Submitted:
05 January 2024
Posted:
08 January 2024
You are already at the latest version
Abstract
Keywords:
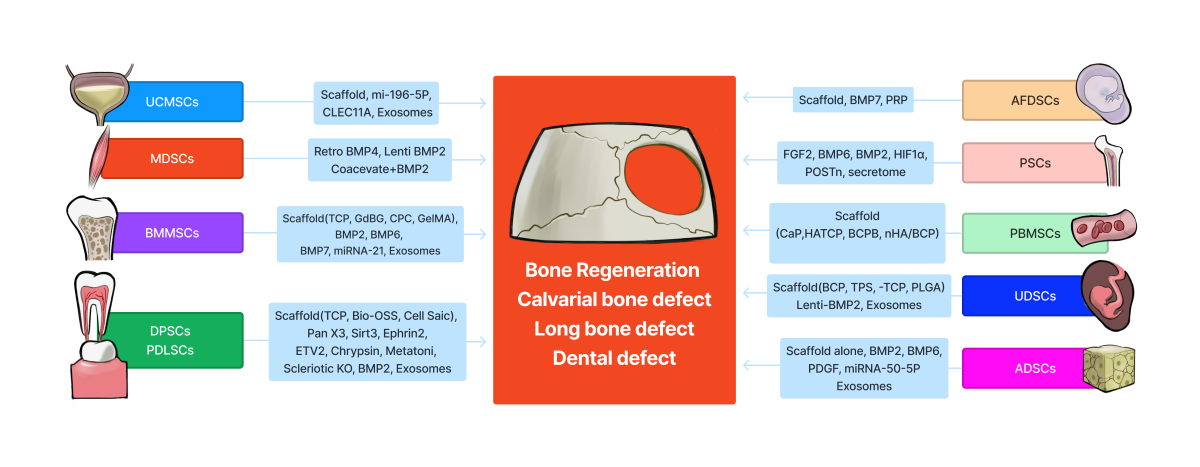
1. Introduction
2. Bone marrow mesenchymal stem(stromal) cells (BMMSCs).
2.1. BMMSCs loaded with different scaffolds for bone tissue engineering.
2.2. BMMSCs delivered with scaffold and bone growth factors for bone tissue engineering.
2.3. BMMSCs derived exosomes or extracellular vesicles in bone tissue engineering.
2.4. Targeting cell senescence to improve BMMSC-mediated bone tissue engineering.
3. Muscle-derived stem cells (MDSCs)
4. Adipose derived stem cells (ASCs).
4.1. ADSCs alone with scaffold for bone tissue engineering
4.2. ADSCs modified with different growth factors for bone tissue engineering.
4.3. ADSC-derived exosomes for bone tissue engineering.
4.4. miRNA regulated ADSCs for bone tissue engineering
5. Dental pulp stem cells and periodontal ligament stem cells
5.1. Unmodified DPSCs loaded with different scaffold for bone tissue engineering.
5.2. DPSCs modified with different genes for bone tissue engineering.
5.3. DPSCs treated with small molecule or its inhibitor enhanced bone repair.
5.4. DPSCs or PDLSCs exosome for bone tissue engineering.
6. Periosteal stem cells (PSCs).
6.1. PSCs alone or combining with bone growth factors for bone tissue engineering.
6.2. PSCs from different anatomic origins demonstrate variable bone regeneration capacities.
6.3. PSC secretomes for bone tissue engineering
7. Amniotic fluid derived stem cells (AFDSCs).
8. Peripheral blood-derived mesenchymal stem cells (PBMSCs).
9. Umbilical cord derived mesenchymal stem cells (UC-MSCs).
9.1. UC-MSCs delivered with different scaffold for bone tissue engineering.
9.2. UC-MSC derived exosomes for bone tissue engineering.
10. Urine derived stem cells (UDSCs).
10.1. UDSCs loaded with different scaffold materials for bone tissue engineering.
10.2. UDSC exosomes for bone tissue engineering.
11. Comparison of bone regenerative potential of different stem cells
12. Advantages and disadvantages of different stem cells for potential clinical applications.
13. Prospective applications of stem cells for bone tissue engineering for human bone tissue repair.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connolly, J.F.; Guse, R.; Tiedeman, J.; Dehne, R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res 1991, 259–270. [Google Scholar] [CrossRef]
- Tiedeman, J.J.; Garvin, K.L.; Kile, T.A.; Connolly, J.F. The role of a composite, demineralized bone matrix and bone marrow in the treatment of osseous defects. Orthopedics 1995, 18, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 2001, 344, 385–386. [Google Scholar] [CrossRef]
- Yang, Y.; Hallgrimsson, B.; Putnins, E.E. Craniofacial defect regeneration using engineered bone marrow mesenchymal stromal cells. J Biomed Mater Res A 2011, 99, 74–85. [Google Scholar] [CrossRef]
- Burastero, G.; Scarfi, S.; Ferraris, C.; Fresia, C.; Sessarego, N.; Fruscione, F.; Monetti, F.; Scarfo, F.; Schupbach, P.; Podesta, M. , et al. The association of human mesenchymal stem cells with BMP-7 improves bone regeneration of critical-size segmental bone defects in athymic rats. Bone 2010, 47, 117–126. [Google Scholar] [CrossRef]
- Scotti, C.; Tonnarelli, B.; Papadimitropoulos, A.; Scherberich, A.; Schaeren, S.; Schauerte, A.; Lopez-Rios, J.; Zeller, R.; Barbero, A.; Martin, I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A 2010, 107, 7251–7256. [Google Scholar] [CrossRef] [PubMed]
- Scotti, C.; Piccinini, E.; Takizawa, H.; Todorov, A.; Bourgine, P.; Papadimitropoulos, A.; Barbero, A.; Manz, M.G.; Martin, I. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci U S A 2013, 110, 3997–4002. [Google Scholar] [CrossRef]
- Long, T.; Zhu, Z.; Awad, H.A.; Schwarz, E.M.; Hilton, M.J.; Dong, Y. The effect of mesenchymal stem cell sheets on structural allograft healing of critical sized femoral defects in mice. Biomaterials 2014, 35, 2752–2759. [Google Scholar] [CrossRef]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef]
- Arthur, A.; Gronthos, S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Stamnitz, S.; Klimczak, A. Mesenchymal Stem Cells, Bioactive Factors, and Scaffolds in Bone Repair: From Research Perspectives to Clinical Practice. Cells 2021, 10. [Google Scholar] [CrossRef]
- Blanco, J.F.; Garcia-Brinon, J.; Benito-Garzon, L.; Pescador, D.; Muntion, S.; Sanchez-Guijo, F. Human Bone Marrow Mesenchymal Stromal Cells Promote Bone Regeneration in a Xenogeneic Rabbit Model: A Preclinical Study. Stem Cells Int 2018, 2018, 7089484. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, X.; Zhao, K.; Zhu, Y.; Hu, B.; Zhou, Y.; Wang, M.; Wu, Y.; Zhang, C.; Xu, J. , et al. miRNA-21 promotes osteogenesis via the PTEN/PI3K/Akt/HIF-1alpha pathway and enhances bone regeneration in critical size defects. Stem Cell Res Ther 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Y.; Lu, B.; Yin, J.H.; Ke, Q.F.; Xu, H.; Zhang, C.Q.; Guo, Y.P.; Gao, Y.S. Gadolinium-doped bioglass scaffolds promote osteogenic differentiation of hBMSC via the Akt/GSK3beta pathway and facilitate bone repair in vivo. Int J Nanomedicine 2019, 14, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Wang, Q.; Ouyang, L.; Wu, H.; Yang, Z.; Fu, X.; Liu, X.; Yan, L.; Cao, Y.; Xiao, R. Comparison of concentrated fresh mononuclear cells and cultured mesenchymal stem cells from bone marrow for bone regeneration. Stem Cells Transl Med 2021, 10, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, R.; Tan, X.; Li, B.; Liu, Y.; Wang, X. Synthesis and Evaluation of BMMSC-seeded BMP-6/nHAG/GMS Scaffolds for Bone Regeneration. Int J Med Sci 2019, 16, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.A.; Monahan, D.S.; Brulin, B.; Gallinetti, S.; Humbert, P.; Tringides, C.; Canal, C.; Ginebra, M.P.; Layrolle, P. Biomimetic versus sintered macroporous calcium phosphate scaffolds enhanced bone regeneration and human mesenchymal stromal cell engraftment in calvarial defects. Acta Biomater 2021, 135, 689–704. [Google Scholar] [CrossRef]
