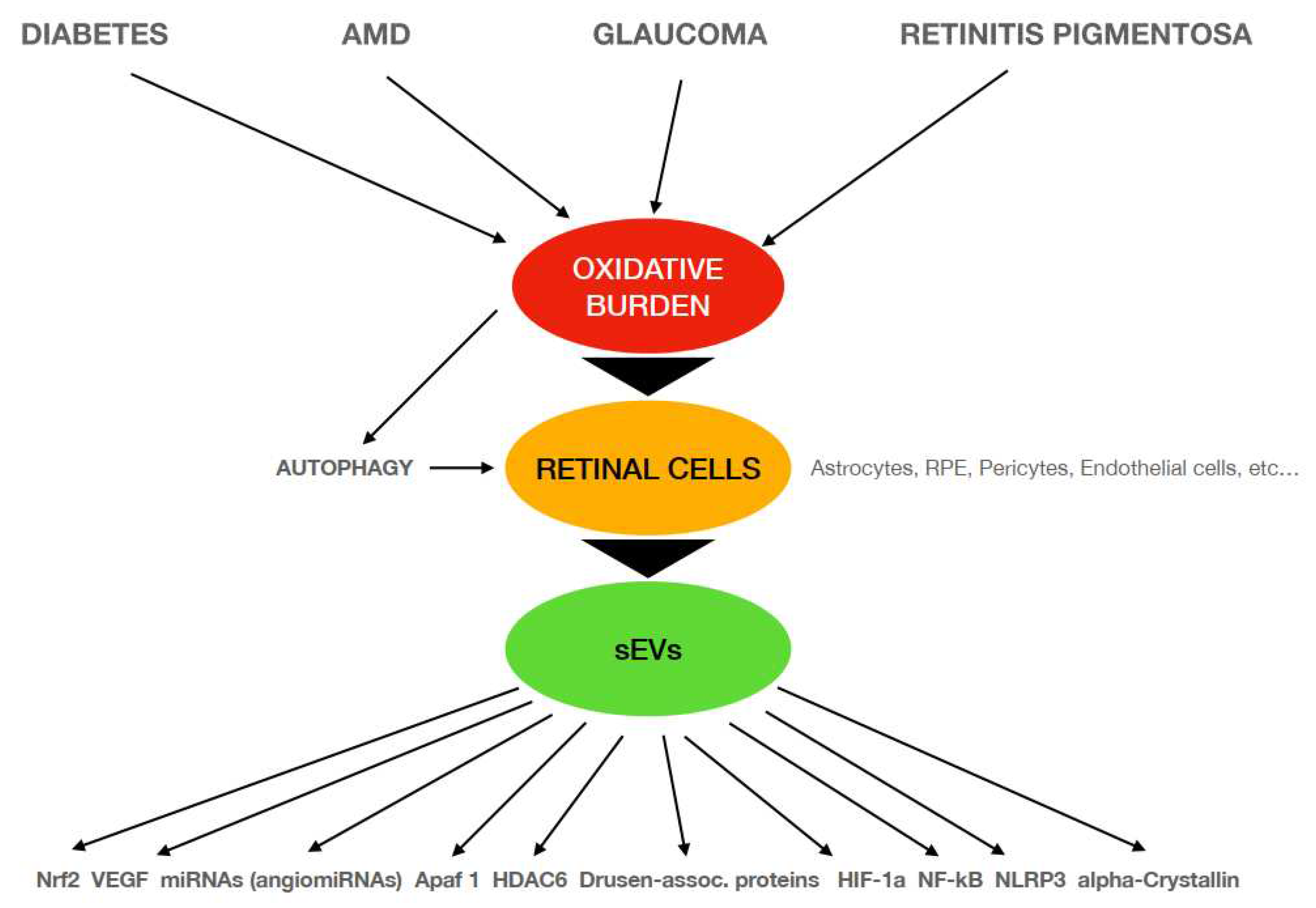
1. Introduction
2. Oxidative Pathophysiological Mechanisms
2.1. Extracellular Vesicles
2.2. Angiogenesis
2.3. Autophagy and sEVs
2.4. Micro RNAs
Funding
References
- Meister, M.; Tessies-Lavigne, M. Low-level visual processing: the retina. In Principles of Neural Sciences; Kandel, E.R., Schwartz, J.M., Jessell, T.M., Siegelbaum, S.A., Hudspeth, A.J., Eds.; McGraw-Hill: New York, 2013; pp. 577-601.
