1. Introduction
Acute myeloid leukemia (AML) is a diverse clonal disorder arising from the hematopoietic stem cell, resulting in compromised hematopoiesis and uncontrolled proliferation of abnormal myeloid clones within the bone marrow [
1]. At diagnosis, the biological characteristic of AML plays a pivotal role in determining the disease prognosis, influencing both the anticipated response to therapy and overall survival.
Recent advancements in comprehending the pathogenic mechanisms have witnessed the introduction of novel targeted therapies, with an increase in the therapeutic arsenal and an improvement of the overall survival in specific patient subgroups. Despite these efforts, frontline therapy for younger, fit patients continues to rely on standard intensive chemotherapy (ICT) followed by allogeneic stem cell transplantation (alloHCT), stratified by the relapse risk at the time of diagnosis [
2]. Also, primary refractory or relapsed acute myeloid patients (R/R AML) continue to face dismal outcomes [
3].
Notably, significant progress has been made in drug development, particularly for elderly patients where traditional intensive chemotherapy regimens may not be viable. Therapeutic modalities such as BCL2-inhibitor venetoclax-based regimens [
4,
5,
6,
7,
8], IDH-1 and IDH-2 inhibitors ivosidenib [
9] and enasidenib [
10], FLT3-inhibitors such as midostaurin [
11] or gilteritinib [
12], CD33 monoclonal antibody gemtuzumab-ozogamicin [
13] or the recent introduction of menin inhibitors like revumenib [
14] for KMT2A and NPM1 AML patients.
In this context, understanding the potential evolution patterns in R/R AML patients becomes crucial to tailor optimal therapeutic strategies, given the inherent limitations. Advanced techniques such as whole-genome sequencing and single-cell analysis have been employed to unravel diverse clonal evolution mechanisms, ranging from linear to branching patterns, all likely within the same disease.
This study delves into the genomic profile of AML patients after relapse or refractoriness to treatment, aiming to elucidate clonal evolution mechanisms shortly after the event. The insights gained from this study might hold some potential to inform subsequent clinical decisions and refine therapeutic approaches for this challenging patient population
2. Endpoint.
The primary endpoint was to analyse the clonal evolution at the time of disease progression in AML, either following initial refractoriness or relapse, in order to identify recurrent mutational patterns associated with specific treatment regimens, and their potential relationship to clinical events.
3. Results
3.1. Baseline characteristics.
In total, 62 patients with paired samples were analyzed in the study. Of these, 28 patients received treatment with ICT, and 34 patients underwent LIT. Notably, patients treated with LIT tended to be older (73y vs. 66y, p = 0.08), had a lower white blood cell count at diagnosis (0.6x109/L vs. 12.1x109/L, p < 0.001) higher frequency of adverse risk according to the ELN 2022 classification (70.6%, vs. 32.1%, p = 0.008), and also more cases of R/R AML (21.2%, vs 0%, p = 0.002). Additionally, patients receiving ICT underwent more alloHCT as consolidation treatment after the initial therapy (42.9% vs. 17.6%, p = 0.04). The detailed baseline characteristics can be found in
Table 1.