- Li, G.; Shen, W.; Tang, X.; Mo, G.; Yao, L.; Wang, J. Combined use of calcium phosphate cement, mesenchymal stem cells and platelet-rich plasma for bone regeneration in critical-size defect of the femoral condyle in mini-pigs. Regen Med 2021, 16, 451–464. [Google Scholar] [CrossRef]
- Li, Z.; Xiang, S.; Lin, Z.; Li, E.N.; Yagi, H.; Cao, G.; Yocum, L.; Li, L.; Hao, T.; Bruce, K.K. , et al. Graphene oxide-functionalized nanocomposites promote osteogenesis of human mesenchymal stem cells via enhancement of BMP-SMAD1/5 signaling pathway. Biomaterials 2021, 277, 121082. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, X.; Fritch, M.R.; Li, Z.; Kuang, B.; Alexander, P.G.; Hao, T.; Cao, G.; Tan, S.; Bruce, K.K. , et al. Engineering pre-vascularized bone-like tissue from human mesenchymal stem cells through simulating endochondral ossification. Biomaterials 2022, 283, 121451. [Google Scholar] [CrossRef]
- Bernhard, J.C.; Marolt Presen, D.; Li, M.; Monforte, X.; Ferguson, J.; Leinfellner, G.; Heimel, P.; Betti, S.L.; Shu, S.; Teuschl-Woller, A.H. , et al. Effects of Endochondral and Intramembranous Ossification Pathways on Bone Tissue Formation and Vascularization in Human Tissue-Engineered Grafts. Cells 2022, 11. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Li, M.; Song, P.; Lei, H.; Gui, X.; Zhou, C.; Liu, L. Biomimetic Methacrylated Gelatin Hydrogel Loaded With Bone Marrow Mesenchymal Stem Cells for Bone Tissue Regeneration. Front Bioeng Biotechnol 2021, 9, 770049. [Google Scholar] [CrossRef]
- Machado-Paula, M.M.; Corat, M.A.F.; de Vasconcellos, L.M.R.; Araujo, J.C.R.; Mi, G.; Ghannadian, P.; Toniato, T.V.; Marciano, F.R.; Webster, T.J.; Lobo, A.O. Rotary Jet-Spun Polycaprolactone/Hydroxyapatite and Carbon Nanotube Scaffolds Seeded with Bone Marrow Mesenchymal Stem Cells Increase Bone Neoformation. ACS Appl Bio Mater 2022, 5, 1013–1024. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Gaihre, B.; Park, S.; Li, Y.; Terzic, A.; Elder, B.D.; Lu, L. Scaffold-Free Spheroids with Two-Dimensional Heteronano-Layers (2DHNL) Enabling Stem Cell and Osteogenic Factor Codelivery for Bone Repair. ACS Nano 2022, 16, 2741–2755. [Google Scholar] [CrossRef]
- Liu, Y.; Kuang, B.; Rothrauff, B.B.; Tuan, R.S.; Lin, H. Robust bone regeneration through endochondral ossification of human mesenchymal stem cells within their own extracellular matrix. Biomaterials 2019, 218, 119336. [Google Scholar] [CrossRef]
- Yang, C.; Li, Z.; Liu, Y.; Hou, R.; Lin, M.; Fu, L.; Wu, D.; Liu, Q.; Li, K.; Liu, C. Single-cell spatiotemporal analysis reveals cell fates and functions of transplanted mesenchymal stromal cells during bone repair. Stem Cell Reports 2022, 17, 2318–2333. [Google Scholar] [CrossRef] [PubMed]
- Pitacco, P.; Sadowska, J.M.; O'Brien, F.J.; Kelly, D.J. 3D bioprinting of cartilaginous templates for large bone defect healing. Acta Biomater 2023, 156, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Herberg, S.; Varghai, D.; Alt, D.S.; Dang, P.N.; Park, H.; Cheng, Y.; Shin, J.Y.; Dikina, A.D.; Boerckel, J.D.; Rolle, M.W. , et al. Scaffold-free human mesenchymal stem cell construct geometry regulates long bone regeneration. Commun Biol 2021, 4, 89. [Google Scholar] [CrossRef]
- Whitehead, J.; Griffin, K.H.; Gionet-Gonzales, M.; Vorwald, C.E.; Cinque, S.E.; Leach, J.K. Hydrogel mechanics are a key driver of bone formation by mesenchymal stromal cell spheroids. Biomaterials 2021, 269, 120607. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Lee, C.S.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Generation of Small RNA-Modulated Exosome Mimetics for Bone Regeneration. ACS Nano 2020, 14, 11973–11984. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Zhu, Y.; Yang, M.; Mao, C. Human Mesenchymal Stem Cell Derived Exosomes Enhance Cell-Free Bone Regeneration by Altering Their miRNAs Profiles. Adv Sci (Weinh) 2020, 7, 2001334. [Google Scholar] [CrossRef]
- Liu, A.; Lin, D.; Zhao, H.; Chen, L.; Cai, B.; Lin, K.; Shen, S.G. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials 2021, 272, 120718. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Kang, M.; Shirazi, S.; Lu, Y.; Cooper, L.F.; Gajendrareddy, P.; Ravindran, S. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater 2021, 126, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xin, X.; Wang, L.; Wang, B.; Chen, L.; Liu, O.; Rowe, D.W.; Xu, M. Senolytics improve bone forming potential of bone marrow mesenchymal stem cells from aged mice. NPJ Regen Med 2021, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Huang, H.; Gao, X.; Yang, J.; Tang, Q.; Xu, X.; Wu, Y.; Li, M.; Liang, C.; Tan, L. , et al. Local Elimination of Senescent Cells Promotes Bone Defect Repair during Aging. ACS Appl Mater Interfaces 2022, 14, 3885–3899. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A 1968, 61, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, D.; Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977, 270, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Blau, H.M.; Chiu, C.P.; Webster, C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell 1983, 32, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Rando, T.A.; Blau, H.M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 1994, 125, 1275–1287. [Google Scholar] [CrossRef]
- Torrente, Y.; Tremblay, J.P.; Pisati, F.; Belicchi, M.; Rossi, B.; Sironi, M.; Fortunato, F.; El Fahime, M.; D'Angelo, M.G.; Caron, N.J. , et al. Intraarterial injection of muscle-derived CD34(+)Sca-1(+) stem cells restores dystrophin in mdx mice. J Cell Biol 2001, 152, 335–348. [Google Scholar] [CrossRef]