- Wässle, H.; Boycott, B.B. Functional architecture of the mammalian retina. Physiol. Rev. 1991, 71, 447-480. [CrossRef]
- Panda-Jonas, S.; Jonas, J.B.; Jakobczyk-Zmija, M. (1996). Retinal pigment epithelial cell count, distribution, and correlations in normal human eyes. Am. J. Ophthalmol. 1996, 121, 181-189. [CrossRef]
- Boulton, M.; Dayhaw-Barker, P. The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye 2001, 15, 384-389. [CrossRef]
- Marmor, M.F. Structure and function of the retinal pigment epithelium. Int. Ophthalmol. Clin. 1975, 15, 115-130. [CrossRef]
- Somasundaran, S.; Constable, I.J.; Mellough, C.B.; Carvalho, L.S. Retinal pigment epithelium and age-related macular degeneration: A review of major disease mechanisms. Clin. Exp. Ophthalmol. 2020, 48, 1043-1056. [CrossRef]
- Tonade, D.; Kern, T.S. Photoreceptor cells and RPE contribute to the development of diabetic retinopathy. Prog. Retin. Eye Res. 2021, 83, 100919. [CrossRef]
- Beatty, S.; Koh, H.-H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [CrossRef]
- Miranda, M.; Johnson, L.E.; Ahuja, S.; Ekstrom, P.A.; Romero, F.J.; van Veen, T. Significant photoreceptor rescue by treatment with a combination of antioxidants in an animal model for retinal degeneration. Neuroscience 2007, 145, 1120–1129. [CrossRef]
- Cantó, A.; Martínez-González, J.; Almansa, I.; López-Pedrajas, R.; Hernández-Rabaza, V.; Olivar, T.; Miranda, M. Time-course changes in oxidative stress and inflammation in the retinas of rds mice: a retinitis pigmentosa model. Antioxidants (Basel) 2022, 11, 1950. [CrossRef]
- van Reyk, D.M.; Gillies, M.C.; Davies, M.J. The retina: oxidative stress and diabetes. Redox Rep. 2003, 8, 187-192. [CrossRef]
- Romero F.J.; Bosch-Morell, F.; Romero M.J.; Jareño, E.J.; Romero, B.; Marín, N.; Romá J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106 (suppl. 5), 1229-1234.
- Puertas, F.J.; Díaz-Llopis, M.; Chipont, E.; Romá, J.; Raya, A.; Romero, F.J. Glutathione system of human retina. Enzymatic conjugation of lipid peroxidation products. Free Radical Biol. Med. 1993, 14, 549-551. [CrossRef]
- Farrar, G.J. On the genetics of retinitis pigmentosa and on mutation-independent approaches to therapeutic intervention. EMBO J. 2002, 21, 857–864.