3.2. Standard intensive chemotherapy (ICT)
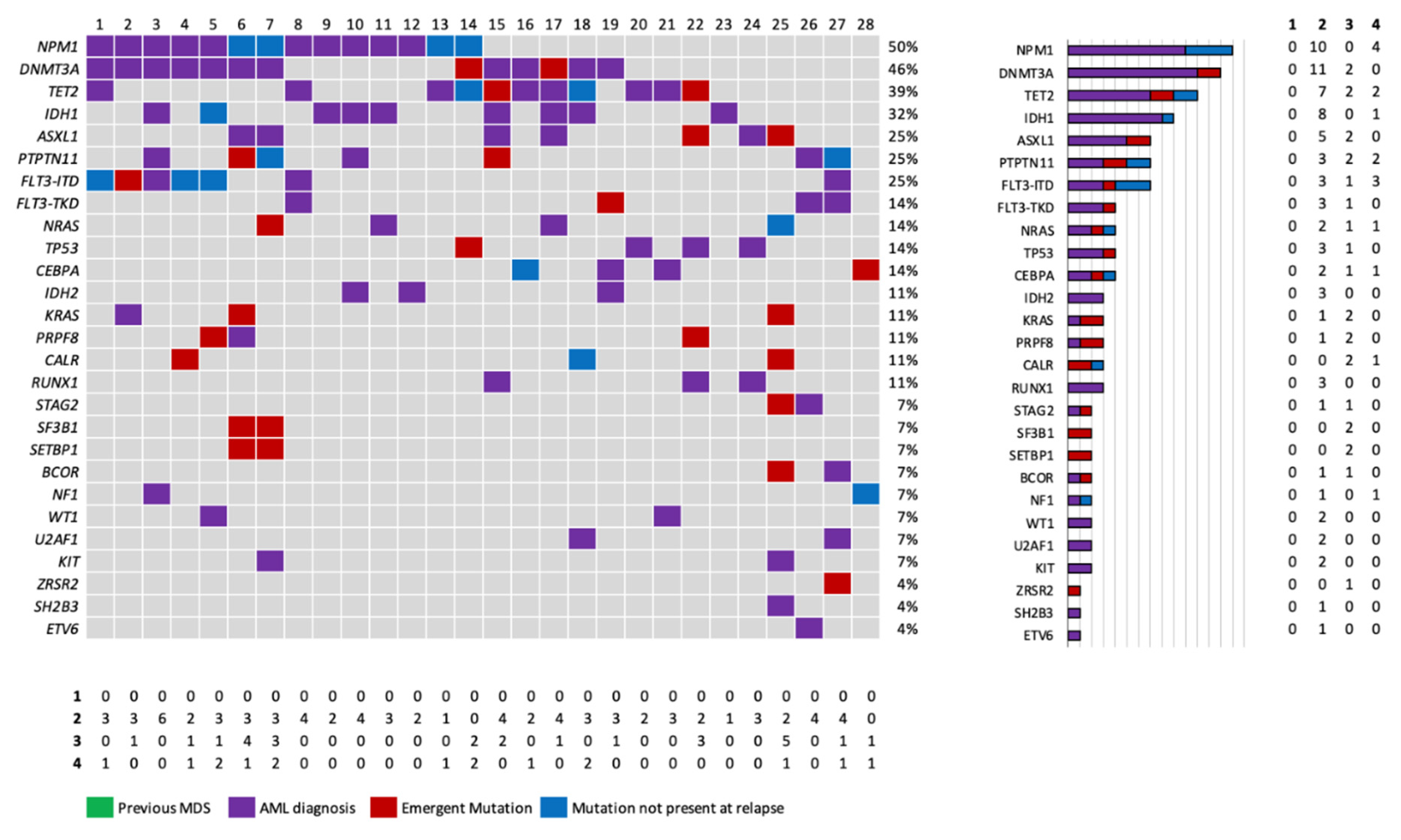
In the ICT group, the most common diagnosis, was AML with mutated NPM1 (50%, 14/28). All patients in this group were treatment-naïve before ICT, with 5 demonstrating initial treatment refractoriness and the remaining 23 relapsing after an initial response. The median number of mutations detected at diagnosis and relapse were 3 (range 1-7) and 4 (range 0-9), respectively (p = 0.24).
Among refractory patients, only one presented emergent mutations after therapy. Among patients who presented a relapse, we observed emergent mutations in 13 out of the 23 patients (56.5%) in a diversity of genes, without a clear pattern of emerging mutations. Thus, emergent mutations were observed in genes related to DNA methylation (DNMT3A, n=2; TET2, n=2), histone modification (ASXL1, n=2), in the RAS-MAPK pathway (KRAS, n=2, NRAS, n = 1), FLT3-ITD, n=1, and splicing factors (SETBP1 (n=2), SF3B1 (n=2), ZRSR and spliceosome PRFR8 (n=1).