- Lee, J.Y.; Musgrave, D.; Pelinkovic, D.; Fukushima, K.; Cummins, J.; Usas, A.; Robbins, P.; Fu, F.H.; Huard, J. Effect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. J Bone Joint Surg Am 2001, 83, 1032–1039. [Google Scholar] [CrossRef]
- Qu-Petersen, Z.; Deasy, B.; Jankowski, R.; Ikezawa, M.; Cummins, J.; Pruchnic, R.; Mytinger, J.; Cao, B.; Gates, C.; Wernig, A. , et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol 2002, 157, 851–864. [Google Scholar] [CrossRef]
- Qu, Z.; Balkir, L.; van Deutekom, J.C.; Robbins, P.D.; Pruchnic, R.; Huard, J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol 1998, 142, 1257–1267. [Google Scholar] [CrossRef]
- Wright, V.; Peng, H.; Usas, A.; Young, B.; Gearhart, B.; Cummins, J.; Huard, J. BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol Ther 2002, 6, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wright, V.; Usas, A.; Gearhart, B.; Shen, H.C.; Cummins, J.; Huard, J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest 2002, 110, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Usas, A.; Olshanski, A.; Ho, A.M.; Gearhart, B.; Cooper, G.M.; Huard, J. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res 2005, 20, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Usas, A.; Hannallah, D.; Olshanski, A.; Cooper, G.M.; Huard, J. Noggin improves bone healing elicited by muscle stem cells expressing inducible BMP4. Mol Ther 2005, 12, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Usas, A.; Proto, J.D.; Lu, A.; Cummins, J.H.; Proctor, A.; Chen, C.W.; Huard, J. Role of donor and host cells in muscle-derived stem cell-mediated bone repair: differentiation vs. paracrine effects. FASEB J 2014, 28, 3792–3809. [Google Scholar] [CrossRef] [PubMed]
- Corsi, K.A.; Pollett, J.B.; Phillippi, J.A.; Usas, A.; Li, G.; Huard, J. Osteogenic potential of postnatal skeletal muscle-derived stem cells is influenced by donor sex. J Bone Miner Res 2007, 22, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, L.B.; Usas, A.; Cooper, G.M.; Huard, J. Effect of host sex and sex hormones on muscle-derived stem cell-mediated bone formation and defect healing. Tissue Eng Part A 2012, 18, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Peng, H.; Usas, A.; Musgrave, D.; Cummins, J.; Pelinkovic, D.; Jankowski, R.; Ziran, B.; Robbins, P.; Huard, J. Enhancement of bone healing based on ex vivo gene therapy using human muscle-derived cells expressing bone morphogenetic protein 2. Hum Gene Ther 2002, 13, 1201–1211. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Derubeis, A.R.; Cancedda, R. Bone and cartilage formation by skeletal muscle derived cells. J Cell Physiol 2005, 204, 594–603. [Google Scholar] [CrossRef]
- Gao, X.; Usas, A.; Lu, A.; Tang, Y.; Wang, B.; Chen, C.W.; Li, H.; Tebbets, J.C.; Cummins, J.H.; Huard, J. BMP2 is superior to BMP4 for promoting human muscle-derived stem cell-mediated bone regeneration in a critical-sized calvarial defect model. Cell Transplant 2013, 22, 2393–2408. [Google Scholar] [CrossRef]
- Gharaibeh, B.; Lu, A.; Tebbets, J.; Zheng, B.; Feduska, J.; Crisan, M.; Peault, B.; Cummins, J.; Huard, J. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc 2008, 3, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Usas, A.; Tang, Y.; Lu, A.; Tan, J.; Schneppendahl, J.; Kozemchak, A.M.; Wang, B.; Cummins, J.H.; Tuan, R.S. , et al. A comparison of bone regeneration with human mesenchymal stem cells and muscle-derived stem cells and the critical role of BMP. Biomaterials 2014, 35, 6859–6870. [Google Scholar] [CrossRef]
- Gao, X.; Lu, A.; Tang, Y.; Schneppendahl, J.; Liebowitz, A.B.; Scibetta, A.C.; Morris, E.R.; Cheng, H.; Huard, C.; Amra, S. , et al. Influences of donor and host age on human muscle-derived stem cell-mediated bone regeneration. Stem Cell Res Ther 2018, 9, 316. [Google Scholar] [CrossRef]
- Usas, A.; Ho, A.M.; Cooper, G.M.; Olshanski, A.; Peng, H.; Huard, J. Bone regeneration mediated by BMP4-expressing muscle-derived stem cells is affected by delivery system. Tissue Eng Part A 2009, 15, 285–293. [Google Scholar] [CrossRef]
- Zheng, B.; Cao, B.; Crisan, M.; Sun, B.; Li, G.; Logar, A.; Yap, S.; Pollett, J.B.; Drowley, L.; Cassino, T. , et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol 2007, 25, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Li, G.; Chen, W.C.; Deasy, B.M.; Pollett, J.B.; Sun, B.; Drowley, L.; Gharaibeh, B.; Usas, A.; Peault, B. , et al. Human myogenic endothelial cells exhibit chondrogenic and osteogenic potentials at the clonal level. J Orthop Res 2013, 31, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.M.; Lozito, T.P.; Djouad, F.; Kuhn, N.Z.; Nesti, L.J.; Tuan, R.S. Differentiation and regeneration potential of mesenchymal progenitor cells derived from traumatized muscle tissue. J Cell Mol Med 2011, 15, 2377–2388. [Google Scholar] [CrossRef]
- Levi, B.; Longaker, M.T. Concise review: adipose-derived stromal cells for skeletal regenerative medicine. Stem Cells 2011, 29, 576–582. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Ricci, G.; D'Andrea, F.; Nicoletti, G.F.; Ferraro, G.A. Human Adipose Stem Cells: From Bench to Bedside. Tissue Eng Part B Rev 2015, 21, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.M.; Shi, Y.Y.; Aalami, O.O.; Chou, Y.F.; Mari, C.; Thomas, R.; Quarto, N.; Contag, C.H.; Wu, B.; Longaker, M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 2004, 22, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Lendeckel, S.; Jodicke, A.; Christophis, P.; Heidinger, K.; Wolff, J.; Fraser, J.K.; Hedrick, M.H.; Berthold, L.; Howaldt, H.P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg 2004, 32, 370–373. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; Levi, B.; Nelson, E.R.; Peng, M.; Commons, G.W.; Lee, M.; Wu, B.; Longaker, M.T. Deleterious effects of freezing on osteogenic differentiation of human adipose-derived stromal cells in vitro and in vivo. Stem Cells Dev 2011, 20, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, S.H.; Kang, B.J.; Kim, W.H.; Yun, H.S.; Kweon, O.K. Comparison of Osteogenesis between Adipose-Derived Mesenchymal Stem Cells and Their Sheets on Poly-epsilon-Caprolactone/beta-Tricalcium Phosphate Composite Scaffolds in Canine Bone Defects. Stem Cells Int 2016, 2016, 8414715. [Google Scholar] [CrossRef]