- Stoorvogel, W.; Kleijmeer, M.J.; Geuze, H.J.; Raposo, G. The biogenesis and functions of exosomes. Traffic 2002, 3, 321-330. [CrossRef]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nature Comm. 2011, 2, 180. [CrossRef]
- Aliotta, J. M., Pereira, M., Johnson, K. W., de Paz, N., Dooner, M. S., Puente, N., Ayala, C., Brilliant, K.; Berz, D.; Lee, D.; Ramratnam, B.; McMillan, P. N.; Hixson, D. C.; Josic, D.; Quesenberry, P. J. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp. Hematol. 2010, 38, 233-245. [CrossRef]
- Baixauli, F.; López-Otín, C.; Mittelbrunn, M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front. Immunol. 2014, 5, 403. [CrossRef]
- Castellana, D.; Zobairi, F.; Martinez, M.C.; Panaro, M.A.; Mitolo, V.; Freyssinet, J.M.; Kunzelmann, C. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009, 69, 785-793. [CrossRef]
- Sadallah, S.; Eken, C.; Schifferli, J.A. Ectosomes as modulators of inflammation and immunity. Clin. Exp. Immunol. 2011, 163, 26-32. [CrossRef]
- Tetta, C.; Bruno, S.; Fonsato, V.; Deregibus, M.C.; Camussi, G. The role of microvesicles in tissue repair. Organogenesis 2011, 7, 105-115. [CrossRef]
- Atienzar-Aroca, S.; Flores-Bellver, M.; Serrano-Heras, G.; Martinez-Gil, N.; Barcia, J. M.; Aparicio, S.; Perez-Cremades, D.; Garcia-Verdugo, J.M.; Diaz-Llopis, M.; Romero, F.J.; Sancho-Pelluz, J. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J. Cell. Mol. Med. 2016, 20, 1457–1466. [CrossRef]
- Biasutto, L.; Chiechi, A.; Couch, R.; Liotta, L.A.; Espina, V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp. Cell Res. 2013, 319, 2113–2123. [CrossRef]
- Freeman, D.W.; Noren Hooten, N.; Eitan, E.; Green, J.; Mode, N.A.; Bodogai, M.; Zhang, Y.; Lehrmann, E.; Zonderman, A.B.; Biragyn, A.; et al. Altered extracellular vesicle concentration, cargo, and function in diabetes. Diabetes 2018, 67, 2377–2388. [CrossRef]
- Oltra, M.; Vidal-Gil, L.; Maisto, R.; Oltra, S.S.; Romero, F.J.; Sancho-Pelluz, J.; Barcia, J. M. MiR302a and 122 are deregulated in small extracellular vesicles from ARPE-19 cells cultured with H2O2. Sci. Rep. 2019, 9, 17954. [CrossRef]
- Greening, D.W.; Simpson, R.J. Understanding extracellular vesicle diversity–current status. Expert Rev. Proteomics 2018, 15, 887–910.
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 2018, 19, 213–228. [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J. D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G. K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [CrossRef]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: from garbage bins to promising therapeutic targets. Int. J. Mol. Sci. 2017, 18, 538. [CrossRef]
- Kao, C.Y.; Papoutsakis, E.T. Extracellular vesicles: exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr. Opin. Biotechnol. 2019, 60, 89–98. [CrossRef]
- Fries, G.R.; Quevedo, J. Exosomal microRNAs as potential biomarkers in neuropsychiatric disorders. Methods Mol. Biol. 2018, 1733, 79-85.
- Roy, S.; Hochberg, F. H.; Jones, P. S. Extracellular vesicles: the growth as diagnostics and therapeutics; a survey. J. Extracell. Vesicles 2018, 7, 1438720. [CrossRef]
- Van der Merwe, Y.; Steketee, M.B. Extracellular vesicles: biomarkers, therapeutics, and vehicles in the visual system. Curr. Ophthalmol. Rep. 2017, 5, 276–28.
- Klingeborn, M.; Dismuke, W.M.; Bowes Rickman, C.; Stamer, W.D. Roles of exosomes in the normal and diseased eye. Prog. Retin. Eye Res. 2017, 59, 158-177. [CrossRef]
- Malik, Z.A.; Kott, K.S.; Poe, A.J.; Kuo, T.; Chen, L.; Ferrara, K.W.; Knowlton, A.A. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H954-H965. [CrossRef]
- Atienzar-Aroca, S.; Serrano-Heras, G.; Freire Valls, A.; Ruiz de Almodovar, C.; Muriach, M.; Barcia, J.M.; Garcia-Verdugo, J.M.; Romero, F.J.; Sancho-Pelluz, J. Role of retinal pigment epithelium-derived exosomes and autophagy in new blood vessel formation. J. Cell. Mol. Med. 2018, 22, 5244-5256. [CrossRef]
- Flores-Bellver, M.; Mighty, J.; Aparicio-Domingo, S.; Li, K.; Shi, C.; Zhou, J.; Cobb, H.; McGrath, P.; Michelis, G.; Lenhart, P.; et al. Extracellular vesicles released by human retinal pigment epithelium mediate increased polarised secretion of drusen proteins in response to AMD stressors. J. Extracell. Vesicles 2021, 10, e12165. [CrossRef]
- Ke, Y.; Fan, X.; Rui, H.; Xinjun, R.; Dejia, W.; Chuanzhen, Z.; Li, X. Exosomes derived from RPE cells under oxidative stress mediate inflammation and apoptosis of normal RPE cells through Apaf1/caspase-9 axis. J. Cell. Biochem. 2020, 121, 4849-4861.