Regarding the subset of patients with NPM1 mutation NPM1, most patients (10/14) maintained NPM1 mutation at relapse with the exception of 4 patients. In 3 / 4 patients with a NPM1wt relapse, a common DNA methylation gene mutation was maintained at relapse (DNMT3A, 2, TET2, n=1), with emergence or not of other mutations (patients in columns 6, 7 & 13), suggesting a common clonal origin ancestor. In a unique patient initially diagnosed (no. 14), genomic characterization at relapse did not identify any common gene mutation between diagnosis (TET2, NPM1) and relapse (DNMT3A, TP53).
Furthermore, FLT3-ITD was not detected at relapse/refractoriness in 42.8% of the patients who had it at initial diagnosis (3/7) (
Figure 1).
Median overall survival after relapse was 10.5 months (range 7.4-31.2). Salvage treatments offered after progressive disease in these patients included: ICT (n = 1), azacitidine (n = 2), venetoclax and azacitidine (n = 3), azacitidine and FLT3 inhibitor sorafenib (n = 1) and enrollment in a clinical trial (n = 4).
3.3. Low-intensity treatments
Low-intensity treatments with paired samples were analyzed in a total number of 34 patients with 36 different LIT. LIT included: hypomethylating agents in monotherapy (n = 12), venetoclax and azacitidine (n = 21), azacitidine in combination with FLT3 inhibitors (n = 2) and azacitidine in combination with IDH2 inhibitor enasidenib (n = 1).
Before starting LIT, 5 patients had previously received standard chemotherapy as frontline therapy. Furthermore, 6 patients received initially hypomethylating agents in monotherapy as first line treatment, and, after an initial progression, they were treated again with another LIT as salvage regimen, being reassessed after each progressive disease. NGS was assessed at both times of progression in 4 out of 10 of these patients. The remaining 23 patients received LIT as first-line for AML (67.6%).
The most common diagnosis was AML with myelodysplasia-related gene mutations (50%), with most of the patients (70.6%) in the adverse risk at initial diagnosis according to the ELN 2022 risk stratification. In correlation with diagnosis, in these patients, mutations related with myelodysplasia were the most common (RUNX1, n=10; STAG2, n=9; ASXL1, n=8), with 2 patients diagnosed with mutated FLT3-ITD at diagnosis. No other mutations related to the RTK/RAS pathway were detected at diagnosis. Baseline characteristics of patients are also displayed in
Table 1.
Median number of mutations at diagnosis and progression were 4 in both time-points (range 1-10 and 0-10, respectively). Emergent mutations at progressive disease in a 64% (22/34) of the patients.
Appearance of mutations in the RAS-MAPK signaling pathway were observed in 35.9% (12/34) of all treated patients, corresponding to 54.5% of all patients with emergent mutations (12/22). Mutations in the RAS-MAPK pathway were found in: NRAS, (n = 5); KRAS, (n = 2); FLT3-ITD, (n = 2); single nucleotide variants in FLT3 (FLT3-SNV), (n = 3), CSF3R (n = 2) and PTPN11, (n = 1). All the emergent in the signaling pathwa mutations emerged individually in each patient except in two patients: one presenting a simultaneous emergency in NRAS, FLT3-SNV and KRAS and another one with mutations occurring in CSF3R and FLT3-SNV. Other new mutations at progression were detected in RUNX1 (n = 2), STAG2 (n = 2), splicing genes such as ZRSR2 (n = 1), SF3B1 (n = 1) or SRSF2 (n = 2), PHF6 (n = 1), IDH1 (n = 1) and IDH2 (n = 1). (
Figure 2)
Splitting the patients according to the different treatments received, emergent mutations were found in 12 out of the 22 patients that received venetoclax in combination with hypomethylating agents. Six of these patients presented mutations in genes related to the RAS-MAPK pathway. In patients receiving hypomethylating agents, half of them presented emergent mutations at progression, with 5 out these 6 presenting mutations related to the RAS-MAPK pathway at progressive disease.
