1. Introduction
Medicinal plants are a rich source of traditional and modern medicine and play an important role in the development of human life. Species in the genus
Polygonatum are perennial herbs of Liliaceae. The species
P. cyrtonema Hua (“Duohua Huangjin” in Chinese), a known medicinal plant, is recorded in China Pharmacopoeia and has a long history of application in Chinese folk medicine [
1,
2]. Modern pharmacological studies have shown that
P. cyrtonema rhizome plays an important role in anti-aging, immune function regulation, control of blood glucose and blood lipid, memory improvement, and antitumor and antibacterial effects [
3,
4,
5]. This function is attributed to the abundant functional medicinal components derived from the rhizome, such as polysaccharides and secondary metabolites (e.g., saponins, flavonoids, and alkaloids) [
6,
7]. Therefore,
P. cyrtonema rhizome is regarded as an important medicinal and edible food resource and has received increasing attention in recent years in China.
Processing, Paozhi in Chinese, is an ancient Chinese pharmaceutic technique to facilitate the clinical use of Chinese herbal medicines according to traditional Chinese medicine theory [
8]. Processing can reduce toxicity, reinforce efficacy, alter the energetic nature and therapeutic direction, and improve the flavor of herbal medicines; these transformations increase the therapeutic effectiveness and applicability of herbal medicines in individualized treatment [
9]. For the purpose of detoxification and enhancing efficacy,
P. cyrtonema rhizome (crude medicine) is usually processed using a traditional method known as “nine-steaming and nine-drying,” which involves nine cycles of steaming and sun-drying [
10,
11]. In recent years, several studies have investigated Polygonatum rhizome treated with nine processing cycles. These works include examining the structural changes of polysaccharides, analyzing polysaccharide and extract contents, and determining the relationship between processing degree and the internal and external quality of rhizoma based on color change [
11,
12,
13]. Some findings suggest that nine processing cycles may lead to the loss and change of polysaccharide in
P. cyrtonema rhizome, which affect factors such as molecular weight, monosaccharide composition, and particle size distribution [
14]. Secondary metabolites (e.g., alkaloids, flavonoids, amines, glycosides, and steroids), a large group of active compounds with low molecular weight are produced at specific stages of the life cycle or morphological differentiation [
15]. These secondary metabolites play an important role in the interaction of plants with their environment and have been used in drug and pharmaceutical industries to treat various disorders [
16,
17,
18,
19]. Therefore, investigating the changes in secondary metabolites of
P. cyrtonema rhizome in abundance and structural transformation is crucial to understand its nutritional value and processing efficacy. However, only a limited number of studies have focused on secondary metabolites in
P. cyrtonema rhizome to date. Thus, the global dynamic changes in this species during processing remain unclear. In addition, whether nine processing cycles can retain the functional components to the greatest extent is uncertain. Accordingly, a comprehensive investigation into the processing-induced dynamic changes in secondary metabolites of
P. cyrtonema rhizome is necessary.
Plant metabolomics is based on the nonbiased and high-throughput analyses of complex metabolites contained in plant extracts by HPLC-MS (for nonvolatile compounds), GC-MS (for volatile oil), or NMR [
20]. Metabolomics is considered an important functional genomics tool because metabolites link genotypes and phenotypes [
21,
22,
23,
24,
25]. In recent years, metabolomics has been widely employed in various fields, such as medicinal plants, biological interaction, fruit nutritional quality, and biological activity [
26]. Our present study focuses on the secondary metabolites derived from
P. cyrtonema rhizome, and metabolomics analysis was performed to investigate the dynamic changes in secondary metabolites during traditional processing. Our objectives were to obtain insights into the processing-induced mechanisms involved in the accumulation of secondary metabolites and provide valuable theoretical and metabolic data to support the pharmacological study on
P. cyrtonema.
2. Materials and Methods
2.1. Plant Material Selection and Pre-Treatment
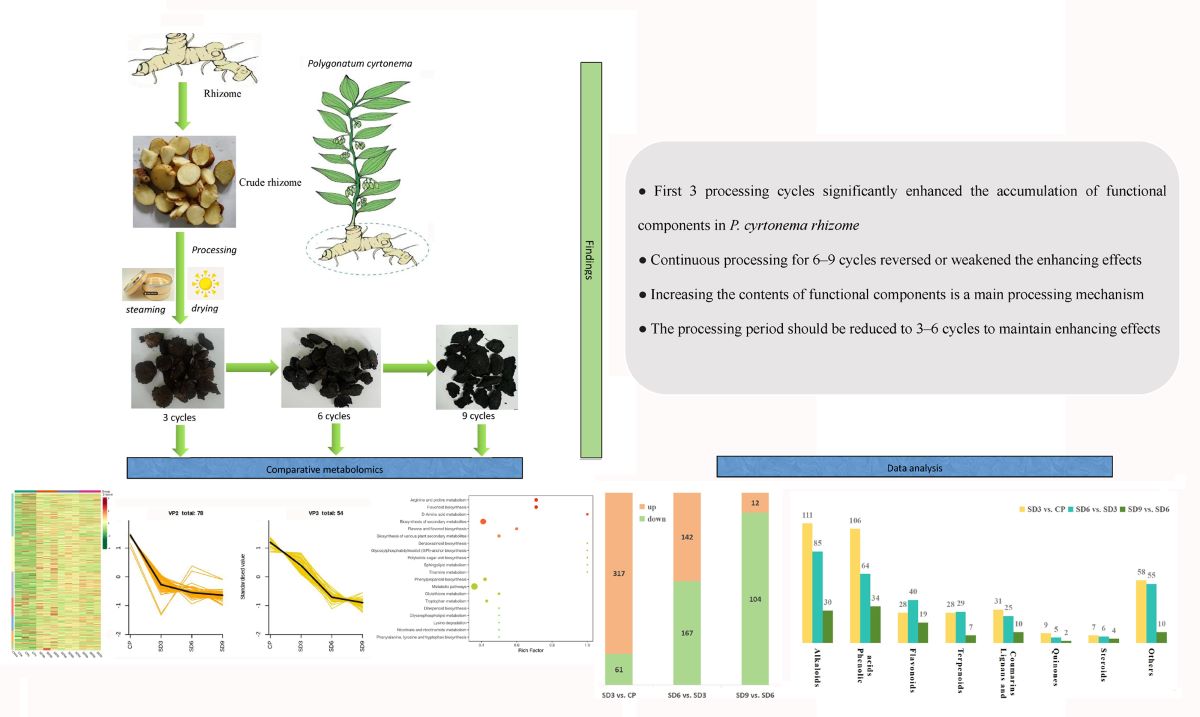
Nine fresh, healthy, five-year-old Polygonatum cyrtonema Hua. plants were collected from plantations in Quzhou (28°37′N, 118°49′E; altitude 446 m), Zhejiang Province (Southeast China), in April 2023. The P. cyrtonema rhizomes were harvested from the plants and cleaned with water, and then, the rhizomes of 3 P. cyrtonema plants were mixed as a biological replicate. The whole rhizomes were divided uniformly into two groups: one as crude rhizome (CP group, non-processed) with three replicates, and the other stored at 4 °C for a short period for follow-up “nine-steaming and nine-drying” as processed rhizome (SD groups) with three replicates. The samples in the CP group were further cut into slices and quickly frozen in liquid nitrogen, which were then stored at −80 °C until further analysis.