- Wang, Y.; Zhang, Q.; Yang, G.; Wei, Y.; Li, M.; Du, E.; Li, H.; Song, Z.; Tao, Y. RPE-derived exosomes rescue the photoreceptors during retina degeneration: an intraocular approach to deliver exosomes into the subretinal space. Drug Deliv. 2021, 28, 218-228.
- 40. Shah N, Ishii M, Brandon C, Ablonczy Z, Cai J, Liu Y, Chou CJ, Rohrer B. Extracellular vesicle-mediated long-range communication in stressed retinal pigment epithelial cell monolayers. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2610-2622. [CrossRef]
- Kannan, R.; Sreekumar, P.G.; Hinton, D.R. Novel roles for α-crystallins in retinal function and disease. Prog. Retin. Eye Res. 2012, 31, 576-604. [CrossRef]
- Sreekumar, P.G.; Kannan, R.; Kitamura, M.; Spee, C.; Barron, E.; Ryan, S.J.; Hinton, D.R. αB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One 2010, 5, e12578. [CrossRef]
- Kannan, R.; Sreekumar, P.G.; Hinton, D.R. Alpha crystallins in the retinal pigment epithelium and implications for the pathogenesis and treatment of age-related macular degeneration. Biochim. Biophys. Acta 2016, 1860, 258-268. [CrossRef]
- Almansa, I.; Fernández, A.; García-Ruiz, C.; Muriach, M.; Barcia, J. M.; Miranda, M.; Fernández-Checa, J.C.; Romero, F.J. Brain mitochondrial alterations after chronic alcohol consumption. J. Physiol. Biochem. 2009, 65, 305-312. [CrossRef]
- Romero, F.J.; Romá, J. Careful consideration of the effects induced by glutathione depletion in rat liver and heart. The involvement of cytosolic and mitochondrial glutathione pools. Chem. Biol. Interact. 1989, 70, 29-37. [CrossRef]
- Romero, F.J.; Sies, H. Subcellular glutathione contents in isolated hepatocytes treated with L-buthionine sulfoximine. Biochem. Biophys. Res. Commun. 1984, 123, 1116-1121. [CrossRef]
- Romero, F.J.; Soboll, S.; Sies, H. Mitochondrial and cytosolic glutathione after depletion by phorone in isolated hepatocytes. Experientia 1984, 40, 365-367. [CrossRef]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Role of mitochondrial DNA damage in ROS-mediated pathogenesis of age-related macular degeneration (AMD). Int. J. Mol. Sci. 2019, 20, 2374. [CrossRef]
- Seong, H.R.; Noh, C.H.; Park, S.; Cho, S.; Hong, S.J.; Lee, A.Y.; Geum, D.; Hong, S.C.; Park, D.; Kim, T.M.; et al. Intraocular pressure-lowering and retina-protective effects of exosome-rich conditioned media from human amniotic membrane stem cells in a rat model of glaucoma. Int. J. Mol. Sci. 2023, 24, 8073. [CrossRef]
- Hernandez, B.J.; Skiba, N.P.; Plössl, K.; Strain, M.; Liu, Y.; Grigsby, D.; Kelly, U.; Cady, M.A.; Manocha, V.; Maminishkis, A.; et al. Polarized desmosome and hemidesmosome shedding via small extracellular vesicles is an early indicator of outer blood-retina barrier dysfunction. J. Extracell. Biol. 2023, 2, e116. [CrossRef]
- Tang, Y.; Kang, Y.; Zhang, X.; Cheng, C. Mesenchymal stem cell exosomes as nanotherapeutics for dry age-related macular degeneration. J. Control Release 2023, 357, 356-370. [CrossRef]
- Du, H.; Huang, Z.; Zhou, X.; Kuang, X.; Long, C.; Tang, H.; Zeng, J.; Huang, H.; Liu, H.; Zhu B.; et al. Oxidative stress-induced lncRNA CYLD-AS1 promotes RPE inflammation via Nrf2/miR-134-5p/NF-κB signaling pathway. FASEB J. 2022, 36, e22577.