- Orbay, H.; Busse, B.; Leach, J.K.; Sahar, D.E. The Effects of Adipose-Derived Stem Cells Differentiated Into Endothelial Cells and Osteoblasts on Healing of Critical Size Calvarial Defects. J Craniofac Surg 2017, 28, 1874–1879. [Google Scholar] [CrossRef]
- Bernhard, J.; Ferguson, J.; Rieder, B.; Heimel, P.; Nau, T.; Tangl, S.; Redl, H.; Vunjak-Novakovic, G. Tissue-engineered hypertrophic chondrocyte grafts enhanced long bone repair. Biomaterials 2017, 139, 202–212. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, P.; Long, Y.; Huang, C.; Chen, D. Repair of bone defects in rat radii with a composite of allogeneic adipose-derived stem cells and heterogeneous deproteinized bone. Stem Cell Res Ther 2018, 9, 79. [Google Scholar] [CrossRef]
- Ruminski, S.; Kalaszczynska, I.; Dlugosz, A.; Lewandowska-Szumiel, M. Osteogenic differentiation of human adipose-derived stem cells in 3D conditions - comparison of spheroids and polystyrene scaffolds. Eur Cell Mater 2019, 37, 382–401. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Yu, N.; Ma, H.; Wang, K.; Liu, J.; Zhang, W.; Cai, Z.; He, Y. Construction of vascularized tissue-engineered bone with polylysine-modified coral hydroxyapatite and a double cell-sheet complex to repair a large radius bone defect in rabbits. Acta Biomater 2019, 91, 82–98. [Google Scholar] [CrossRef]
- Probst, F.A.; Fliefel, R.; Burian, E.; Probst, M.; Eddicks, M.; Cornelsen, M.; Riedl, C.; Seitz, H.; Aszódi, A.; Schieker, M. , et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci Rep 2020, 10, 2062. [Google Scholar] [CrossRef]
- Peterson, B.; Zhang, J.; Iglesias, R.; Kabo, M.; Hedrick, M.; Benhaim, P.; Lieberman, J.R. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng 2005, 11, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.N.; Yu, F.J.; Chang, Y.H.; Huang, K.L.; Pham, N.N.; Truong, V.A.; Lin, M.W.; Kieu Nguyen, N.T.; Hwang, S.M.; Hu, Y.C. CRISPR interference-mediated noggin knockdown promotes BMP2-induced osteogenesis and calvarial bone healing. Biomaterials 2020, 252, 120094. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.F.; Zuk, P.A.; Chang, T.L.; Benhaim, P.; Wu, B.M. Adipose-derived stem cells and BMP2: part 1. BMP2-treated adipose-derived stem cells do not improve repair of segmental femoral defects. Connect Tissue Res 2011, 52, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, B.J.; Kim, W.H.; Yun, H.S.; Kweon, O.K. Evaluation of Mesenchymal Stem Cell Sheets Overexpressing BMP-7 in Canine Critical-Sized Bone Defects. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef]
- Osinga, R.; Di Maggio, N.; Todorov, A.; Allafi, N.; Barbero, A.; Laurent, F.; Schaefer, D.J.; Martin, I.; Scherberich, A. Generation of a Bone Organ by Human Adipose-Derived Stromal Cells Through Endochondral Ossification. Stem Cells Transl Med 2016, 5, 1090–1097. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Ahmad, T.; Madhurakkat Perikamana, S.K.; Lee, J.; Kim, E.M.; Shin, H. Human adipose-derived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials 2020, 255, 120192. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; He, R.Z.; Tu, B.; He, J.S.; Cao, X.; Xia, H.S.; Ba, H.L.; Wu, S.; Peng, C.; Xiong, K. Drilling Combined with Adipose-derived Stem Cells and Bone Morphogenetic Protein-2 to Treat Femoral Head Epiphyseal Necrosis in Juvenile Rabbits. Curr Med Sci 2018, 38, 277–288. [Google Scholar] [CrossRef]
- Fan, J.; Im, C.S.; Cui, Z.K.; Guo, M.; Bezouglaia, O.; Fartash, A.; Lee, J.Y.; Nguyen, J.; Wu, B.M.; Aghaloo, T. , et al. Delivery of Phenamil Enhances BMP-2-Induced Osteogenic Differentiation of Adipose-Derived Stem Cells and Bone Formation in Calvarial Defects. Tissue Eng Part A 2015, 21, 2053–2065. [Google Scholar] [CrossRef]
- Yao, W.; Lay, Y.E.; Kot, A.; Liu, R.; Zhang, H.; Chen, H.; Lam, K.; Lane, N.E. Improved Mobilization of Exogenous Mesenchymal Stem Cells to Bone for Fracture Healing and Sex Difference. Stem Cells 2016, 34, 2587–2600. [Google Scholar] [CrossRef] [PubMed]
- Negri, S.; Wang, Y.; Sono, T.; Qin, Q.; Hsu, G.C.; Cherief, M.; Xu, J.; Lee, S.; Tower, R.J.; Yu, V. , et al. Systemic DKK1 neutralization enhances human adipose-derived stem cell mediated bone repair. Stem Cells Transl Med 2021, 10, 610–622. [Google Scholar] [CrossRef]
- Levi, B.; James, A.W.; Nelson, E.R.; Li, S.; Peng, M.; Commons, G.W.; Lee, M.; Wu, B.; Longaker, M.T. Human adipose-derived stromal cells stimulate autogenous skeletal repair via paracrine Hedgehog signaling with calvarial osteoblasts. Stem Cells Dev 2011, 20, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl Mater Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, Y.; Liu, Y.; Zhang, P.; Lv, L.; Zhang, X.; Jia, L.; Zhou, Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif 2019, 52, e12669. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Xu, C.; Meng, L.; Dong, X.; Qi, M.; Jiang, D. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact Mater 2022, 18, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Fu, X.; Li, X.; Li, J.; Han, W.; Wang, Y. Modification of adipose mesenchymal stem cells-derived small extracellular vesicles with fibrin-targeting peptide CREKA for enhanced bone repair. Bioact Mater 2023, 20, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Fan, J.; Liu, Y.; Li, T.; Xu, H.; Yang, Y.; Deng, L.; Li, H.; Zhao, R.C. miR-450b Promotes Osteogenic Differentiation In Vitro and Enhances Bone Formation In Vivo by Targeting BMP3. Stem Cells Dev 2018, 27, 600–611. