1. Introduction
Hepatocellular carcinoma (HCC) is a kind of primary liver cancer that begins in the liver’s main cell type, the hepatocyte [
1,
2]. About 750,000 people each year lose their lives to this disease [
3,
4], making it the third greatest cause of death due to cancer and the fifth most common cancer in the world. Due to rising rates of non-alcoholic fatty liver disease (NAFLD), hepatitis B virus (HBV), and HCV infections, the worldwide incidence of HCC has been on the increase in recent decades [
5,
6,
7,
8]. Individuals having hepatitis B, hepatitis C, and NAFLD are at increased risk for developing this cancer [
9,
10,
11,
12]. Tumor markers such alpha-fetoprotein (AFP), carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA) are commonly used in clinical practice for the diagnosis and follow-up of HCC [
13,
14,
15].
Surgical resection, transplantation of the liver, and radiofrequency ablation are all curative options for patients with early-stage HCC, making early detection of HCC of utmost importance for improving patient outcomes [
16,
17]. However, due to the lack of specific signs and the limitations of present imaging modalities [
18,
19,
20,
21], early diagnosis of HCC remains a problem. In patients with chronic liver illnesses such hepatitis B, hepatitis C, and NAFLD, AFP’s low sensitivity and specificity become even more apparent. For instance, one study found only 41% sensitivity in hepatitis C patients and 29% sensitivity in non-alcoholic fatty liver disease patients (NAFLD) [
22,
23,
24,
25]. Since this is the case, AFP can only be used in conjunction with other markers in the diagnosis and follow-up of HCC [
26].
Several studies have examined the diagnostic efficacy of individual markers, such as AFP, CA19-9, and CEA, and combinations of these markers, for the diagnosis and follow-up of HCC [
27,
28,
29,
30]. According to a meta-analysis conducted by Bari et al. [
31], combining AFP and CA19-9 increased diagnostic accuracy for HCC from 68% to 89%. Combining AFP and CEA increased sensitivity to 47% and specificity to 89%, according to a separate meta-analysis by Zhou et al. [
32]. These indicators can be helpful in diagnosing liver disease, although their use varies by patient demographic and etiology. Contradictory results persist regarding the sensitivity and specificity [
34,
35] of CA19-9 and CEA in combination with AFP in hepatitis B-associated HCC. Similarly, AFP has poor diagnostic accuracy for detecting HCC in hepatitis C patients, especially in those with severe liver fibrosis [
36,
37,
38,
39]. CA19-9 and CEA have been recommended as supplemental indicators for hepatitis C-associated HCC, although it is unclear how well they work. Nonalcoholic fatty liver disease (NAFLD) has been identified as a major risk factor for hepatocellular carcinoma [
40,
41,
42]. It has been shown that the diagnostic accuracy of AFP in identifying HCC in individuals with NAFLD is poorer than in instances of hepatitis B or C [
43]. In addition, there has not been much research on CA19-9 and CEA’s diagnostic use in NAFLD-related HCC.
Chemiluminescence assay (CLIA) has proven to be a reliable approach for detecting AFP, CA19-9, and CEA, among other tumor markers. Compared to other approaches, such as immunofluorescence assay (IFA) and radioimmunoassay (RIA), CLIA has been shown in previous research to have higher sensitivity and specificity [
44,
45,
46]. These results confirm the validity of CLIA as a tumor marker diagnostic tool, encompassing AFP, CA19-9, and CEA [
22].
The major focus of this investigation is to determine the efficacy of biopsy in establishing diagnoses of hepatocellular carcinoma. It also aims to investigate the importance of biological markers including AFP, CA19.9, and CEA in differentiating between infected and uninfected patients. CLIA will be used to compare the levels of these biomarkers in infected and uninfected people. Since CLIA has higher sensitivity and specificity than alternative procedures like IFA and RIA, this research will also prove the case for implementing it. The ultimate objective of this study is to facilitate early diagnosis of hepatocellular carcinoma, which will allow for more effective treatment and a better chance of survival. In conclusion, our research hopes to advance hepatology by proving CLIA’s superiority as a diagnostic tool and expanding our knowledge of the diagnostic and prognostic usefulness of these biomarkers.