- Ebrahimi, K.B.; Fijalkowski, N.; Cano, M.; Handa, J.T. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J. Pathol. 2013, 229, 729-742. [CrossRef]
- Ebrahimi, K.B.; Fijalkowski, N.; Cano, M.; Handa, J.T. Oxidized low-density-lipoprotein-induced injury in retinal pigment epithelium alters expression of the membrane complement regulatory factors CD46 and CD59 through exosomal and apoptotic bleb release. Adv. Exp. Med. Biol. 2014, 801, 259-65.
- Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971, 285, 1182-1186. [CrossRef]
- Huang, H. Pericyte-endothelial interactions in the retinal microvasculature. Int. J. Mol. Sci. 2020, 21, 7413. [CrossRef]
- Mayo, J.N.; Bearden, S.E. Driving the hypoxia-inducible pathway in human pericytes promotes vascular density in an exosome-dependent manner. Microcirculation 2015, 22, 711–723. [CrossRef]
- Xu, B.; Zhang, Y.; Du, X.F.; Li, J.; Zi, H.X.; Bu, J.W.; Yan, Y.; Han, H.; Du, J.L. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 2017, 27, 882–897. [CrossRef]
- Huang, C.; Fisher, K.P.; Hammer, S.S.; Navitskaya, S.; Blanchard, G.J.; Busik, J.V. Plasma exosomes contribute to microvascular damage in diabetic retinopathy by activating the classical complement pathway. Diabetes 2018, 67, 1639–1649. [CrossRef]
- Zagrean, A.M.; Hermann, D.M.; Opris, I.; Zagrean, L.; Popa-Wagner, A. Multicellular crosstalk between exosomes and the neurovascular unit after cerebral ischemia. Therapeutic implications. Front. Neurosci. 2018, 12, 811. [CrossRef]
- Bergers, G., Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nature Rev. Cancer 2003, 3, 401-410. [CrossRef]
- He, W.; Lin, A.; Wang, C. Adipocyte-derived exosomal LINC00968 promotes mouse retina microvascular endothelial cell dysfunction in a high-glucose environment by modulating the miR-361-5p/TRAF3 axis. Horm. Metab. Res. 2023, 55, 124-135. [CrossRef]
- Hajrasouliha, A.R.; Jiang, G.; Lu, Q.; Lu, H.; Kaplan, H. J.; Zhang, H. G.; Shao, H. Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J. Biol. Chem. 2013, 288, 28058-28067. [CrossRef]
- D’Amore P.A. Mechanisms of retinal and choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 1994, 35, 3974-3979.
- Campochiaro, P.A. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog. Retin. Eye Res. 2015, 49, 67-81. [CrossRef]
- Ebrahim, N.; El-Halim, H.E.A.; Helal, O.K.; El-Azab, N.E.; Badr, O.A.M.; Hassouna, A.; Saihati, H.A.A.; Aborayah, N.H.; Emam, H.T.; El-Wakeel, H.S.; et al. Effect of bone marrow mesenchymal stem cells-derived exosomes on diabetes-induced retinal injury: Implication of Wnt/ b-catenin signaling pathway. Biomed. Pharmacother. 2022, 154, 113554. [CrossRef]
- Liu, J.; Copland, D.A.; Theodoropoulou, S.; Chiu, H. A.A.; Barba, M.D.; Mak, K.W.; Dick, A.D. Impairing autophagy in retinal pigment epithelium leads to inflammasome activation and enhanced macrophage-mediated angiogenesis. Sci. Rep. 2016, 6, 20639. [CrossRef]
- Bhattacharya, S.; Pal, K.; Sharma, A.K.; Dutta, S.K.; Lau, J.S.; Yan, I.K.; Patel, T.C. GAIP interacting protein C-terminus regulates autophagy and exosome biogenesis of pancreatic cancer through metabolic pathways. PloS One 2014, 9, e114409. [CrossRef]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One 2009, 4, e4160. [CrossRef]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy, exosomes and drusen formation in age-related macular degeneration. Autophagy 2009, 5, 563–564. [CrossRef]
