1. Introduction
From its’ first detection in China during January 2019, the fall armyworm (FAW),
Spodoptera frugiperda (Lepidoptera, Noctuidae), a notorious migratory agricultural pest, spread to 26 provinces (autonomous regions and municipalities) by October 2019 damaging more than 106.5 million hectares of maize fields in that year [
1]. FAW effectively completed all stages of the invasion process during 2019; from introduction, colonization, incubation, to spread and causing damage. South of 28°N in China the species can continue to reproduce and could survive winter between 28°-31°N [
1].
FAW is potentially a new major pest to Chinese agriculture [
2], as it is reputedly polyphagous being recorded on more than 353 plants, of which 180 were agricultural crops [
3,
4,
5], including maize, sorghum, peanut; but see [
6]. Two genetically-distinct sub-populations or strains are recognized: “corn-strain” and “rice-strain”, based on their host preference, mating behavior and sex pheromone [
7,
8]. From a genomic analysis of snips and gene sequences of 318
S. frugiperda moths collected from 13 provinces researchers concluded that the
S. frugiperda strain that invaded China was derived from “rice-strain” female parent and “corn-strain” male parent [
9,
10]. Most field populations have been recorded on corn and other Poaceae, as has been found Africa [
11], India [
12] and Australia [
6]. Some 83% of FAW moths trapped by searchlight on Yongxing island, had fed on C4 plants such as corn and not rice [
13]. The
S. frugiperda strain in China mainly damages corn, especially fresh corn. Larvae can tunnel and destroy the corn ear during milk maturation stage [
14]. FAW has the potential to reduce annual maize production by 21-53% in the absence of pest management [
11]. Guangdong, Guangxi and Hainan provinces in southern China are suitable for year-round populations of FAW [
15] with about 300,000 hectares of corn [
16] at risk.
Temperature influences the life of an ectotherm, determining its body temperature and consequently influencing the rate of physiological functions affecting growth, development, and fitness components [
17,
18,
19]. The global mean surface air temperature has risen substantially during the 20th century [
20], with night temperature increases greater than day temperatures [
21,
22]. The low temperature phase of the thermocycle (daily cycle of temperature) appears to play a major role in determining an insect’s performance [
23]. Here our primary goal was to evaluate the effect of increasing night temperature on
S. frugiperda growth and development relevant to corn planting area in southern China. Most work on temperature effects on insects use constant temperatures and FAW is no exception [
24,
25,
26,
27]. Here we test the effects of day-night temperature regimes relevant to the corn growing area of southern China on
S. frugiperda by evaluating various life history traits. Constant temperature studies do not predict the development under our changing temperature. Under some fluctuations FAW develops faster than expected. Our results will better inform attempts at predicting the timing of FAW stages in the field.
2. Materials and Methods
2.1. Insect Rearing
The
S. frugiperda used in this study was acquired from Tobacco Research Institute of Beijing Academy of Agricultural Sciences, reared at 27±1℃, humidity 70±5% for more than 20 generations in the insectary. First three (1-3) larval stages were fed fresh maize seedlings (variety: ZD958, height: 30cm) in cages (40cm×30cm×45cm), late larval stages (4-6) were fed with artificial diet individually in 6 well plates [
28]. Pupae were kept in Petri dishes until emergence. Adults were kept in mesh cages (40 cm×30 cm×45 cm), for mating and oviposition, and honey water solution (10%) was provided.
Egg masses (n=45) each with at least 30 eggs (≤12h old) were put on fresh corn leaves and placed in 9 climate chambers (5 egg masses/ chamber). Larvae were transferred (twice a day) to fresh corn leaves when eggs hatched, and the time of each transfer noted. Third instar larvae were reared individually on fresh corn leaves in finger tubes (2cm diameter) covered with nylon gauze (120 mesh) until they pupated. Pupae were moved to Petri dishes 30% filled with soil until emergence. We checked the insects twice a day and recorded the instar. Emerging adults were put into cages (40cm×30cm×45cm), with honey water (10%) and the number alive recorded daily until all the moths had died.
2.2. Experimental Design
FAW can survive and develop in the ambient temperature range of 14-32℃ although morality is high at temperatures below 18℃ [
29]. Temperatures above 32℃ are less suitable for the species, and largest pupal body weight and highest fecundity were recorded at 20-28℃ suggesting these conditions are optimal [
26,
29,
30,
31]. Daily temperature data of five years (2018-2022) were downloaded from a weather website (
https://tianqi.2345.com/). Daily maximum temperature and diurnal temperature variation/ range during the corn growing season, October to March, was summarized for southern Provinces in China. Based on the results, we selected three high day temperatures of 27℃, 24℃, 20℃ and three night temperatures regimes that differed by 6℃ to 4℃ and 2℃to give three treatment groups; Group I: 27-21℃, 27-23℃, 27-25℃; Group II: 24-18℃, 24-20℃, 24-22℃; Group III: 20-14℃, 20-16℃, 20-18℃ (see support Figure 1). The temperature cycle was set to 12h day temperature and 12h night. The time needed for temperature to change from one to the other was 30min. The climate chambers temperatures were confirmed with thermometers and checked daily during the study.