Median overall survival in treated patients was 19.5 months (range 17.4-38.3) and median overall survival after progressive disease was 6 months (range 3.7-13.3). Patients who presented an emergent mutation in the RAS-MAPK pathway did not have a shorter survival (6.1 vs. 4.8 months, p = 0.68, Supplementary Material). Salvage treatments offered after progressive disease in these patients included: Venetoclax and azacitidine in 3 patients initially treated with hypomethylating agents in monotherapy, with no response and no further analysis, targeted therapy (1 patient with FLT3 inhibitor sorafenib after an emergent mutation and another one with IDH2 inhibitor enasidenib) and enrollment in clinical trials in 4 cases.
3.4. Subgroup analysis of patients with AML with mutated TP53
Interestingly, we performed parallel analysis on the patients harboring TP53 mutation at treatment start (n=6), independently of the offered treatment due to the dismal outcome of this subgroup. They maintained this mutation at progression in both types of treatment. Two additional patients presented with an emerging TP53 mutation at relapse, one after standard chemotherapy consolidated with an allogeneic stem cell transplantation and the remaining after venetoclax-based LIT. Despite this initial response, only one patient was alive at the moment of data analysis
4. Discussion
Our examination of the mutational landscape after relapse or refractoriness to treatment in sequential samples, holds promise for advancing our understanding of clonal evolution and escape mechanisms in AML patients. The insights derived from this study could significantly contribute to informed clinical decision regarding salvage therapies, using the benefits of accessibility and sensitivity. It is essential to acknowledge the limitations of the study, including a small patient cohort and inherent technical limitations. Despite these limitations, the findings offer valuable insights into clonal selection in AML after therapy.
In the subgroup of patients treated with ICT, the results underscore the disease's heterogeneity, not only at the time of diagnosis but also in its behavior during follow-up, showing diverse patterns across all AML subtypes. The observed clonal evolution exhibits both linear evolution, marked by the addition of mutations, as well as branching evolution, characterized by the loss of driver mutations post-initial treatment or the emergence of mutations in signaling pathways. These results echo the broad concept of clonal evolution in this setting and align with previous studies employing various molecular techniques to analyze clonal evolution, such as whole exome sequencing (WES), RNA-sequencing, or single-cell sequencing [
15,
16,
17,
18,
19].
We also observed, as previously described, the need of time for a clonal evolution and therapy selection, since the biological architecture was only modified in one of the patients that showed an initial refractoriness to treatment.
An example of the variety in the evolution mechanisms previously mentioned, is shown in our series as the development of a second NPM1wt myeloid neoplasm patients after an initial AML with NPM1: in our study, these cases presented as late relapses with the presence of mutations in age-related clonal hematopoiesis genes, as previously described [
20]. These situations offer a challenge for clinicians to choose the best therapeutical option at the moment of relapse, having to consider the previous treatments in this new setting.
On the other hand, in patients treated with LIT, we have observed emergent mutations in 62% of the patients, with a consisting profile of emerging mutations in the RAS-MAPK signaling pathways.
In patients treated with single hypomethylating agents, secondary resistance to azacitidine has been found to be heterogeneous, describing linear and branching clinical patterns without a distinguished mechanism [
21].
Interestingly, we have also found a significant number of patients with emergent RAS-MAPK mutations at relapse; however, given the small number of treated patients, larger cohorts are needed to confirm our results.
The consistence of the clonal selection of mutations in the RAS-MAPK signaling pathways earns relevance in the pathogenesis of secondary clinical resistance of patients treated with hypomethylating agents in combination with targeted therapy in this study, including FLT3 inhibitors and BCL2 inhibitor venetoclax.
Our results are akin to the predominant selection of RAS-MAPK mutated clones after treatment with midostaurin [
22], where whole genome sequencing was used to study R/R AML patients after therapy of the RATIFY. In this study, mutations in the RAS-MAPK pathway were more frequent as a relapse mechanism than resistant FLT3-ITD clones, and gilteritinib, where it was seen using single-cell that the emergency of these mutations at relapse was eventually due to the selection of these subclones that were already present at diagnosis [
23].