- Du, J.; Teng, R.J.; Guan, T.; Eis, A.; Kaul, S.; Konduri, G.G.; Shi, Y. Role of autophagy in angiogenesis in aortic endothelial cells. Am. J. Physiol.-Cell Physiol. 2012, 302, C383-C391. [CrossRef]
- Zhu, L.; Zang, J.; Liu, B.; Yu, G.; Hao, L.; Liu, L.; Zhong, J. Oxidative stress-induced RAC autophagy can improve the HUVEC functions by releasing exosomes. J. Cell Physiol. 2020, 235, 7392-7409. [CrossRef]
- Lu, W.H.; Shi, Y.X.; Ma, Z.L.; Wang, G.; Liu, L.; Chuai, M.; Yang, X. Proper autophagy is indispensable for angiogenesis during chick embryo development. Cell Cycle 2016, 15, 1742-1754. [CrossRef]
- Li, R.; Du, J.; Chang, Y. Role of autophagy in hypoxia-induced angiogenesis of RF/6A cells in vitro. Curr. Eye Res. 2016, 41, 1566-1570. [CrossRef]
- Carozza, G.; Tisi, A.; Capozzo, A.; Cinque, B.; Giovanelli, A.; Feligioni, M.; Floti, V.; Maccarone, R. New insights into dose-dependent effects of curcumin on ARPE-19 cells. Int. J. Mol. Sci. 2022, 23, 14771. [CrossRef]
- Zhang, W.; Ma, Y.; Zhang, Y.; Yang, J.; He, G.; Chen, S. Photo-oxidative blue-light stimulation in retinal pigment epithelium cells promotes exosome secretion and increases the activity of the NLRP3 inflammasome. Curr. Eye Res. 2019, 44, 67-75. [CrossRef]
- Xue, Z.; Zhang, Z.; Liu, H.; Li, W.; Guo, X.; Zhang, Z.; Liu, Y.; Jia, L.; Li, Y.; Ren, Y.; et al. lincRNA-Cox2 regulates NLRP3 inflammasome and autophagy mediated neuroinflammation. Cell Death Differ. 2019, 26, 130-145.
- Kang, G.Y.; Bang, J.Y.; Choi, A.J.; Yoon, J.; Lee, W.C.; Choi, S.; Yoon, S.; Kim, H. C.; Baek, J. H.; Park, H. S.; Lim, H. J.; Chung, H. Exosomal proteins in the aqueous humor as novel biomarkers in patients with neovascular age related macular degeneration. J. Proteome Res. 2014, 13, 581–595. [CrossRef]
- Lee, J.K.; Park, S.R.; Jung, B.K.; Jeon, Y.K.; Lee, Y.S.; Kim, M.K.; Kim, C.W. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PloS One 2013, 8, e84256. [CrossRef]
- Flores-Bellver, M.; Bonet-Ponce, L.; Barcia, J.M.; Garcia-Verdugo, J.M.; Martinez-Gil, N.; Saez-Atienzar, S.; Romero, F.J. Autophagy and mitochondrial alterations in human retinal pigment epithelial cells induced by ethanol: implications of 4-hydroxy-nonenal. Cell Death Dis. 2014, 5, e1328. [CrossRef]
- Kannan, R.; Zhang, N.; Sreekumar, P.G.; Spee, C.K.; Rodriguez, A.; Barron, E.; Hinton, D.R. Stimulation of apical and basolateral VEGF-A and VEGF-C secretion by oxidative stress in polarized retinal pigment epithelial cells. Mol. Vis. 2006, 12, 1649-1659.
- Maisto, R.; Oltra, M.; Vidal-Gil, L.; Martínez-Gil, N.; Sancho-Pellúz, J.; Filippo, C.D.; Rossi, S.; D’Amico, M.; Barcia, J.M.; Romero, F.J. ARPE-19-derived VEGF-containing exosomes promote neovascularization in HUVEC: the role of the melanocortin receptor 5. Cell Cycle 2019, 18, 413-424. [CrossRef]
- Vidal-Gil, L.; Sancho-Pelluz, J.; Zrenner, E.; Oltra, M.; Sahaboglu, A. Poly ADP ribosylation and extracellular vesicle activity in rod photoreceptor degeneration. Sci. Rep. 2019, 9, 3758. [CrossRef]
- Sahaboglu, A.; Vidal-Gil, L.; Sancho-Pelluz, J. Release of retinal extracellular vesicles in a model of retinitis pigmentosa. Adv. Exp. Med. Biol. 2019, 1185, 431-436.