2.2. Polygonatum Rhizome Processing
The processing of
P. cyrtonema rhizome followed a previously described method [
13]. In brief, the fresh
P. cyrtonema rhizome was placed in a steamer to steam for 6 h, subsequently moisturized overnight, and then dried in an oven at 50 °C for 8 h with 1–9 cycles. Next, rhizomes subjected to 3, 6, and 9 cycles of processing were collected as SD3, SD6, and SD9 groups, respectively. Similarly, the samples in the SD groups were further cut into slices and quickly frozen in liquid nitrogen, which were then stored at −80 °C until further analysis (
Figure 1).
2.3. Sample Preparation and Extraction for Widely Targeted Metabolomic Analysis
Sample preparation and extraction followed the methods provided by Metware Biotechnology Co., Ltd. (Wuhan, China). Briefly, the P. cyrtonema rhizome samples were freeze-dried in a lyophilizer (Scientz-100F) using vacuum freeze-drying technology, followed by grinding for 1.5 min at 30 Hz with a grinder (MM 400, Retsch, Germany). Then, 50 mg of powder from each sample was weighted and then dissolved in 1.2 mL of 70% methanolic aqueous pre-cooled at −20 °C. After extraction, the mixtures were centrifuged at 12000 rpm for 3 min, and the supernatant was collected and filtered through a microporous membrane (SCAA-104, 0.22 μm pore size; ANPEL, Shanghai, China). Finally, the filtered samples were stored in injection vials for UPLC-MS/MS analysis.
2.4. UPLC Conditions
The
P. cyrtonema rhizome sample extracts were analyzed using the ultra-performance liquid chromatography–electrospray ionization–tandem mass spectrometry system (UPLC-ESI-MS/MS, UPLC, ExionLC™ AD,
https://sciex.com.cn). The UPLC analytical conditions were as follows: column, Agilent SB-C18 (1.8 µm, 2.1 mm×100 mm); the mobile phase consisted of solvent A, pure water with 0.1% formic acid, and solvent B, acetonitrile with 0.1% formic acid. Sample measurements were performed with a gradient program that employed the starting conditions of 95% A, 5% B. Within 9 min, a linear gradient to 5% A, 95% B was programmed, and a composition of 5% A, 95% B was kept for 1 min. Subsequently, a composition of 95% A, 5.0% B was adjusted within 1.1 min and kept for 2.9 min. The flow velocity was set at 0.35 mL per minute, the column oven was set to 40 °C, and the injection volume was 2 μL. The effluent was alternatively connected to an ESI-triple quadrupole-linear ion trap (QTRAP)-MS.
2.5. ESI-Q TRAP-MS/MS
The ESI source operation parameters were as follows: source temperature of 500 °C; ion spray voltage (IS) of 5500 V (positive ion mode)/ −4500 V (negative ion mode); ion source gas I (GSI), gas II (GSII), and curtain gas were set at 50, 60, and 25 psi, respectively; the collision-activated dissociation was set to high. Triple quadrupole (QQQ) scans were acquired as multiple reaction monitoring (MRM) experiments, with collision gas (nitrogen) set to medium. Declustering potential (DP) and collision energy (CE) for individual MRM transitions were obtained with further DP and CE optimization. A specific set of MRM transitions was monitored for each period according to the metabolites eluted within that period.
2.6. Qualitative and Quantitative Analysis of Metabolites
Based on the reference library Metware database MWDB v2.0 (Metware Biotechnology Co., Ltd. Wuhan, China) and publicly available metabolite databases, primary and secondary mass spectrometry data obtained using targeted MRM were subjected to qualitative and quantitative analysis. In brief, high-resolution mass spectrometry AB Sciex TripleTOF 6600 was used for qualitative detection of mixed samples in widely target metabolomics, followed by accurate metabolite quantification using AB Sciex 6500 QTRAP for accurate metabolite quantification. This approach combines the advantages of non-targeted and targeted metabolomics by using high-resolution QQQ mass spectrometry with high sensitivity, high specificity, and excellent quantitation capabilities.
During the qualitative analysis of metabolites, repeat signals of K+, Na+, NH4+, and other substances with large molecular weights were eliminated based on the information of secondary spectrum [
27,
28]. Quantitative analysis was conducted in the MRM mode, where characteristic ions of each metabolite were screened through the QQQ mass spectrometry to obtain signal strengths. After the metabolite profile data of different samples were obtained, integration and correction of chromatographic peaks were performed using MultiQuant v3.0.2 (AB Sciex, Concord, Ontario, Canada). All chromatographic peak area integral data were derived, and the relative content of the corresponding metabolites was calculated based on the peak area integrals [
29,
30].
2.7. Multivariate Statistical Analysis
Metabolite data were log
2-transformed and underwent autoscaling before any statistical analysis. Metabolite data from 12
P. cyrtonema rhizome samples were used for unsupervised principal component analysis (PCA), hierarchical clustering analysis (HCA), and orthogonal partial least squares discriminant analysis (OPLA-DA) using the Metware Cloud, which is a free online platform for data analysis (
https://cloud.metware.cn). Briefly, unsupervised PCA was performed using the “prcomp” function within R v3.5.1 (base package,
https://www.r-project.org). The HCA results of samples and metabolites were presented as heatmaps with dendrograms. Pearson’s correlation coefficients (PCC) between samples were calculated using the “cor” function in R v3.5.1 and v4.4.0 (base package; Hmisc) and presented as only heatmaps. HCA and PCC were obtained using R v2.8.0 (Complex Heatmap). For HCA, normalized signal intensities of metabolites (unit variance scaling, UV scaling) were visualized as a color spectrum. OPLS-DA was performed using R v1.0.1 (MetaboAnalystR). Before OPLS-DA, the data were log2-transformed and underwent mean centering. A permutation test (200 permutations) was performed to avoid overfitting. The OPLS-DA results also include score and permutation plots [
31].
For the two-group analysis, differentially accumulated metabolites were determined by variable importance in project scores (VIP ≥ 1) and fold change ≥ 2 or fold change ≤ 0.5, with VIP values extracted from the OPLS-DA results. The identified differential metabolites were annotated using the Kyoto Encyclopedia of Genes and Genomes (KEGG) compound database (
http://www.kegg.jp/kegg/compound), and annotated metabolites were then mapped to the KEGG pathway database (
http://www.kegg.jp/kegg/pathway.html). Pathways with significantly regulated metabolites were subjected to metabolite set enrichment analysis, and their significance was determined by hypergeometric test’s p-values.
4. Discussion
Processing often leads to the structural transformation of herbal components. During processing, herbal components may undergo oxidation, decomposition, isomerization, hydrolysis, and/or reaction with other constituents to eventually form novel compounds [
32]. Some herbal medicines, such as Aconitum root, Ginseng Radix et Rhizome, and Rhei Radix et Rhizoma, have been demonstrated to possess distinct chemical profiles after processing and showed reduced toxicity or altered therapeutic activities [
9]. Understanding these complicated processing mechanisms provides a theoretical and metabolic data basis for improving processing techniques and for the pharmacological study of
P. cyrtonema.
In the processed rhizome, we found numerous newly added secondary metabolites and a small number of disappeared secondary metabolites, which might result from the formation of novel secondary metabolites and the decomposition of the original secondary metabolites induced by processing. Liang et al. found that, with an increase in processing time, drastic changes occurred in the chemical components of
P. cyrtonema rhizome, such as component isomerization, decreased type and content of primary glycosides, and increased content of aglycones [
33]. Therefore, besides the increased contents of functional components, the structural transformation of secondary metabolites in crude
P. cyrtonema rhizome might also be an important processing mechanism related to the enhancing effect. In this mechanism, the synthesis of novel compounds and decomposition of original secondary metabolites did not occur synergistically: one mainly in the first 3 processing cycles, and the other mainly in the last 6–9 processing cycles.