All the studies were conducted simultaneously in 9 climate chambers (Model: RGC-500B, Anhui Youke Instrument and Equipment Company): the variation in temperature is ±1℃ and ±3-7% in humidity. We set the climate chambers on the temperature change mode with the following photoperiod: L:D=14h/10h, humidity:75%, light strength:15000lx, respectively.
2.3. Data Analysis
Mortality rate and development time data were calculated using SPSS (version: 23.0). The effects of temperature treatments on the development time and survival rate were analyzed using General Liner Model (GLM). Survival rate (Binomial) and development time (Poisson) are the dependent variables. Day temperature, night temperature and the interaction of day temperature and night temperature are the independent variables, respectively. Tukey–Kramer HSD test was used for the significant differences.
Linear regression model (Equation 1) was used to describe the relationship between the developmental rate and temperature, R
2 indicate the degree of fit:
Where a, b are the model parameters, V(T) is the developmental rate of selected stage (1/time in stage) and T is the temperature.
According to the effective accumulated temperature rule, the least squares method is used to calculate the developmental starting temperature and effective accumulated temperature of the armyworm at each developmental stage. The calculation formula (2) (3) is as follows:
In the formula, T is the treatment temperature; C is the developmental threshold temperature; K is the effective accumulated temperature constant; V is the developmental rate (1/developmental days); n is the number of experimental temperature groups, in this study n=9.
We used results from previous studies to compare development among populations (see
Table 1).
3. Results
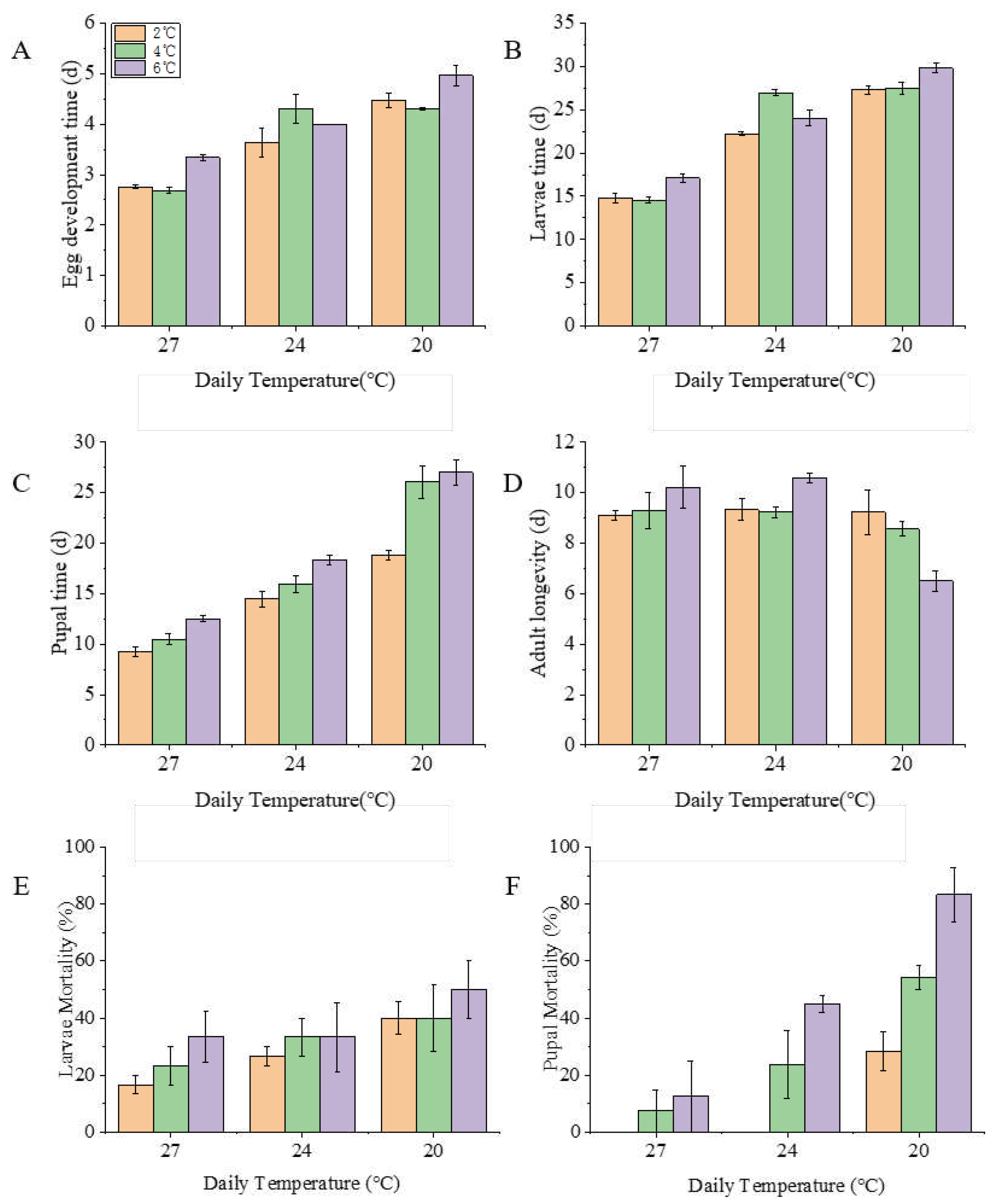
3.1. Development Duration
Development duration of eggs (
Figure 2A), larvae (
Figure 2B) and pupae (
Figure 2C) increased with the decreased daily temperature and night temperature. Moth longevity was reduced with declining daily temperature and night temperature increasing (
Figure 2D). Daily temperature treatments levels had significant effects on duration of all stages except for adults (Egg,
χ2=9.905;
df=2;
P=0.000; Larvae,
χ2=483.963;
df=2;
P=0.000; Pupae,
χ2=358.403;
df=2;