Finally, in patients treated with venetoclax in combination with hypomethylating agents (VenH) the evidence found in our results enlightens the critical role of the RAS-MAPK pathway in the resistance to venetoclax and azacitidine. At diagnosis, the significant prognosis of mutations in signaling pathway regulators such as PTPN11 and FLT3 has already been shown in several studies [
24,
25]. This relevance has been also highlighted in the recent risk stratification proposed by Döhner et al. [
26] based on the Viale-A4 study, where patients with FLT3-ITD and NRAS or KRAS mutations have an intermediate risk with a shorter survival than most of the treated patients.
At relapse, some resistance mechanisms have been described, including the loss of dependence of oxidative phosphorylation of the AML cells, the monocytic phenotype [
30] of the clone or the upstream regulation of other BCL-2 family proteins such as MCL-131,. In these last two resistance mechanisms, the RAS-MAPK pathway has turned out to be essential, due to the presence of mutations in NRAS/KRAS in the monocytic clones and the upstream regulation of MCL-1. Since this evidence is based on a small number of patients, further knowledge y is needed to know whose patients may benefit from new combinations. For instance, triplets with FLT3 inhibitors in FLT3mut patients32 or FLT3wt patients [
31] might be useful as they have been shown to restructure the upstream regulation of MCL-1 in the AML cells.
5. Materials and Methods
A retrospective, single-center study was undertaken to evaluate the mutation profile in paired samples obtained at the time of diagnosis and at the onset of progressive or refractory disease in patients diagnosed with AML who received treatment at Hospital Clinic de Barcelona, Barcelona, Spain.
A commercial next-generation sequencing (NGS) panel, specifically the Oncomine Myeloid Research Assay® (ThermoFisher), employing Ion Torrent® technology, was used for mutational analysis. This NGS panel comprehensively assesses the "hotspot" regions of 23 genes and the complete coding sequence of 17 genes via DNA analysis; and the rearrangements of 29 fusion driver genes through RNA analysis. These genes are recurrently associated with the pathogenesis of myeloid neoplasms (
Appendix B). AML was classified accordingly with the ICC 2022 and the WHO 5
th classification of myeloid neoplasms [
32,
33], and previous treatment response was evaluated based on the 2022 European LeukemiaNet Risk Classification (ELN 2022)2, following Cheson criteria [
34].
Patients were categorized based on the received treatment: Standard chemotherapy (CETLAM-12 or CETLAM-22 protocols) or low-intensity therapy (LIT), encompassing hypomethylating agents in monotherapy (azacitidine or decitabine), venetoclax and azacitidine, azacitidine in combination with FLT3 inhibitors (sorafenib or gilteritinib), and azacitidine in combination with IDH-2 inhibitor enasidenib. AlloHCT, following ELN 2022 recommendations based on relapse risk after initial therapy, was considered for patients in both subgroups. Clinical and biological patient characteristics were obtained from medical records, and the study adhered to the principles of the Declaration of Helsinki.
Statistical analyses included descriptive statistics for all patients, using median and range for continuous variables and frequency and percentage for categorical variables. Univariate analysis of categorical variables employed Fisher’s exact test or χ2 test, while the t-test was used to compare continuous variables. All p-values were two-sided, with statistical significance evaluated at the 0.05 alpha level. Statistical analyses were conducted using R statistics version 4.0.3 (R core Team, R Foundation for Statistical Computing, Vienna, Austria).
6. Conclusions
We conclude that, emerging mutations are frequently observed at progressive AML disease after therapy. In pa6resistance, not only if they are present at diagnosis, but also as emergent escape mechanisms during response, becoming a potential target for the development of new drugs.
Author Contributions
CJV designed the study, performed the statistical analysis and wrote the manuscript; JE supervised the study and contributed to manuscript writing; MLG, FG and DC performed molecular analysis; AIPV, DM, SCD, AMR, ACB, DE, AT, NT, IH and MDB collected data. All authors revised and approved the final version of the manuscript.