Apart from the structural transformation of components, directly reducing the contents of toxic components is also a main processing mechanism in Chinese herbal medicines [
9]. Alkaloids are one of the largest groups of plant secondary metabolites, present in several economically relevant plant families [
34]. Extracts from alkaloid-containing plants have been used to treat several ailments such as fever, snakebite, and insanity. However, despite their significant benefits to health and pharmaceutical industries, the toxicity of some types of plant alkaloids, such as pyrrolizidine, tropane, piperidine, indolizidine, and steroidal alkaloids, has been observed in animals and humans [
35,
36]. Alkaloid compounds were found to be the most abundant secondary metabolites in
P. cyrtonema rhizome. Among the 206 alkaloids identified, 20 of them were regarded as potential toxic alkaloids according to the above mentioned reported literature, including 7 piperidine alkaloids, 7 pyridine alkaloids, 2 isoquinoline alkaloids, 2 tropan alkaloids, and 2 steroidal alkaloids. Continuous processing did not lead to the complete decomposition of these alkaloids, and only the relative abundance of piperidine and 6-Deoxyfagomine (piperidine alkaloids) in rhizomes processed for 6 cycles were significantly decreased unlike in crude rhizomes (vp 3 in
Figure 7). Although the toxicity of crude
P. cyrtonema rhizome and related toxic components in it were unclear, our present study suggested that reducing the contents of potential toxic alkaloids and decomposing toxic alkaloids may not be the main mechanisms for the detoxification of
P. cyrtonema rhizome. Therefore, some chemical changes or physically structural alterations may have occurred during processing, which were related to the detoxification of
P. cyrtonema rhizome.
Flavonoids comprise a large and diverse group of polyphenolic compounds with antioxidant, hypoglycemic, hypolipidemic, and anticancer properties [
37]. Flavonoids and their glycosides are important bioactive constituents of the rhizomes of Polygonatum plants. Currently, 61 flavonoids have been isolated from Polygonatum plants, with 13 of them identified in
P. cyrtonema [
4]. The stability of different flavonoids, such as flavanones, flavonols, and flavones, is influenced not only by the processing but also by the flavonoid structure [
38]. A total of 86 flavonoids were identified in this study, which were mainly flavones, flavonols, isoflavones, chalcones, and other types of flavonoids. Continuous processing induced diversified variation patterns in the relative abundance of different flavonoids, as well as the synthesis of novel secondary metabolites and the decomposition of original secondary metabolites. The change profiles observed in most flavonoids suggest that 3–6 processing cycles can enhance efficacy by increasing their contents and inducing the synthesis of 85% newly added secondary metabolites. Continuous processing until 9 cycles will unavoidably weaken the enhancing effect by decreasing the increased contents during 3–6 processing cycles and further decomposing more flavonoids.
Phenolic acids, which are a subclass of plant phenolics, exhibit tremendous antioxidant activity and protective effects, including antimicrobial, anticancer, anti-inflammatory, and anti-mutagenic [
39]. As the main secondary metabolites rich in
P. cyrtonema rhizome, the variation patterns exhibited by most of them suggest that 3 processing cycles induced their significant accumulation and the addition of 91% newly formed secondary metabolites. Saponins in medicinal plants exhibit essential medicinal properties, such as anti-inflammatory, antiviral, insecticidal, and anticancer actions [
40]. Saponins are primarily classified into steroid and triterpenoid saponins based on the structure of the hydrophobic aglycone unit [
41]. As the primary active ingredient, Polygonatum plants are rich in steroidal saponins, and 10 steroidal saponins have been isolated and identified from
P. cyrtonema to date [
42]. A total of 13 steroidal saponins and 3 triterpene saponins were identified in this study. The variation patterns suggest that 3 processing cycles were sufficient to increase the contents of saponins and induce 100% newly added secondary metabolites. Conversely, continuous processing for more than 3 cycles will unavoidably weaken the enhancing effects induced by the first 3 cycles. In addition, saponins might have higher stability than other secondary metabolites in
P. cyrtonema rhizome because the decomposition of only 1 steroidal saponin in crude rhizome was found during the entire processing. A total of 17 kinds of amino acids were detected in crude Polygonatum rhizome, which were important flavor and nutritional components [
43]. However, the effects of traditional processing on amino acid composition, contents, and nutritional value of Polygonatum plants have not been fully understood to date. KEGG enrichment analysis suggests that processing has significant effects on amino acid metabolisms. Therefore, in future work, changes in amino acid metabolisms during processing will be explored to further provide metabolic data support for the nutritional and healthy values of processed
P. cyrtonema rhizome from the aspect of amino acid nutrients.
Our present study reveals complicated changes in secondary metabolites of
P. cyrtonema rhizome during traditional processing with “nine-steaming and nine-drying.” Undoubtedly, processing produces beneficial effects on reinforcing efficacy by promoting the accumulation of functional components and on reducing toxicity to a certain extent by decreasing the contents of toxic components, which is meaningful for edible safety and increasing the medicinal values of
P. cyrtonema rhizome. However, processing also brings adverse effects given that it can significantly decrease the contents of a small number of functional components in the rhizome, even after only 3 cycles. Apart from these functional components belonging to secondary metabolites, polysaccharides are also the main functional component in
P. cyrtonema rhizome. Previous studies have found the highest content of polysaccharide in crude rhizome, with decreasing content in processed rhizome with increasing processing cycles, and 4 processing cycles may be the ideal processing method for Polygonatum to retain more active ingredients [
14,
44]. Based on the comprehensive quality evaluation index, 5 processing cycles were regarded as a turning point with the highest index [
11]. Therefore, achieving the purpose of reinforcing efficacy, reducing toxicity by processing, and preserving functional components as much as possible in processed
P. cyrtonema rhizome should be comprehensively considered. Thus, we propose that the traditional processing method should be improved in the processing period. Furthermore, the 9 processing cycles of steaming and drying used in
P. cyrtonema rhizome should be reduced to 3–6 cycles.
Despite the extensive use of processed Chinese herbal medicines, the underlying scientific mechanism of processing remain unclear for most of them, including Polygonatum plants to date. During processing with heating and drying, complicated changes in functional components of P. cyrtonema rhizome may occur not only in regulated contents but also in formed novel compounds. In many cases, the contents and structures of components may be altered simultaneously. Future studies should be devoted to comprehensively elucidating the processing-induced scientific mechanisms of reinforcing efficacy and reducing toxicity. Thus, additional efforts should be made to investigate the association of chemical and pharmacological changes using advanced technologies and assess the contribution of processing-induced chemical alteration to the changed bioactivities of P. cyrtonema. Furthermore, a unified and scientific processing technology should be established in the future to improve the traditional processing method used in P. cyrtonema.