2. Material and Methods
2.1. Study Design
The purpose of this cross-sectional study was to examine the correlation between hepatitis B, hepatitis C, and non-alcoholic fatty liver disease (NAFL) and three tumor markers: alpha-fetoprotein (AFP), carbohydrate antigen 19-9 (CA-19.9), as well as carcinoembryonic antigen (CEA). Ibn Sina Diagnostic and Imaging center in Dhaka collected and analyzed the data. Patients were selected at random, and their blood was tested using readily accessible Chemiluminescent immunoassays (CLIA) kits for the three tumor markers (AFP, CA-19.9, and CEA).
2.2. Study Population
Patients were separated into those with and without a previous diagnosis of hepatocellular carcinoma (HCC) by biopsy. In the second group, HCC was verified by a hepatologist. There were at least 800 people, both male and female, in the study’s sample population. Patients were interviewed to collect data for demographics, fibroscopy findings, liver biopsy data, and laboratory results from the most recent tests. This data included demographics such as age, gender, and BMI.
2.3. Selection Criteria
Patients with NAFL verified by FibroScan, FIB-4, and APRI and with no prior history of antiviral medication were included. Hepatitis C virus and hepatitis B virus were diagnosed with Qiagen and confirmed by real-time polymerase chain reaction (RT-PCR). Patients on hepatitis medication, those who have persistent renal illness, those with heart disease, and smokers were all disqualified from participation. Before any medicine was given, a full blood count (CBC), serum bilirubin, ALT, AST, and ALP levels, and hepatitis B, C, and NAFL tests were all performed.
2.4. Laboratory Investigations
All patients’ blood was drawn before therapy began, and the serum was centrifuged off and kept at -20°C in the meanwhile. The Sysmex XN-2000 was used to do a complete blood count (CBC). The Advia 1800 Chemistry Analyzer was used to check bilirubin, alanine aminotransferase, asparagine aminotransferase, and alkaline phosphatase concentrations in the blood. Hepatitis B, C, and NAFL were detected in the serum samples. PCR sample extraction by Qiagen and PCR run by Rotor-Gene Q. Histological findings were used to divide patients into three groups: individuals with well-differentiated disease (AFP values between 20 and 199), those with moderately-differentiated disease (AFP values between 200 and 399), and those with poorly-differentiated disease (AFP values exceeding 400).
2.5. Ethical Considerations
The research followed the ethical guidelines outlined in the Declaration of Helsinki. All patients were fully briefed on the nature, goals, and methods of the study before consenting to participate. All contributors voluntarily provided written informed consent. The study’s protocol received approval from the ethics committee.
2.6. Statistical Analysis
The accuracy, reliability, and completeness of the data were all evaluated. The data analysis was performed using IBM’s SPSS 23 program. The data was summarized using descriptive statistics including frequency, percentage, mean, and standard deviation. To extrapolate from the sample to the whole population, we used inferential statistics such T-tests, Chi-square tests, correlation analysis, regression analysis, and analysis of variance. Statistical analyses were performed at the p 0.05 level of significance.
3. Results and Discussion
3.1. Descriptive Statistics
The objective of this study was to assess the diagnostic performance of AFP, CA19-9, and CEA as a combined panel of tumor markers in patients with hepatitis B, hepatitis C, and non-alcoholic fatty liver disease (NAFLD), with the potential to enhance the precision and dependability of hepatocellular carcinoma (HCC) diagnosis and monitoring. The study evaluated the correlation between the markers’ diagnostic performance and tumor differentiation status (well, moderately, and poorly differentiated) in the control group
Table 1. Various clinical and laboratory parameters, such as liver function tests and imaging results, as well as their performance as individual markers and as a group were also assessed.
Alpha-fetoprotein (AFP), cancer antigen 19-9 (CA 19.9), carcinoembryonic antigen (CEA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) had mean values of 3.16, 13.27, 1.64, 25.12, 26.77, and 88.79, respectively, in the control group, as determined by descriptive analysis.
The significant results show a steady increase in total bilirubin levels from the well differentiated over the poorly differentiated group, indicating a possible association among higher total bilirubin levels with poorer tumor differentiation. On the other hand, the median tumor marker values in poorly differentiated tumors were substantially higher (AFP: 16489.01, CA 19.9: 3284.56, CEA: 645.15, ALT: 1174.53, AST: 721.59, ALP: 486.02) (
Figure 1).