P=0.000; Adult,
χ2=6.466;
df=2;
P=0.000; Generation,
χ2=1354.096;
df=2;
P=0.000). Night temperature levels had significant effects on duration of all stages except for adults (Egg,
χ2=1.083;
df=2;
P=0.000; Larvae,
χ2=20.248;
df=2;
P=0.000; Pupae,
χ2=58.729;
df=2;
P=0.000; Adult,
χ2=0.125;
df=2;
P=0.875; Generation,
χ2=110.932;
df=2;
P=0.000). There were significant interaction effects caused by the night temperature increasing in larvae (
χ2=9.152;
df=4;
P=0.000), pupae (
χ2=8.885;
df=4;
P=0.014) and adults (
χ2=3.451;
df=4;
P=0.025).. Generation time was 41.4 d, 35.6 d and 35.5 d in the group of 27℃ day temperature, night temperature 21℃, 23℃ and 25℃, respectively. Generation time was 57.6 d, 55.9 d and 50 d with 24℃ day temperature, night temperature of 18℃, 20℃ and 22℃, respectively. While generation time was 70.3 d, 65.5 d and 60 d at 20℃ day temperature, with night temperatures of 14℃, 16℃ and 18℃, respectively.
3.2. Survival Rate
All eggs survived and hatched and there were no moth deaths over the first three days of adult life. Mortality rate of larvae (
Figure 2E) and pupa (
Figure 2F) increased with the decreased daily temperature and night temperature range, much more so for pupae (
Figure 2F). Significant effects were observed between day temperature regime and mortality in larvae (
χ2=0.0978;
df=2;
P=0.0048) and pupae (
χ2=0.5957;
df=2;
P=0.0003).
3.3. Development of Larvae, Pupae and Adults
Compared to previous studies, estimated lower temperature threshold of larvae and generation in this study are lower, and higher for the pupal stage. The estimated thermal requirement in degree-days of larvae is higher, but the pupae and generation are lower than others (
Table 1).
Developmental rate, V(T) increased with increased day and night temperatures. Slope of the linear regression model indicates the development speed of insects. The linear equation for larvae was V(T) =0.0033T-0.0423 (R
2=0.8389), for pupae V(T) =0.0113T-0.154 (R
2=0.9402) and generation V(T) =0.0016T-0.0123 (R
2=0.6582), where T is the average temperature experienced. Compared to results conducted under constant temperature previously [
26,
29,
30,
31], observed slope of linear regression model of larvae and adults in this study were similar. The slope for pupae in our study is higher than in previous work.