5. Conclusions
This study applied a comparative metabolomics strategy to reveal changes in secondary metabolites during the processing of Polygonatum cyrtonema rhizome. The rhizome samples underwent traditional processing involving “nine cycles of steaming and sun-drying.” After being subjected to 3, 6, and 9 processing cycles, the extracts from 12 processed rhizome samples were used for the qualitative and quantitative analysis of secondary metabolites. The results are as obtained as follows:
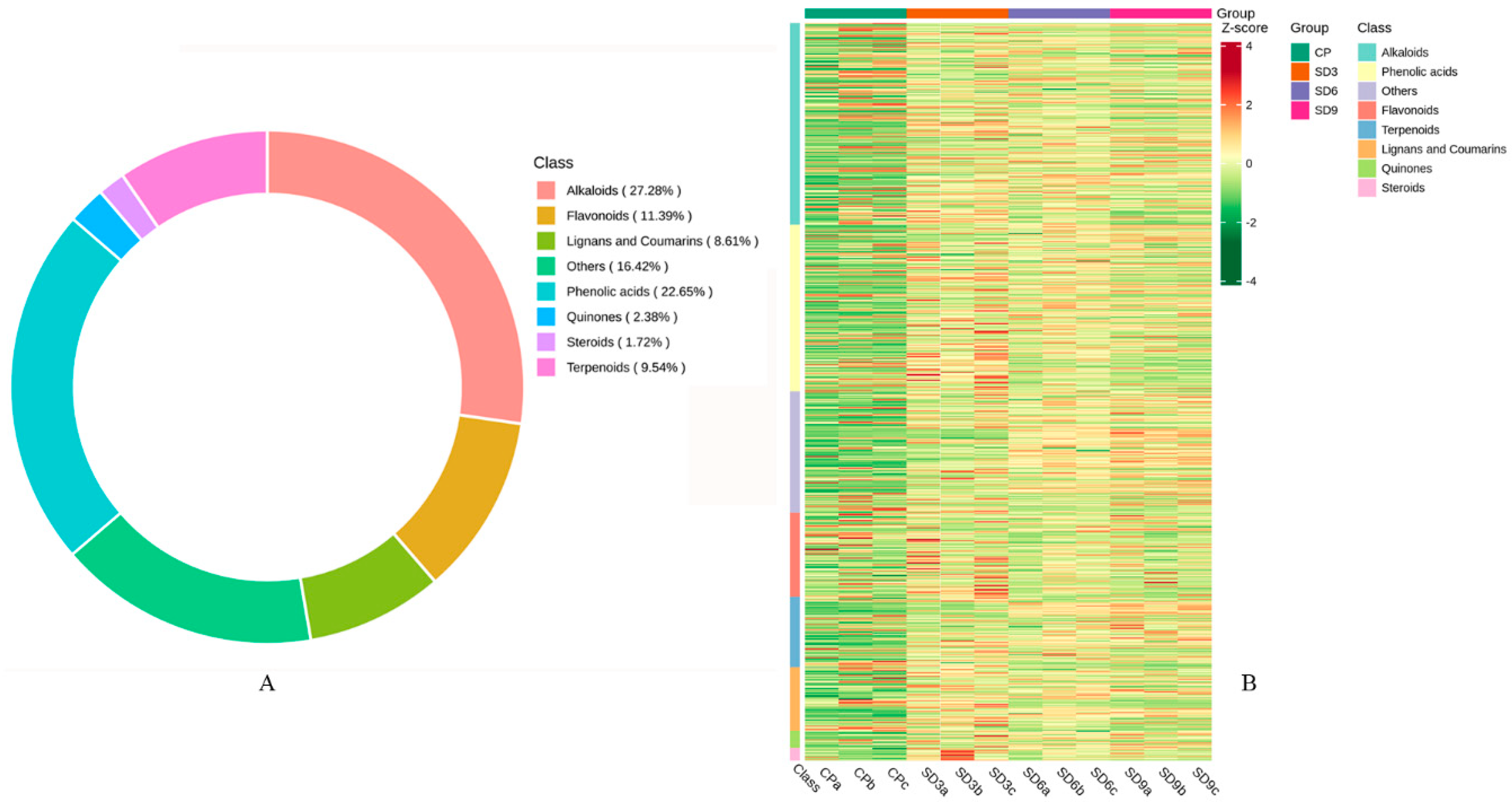
1) The major secondary metabolites in P. cyrtonema rhizome were polyphenols (phenolic compounds), including phenolic acids and flavonoids, followed by alkaloids, terpenoids, lignans, and coumarins, and finally quinones and steroids.
2) Processing significantly increased the global accumulation of secondary metabolites in processed rhizome unlike in unprocessed crude rhizome. In all 9 processing cycles, drastic changes in the relative abundance of secondary metabolites from crude rhizome to processed rhizome occurred after the first 6 cycles, and the last 3 cycles did not induce further significant changes. For most secondary metabolites, the first 3 processing cycles significantly enhanced their accumulation in relative abundance, while significantly decreasing their accumulation in a small number of secondary metabolites. Processing also led to numerous newly added secondary metabolites mainly occurring in the first 3 cycles, with a small number of disappeared secondary metabolites mainly occurring in the last 6–9 cycles.
3) Processing induced some significantly enriched KEGG pathways, in which the first 3 processing cycles enhanced the biosynthesis of various secondary metabolites and had significant effects on amino acid metabolisms.
4) Processing greatly influenced the relative abundance of functional components, and a short period of processing was helpful for enhancing their biosynthesis in P. cyrtonema rhizome. However, the enhancing effects will unavoidably be reversed or weakened under continuous processing for a long period. Therefore, the processing period of steaming and drying should be reduced to 3–6 cycles to maintain an enhancing effect on the accumulation of functional components.
5) Different functional components, even if belonging to the same class, had different response mechanisms toward processing, which were presented as up-regulated, down-regulated, or stable in relative abundance. 6) Future studies should be devoted to comprehensively elucidating the processing-induced scientific mechanisms of reinforcing efficacy and reducing toxicity. Furthermore, a unified and scientific processing technology should be established in the future to improve the traditional processing method used in P. cyrtonema.
Figure 1.
The P. cyrtonema rhizome samples during 9 processing cycles of steaming and drying. CP represents crude rhizome samples and SD3, SD6 and SD9 represent processed samples subjected to 3, 6 and 9 processing cycles, respectively.
Figure 1.
The P. cyrtonema rhizome samples during 9 processing cycles of steaming and drying. CP represents crude rhizome samples and SD3, SD6 and SD9 represent processed samples subjected to 3, 6 and 9 processing cycles, respectively.
Figure 2.
Basic information of secondary metabolites detected in P. cyrtonema rhizome. (A) quantity and classification of secondary metabolites. (B) clustered heatmap of secondary metabolites in crude rhizome samples (CP group) and in processed rhizome samples (SD3, SD6 and SD9 groups). The shades of color indicate more or less metabolites, with redder representing more and greener representing less.
Figure 2.
Basic information of secondary metabolites detected in P. cyrtonema rhizome. (A) quantity and classification of secondary metabolites. (B) clustered heatmap of secondary metabolites in crude rhizome samples (CP group) and in processed rhizome samples (SD3, SD6 and SD9 groups). The shades of color indicate more or less metabolites, with redder representing more and greener representing less.