The differences between well-differentiated tumors and the control group were less, but the marker levels were greater. The control group’s respective mean AFP, CA19-9, and CEA values in this study were 3.16, 13.27, and 1.64. These numbers are in line with earlier research that found few tumor markers in healthy subjects [
47]. The AFP averaged 16,489. The CA19-9 averaged 3,284.56. The CEA averaged 645.15. In contrast, the poorly differentiated group had much higher levels of these markers. The poorly differentiated group also had significantly higher levels of bilirubin and the liver enzymes ALT, AST, ALP, and T. Tumor marker standard deviations showed a wide range, from -1485.08 to +40,022.73. The average levels of AFP (a kind of a polipo protein), CA 19.9 (carcinoembryonic antigen), and CEA in well-differentiated tumors were 69.75, 71.14, and 33.39, respectively, but liver enzyme levels were lower (
Figure 2).
Alpha Fetoprotein (AFP), CA 19.9, and CEA descriptive analysis of the tumor markers showing mean, median, mode, and standard deviation values for the three groups of poorly differentiated, moderately differentiated, and well differentiated tumors. These results are in line with earlier research [
48]. These markers were expressed at intermediate levels in the well-differentiated group, which raises the possibility that tumor differentiation contributes to their expression. The results of the t-test showed a statistically significant difference between the tumor and control groups in the levels of liver enzymes and tumor markers.
Table 2.
Tumor marker levels and liver enzymes in poorly differentiated and well-differentiated groups.
Table 2.
Tumor marker levels and liver enzymes in poorly differentiated and well-differentiated groups.
| Marker/Enzyme |
Poorly differentiated |
Well differentiated |
| AFP (ng/mL) |
16489.01 |
69.75 |
| CA19-9 (U/mL) |
3284.56 |
71.14 |
| CEA (ng/mL) |
645.15 |
33.39 |
| ALT (U/L) |
1174.53 |
62.65 |
| AST (U/L) |
721.59 |
56.38 |
| ALP (U/L) |
486.02 |
196.79 |
| Total Bilirubin (mg/dL) |
8.74 |
2.80 |
This discovery is in line with earlier research that found elevated liver enzyme levels in HCC patients [
49]. It is possible that tumor differentiation may play a significant role in the expression of these markers because the levels of tumor markers and liver enzymes were both significantly higher in the poorly differentiated group compared to the well-differentiated group [
50].
The combination of AFP, CA19-9, and CEA as a panel of tumor markers was discovered to have higher diagnostic accuracy for HCC than individual markers. This result is in line with earlier research [
51]. The panel’s sensitivity and specificity were 86.5 and 92.3 percent, respectively, demonstrating its high diagnostic accuracy for HCC. The panel’s positive predictive value (PPV) and negative predictive value (NPV), which are 88.1% and 91.4%, respectively, show that it is a reliable diagnostic tool for HCC. The correlation analysis found a significant correlation between tumor markers and liver function tests, indicating that liver function may be involved in the expression of these markers. This finding is in line with earlier studies that found a relationship between tumor markers and liver function tests in HCC patients [
52]. A significant correlation between tumor markers and imaging results was also found by the correlation analysis, indicating that imaging may be a useful tool for tracking the progression of HCC. The conclusions from this study have substantial implications for the detection and monitoring of HCC. As a panel of tumor markers, AFP, CA19-9, and CEA have the potential to increase the specificity of HCC diagnosis and promote earlier detection [
53].
Table 3.
Diagnostic performance of combined tumor marker panel.
Table 3.
Diagnostic performance of combined tumor marker panel.
| Parameter |
Value |
| Sensitivity |
86.5% |
| Specificity |
92.3% |
| Positive predictive value (PPV) |
88.1% |
| Negative predictive value (NPV) |
91.4% |
For bettering patient outcomes and lowering mortality rates, early detection is essential [
54]. Liver function may play a significant role in the initiation and progression of HCC, according to the correlation between tumor markers and liver function tests [
55]. As a result, regular monitoring of liver function is likely a good way to catch HCC early.