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Q.; Zhao, Y.; Tian, Z.; Chang, S.; Tong, H.; Liu, N.; Bai, S.; Li, X.; Fan, J. Adipose-derived stem cells with miR-150-5p inhibition laden in hydroxyapatite/tricalcium phosphate ceramic powders promote osteogenesis via regulating Notch3 and activating FAK/ERK and RhoA. Acta Biomater 2023, 155, 644–653. [Google Scholar] [CrossRef]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 2015, 33, 627–638. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Shakesheff, K.M.; White, L.J. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med 2015, 9, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Mayo, V.; Sawatari, Y.; Huang, C.Y.; Garcia-Godoy, F. Neural crest-derived dental stem cells--where we are and where we are going. J Dent 2014, 42, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- La Noce, M.; Paino, F.; Spina, A.; Naddeo, P.; Montella, R.; Desiderio, V.; De Rosa, A.; Papaccio, G.; Tirino, V.; Laino, L. Dental pulp stem cells: state of the art and suggestions for a true translation of research into therapy. J Dent 2014, 42, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Laino, G.; d'Aquino, R.; Graziano, A.; Lanza, V.; Carinci, F.; Naro, F.; Pirozzi, G.; Papaccio, G. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res 2005, 20, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Walboomers, X.F.; van Osch, G.J.; van den Dolder, J.; Jansen, J.A. Hard tissue formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Eng Part A 2008, 14, 285–294. [Google Scholar] [CrossRef]
- Alge, D.L.; Zhou, D.; Adams, L.L.; Wyss, B.K.; Shadday, M.D.; Woods, E.J.; Gabriel Chu, T.M.; Goebel, W.S. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med 2010, 4, 73–81. [Google Scholar] [CrossRef]
- Maraldi, T.; Riccio, M.; Pisciotta, A.; Zavatti, M.; Carnevale, G.; Beretti, F.; La Sala, G.B.; Motta, A.; De Pol, A. Human amniotic fluid-derived and dental pulp-derived stem cells seeded into collagen scaffold repair critical-size bone defects promoting vascularization. Stem Cell Res Ther 2013, 4, 53. [Google Scholar] [CrossRef]
- Huojia, M.; Wu, Z.; Zhang, X.; Maimaitiyiming, M.; Rong, M. Effect of Dental Pulp Stem Cells (DPSCs) in Repairing Rabbit Alveolar Bone Defect. Clin Lab 2015, 61, 1703–1708. [Google Scholar] [CrossRef]
- Jahanbin, A.; Rashed, R.; Alamdari, D.H.; Koohestanian, N.; Ezzati, A.; Kazemian, M.; Saghafi, S.; Raisolsadat, M.A. Success of Maxillary Alveolar Defect Repair in Rats Using Osteoblast-Differentiated Human Deciduous Dental Pulp Stem Cells. J Oral Maxillofac Surg 2016, 74, 829–e821. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Chen, S.; Macri, L.; Kohn, J.; Yelick, P.C. Mandibular Jaw Bone Regeneration Using Human Dental Cell-Seeded Tyrosine-Derived Polycarbonate Scaffolds. Tissue Eng Part A 2016, 22, 985–993. [Google Scholar] [CrossRef]
- Zhang, W.; Saxena, S.; Fakhrzadeh, A.; Rudolph, S.; Young, S.; Kohn, J.; Yelick, P.C. Use of Human Dental Pulp and Endothelial Cell Seeded Tyrosine-Derived Polycarbonate Scaffolds for Robust in vivo Alveolar Jaw Bone Regeneration. Front Bioeng Biotechnol 2020, 8, 796. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Nan, X.; Wei, H.; Shi, J.; Li, A.; Gou, J. Repair of human periodontal bone defects by autologous grafting stem cells derived from inflammatory dental pulp tissues. Stem Cell Res Ther 2016, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cao, Y.; Xie, Y.; Wang, H.; Fan, Z.; Wang, J.; Zhang, C.; Wang, J.; Wu, C.T.; Wang, S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res Ther 2016, 7, 130. [Google Scholar] [CrossRef]
- Lyu, J.; Hashimoto, Y.; Honda, Y.; Matsumoto, N. Comparison of Osteogenic Potentials of Dental Pulp and Bone Marrow Mesenchymal Stem Cells Using the New Cell Transplantation Platform, CellSaic, in a Rat Congenital Cleft-Jaw Model. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, E.E.A.; Beherei, H.H.; El-Zawahry, M.; Farrag, A.R.H.; Kholoussi, N.; Helwa, I.; Mabrouk, M.; Abdel Aleem, A.K. Osteogenic enhancement of modular ceramic nanocomposites impregnated with human dental pulp stem cells: an approach for bone repair and regenerative medicine. J Genet Eng Biotechnol 2022, 20, 123. [Google Scholar] [CrossRef] [PubMed]
- Barbier, L.; Ramos, E.; Mendiola, J.; Rodriguez, O.; Santamaria, G.; Santamaria, J.; Arteagoitia, I. Autologous dental pulp mesenchymal stem cells for inferior third molar post-extraction socket healing: A split-mouth randomised clinical trial. Med Oral Patol Oral Cir Bucal 2018, 23, e469–e477. [Google Scholar] [CrossRef]
- Song, F.; Sun, H.; Huang, L.; Fu, D.; Huang, C. The Role of Pannexin3-Modified Human Dental Pulp-Derived Mesenchymal Stromal Cells in Repairing Rat Cranial Critical-Sized Bone Defects. Cell Physiol Biochem 2017, 44, 2174–2188. [Google Scholar] [CrossRef]
- Song, D.; Xu, P.; Liu, S.; Wu, S. Dental pulp stem cells expressing SIRT1 improve new bone formation during distraction osteogenesis. Am J Transl Res 2019, 11, 832–843. [Google Scholar]
- Wang, W.; Yuan, C.; Geng, T.; Liu, Y.; Zhu, S.; Zhang, C.; Liu, Z.; Wang, P. EphrinB2 overexpression enhances osteogenic differentiation of dental pulp stem cells partially through ephrinB2-mediated reverse signaling. Stem Cell Res Ther 2020, 11, 40. [Google Scholar] [CrossRef]
- Li, J.; Du, H.; Ji, X.; Chen, Y.; Li, Y.; Heng, B.C.; Xu, J. ETV2 promotes osteogenic differentiation of human dental pulp stem cells through the ERK/MAPK and PI3K-Akt signaling pathways. Stem Cell Res Ther 2022, 13, 495. [Google Scholar] [CrossRef]