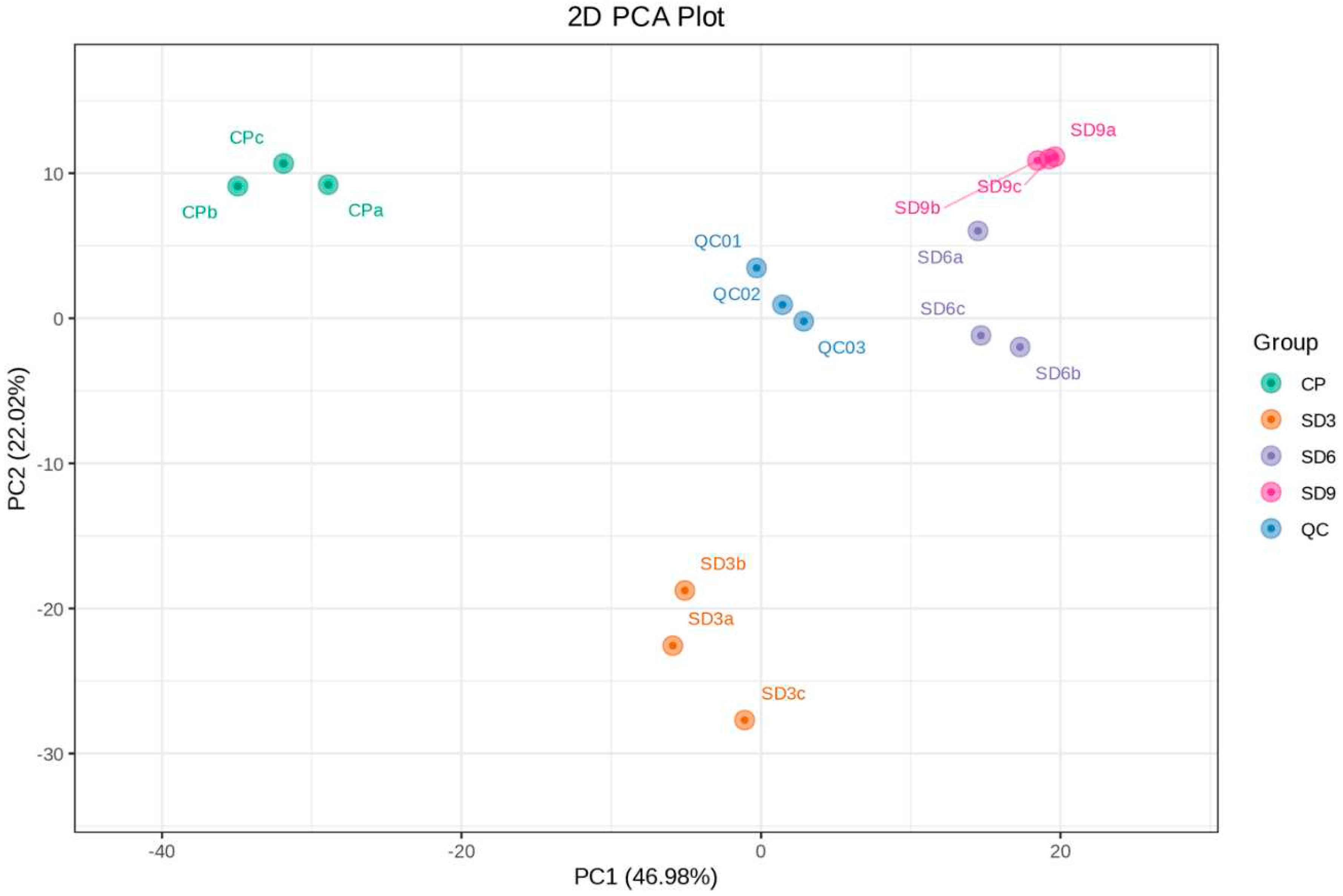
Figure 3.
Principal component analysis (PCA) plot of secondary metabolites from 12 P. cyrtonema samples. The figure in brackets refer to the explained variance ratio.
Figure 3.
Principal component analysis (PCA) plot of secondary metabolites from 12 P. cyrtonema samples. The figure in brackets refer to the explained variance ratio.
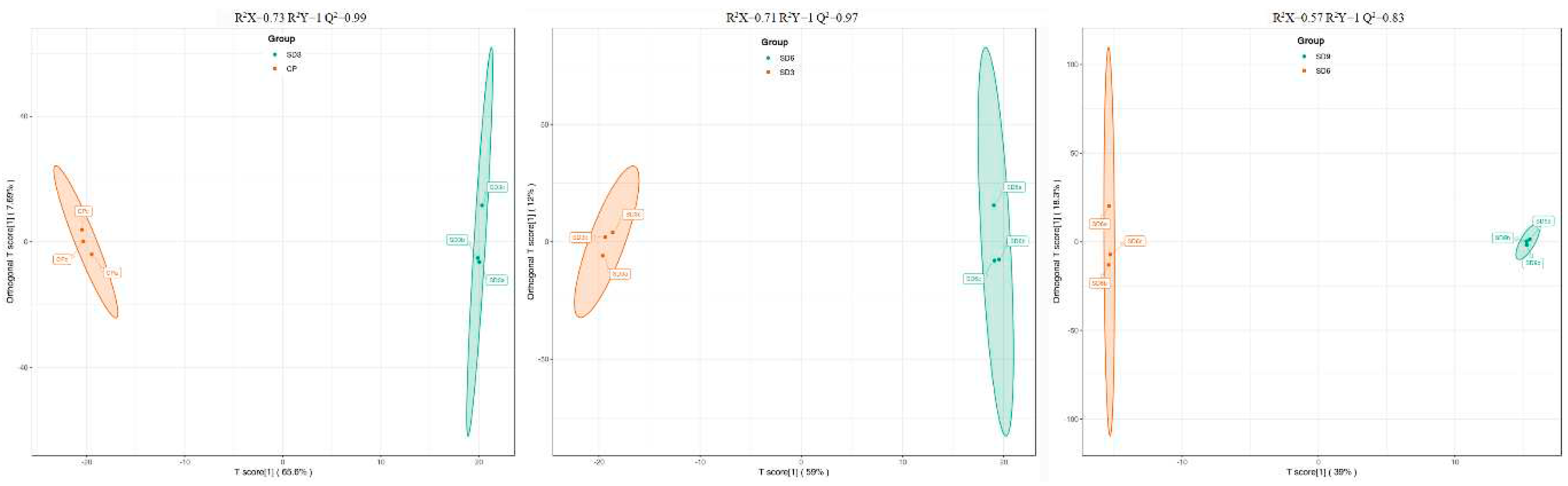
Figure 4.
The OPLS-DA score plots of the pairwise comparison on SD3 vs. CP, SD6 vs. SD3 and SD9 vs. SD6.
Figure 4.
The OPLS-DA score plots of the pairwise comparison on SD3 vs. CP, SD6 vs. SD3 and SD9 vs. SD6.
Figure 5.
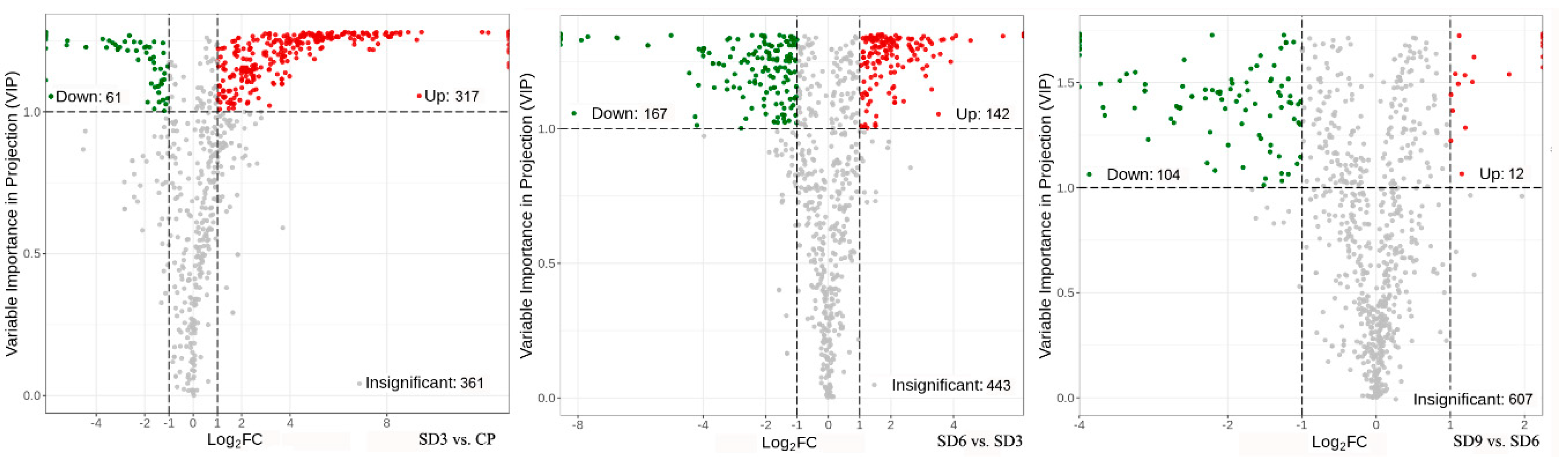
Volcano plots of differential accumulated secondary metabolites. The dots in plots represent differential accumulated secondary metabolites, with red representing up-regulated, green representing down-regulated, and gray indicating insignificant.