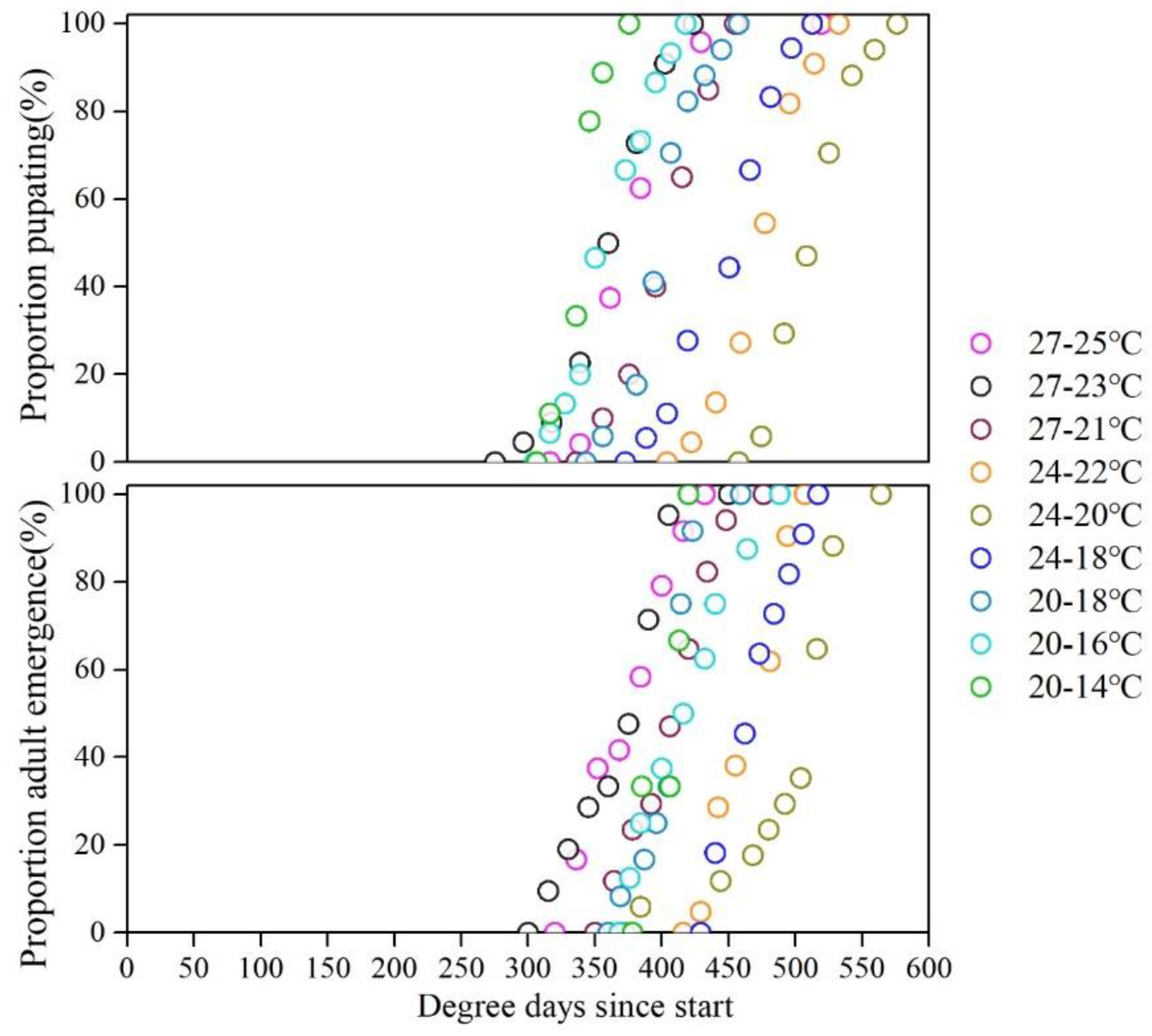
3.4. Pupation and Emergence Uniformity
Higher uniformity of S. frugiperda pupation and emergence are observed in high daily temperature and night temperature. The start of pupation and emergence in the treatment of 27-25℃ were 20d and 27d while 40d and 60d in the treatment of 20-14℃.
Figure 3.
Cumulative proportion pupation (A) and adult emergence (B) for each temperature regime plotted against degree-days experienced.
Figure 3.
Cumulative proportion pupation (A) and adult emergence (B) for each temperature regime plotted against degree-days experienced.