- Yamakawa, D.; Kawase-Koga, Y.; Fujii, Y.; Kanno, Y.; Sato, M.; Ohba, S.; Kitaura, Y.; Kashiwagi, M.; Chikazu, D. Effects of Helioxanthin Derivative-Treated Human Dental Pulp Stem Cells on Fracture Healing. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Huo, J.F.; Zhang, M.L.; Wang, X.X.; Zou, D.H. Chrysin induces osteogenic differentiation of human dental pulp stem cells. Exp Cell Res 2021, 400, 112466. [Google Scholar] [CrossRef]
- Chan, Y.H.; Ho, K.N.; Lee, Y.C.; Chou, M.J.; Lew, W.Z.; Huang, H.M.; Lai, P.C.; Feng, S.W. Melatonin enhances osteogenic differentiation of dental pulp mesenchymal stem cells by regulating MAPK pathways and promotes the efficiency of bone regeneration in calvarial bone defects. Stem Cell Res Ther 2022, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Maillard, S.; Sicard, L.; Andrique, C.; Torrens, C.; Lesieur, J.; Baroukh, B.; Coradin, T.; Poliard, A.; Slimani, L.; Chaussain, C. Combining sclerostin neutralization with tissue engineering: An improved strategy for craniofacial bone repair. Acta Biomater 2022, 140, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Li, M.; Lin, T.; Zhou, H.; Wang, F.; Su, X. Treatment of inflammatory bone loss in periodontitis by stem cell-derived exosomes. Acta Biomater 2022, 141, 333–343. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, Y.; Liu, X.; He, J.; Zheng, B.; Liu, Y. The Experimental Study of Periodontal Ligament Stem Cells Derived Exosomes with Hydrogel Accelerating Bone Regeneration on Alveolar Bone Defect. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Perrin, S.; Colnot, C. Periosteal Skeletal Stem and Progenitor Cells in Bone Regeneration. Curr Osteoporos Rep 2022, 20, 334–343. [Google Scholar] [CrossRef]
- van Gastel, N.; Stegen, S.; Stockmans, I.; Moermans, K.; Schrooten, J.; Graf, D.; Luyten, F.P.; Carmeliet, G. Expansion of murine periosteal progenitor cells with fibroblast growth factor 2 reveals an intrinsic endochondral ossification program mediated by bone morphogenetic protein 2. Stem Cells 2014, 32, 2407–2418. [Google Scholar] [CrossRef]
- Ji, W.; Kerckhofs, G.; Geeroms, C.; Marechal, M.; Geris, L.; Luyten, F.P. Deciphering the combined effect of bone morphogenetic protein 6 and calcium phosphate on bone formation capacity of periosteum derived cells-based tissue engineering constructs. Acta Biomater 2018, 80, 97–107. [Google Scholar] [CrossRef]
- Lammens, J.; Marechal, M.; Delport, H.; Geris, L.; Oppermann, H.; Vukicevic, S.; Luyten, F.P. A cell-based combination product for the repair of large bone defects. Bone 2020, 138, 115511. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhao, Z.; Cheng, M.; Li, M.; Si, J.; Lin, K.; Yu, H. HIF-1alpha Regulates Osteogenesis of Periosteum-Derived Stem Cells Under Hypoxia Conditions via Modulating POSTN Expression. Front Cell Dev Biol 2022, 10, 836285. [Google Scholar] [CrossRef] [PubMed]
- Groeneveldt, L.C.; Herpelinck, T.; Marechal, M.; Politis, C.; van, I.W.F.J.; Huylebroeck, D.; Geris, L.; Mulugeta, E.; Luyten, F.P. The Bone-Forming Properties of Periosteum-Derived Cells Differ Between Harvest Sites. Front Cell Dev Biol 2020, 8, 554984. [Google Scholar] [CrossRef]
- Yufei, T.; Bingfeng, W.; Jiayi, L.; Hu, L.; Wenli, L.; Lin, X. Distinct osteogenic effect of different periosteum derived cells via Hippo-YAP cascade signaling. Cell Cycle 2023, 22, 183–199. [Google Scholar] [CrossRef]
- Pranskunas, M.; Simoliunas, E.; Alksne, M.; Martin, V.; Gomes, P.S.; Puisys, A.; Kaupinis, A.; Juodzbalys, G. Assessment of the Bone Healing Process Mediated by Periosteum-Derived Mesenchymal Stem Cells' Secretome and a Xenogenic Bioceramic-An In Vivo Study in the Rabbit Critical Size Calvarial Defect Model. Materials (Basel) 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Feng, K.; Hu, J.; Soker, S.; Atala, A.; Ma, P.X. Osteogenic differentiation of human amniotic fluid-derived stem cells induced by bone morphogenetic protein-7 and enhanced by nanofibrous scaffolds. Biomaterials 2010, 31, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeong, S.Y.; Ju, Y.M.; Yoo, J.J.; Smith, T.L.; Khang, G.; Lee, S.J.; Atala, A. In vitro osteogenic differentiation of human amniotic fluid-derived stem cells on a poly(lactide-co-glycolide) (PLGA)-bladder submucosa matrix (BSM) composite scaffold for bone tissue engineering. Biomed Mater 2013, 8, 014107. [Google Scholar] [CrossRef]
- Mohammed, E.E.A.; Beherei, H.H.; El-Zawahry, M.; Farrag, A.R.H.; Kholoussi, N.; Helwa, I.; Gaber, K.; Allam, M.A.; Mabrouk, M.; Aleem, A.K.A. Combination of Human Amniotic Fluid Derived-Mesenchymal Stem Cells and Nano-hydroxyapatite Scaffold Enhances Bone Regeneration. Open Access Maced J Med Sci 2019, 7, 2739–2750. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Wang, Y.; Xu, J.; Huang, T.; Luo, X. Construction of biomimetic cell-sheet-engineered periosteum with a double cell sheet to repair calvarial defects of rats. J Orthop Translat 2023, 38, 1–11. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Si, J.; Dai, J.; Shi, J.; Wang, X.; Guo, L.; Shen, G. Amniotic fluid-derived stem cells mixed with platelet rich plasma for restoration of rat alveolar bone defect. Acta Biochim Biophys Sin (Shanghai) 2017, 49, 197–207. [Google Scholar] [CrossRef]
- Ghaffarinovin, Z.; Soltaninia, O.; Mortazavi, Y.; Esmaeilzadeh, A.; Nadri, S. Repair of rat cranial bone defect by using amniotic fluid-derived mesenchymal stem cells in polycaprolactone fibrous scaffolds and platelet-rich plasma. Bioimpacts 2021, 11, 209–217. [Google Scholar] [CrossRef]
- Wan, C.; He, Q.; Li, G. Allogenic peripheral blood derived mesenchymal stem cells (MSCs) enhance bone regeneration in rabbit ulna critical-sized bone defect model. J Orthop Res 2006, 24, 610–618. [Google Scholar] [CrossRef]
- Zheng, R.C.; Park, Y.K.; Kim, S.K.; Cho, J.; Heo, S.J.; Koak, J.Y.; Lee, S.J.; Park, J.M.; Lee, J.H.; Kim, J.H. Bone Regeneration of Blood-derived Stem Cells within Dental Implants. J Dent Res 2015, 94, 1318–1325. [Google Scholar] [CrossRef]