Conflicts of Interest
C.J-V. serves as speaker for AbbVie and received travel grants from AbbVie and Pfizer. F.G serves as educational speaker for AbbVie. A.M-R. serves as a consultant or in an advisory role, for Bristol Myers Squibb (BMS), AbbVie, and Kite Gilead; received travel grants from Kite Gilead, Roche, Takeda, Janssen, and AbbVie; and serves as a speaker for AbbVie and Gilead. M.D-B. serves as a consultant for, in an advisory role for, received travel grants from, or served as speaker for BMS, AbbVie, Astellas, JazzPharma, Takeda, and Novartis. J.E. declares consultancy honoraria from AbbVie, Novartis, Astellas, Jazz Pharmaceuticals, BMS-Celgene, Pfizer, and Daichii-Sankyo, and received research grants from Novartis, Jazz Pharmaceuticals, and Pfizer. The remaining authors declare no competing financial interests.
Appendix A
Figure A1.
Overall survival after relapse of patients treated with low-intensity treatments depending on the emergency of mutations in the RAS-MAPK pathway after treatment.
Figure A1.
Overall survival after relapse of patients treated with low-intensity treatments depending on the emergency of mutations in the RAS-MAPK pathway after treatment.
Appendix B
Hotspot regions: ABL1, BRAF, CBL, CSF3R, DNMT3A, FLT3, GATA2, HRAS, IDH1, IDH2, JAK2, KIT, KRAS, MPL, MYD88, NPM1, NRAS, PTPN11, SETBP1, SF3B1, SRSF2, U2AF1, WT1
Full coding region genes: ASXL1, BCOR, CALR, CEBPA, ETV6, EZH2, IKZF1, NF1, PHF6, PRPF8, RB1, RUNX1, SH2B3, STAG2, TET2, TP53, ZRSR2
Fusion driver genes: ABL1, ALK, BCL2, BRAF, CCND1, CREBBP, EGFR, ETV6, FGFR1, FGFR2, FUS, HMGA2, JAK2, KMT2A, MECOM, MET, MLLT10, MLLT3, MYBL1, MYH11, NTRK3, NUP214, PDGFRA, PDGFRB, RARA, RBM15, RUNX1, TCF3, TFE3
Figure B1. Genes included in the targeted-NGS panel used for the study Oncomine Myeloid Research Assay®, ThermoFisher Scientific)
References
- Döhner, H., Weisdorf, D. J. & Bloomfield, C. D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152.
- Döhner, H. et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood vol. 140 (2022).
- Ganzel, C. et al. Very poor long-term survival in past and more recent studies for relapsed AML patients: The ECOG-ACRIN experience. Am. J. Hematol. 2018, 93, 1074–1081. [CrossRef]
- DiNardo, C. D. et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [CrossRef]
- DiNardo, C. D. et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol. 2020, 7, e724–e736. [CrossRef]
- DiNardo, C. D. et al. Venetoclax combined with FLAG-IDA induction and consolidation in newly diagnosed acute myeloid leukemia. Am. J. Hematol. 2022, 97, 1035–1043. [CrossRef]
- Chua, C. C. et al. Chemotherapy and Venetoclax in Elderly Acute Myeloid Leukemia Trial (CAVEAT): A Phase Ib Dose-Escalation Study of Venetoclax Combined with Modified Intensive Chemotherapy. J. Clin. Oncol. 2020, 38, 3506–3517. [CrossRef]
- Kadia, T. M. et al. Venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a cohort from a single-centre, single-arm, phase 2 trial. Lancet Haematol. 2021, 8, e552–e561. [CrossRef]
- Montesinos, P. et al. Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [CrossRef]
- DiNardo, C. D. et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-<em>IDH2</em> acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021, 22, 1597–1608. [CrossRef]
- Stone, R. M. et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [CrossRef]
- Perl, A. E. et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [CrossRef]
- Juliette Lambert et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119.