4. Discussion
In ectotherms body temperature more or less tracks changes in ambient temperature, although behavior can greatly modify the temperature experienced —e.g., [
32,
33,
34,
35]. The temperature experienced in the organism’s physical environment will strongly influence development, survival, potential and realized fecundity, and migration; indeed, virtually all aspects of its physiological ecology [
36]. Invariably when an insect species invades a new area there are a flood of papers reporting these temperature effects, and
Spodoptera frugiperda is no exception; e.g. China [
24,
25,
26,
30,
37,
38], India [
31,
32,
33,
34,
35,
36,
37,
38,
39] and Kenya [
40]. These papers are locally based and essentially reinvent a wheel, as they are generally based on constant temperatures. The premise and indeed promise is that using these data we can predict development based on some fitted model to the laboratory results [
41]. Surprisingly constant temperature results and even simple degree day or linear models and rate summing ‘work’, although they can be right for the wrong reasons. Much of the ability to predict depends on what temperatures are experienced and in practice real temperatures fluctuate and various studies have reported such effects [
42,
43,
44,
45]. Here we tested simple changes in temperature experienced based on day-night temperature changes relevant to the invaded range in tropical parts of southern China. In these regions the shape of the temperature curve can indeed be more of less flat, between small day-night changes.
As expected, significant effects on development were observed in all temperature treatments – generally the higher the temperature the faster the development, but there were significant interactions effects of temperature changes. The greatest effect of fluctuation was to slow down development at intermediate temperatures (24℃) relative to the simple expectations of a degree-day model. Fluctuations increase rate of development at low temperature treatments (20 ℃), consistently so in this treatment, the greater the fluctuation the faster the development. Similar fluctuating temperature effects, namely increasing rates of development, have been observed in other studies — e.g., [
46]. There was little to no effect at near optimal temperatures (27℃). Linear degree day models are meant to apply well to the intermediate temperature range in which our experimental treatments fell. However, predictions are likely to be out by potentially up to 5 days for larvae. The timing of management decisions may need to be adjusted for such effects of day-night temperature changes.
Other studies too have shown that larval growth rate, survival rate, adult longevity and fecundity are increased with the moderate increase of night temperature [
47] and for the red scale population density in the Covina Valley is inversely correlated with the number of nights with the temperature dropped [
48]. Whitney and Johnson (2005) showed that increasing night temperature increased the intrinsic rate of increase in
Pieris rapae [
49]. The effects of night temperatures on adult lifespan of the Finnish Glanville fritillary butterfly (
Melitaea cinxia) [
50] were similar with this study. The larval and pupal survival rate was only significantly affected by the daily highest temperature. Low nighttime temperatures had little effect on the survival and the total amount of maturation feeding of
Monochamus alternatus adults [
51].
Geographical ranges, population dynamics and phenologies of many organisms are altered by climate change. For ectotherms, increased ambient temperatures frequently have direct consequences for metabolic rates, activity patterns and developmental rates. Consequently, in many insect species, earlier and prolonged seasonal duration occur with recent global warming. However, from an ecological and evolutionary perspective, the voltinism and investment into each generation may be even more important than seasonality, since an additional generation per unit time may accelerate population growth and the potential for adaptation [
52,
53,
54]. Pupation and emergence of
S. frugiperda increased along with the increased daily temperature and night temperature. The uniformity of pupation and emergence is higher in the warmer night in this study indicating that the growth, development, population and voltinism will increase. We suggest management of
S. frugiperda will become more costly to the economy and the environment.
Chinese government has announced the rules on
S. frugiperda management, for Hainan, Guangdong, Guangxi and Yunnan provinces which are the winter reproducing areas of
S. frugiperda. Monitoring the invaded population and decreasing the number of pest sources which may migrate to northern China from this area are two critical objectives [
55,
56,
57]. However, as mentioned earlier, with the huge demand of fresh corn consumption in China, winter corn planting area has increased rapidly in Guangxi, Guangdong and Hainan provinces. The population of
S. frugiperda in this area may provide a source population for annual migration and invasion of northern China, but transgenic varieties and pesticide cannot be used as sweet corn is consumed by people directly. It will be a big challenge to policy makers to balance the in’s and out’s of
S. frugiperda management. In conclusion, the results of this study could help the local authorities to better time biological and physical control interventions.
Author Contributions
Conceptualization, Z.Z.L., H.P.C and Y.C.X; methodology, H.P.C, Y.C.X and M.P.Z.; investigation, H.P.C and M.Y.S.; resources, Z.Z.L.; data curation, Y.C.X; writing—original draft preparation, Y.C.X.; writing—review and editing, Z.Z.L and M.P.Z.; supervision and project administration, Z.Z.L. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research was funded by Shandong Province “Double-Hundred Talent Plan”on 100 Foreign Experts and 100 Foreign Expert Teams Program (WSG2021035) titled “Research on Regional Pest Occuring Mechanisms and Early Warning Technologies development for Major Pests in Grain and Cotton in Shandong”.
Data Availability Statement
Data used in this study are available from the corresponding authors upon reasonable request.