Figure 5.
Volcano plots of differential accumulated secondary metabolites. The dots in plots represent differential accumulated secondary metabolites, with red representing up-regulated, green representing down-regulated, and gray indicating insignificant.
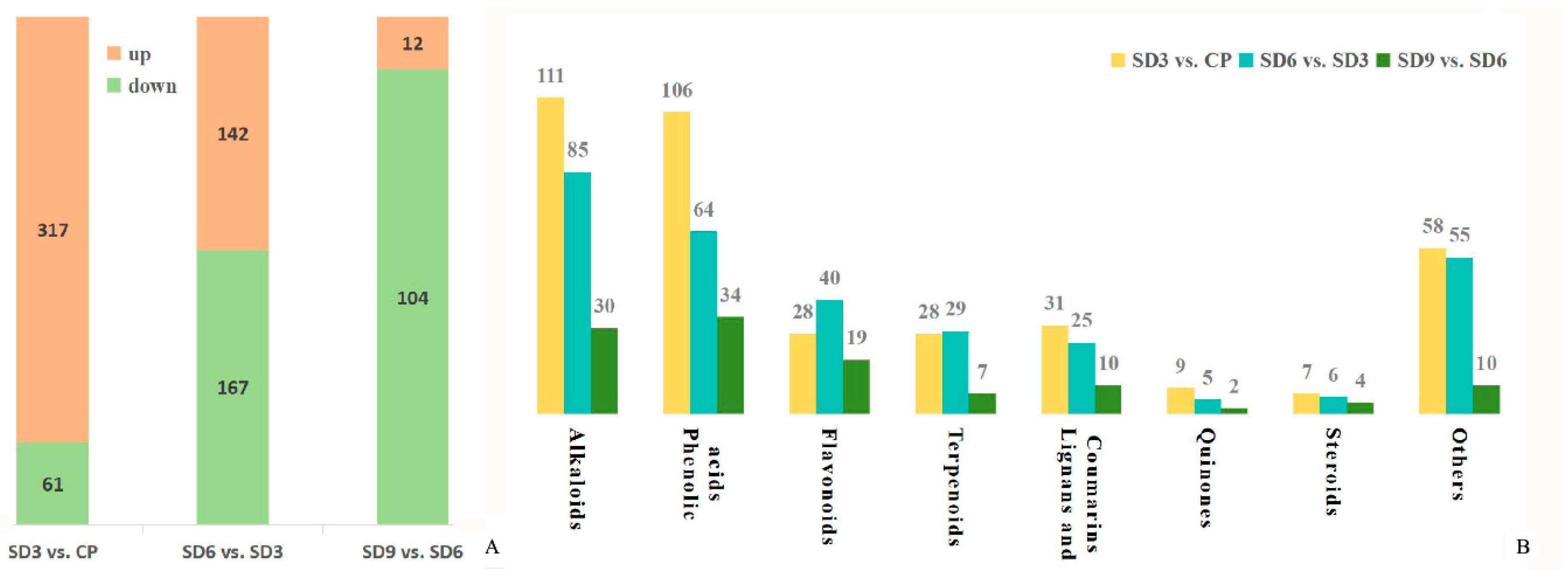
Figure 6.
Quantity and class of differential accumulated secondary metabolites from three comparison groups. (A) total quantity of differential accumulated secondary metabolites. (B) specific quantity of differential accumulated secondary metabolites within each class.
Figure 6.
Quantity and class of differential accumulated secondary metabolites from three comparison groups. (A) total quantity of differential accumulated secondary metabolites. (B) specific quantity of differential accumulated secondary metabolites within each class.
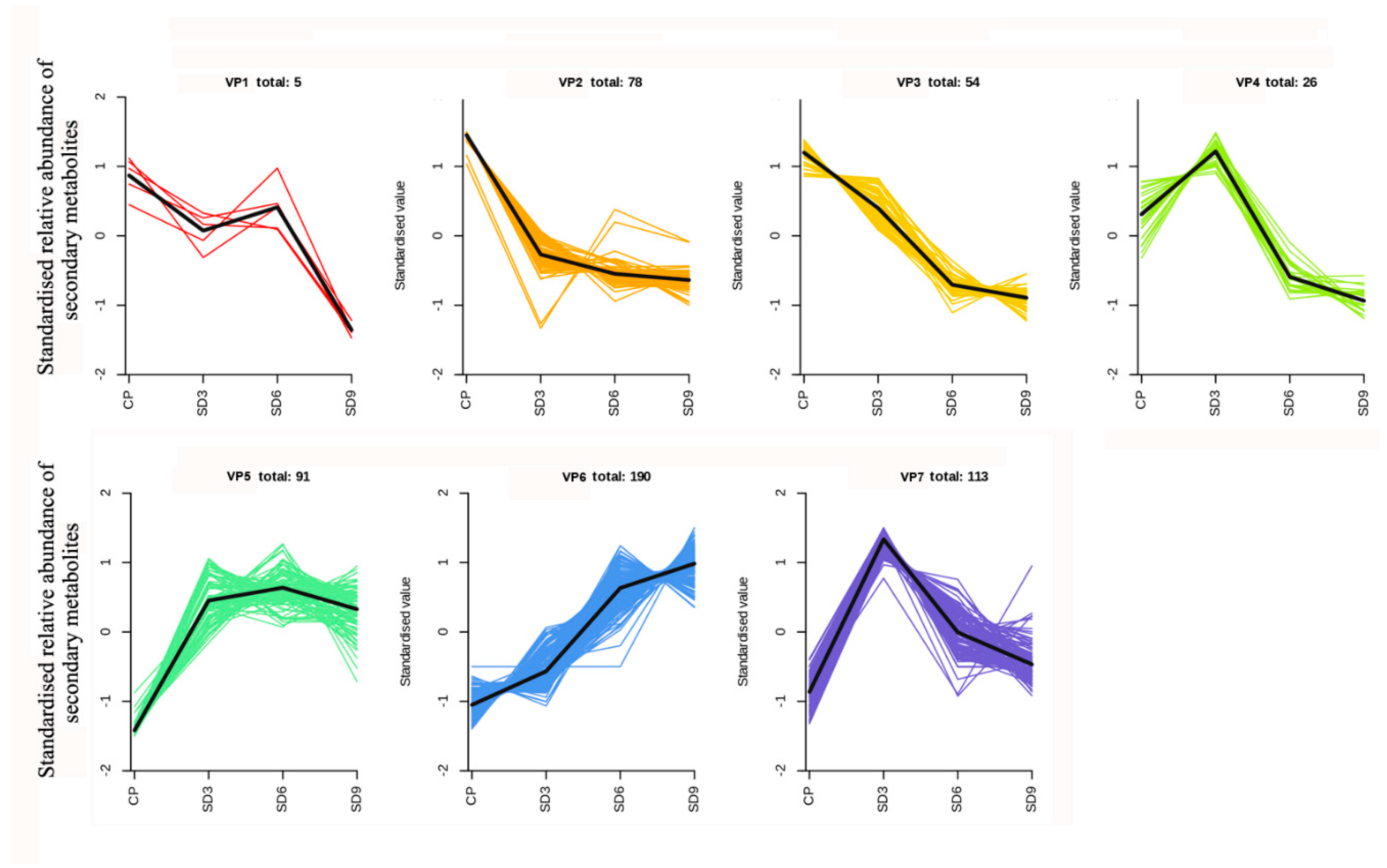
Figure 7.