- Issa, G. C. et al. The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia. Nature 2023, 615, 920–924. [CrossRef]
- Christen, F. et al. Genomic landscape and clonal evolution of acute myeloid leukemia with t(8;21): an international study on 331 patients. Blood 2019, 133, 1140–1151. [CrossRef]
- Vosberg, S. Clonal evolution of acute myeloid leukemia from diagnosis to relapse. 839–849 (2019). [CrossRef]
- Greif, P. A. et al. Evolution of Cytogenetically Normal Acute Myeloid Leukemia During Therapy and Relapse: An Exome Sequencing Study of 50 Patients. Clin. Cancer Res. 2018, 24, 1716–1726. [CrossRef]
- Morita, K. et al. Clonal evolution of acute myeloid leukemia revealed by high-throughput single-cell genomics. Nat. Commun. 2020, 11, 1–17. [CrossRef]
- Cocciardi, S. et al. Clonal evolution patterns in acute myeloid leukemia with NPM1 mutation. Nat. Commun. 2019, 10, 2031. [CrossRef]
- Miles, L. A. et al. Single-cell mutation analysis of clonal evolution in myeloid malignancies. Nature 587, (2020). [CrossRef]
- Höllein, A. et al. NPM1 mutated AML can relapse with wild-type NPM1: persistent clonal hematopoiesis can drive relapse. Blood Adv. 2018, 2, 3118–3125. [CrossRef]
- Symeonidou, V. et al. Heterogeneous genetic and non-genetic mechanisms contribute to response and resistance to azacitidine monotherapy. 794–803 (2022). [CrossRef]
- Schmalbrock, L. K. et al. Clonal evolution of acute myeloid leukemia with FLT3 -ITD mutation under treatment with midostaurin. 2021, 137, 3093–3104. [CrossRef]
- Mcmahon, C. M. et al. Clonal Selection with RAS Pathway Activation Mediates Secondary Clinical Resistance to Selective FLT3 Inhibition in Acute Myeloid Leukemia. (2019). [CrossRef]
- 25. Chyla, B. et al. Genetic biomarkers of sensitivity and resistance to venetoclax monotherapy in patients with relapsed acute myeloid leukemia. Am. J. Hematol. 2018, 93, E202–E205. [CrossRef]
- DiNardo, C. D. et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 2020, 135, 791–803. [CrossRef]
- Döhner, H. et al. ELN Risk Stratification Is Not Predictive of Outcomes for Treatment-Naïve Patients with Acute Myeloid Leukemia Treated with Venetoclax and Azacitidine. Blood 2022, 140, 1441–1444. [CrossRef]
- Pei, S. et al. Monocytic Subclones Confer Resistance to Venetoclax-Based Therapy in Patients with Acute Myeloid Leukemia. Cancer Discov. 2020, 10, 536–551. [CrossRef]
- Zhang, Q. et al. Activation of RAS/MAPK pathway confers MCL-1 mediated acquired resistance to BCL-2 inhibitor venetoclax in acute myeloid leukemia. Signal Transduct. Target. Ther. 2022, 7, 51.
- Daver, N. et al. Venetoclax Plus Gilteritinib for FLT3-Mutated Relapsed/Refractory Acute Myeloid Leukemia. J. Clin. Oncol. 2022, 40, 4048–4059. [CrossRef]
- Janssen, M. et al. Venetoclax synergizes with gilteritinib in FLT3 wild-type high-risk acute myeloid leukemia by suppressing MCL-1. Blood 2022, 140, 2594–2610. [CrossRef]
- Khoury, J. D. et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia (2022). [CrossRef]
- Arber, D. et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemia: Integrating Morphological, Clinical, and Genomic Data. Blood (2022).
- Cheson, B. D. et al. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [CrossRef]
- Bataller, A. et al. European LeukemiaNet 2017 risk stratification for acute myeloid leukemia: validation in a risk-adapted protocol. Blood Adv. 2022, 6, 1193–1206. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).