Acknowledgments
We thank Dr. Zhang Ping for the supports of data analysis and mapping.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, Y.; Liu, J.; Wu, Q.; Tsering, Z.; Zeng, J. Investigation of overwintering and wintering areas of fall armyworm in China. China Plant Prot 2021, 47, 212–217. [Google Scholar] [CrossRef]
- Guo, J.F.; Zhang, Y.J.; Wang, Z.Y. Major progress in coping with the invasion of fall armyworm in China. China Plant Prot 2022, 48, 79–87. [Google Scholar] [CrossRef]
- Casmuz, A.; Juarez, M.L.; Socias, M.G.; Murua, M.G.; Prieto, S.; Medina, S.; Willink, E.; Gastaminza, G. Review of the host plants of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev Soc Entomol Arge 2010, 69, 209–231. [Google Scholar]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.D.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr Entomol 2018, 26, 286–301. [Google Scholar] [CrossRef]
- Tambo, J.A.; Kansiime, M.K.; Mugambi, I.; Rwomushana, I.; Kenis, M.; Day, R.K.; Julien, L.G. Understanding smallholders’ responses to fall armyworm (Spodoptera frugiperda) invasion: evidence from five African countries. Sci Total Environ 2020, 740, 140015. [Google Scholar] [CrossRef] [PubMed]
- Volp, T.; Zalucki, M.P.; Furlong, M.P. What defines a host? Oviposition behavior and larval performance of Spodoptera frugiperda (Lepidoptera: Noctuidae) on five putative host plants. J Econ Entomol 2022, 115, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.P. Quantitative genetics, development, and physiological adaptation in host strains of fall armyworm. Evolution 1988, 42, 93–102. [Google Scholar] [CrossRef]
- Hafeez, M.; Li, X.W.; Zhang, J.M.; Zhang, Z.J.; Huang, J.; Wang, L.K.; Khan, M.M.; Shah, S.; Fernández-Grandon, G.M.; Lu, Y.B. Role of digestive protease enzymes and related genes in host plant adaptation of a polyphagous pest, Spodoptera frugiperda. Insect Sci 2021, 28, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, B.; Jiang, Y.Y.; Liu, J.; Wu, K.M.; Xiao, Y.T. Molecular characterization analysis of fall armyworm populations in China. China Plant Prot 2019, 45, 20–27. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, M.H.; Zhang, D.D.; Jiang, Y.Y.; Liu, J.; Wu, K.M.; Xiao, Y.T. Molecular identification of invasive fall armyworm Spodoptera frugiperda in Yunnan Province. China Plant Prot 2019, 45, 19-24+56. [Google Scholar] [CrossRef]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J. Fall armyworm: impacts and implications for Africa. Outlooks Pest Manage 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Ganiger, P.C.; Yeshwanth, H.M.; Muralimohan, K.; Vinay, N.; Kumar, A.R.; Chandrashekara, K. Occurrence of the New Invasive Pest, Fall Armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), in the Maize Fields of Karnataka, India. Current Science 2018, 115, 621–623. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wu, Q.L.; Jia, H.R.; Wu, K.M. Searchlight trapping reveals seasonal cross-ocean migration of fall armyworm over the South China Sea. J Integr Agr 2021, 20, 673–684. [Google Scholar] [CrossRef]
- Li, X.J.; Wu, J.Y.Z.; Dai, X.C.; Wang, Y.Q.; Wang, R.F.; Zhang, Z.Z.; Xu, H.H. Study on fruit ears of different maize varieties by autumn armyworm. Journal of South China Agricultural University 2021, 42, 71–79. [Google Scholar] [CrossRef]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.; Day, R.K.; Desneux, N.; Harrison, R.D.; Kriticos, D.J.; Rwomushana, I.; van den Berg, J.; Verheggen, F.J.; Zhang, Y.; Agboyi, L.K.; Ahissou, R.B.; Ba, M.N.; Bernal, J.S. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol Gen 2022, 10, 1127. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B. The development and prospect of fresh corn - explore the northern market of sweet corn in China. China Vegetables 2016, 12, 1–6. [Google Scholar]
- Huey, R.; Kingsolver, J.G. Evolution of resistance to high temperature in ectotherms. Am. Nat 1993, 142, S1–S46. [Google Scholar] [CrossRef]
- Petersen, C.; Woods, H.A.; Kingsolver, J.G. Stage-specific effects of temperature and dietary protein on growth and survival of Manduca sexta caterpillars. Physiol Entomol 2000, 25, 35–40. [Google Scholar] [CrossRef]
- Du, Y.; Ma, C.S.; Zhao, Q.H.; Ma, G.; Yang, H.P. Research progress on physiological and biochemical mechanism of high temperature on insects. Acta Ecologica Sinica 2007, 1565–1572. [Google Scholar]
- The Intergovernmental Panel on Climate Change. Available online: ipcc.ch/site/assets/uploads/2018/03/SREX_Full_Report-1.pdf (accessed on 22 December 2023).