K-Means clustering analysis of secondary metabolites. The number represent the total quantity of secondary metabolites with same variation pattern.
Figure 7.
K-Means clustering analysis of secondary metabolites. The number represent the total quantity of secondary metabolites with same variation pattern.
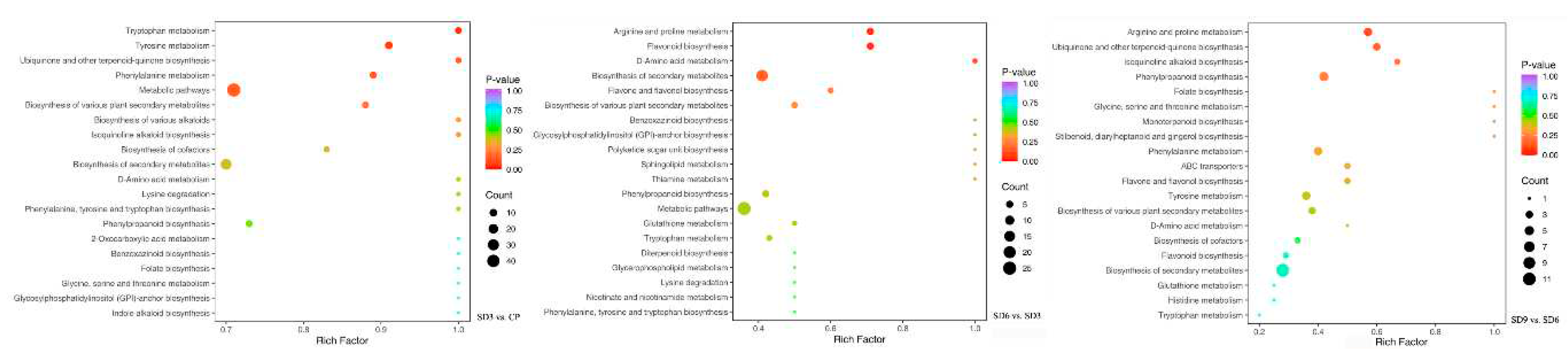
Figure 8.
The top 20 significantly enriched KEGG pathways. The colour of dots in plots refer to higher or lower P-value, with bluer representing higher, redder representing lower. The size of a dot represents more or less secondary metabolites, with bigger representing more and smaller representing less.
Figure 8.
The top 20 significantly enriched KEGG pathways. The colour of dots in plots refer to higher or lower P-value, with bluer representing higher, redder representing lower. The size of a dot represents more or less secondary metabolites, with bigger representing more and smaller representing less.
Figure 9.
Changes in relative abundance of functional components in P. cyrtonema rhizome during 9 processing cycles. The shades of color and arrows represent up- or down-regulated components, with orange and ↑ representing up-regulated, green and ↓ representing down-regulated, and gray representing unchanged. The number represent the quantity of up- or down-regulated and unchanged components.
Figure 9.
Changes in relative abundance of functional components in P. cyrtonema rhizome during 9 processing cycles. The shades of color and arrows represent up- or down-regulated components, with orange and ↑ representing up-regulated, green and ↓ representing down-regulated, and gray representing unchanged. The number represent the quantity of up- or down-regulated and unchanged components.
Table 1.
The detail information of top 20 differential accumulated secondary metabolites with maximum values of Log2FC.
Table 1.
The detail information of top 20 differential accumulated secondary metabolites with maximum values of Log2FC.
| Class |
DASM//Log2FC |
| SD3 vs. CP |
SD6 vs. SD3 |
SD9 vs.SD6 |
| Alkaloids |
Cephalanthrin A//12.57
1-Acetyl-β-carboline//8.98
(R)-1,2,3,4-Tetrahydro-3-carboxy-2-carboline//8.52
N-benzoyl-2-aminoethyl-β-D-glucopyranoside//8.18
Valerine//7.27
(1R,3S)-1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid//6.6
|
4′-O-Methylnorbelladine//4.14
Casuarine Analogue//-3.2
Folicanthine//-3.66
|
|
| Phenolic acids |
Chlorogenic acid methyl ester//9.32
4-O-Caffeoylquinic acid methyl ester//7.92
3-hydroxyphenylacetic acid//7.38
methyl 5-caffeoylquinate//6.91
4-Hydroxybenzoic acid//6.87
1-O-Feruloylquinic acid//6.8
2,3-Dihydroxybenzoic Acid//6.54
|
Ethyl malto//4.14
Antiarol; 3,4,5-Trimethoxyphenol//3.64
methyl 5-caffeoylquinate//-3.33
1-O-Feruloylquinic acid//-3.41
3-O-Feruloylquinic acid//-3.48
4-O-Caffeoylquinic acid methyl ester//-3.64
p-Hydroxyphenyl 6-O-(E)-caffeoyl-β-D-allopyranoside//-4.7
|
1-O-p-Coumaroylquinic acid//-2.64
4-O-Caffeoylquinic acid methyl ester//-2.8
Chlorogenic acid methyl ester//-2.85
1-O-Feruloylquinic acid//-2.9
2-Hydroxycinnamic acid//-3.04
2-(Formylamino)benzoic acid//-3.13
α-Hydroxycinnamic Acid//-3.28
3-Hydroxycinnamic Acid//-3.33
Methyl 5-caffeoylquinate//-3.43
3,4,5-Trimethoxycinnamic acid//-3.73
|
| Flavonoids |
Sesuvioside A//7.41
|
3-Hydroxy-4′,5,7-Trimethoxyflavanone//4.03
Butin; ,3′,4′-Trihydroxyflavanone//3.41
Sesuvioside A//-3.25
Isorhamnetin-3-O-neohesperidoside//-3.73
3,5,7-Trihydroxy-6,8-dimethyl-3-(4′-hydroxybenzyl)-chroman-4-one (Polygonatone C)//-4.23
|
3-[(3,4-dihydroxyphenyl)methylidene]-5,7-dihydroxy-6-methoxy-2h-1-benzopyran-4-one glucosyl rhamnoside//-2.72
Apigenin-6-C-(2″-glucosyl)arabinoside//-2.78
Tricin (5,7,4′-Trihydroxy-3′,5′-dimethoxyflavone)//-2.95
|
| Steroids |
|
Spirost-5-en-12-one-3-O-glucosyl(1→2)glucosyl(1→4)galactoside (Pratioside D1)//-4.0
|
Spirost-5-ene-3,27-diol-27-O-glucoside-3-O-[rhamnosyl(1→4)]glucoside (Polygonatoside D)//-2.86
27-Hydroxyspirost-5-en-3-yl-O-rhamnosyl-(1→2)-O-[glucosyl-(1→6)]-glucoside//-3.17
Spirost-5-en-3-ol-3-O-glucosyl(1→2)glucosyl(l→4)galactoside (Neosibiricoside D)//-3.32
|
| Lignans and Coumarins |
Phellodenol E//8.25
Guaiacylglycerol-β-Guaiacyl Ether//6.69
|
7,8-Dihydroxy-4-methylcoumarin//5.33
7-Hydroxycoumarin;Umbelliferone//3.38
5,7-Dihydroxy-4-Phenylcoumarin//3.22
|
Phellodenol E//-3.12
|
| Others |
2,5-Dihydroxybenzaldehyde//7.4
Protocatechualdehyde//6.96
4-Methyl-5-thiazoleethanol//6.75
4-Hydroxybenzaldehyde//6.46
|
Squamocin K//3.56
|
4-hydroxyphenyl acrylaldehyde//-3.67
4-Methylbenzaldehyde//-3.92
3-Methylbenzaldehyde//-4.11
|
| Total |
Up: 20; down: 0 |
Up: 9; down: 11 |
Up: 0; down: 20 |
Table 2.