- Greco, M.; Chiefari, E.; Accattato, F.; Corigliano, D. M.; Arcidiacono, B.; Mirabelli, M.; Liguori, R.; Brunetti, F.S.; Pullano, S.A.; Scorcia, V.; et al. Microrna-1281 as a novel circulating biomarker in patients with diabetic retinopathy. Front. Endocrinol. 2020, 11, 1–12. [CrossRef]
- Li, X.; Yu, Z. W.; Wang, Y.; Fu, Y.H.; Gao, X.Y. MicroRNAs: potential targets in diabetic retinopathy. Horm. Metab. Res. 2020, 52, 142–148. [CrossRef]
- Ren, C.; Liu, Q.;Wei, Q.; Cai, W.; He, M.; Du, Y.; Xu, D.;Wu, Y.; Yu, J. Circulating miRNAs as potential biomarkers of age-related macular degeneration. Cell Physiol. Biochem. 2017, 41, 1413–1423. [CrossRef]
- ElShelmani, H.; Wride, M.A.; Saad, T.; Rani, S.; Kelly, D.J.; Keegan, D. The role of deregulated microRNAs in age-related macular degeneration pathology. Transl. Vis. Sci. Technol. 2021, 10, 12. [CrossRef]
- Morris, D.R.; Bounds, S.E.; Liu, H.; Ding, W.Q.; Chen, Y.; Liu, Y.; Cai, J. Exosomal miRNA transfer between retinal microglia and RPE. Int. J. Mol. Sci. 2020, 21, 3541. [CrossRef]
- Li, W.; Jin, L.Y.; Cui, Y.B.; Xie, N. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-17-3p ameliorates inflammatory reaction and antioxidant injury of mice with diabetic retinopathy via targeting STAT1. Int. Immunopharmacol. 2021, 90, 107010. [CrossRef]
- Wooff, Y.; Cioanca, A.V.; Chu-Tan, J.A.; Aggio-Bruce, R.; Schumann, U.; Natoli, R. Small-medium extracellular vesicles and their miRNA cargo in retinal health and degeneration: mediators of homeostasis, and vehicles for targeted gene therapy. Front. Cell. Neurosci. 2020, 14, 160. [CrossRef]
- Tong, Y.; Zhou, Y.L.; Wang, Y.X.; Zhao, P.Q.; Wang, Z.Y. Retinal pigment epithelium cell-derived exosomes: Possible relevance to CNV in wet-age related macular degeneration. Med. Hypotheses 2016, 97, 98-101. [CrossRef]
- Oltra, M.; Vidal-Gil, L.; Maisto, R.; Sancho-Pelluz, J.; Barcia J. M. Oxidative stress-induced angiogenesis is mediated by miR-205-5p. J. Cell. Mol. Med. 2020, 24, 1428-1436.
- Oltra, M.; Martínez-Santos, M.; Ybarra, M.; Rowland, H.; Muriach, M.; Romero F.J.; Sancho-Pelluz, J.; Barcia, J.M. Oxidative-induced angiogenesis is modulated by small extracellular vesicle miR-302a-3p cargo in retinal pigment epithelium cells. Antioxidants (Basel) 2022, 11, 818. [CrossRef]
- Liu, C.-H.; Huang, S.; Britton, W. R.; Chen, J. MicroRNAs in vascular eye diseases. Int. J. Mol. Sci. 2020, 21, 649. [CrossRef]
- Ebrahim, N.; El-Halim, H.E.A.; Helal, O.K.; El-Azab, N.E.; Badr, O.A.M.; Hassouna, A.; Saihati, H.A.A.; Aborayah, N.H.; Emam, H.T.; El-Wakeel, H.S.; et al. Effect of bone marrow mesenchymal stem cells-derived exosomes on diabetes-induced retinal injury: Implication of Wnt/ b-catenin signaling pathway. Biomed. Pharmacother. 2022, 154, 113554. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