- Easterling, D.R.; Karl, T.R.; Gallo, K.P.; Robinson, D.A.; Trenberth, K.E.; Dai, A. Observed climate variability and change of relevance to the biosphere. J Geophys Res 2000, 105, 194. [Google Scholar] [CrossRef]
- Caesar, J.; Alexander, L.; Vose, R. Large-scale changes in observed daily maximum and minimum temperatures: creation and analysis of a new gridded data set. J Geophys Res 2006, 111, D05101. [Google Scholar] [CrossRef]
- Beck, S.D. Insect thermoperiodism. Annu Rev Entomol 1983, 28, 91–108. [Google Scholar] [CrossRef]
- Xie, D.J.; Zhang, L.; Cheng, Y.X.; Jiang, X.F. Construction of amphoteric life table in age-stage experimental populations of fall armyworm at different temperatures. China Plant Prot 2019, 45, 20–27. [Google Scholar] [CrossRef]
- Lu, Z.H.; He, S.Q.; Yan, N.S.; Zhao, W.J.; Yao, W.F.; Chen, Y.P.; Yang, T.; Jiang, Y.Y.; Gui, F.R. Effects of temperature on growth, development and reproduction of fall armyworm. China Plant Prot 2019, 45, 27-31+53. [Google Scholar] [CrossRef]
- Zhang, H.H.; Yin, Y.Q.; Zhao, X.Q.; Li, X.Y.; Wang, Y.; Liu, Y.; Chen, F.S.; Chen, A.D. Growth and development characteristics of fall armyworm under different temperature conditions. China Acta Environmental Entomological Sinica 2020, 42, 52–59. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, D.F.; Yang, M.F.; Liu, J.F. The Effect of Temperatures and Hosts on the Life Cycle of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Zhang, Y.P.; Huang, S.H.; Liu, W.L.; Zhang, Y.P. Study on indoor captive rearing technology of fall armyworm. China Journal of Environmental Entomology 2019, 41, 986–991. [Google Scholar] [CrossRef]
- Du Plessis, H.; Schlemmer, M.L.; Van, d.B.J. The Effect of Temperature on the Development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- He, L.M.; Ge, S.S.; Chen, Y.; Wu, Q.L.; Jiang, Y.Y.; Wu, K.M. Prediction model of developmental starting temperature, effective accumulated temperature, and developmental duration of fall armyworm. China Plant Prot 2019, 45, 18–26. [Google Scholar] [CrossRef]
- Prasad, T.V.; Srinivasa, R.M.; Rao, K.V. Temperature-based phenology model for predicting the present and future establishment and distribution of recently invasive Spodoptera frugiperda (J. E. Smith) in India. B Entomol Res 2021, 112, 1–15. [Google Scholar] [CrossRef]
- Serratore, V.R.; Zalucki, M.P.; Carter, P.A. Thermoregulation in moulting and feeding Danaus plexippus L. (Lepidoptera: Nymphalidae) caterpillars. Aust J Entomol 2013, 52, 8–13. [Google Scholar] [CrossRef]
- Ma, G.; Ma, C.S. Effect of acclimation on heat-escape temperatures of two aphid species: Implications for estimating behavioral response of insects to climate warming. J Insect Physiol 2012, 58, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Bai, C.; Wang, X.; Majeed, M.Z.; Ma, C. Behavioural thermoregulation alters microhabitat utilization and demographic rates in ectothermic invertebrates. Anim Behav 2018, 142, 49–57. [Google Scholar] [CrossRef]
- Bai, X.; Wang, X.J.; Ma, C.S.; Ma, G. Heat-avoidance behavior associates with thermal sensitivity rather than tolerance in aphid assemblages. J Therm Biol 2023, 114, 103550. [Google Scholar] [CrossRef]
- Hoffmann, K.H. Environmental Physiology and Biochemistry of Insects, 1st ed.; Springer: Berlin, German, 1984; pp. 1–32. [Google Scholar]
- Huang, L.L.; Xue, F.S.; Chen, C. Effects of temperature on life-history traits of the newly invasive fall armyworm, Spodoptera frugiperda in Southeast China. Ecol Evol 2021, 11, 5255–5264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, D.F.; Yang, M.F.; Liu, J.F. The Effect of Temperatures and Hosts on the Life Cycle of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 211. [Google Scholar] [CrossRef]