- Chen, L.; Wu, J.; Wu, C.; Xing, F.; Li, L.; He, Z.; Peng, K.; Xiang, Z. Three-Dimensional Co-Culture of Peripheral Blood-Derived Mesenchymal Stem Cells and Endothelial Progenitor Cells for Bone Regeneration. J Biomed Nanotechnol 2019, 15, 248–260. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Lai, S.; Cao, Q.; Liu, Y.; Li, J.; Zhu, X.; Fu, W.; Zhang, X. Construction of Vascularized Tissue Engineered Bone with nHA-Coated BCP Bioceramics Loaded with Peripheral Blood-Derived MSC and EPC to Repair Large Segmental Femoral Bone Defect. ACS Appl Mater Interfaces 2023, 15, 249–264. [Google Scholar] [CrossRef]
- Li, S.; Huang, K.J.; Wu, J.C.; Hu, M.S.; Sanyal, M.; Hu, M.; Longaker, M.T.; Lorenz, H.P. Peripheral blood-derived mesenchymal stem cells: candidate cells responsible for healing critical-sized calvarial bone defects. Stem Cells Transl Med 2015, 4, 359–368. [Google Scholar] [CrossRef]
- Lin, W.; Xu, L.; Lin, S.; Shi, L.; Wang, B.; Pan, Q.; Lee, W.Y.W.; Li, G. Characterisation of multipotent stem cells from human peripheral blood using an improved protocol. J Orthop Translat 2019, 19, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Ma, Q.; Cui, F.; Zhong, Y. Human umbilical cord mesenchymal stem cells: osteogenesis in vivo as seed cells for bone tissue engineering. J Biomed Mater Res A 2009, 91, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Qu, Z.; Yin, X.; Shang, C.; Ao, Q.; Gu, Y.; Liu, Y. Efficacy of umbilical cord-derived mesenchymal stem cell-based therapy for osteonecrosis of the femoral head: A three-year follow-up study. Mol Med Rep 2016, 14, 4209–4215. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, M.; Figiel-Dabrowska, A.; Lech, W.; Wieprzowski, L.; Strzalkowski, R.; Strzemecki, D.; Cheda, L.; Lenart, J.; Domanska-Janik, K.; Sarnowska, A. Bone Defect Repair Using a Bone Substitute Supported by Mesenchymal Stem Cells Derived from the Umbilical Cord. Stem Cells Int 2020, 2020, 1321283. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Yang, H.; Cao, Y.; Yu, D.; Zhao, Y.; Cao, Y. MicroRNA-196a-5p overexpression in Wharton's jelly umbilical cord stem cells promotes their osteogenic differentiation and new bone formation in bone defects in the rat calvarium. Cell Tissue Res 2022, 390, 245–260. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, B.; Yin, P.; Zhao, L.; Wang, Y.; Fu, Z.; Dang, R.; Xu, J.; Zhang, J.; Wen, N. Integration of Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomes with Hydroxyapatite-Embedded Hyaluronic Acid-Alginate Hydrogel for Bone Regeneration. ACS Biomater Sci Eng 2020, 6, 1590–1602. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Ni, C.Y.; Chen, C.Y.; Rao, S.S.; Yin, H.; Huang, J.; Tan, Y.J.; Wang, Z.X.; Cao, J. , et al. Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism. Theranostics 2020, 10, 2293–2308. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Hao, Z.; Zhou, P.; Wang, P.; Fang, S.; Li, L.; Xu, S.; Xia, Y. Umbilical Mesenchymal Stem Cell-Derived Exosome-Encapsulated Hydrogels Accelerate Bone Repair by Enhancing Angiogenesis. ACS Appl Mater Interfaces 2021, 13, 18472–18487. [Google Scholar] [CrossRef]
- Bahar, D.; Gonen, Z.B.; Gumusderelioglu, M.; Onger, M.E.; Tokak, E.K.; Ozturk-Kup, F.; Ozkan, B.B.; Gokdemir, N.S.; Cetin, M. Repair of Rat Calvarial Bone Defect by Using Exosomes of Umbilical Cord-Derived Mesenchymal Stromal Cells Embedded in Chitosan/Hydroxyapatite Scaffolds. Int J Oral Maxillofac Implants 2022, 37, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhang, J.; Li, H.; Zhu, Z.; Guo, S.; Niu, X.; Wang, Y.; Zhang, C. Human Urine Derived Stem Cells in Combination with beta-TCP Can Be Applied for Bone Regeneration. PLoS One 2015, 10, e0125253. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, J.; Zhu, Z.; Niu, X.; Guo, S.; Wang, Y.; Zhang, C. Bone morphogenetic protein 2 gene transduction enhances the osteogenic potential of human urine-derived stem cells. Stem Cell Res Ther 2015, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhang, J.; Guo, S.; Zhu, H.; Zhu, Z.; Li, H.; Wang, Y.; Zhang, C.; Chang, J. Human urine-derived stem cells can be induced into osteogenic lineage by silicate bioceramics via activation of the Wnt/beta-catenin signaling pathway. Biomaterials 2015, 55, 1–11. [Google Scholar] [CrossRef]
- Xing, F.; Li, L.; Sun, J.; Liu, G.; Duan, X.; Chen, J.; Liu, M.; Long, Y.; Xiang, Z. Surface mineralized biphasic calcium phosphate ceramics loaded with urine-derived stem cells are effective in bone regeneration. J Orthop Surg Res 2019, 14, 419. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, L.; Xing, F.; Yang, Y.; Gong, M.; Liu, G.; Wu, S.; Luo, R.; Duan, X.; Liu, M. , et al. Graphene oxide-modified silk fibroin/nanohydroxyapatite scaffold loaded with urine-derived stem cells for immunomodulation and bone regeneration. Stem Cell Res Ther 2021, 12, 591. [Google Scholar] [CrossRef]
- Liu, G.; Sun, J.; Gong, M.; Xing, F.; Wu, S.; Xiang, Z. Urine-derived stem cells loaded onto a chitosan-optimized biphasic calcium-phosphate scaffold for repairing large segmental bone defects in rabbits. J Biomed Mater Res B Appl Biomater 2021, 109, 2014–2029. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Z.; Yu, X.; Duan, X.; Chen, J.; Liu, G.; Gong, M.; Xing, F.; Sun, J.; Huang, S. , et al. A sustained release of BMP2 in urine-derived stem cells enhances the osteogenic differentiation and the potential of bone regeneration. Regen Biomater 2022, 9, rbac015. [Google Scholar] [CrossRef]