The number of newly added and disappeared secondary metabolites identified from three comparison groups.
Table 2.
The number of newly added and disappeared secondary metabolites identified from three comparison groups.
| Secondary metabolites |
Comparison group |
Total number |
Class |
| Alkaloids |
Phenolic acids |
Flavonoids |
Terpenoids |
Lignans and Coumarins |
Quinones |
Steroids |
Others |
| Newly added |
SD3 vs. CP |
164 |
45 |
42 |
23 |
13 |
15 |
8 |
5 |
13 |
| SD6 vs. SD3 |
16 |
6 |
3 |
4 |
|
1 |
|
|
2 |
| SD9 vs. SD6 |
4 |
|
1 |
|
1 |
|
|
|
2 |
| Disappeared |
SD3 vs. CP |
3 |
1 |
|
1 |
|
1 |
|
|
|
| SD6 vs. SD3 |
33 |
10 |
6 |
11 |
1 |
1 |
|
1 |
3 |
| SD9 vs. SD6 |
30 |
9 |
10 |
4 |
2 |
3 |
1 |
|
1 |
Table 3.
Quantity and class of secondary metabolites clustered into seven variation patterns.
Table 3.
Quantity and class of secondary metabolites clustered into seven variation patterns.
| Variation pattern |
Number (%) |
Class |
| Alkaloids |
Phenolic acids |
Flavonoids |
Terpenoids |
Lignans and Coumarins |
Quinones |
Steroids |
Others |
| 1 |
5 (0.90) |
3 |
1 |
1 |
|
|
|
|
|
| 2 |
78 (14.00) |
26 |
17 |
13 |
8 |
6 |
1 |
|
7 |
| 3 |
54 (9.69) |
19 |
10 |
6 |
6 |
6 |
|
|
7 |
| 4 |
26 (4.67) |
7 |
5 |
4 |
1 |
1 |
2 |
2 |
4 |
| 5 |
91 (16.34) |
36 |
22 |
5 |
7 |
4 |
2 |
1 |
14 |
| 6 |
190 (34.11) |
39 |
47 |
14 |
26 |
14 |
4 |
2 |
44 |
| 7 |
113 (20.29) |
24 |
33 |
13 |
6 |
14 |
4 |
5 |
14 |
| Total |
557 |
154 |
135 |
56 |
54 |
45 |
13 |
10 |
90 |
Table 4.
Significantly enriched KEGG pathways related to biosynthesis of secondary metabolites and amino acid metabolisms.
Table 4.
Significantly enriched KEGG pathways related to biosynthesis of secondary metabolites and amino acid metabolisms.
| Comparison group |
Biosynthesis of secondary metabolites |
Number of DASMs |
Amino acid metabolism |
Number of DASMs |
| SD3 vs. CP |
ko00130: Ubiquinone and other terpenoid-quinone biosynthesis |
4↑; 1↓ |
ko00380: Tryptophan metabolism |
4↑; 3↓ |
|
ko00999: Biosynthesis of various plant secondary metabolites |
5↑; 2↓ |
ko00350: Tyrosine metabolism |
7↑; 3↓ |
|
ko00996: Biosynthesis of various alkaloids |
3↑ |
ko00360: Phenylalanine metabolism |
8↑ |
|
ko00950: Isoquinoline alkaloid biosynthesis |
3↑ |
ko00470: D-Amino acid metabolism |
2↓ |
|
ko01110: Biosynthesis of secondary metabolites |
23↑; 5↓ |
ko00310: Lysine degradation |
1↑; 1↓ |
|
ko00901: Indole alkaloid biosynthesis |
1↑ |
ko00400: Phenylalanine, tyrosine and tryptophan biosynthesis |
1↑; 1↓ |
|
ko00902: Monoterpenoid biosynthesis |
1↑ |
ko00260: Glycine, serine and threonine metabolism |
1↑ |
|
ko00904: Diterpenoid biosynthesis |
1↓ |
ko00300: Lysine biosynthesis |
1↑ |
|
ko00943: Isoflavonoid biosynthesis |
1↑ |
ko01230: Biosynthesis of amino acids |
1↑ |
|
ko00941: Flavonoid biosynthesis |
2↑ |
ko00340: Histidine metabolism |
2↑ |
|
ko00960: Tropane, piperidine and pyridine alkaloid biosynthesis |
1↑; 2↓ |
ko00330: Arginine and proline metabolism |
2↓ |
| SD6 vs. SD3 |
ko00941: Flavonoid biosynthesis |
5↑ |
ko00470: D-Amino acid metabolism |
2↓ |
|
ko01110: Biosynthesis of secondary metabolites |
9↑; 9↓ |
ko00380: Tryptophan metabolism |
3↓ |
|
ko00944: Flavone and flavonol biosynthesis |
3↓ |
ko00400: Phenylalanine, tyrosine and tryptophan biosynthesis |
1↓ |
|
ko00999: Biosynthesis of various plant secondary metabolites |
1↑; 3↓ |
ko00340: Histidine metabolism |
1↑ |
|
ko00904: Diterpenoid biosynthesis |
1↑ |
ko00350: Tyrosine metabolism |
1↑; 1↓ |
|
ko00960: Tropane, piperidine and pyridine alkaloid biosynthesis |
2↓ |
ko00360: Phenylalanine metabolism |
1↑ |
|
ko00943: Isoflavonoid biosynthesis |
1↑ |
|
|
|
ko00950: Isoquinoline alkaloid biosynthesis |
1↓ |
|
|
|
ko00130: Ubiquinone and other terpenoid-quinone biosynthesis |
1↓ |
|
|
| SD9 vs. SD6 |
ko00130: Ubiquinone and other terpenoid-quinone biosynthesis |
3↓ |
ko00330: Arginine and proline metabolism |
4↓ |
|
ko00950: Isoquinoline alkaloid biosynthesis |
2↓ |
ko00260: Glycine, serine and threonine metabolism |
1↓ |
|
ko00902: Monoterpenoid biosynthesis |
1↓ |
ko00360: Phenylalanine metabolism |
4↓ |
|
ko00944: Flavone and flavonol biosynthesis |
2↓ |
ko00350: Tyrosine metabolism |
4↓ |
|
ko00999: Biosynthesis of various plant secondary metabolites |
1↑; 2↓ |
ko00470: D-Amino acid metabolism |
1↓ |
|
ko00941: Flavonoid biosynthesis |
1↑; 1↓ |
ko00340: Histidine metabolism |
1↓ |
|
ko01110: Biosynthesis of secondary metabolites |
2↑; 10↓ |
ko00380: Tryptophan metabolism |
1↓ |
|
ko00960: Tropane, piperidine and pyridine alkaloid biosynthesis |
1↓ |
|
|