- Kumar, S.; Suby, S.B.; Vasmatkar, P. Influence of temperature on insecticidal toxicity and detoxifying enzymes to Spodoptera frugiperda. Phytoparasitica 2023, 51, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Sokame, B.M.; Rebaudo, F.; Malusi, P.; Subramanian, S.; Kilalo, D.C.; Juma, G.; Calatayud, P.A. Influence of Temperature on the Interaction for Resource Utilization between Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), and a Community of Lepidopteran Maize Stemborers Larvae. Insects 2020, 11, 73. [Google Scholar] [CrossRef]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Annu Rev Entomol 2015, 60, 123–140. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, W.; Hoffmann, A.A.; Ma, C.S. Night warming on hot days produces novel impacts on development, survival and reproduction in a small arthropod. J Anim Ecol 2014, 83, 769–778. [Google Scholar] [CrossRef]
- Ma, G.; Rudolf, V.H.W.; Ma, C.S. Extreme temperature events alter demographic rates, relative fitness, and community structure. Glob Change Bio l 2015, 21, 1794–1808. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Xing, K.; Hoffmann, A.A.; Ma, C.S. The importance of timing of heat events for predicting the dynamics of aphid pest populations. Pest Manag Sci 2019, 75, 1866–1874. [Google Scholar] [CrossRef]
- Wang, X.J.; Ma, C.S. Can laboratory-reared aphid populations reflect the thermal performance of field populations in studies on pest science and climate change biology? J Pest Sci 2023, 96, 509–522. [Google Scholar] [CrossRef]
- Lv, W.; Xie, X. Effect of fluctuating temperatures on development, reproduction and energy of oriental armyworm populations, Mythimna separata. J Appl Entomol 2022, 146, 511–524. [Google Scholar] [CrossRef]
- Li, Y.T. Response of bird cherry-oat aphid, Rhopalosiphum padi (L.) to thermal stress and molecular mechanisms. Doctor, Northwest A&F University, Xianyang, China, June 2011.
- Hutchinson, R.N. Influence of winter night temperatures on the California Red Scale. J Econ Entomol 1947, 40, 921–922. [Google Scholar] [CrossRef] [PubMed]
- Whitney-Johnson, A.; Thompson, M.; Hon, E. Responses to predicted Global warming in Pierisrapae L. (Lepidoptera): consequences of nocturnal versus diurnal temperature change on fitness components. Environ Entomol 2005, 34, 535–540. [Google Scholar] [CrossRef]
- Rosa, E.; Saastamoinen, M. Warm-night temperature alters paternal allocation strategy in a North temperate-zone butterfly. Ecol Evol 2021, 11, 16514–16523. [Google Scholar] [CrossRef] [PubMed]
- Maehara, N.; Nakamura, K. Effects of low-temperature summer nights on adults of Monochamus alternatus (Coleoptera: Cerambycidae). J Forest Res-JPN 2018, 23, 237–241. [Google Scholar] [CrossRef]
- Estay, S.A.; Lima, M.; Labra, F.A. Predicting insect pest status under climate change scenarios: Combining experimental data and population dynamics modelling. J Appl Entomol 2009, 133, 491–499. [Google Scholar] [CrossRef]
- Altermatt, F. Climatic warming increases voltinism in European butterflies and moths. P Roy Soc B-Biol Sci 2009, 277, 1281–1287. [Google Scholar] [CrossRef]
- Gagnon, A.-È.; Bourgeois, G.; Bourdages, L.; Grenier, P.; Blondlot, A. Impact of climate change on Ostrinia nubilalis (Lepidoptera: Crambidae) phenology and its implications on pest management. Agr Forest Entomol 2019, 21, 253–264. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Liu, J.; Wu, Q.L.; Tsering, Z.; Zeng, J. Investigation on winter breeding and overwintering areas of Spodoptera frugiperda in China. China Crop prot 2021, 47, 212–217. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wu, Q.L.; Jia, H.R.; Wu, K.M. Searchlight trapping reveals seasonal cross-ocean migration of fall armyworm over the South China Sea. J Integr Agr 2021, 20, 673–684. [Google Scholar] [CrossRef]
- Ge, S.S.; Zhang, H.W.; Liu, D.Z.; Lv, C.Y.; Cang, X.Z.; Sun, X.X.; Song, Y.F.; He, W.; Chu, B.; Zhao, S.Y.; Wu, Q.L.; Yang, X.M.; Wu, K.M. Seasonal migratory activity of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) across China and Myanmar. Pest Manag Sci 2022, 78, 4975–4982. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).