- Xing, F.; Yin, H.M.; Zhe, M.; Xie, J.C.; Duan, X.; Xu, J.Z.; Xiang, Z.; Li, Z.M. Nanotopographical 3D-Printed Poly(epsilon-caprolactone) Scaffolds Enhance Proliferation and Osteogenic Differentiation of Urine-Derived Stem Cells for Bone Regeneration. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.L.; Xing, F.; Duan, X. Three-dimensional printed polylactic acid and hydroxyapatite composite scaffold with urine-derived stem cells as a treatment for bone defects. J Mater Sci Mater Med 2022, 33, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, X.L.; Wang, Y.N.; Lu, W.; Wang, H.; Liao, R.; Zeng, M.; Yang, J.X.; Hu, Y.; Xie, J. Extracellular Vesicles from Human Urine-Derived Stem Cells Ameliorate Particulate Polyethylene-Induced Osteolysis. Int J Nanomedicine 2021, 16, 7479–7494. [Google Scholar] [CrossRef]
- Lu, W.; Zeng, M.; Liu, W.; Ma, T.; Fan, X.; Li, H.; Wang, Y.; Wang, H.; Hu, Y.; Xie, J. Human urine-derived stem cell exosomes delivered via injectable GelMA templated hydrogel accelerate bone regeneration. Mater Today Bio 2023, 19, 100569. [Google Scholar] [CrossRef]
- Hayashi, O.; Katsube, Y.; Hirose, M.; Ohgushi, H.; Ito, H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif Tissue Int 2008, 82, 238–247. [Google Scholar] [CrossRef]
- Stockmann, P.; Park, J.; von Wilmowsky, C.; Nkenke, E.; Felszeghy, E.; Dehner, J.F.; Schmitt, C.; Tudor, C.; Schlegel, K.A. Guided bone regeneration in pig calvarial bone defects using autologous mesenchymal stem/progenitor cells - a comparison of different tissue sources. J Craniomaxillofac Surg 2012, 40, 310–320. [Google Scholar] [CrossRef]
- Niemeyer, P.; Fechner, K.; Milz, S.; Richter, W.; Suedkamp, N.P.; Mehlhorn, A.T.; Pearce, S.; Kasten, P. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials 2010, 31, 3572–3579. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Wang, B. , et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther 2017, 8, 275. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, S.; Yassin, M.A.; Rashad, A.; Espedal, H.; Idris, S.B.; Finne-Wistrand, A.; Mustafa, K.; Vindenes, H.; Fristad, I. Comparison of bone regenerative capacity of donor-matched human adipose-derived and bone marrow mesenchymal stem cells. Cell Tissue Res 2021, 383, 1061–1075. [Google Scholar] [CrossRef]
- Rodrigues, M.T.; Lee, S.J.; Gomes, M.E.; Reis, R.L.; Atala, A.; Yoo, J.J. Amniotic fluid-derived stem cells as a cell source for bone tissue engineering. Tissue Eng Part A 2012, 18, 2518–2527. [Google Scholar] [CrossRef]
- Mohammed, E.E.A.; El-Zawahry, M.; Farrag, A.R.H.; Aziz, N.N.A.; Sharaf-ElDin, W.; Abu-Shahba, N.; Mahmoud, M.; Gaber, K.; Ismail, T.; Mossaad, M.M. , et al. Osteogenic Differentiation Potential of Human Bone Marrow and Amniotic Fluid-Derived Mesenchymal Stem Cells in Vitro & in Vivo. Open Access Maced J Med Sci 2019, 7, 507–515. [Google Scholar] [CrossRef]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M. , et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem Biophys Res Commun 2018, 497, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chan, Y.H.; Hsieh, S.C.; Lew, W.Z.; Feng, S.W. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.; Mannel, C.; Bolte, J.; Tian, X.; Goodman, S.B.; Zwingenberger, S. Effectiveness of Dental Pulp-derived Stem Cells and Bone Marrowderived Mesenchymal Stromal Cells Implanted into a Murine Critical Bone Defect. Curr Stem Cell Res Ther 2022, 17, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.T.; Lee, W.F.; Chen, S.M.; Hao, L.T.; Hung, Y.T.; Lai, P.C.; Feng, S.W. Effect of Different Bone Grafting Materials and Mesenchymal Stem Cells on Bone Regeneration: A Micro-Computed Tomography and Histomorphometric Study in a Rabbit Calvarial Defect Model. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wei, S.M.; Yan, K.X.; Gu, Y.X.; Lai, H.C.; Qiao, S.C. Bovine-Derived Xenografts Immobilized With Cryopreserved Stem Cells From Human Adipose and Dental Pulp Tissues Promote Bone Regeneration: A Radiographic and Histological Study. Front Bioeng Biotechnol 2021, 9, 646690. [Google Scholar] [CrossRef]
- Nyberg, E.; Farris, A.; O'Sullivan, A.; Rodriguez, R.; Grayson, W. Comparison of Stromal Vascular Fraction and Passaged Adipose-Derived Stromal/Stem Cells as Point-of-Care Agents for Bone Regeneration. Tissue Eng Part A 2019, 25, 1459–1469. [Google Scholar] [CrossRef]
- Zhang, Y.; Grosfeld, E.C.; Camargo, W.A.; Tang, H.; Magri, A.M.P.; van den Beucken, J. Efficacy of intraoperatively prepared cell-based constructs for bone regeneration. Stem Cell Res Ther 2018, 9, 283. [Google Scholar] [CrossRef]
- Lough, D.; Swanson, E.; Sopko, N.A.; Madsen, C.; Miller, D.; Wang, H.; Guo, Q.; Sursala, S.M.; Kumar, A.R. Regeneration of Vascularized Corticocancellous Bone and Diploic Space Using Muscle-Derived Stem Cells: A Translational Biologic Alternative for Healing Critical Bone Defects. Plast Reconstr Surg 2017, 139, 893–905. [Google Scholar] [CrossRef]
- Agata, H.; Asahina, I.; Yamazaki, Y.; Uchida, M.; Shinohara, Y.; Honda, M.J.; Kagami, H.; Ueda, M. Effective bone engineering with periosteum-derived cells. J Dent Res 2007, 86, 79–83. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.B.; Lamo-Espinosa, J.M.; Muinos-Lopez, E.; Ripalda-Cemborain, P.; Abizanda, G.; Valdes-Fernandez, J.; Lopez-Martinez, T.; Flandes-Iparraguirre, M.; Andreu, I.; Elizalde, M.R. , et al. Periosteum-derived mesenchymal progenitor cells in engineered implants promote fracture healing in a critical-size defect rat model. J Tissue Eng Regen Med 2019, 13, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Gan, Y.; Shi, D.; Zhao, J.; Tang, T.; Dai, K. A novel cytotherapy device for rapid screening, enriching and combining mesenchymal stem cells into a biomaterial for promoting bone regeneration. Sci Rep 2017, 7, 15463. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hwang, M.P.; Wright, N.; Lu, A.; Ruzbarsky, J.J.; Huard, M.; Cheng, H.; Mullen, M.; Ravuri, S.; Wang, B. , et al. The use of heparin/polycation coacervate sustain release system to compare the bone regenerative potentials of 5 BMPs using a critical sized calvarial bone defect model. Biomaterials 2022, 288, 121708. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