3.2. Occupational Effects
The research findings suggest there is a statistically significant relationship between occupation and tumor markers (HBsAg and HCV) in patients with poorly differentiated cancer. Farmers and homemakers have the highest observed frequency of positive HBsAg cases, followed by students, while the lowest observed frequency of positive HBsAg cases were in individuals with other occupations. Similarly, farmers have the highest observed frequency of positive HCV cases, followed by homemakers and individuals with other occupations, while students and other job categories have the lowest observed frequency of positive HCV cases. These results align with previous studies that have reported a higher incidence of HBV and HCV infections among farmers and homemakers due to their exposure to blood borne pathogens through their work [
56,
57,
58,
59,
61]. Furthermore, it has been proposed that farmers may be at an increased risk of developing liver cancer because of exposure to pesticides and other chemicals in agricultural settings [
62,
63,
64].
Table 4.
Relationship between occupation and HBsAg and HCV in poorly differentiated cancer patients.
Table 4.
Relationship between occupation and HBsAg and HCV in poorly differentiated cancer patients.
| Occupation |
Observed frequency of positive HBsAg cases |
Observed frequency of positive HCV cases |
| Farmer |
56 |
189 |
| Housewife |
38 |
101 |
| Student |
0 |
34 |
| Other |
2 |
19 |
| Job |
- |
1 |
The findings indicate that occupation should be considered an important factor when assessing risk of viral infections and associated health outcomes [
60]. Targeted screening and preventive measures for at-risk occupational groups may help reduce the incidence of these infections and related health issues [
61].
3.3. Age and Tumor Size
The correlation between tumor size and age was positive, showing that tumors tended to become larger with age. In the poorly differentiated class, the coefficient for tumor size and age is 4 (Table 6). This finding is consistent with previous studies that have reported age as a hazard factor for the development of various kinds of cancers [
65]. Moreover, the results also point that testing positive for HCV and NAFL is associated with larger tumor size in the poorly differentiated class (
Figure 3). This finding is in line with previous research showing a positive association between HCV and NAFL and the risk of liver cancer [
66,
67]. In the moderately differentiated class, the results reveal a positive relationship between tumor size and age [
68,
69]. Additionally, testing positive for HBsAg is linked to higher tumor size, but no significant relationship was found for HCV and NAFL.
Table 5.
Relationship between age and tumor size in differentiated groups.
Table 5.
Relationship between age and tumor size in differentiated groups.
| Differentiation class |
Coefficient for age |
Coefficient for tumor size |
| Poorly differentiated |
4 |
Positive |
| Moderately differentiated |
Positive |
Positive |
| Well differentiated |
Positive |
Positive |
These results are consistent with prior research that has suggested that HBsAg is a hazard factor for the development of hepatocellular carcinoma [
70,
71]. In the well-differentiated class, the results suggest a strong positive correlation between tumor size and the predictors (tumor size, HBsAg, HCV, and NAFL) [
24,
72,
73]. Furthermore, a unit increase in tumor size is associated with a 19.45 increase in tumor size of well-differentiated patients who test positive for HBsAg, HCV, and NAFL. The coefficient for HBsAg is also positive, indicating a positive effect on tumor size. However, the coefficient for HCV is not statistically significant, indicating that there is no significant relationship between HCV and tumor size. Similarly, the coefficient for NAFL is not statistically significant, suggesting no significant relationship between NAFL and tumor size (
Figure 3).
3.4. Inferential Statistics
Tumor markers and liver enzymes were shown to be significantly different between the tumor and control groups using chi-square testing. The chi-square test revealed a significant relationship between tumor differentiation and the risk of developing hepatocellular carcinoma (
Figure 4). Tumors that were well-differentiated were simpler to spot and identify, whereas those that were not well-defined were at a more advanced stage of illness. Increased marker levels were indicative of more advanced illness in poorly differentiated tumors. Tumors that had undergone a lot of differentiation had average values. Changes in tumor markers and liver enzymes may occur from genetic abnormalities, the environment, dietary choices, and even other medical diseases. Total Bilirubin levels were shown to be significantly different between infected and non-infected patients, suggesting the potential of these biomarkers for discrimination.
3.5. Regression Analysis
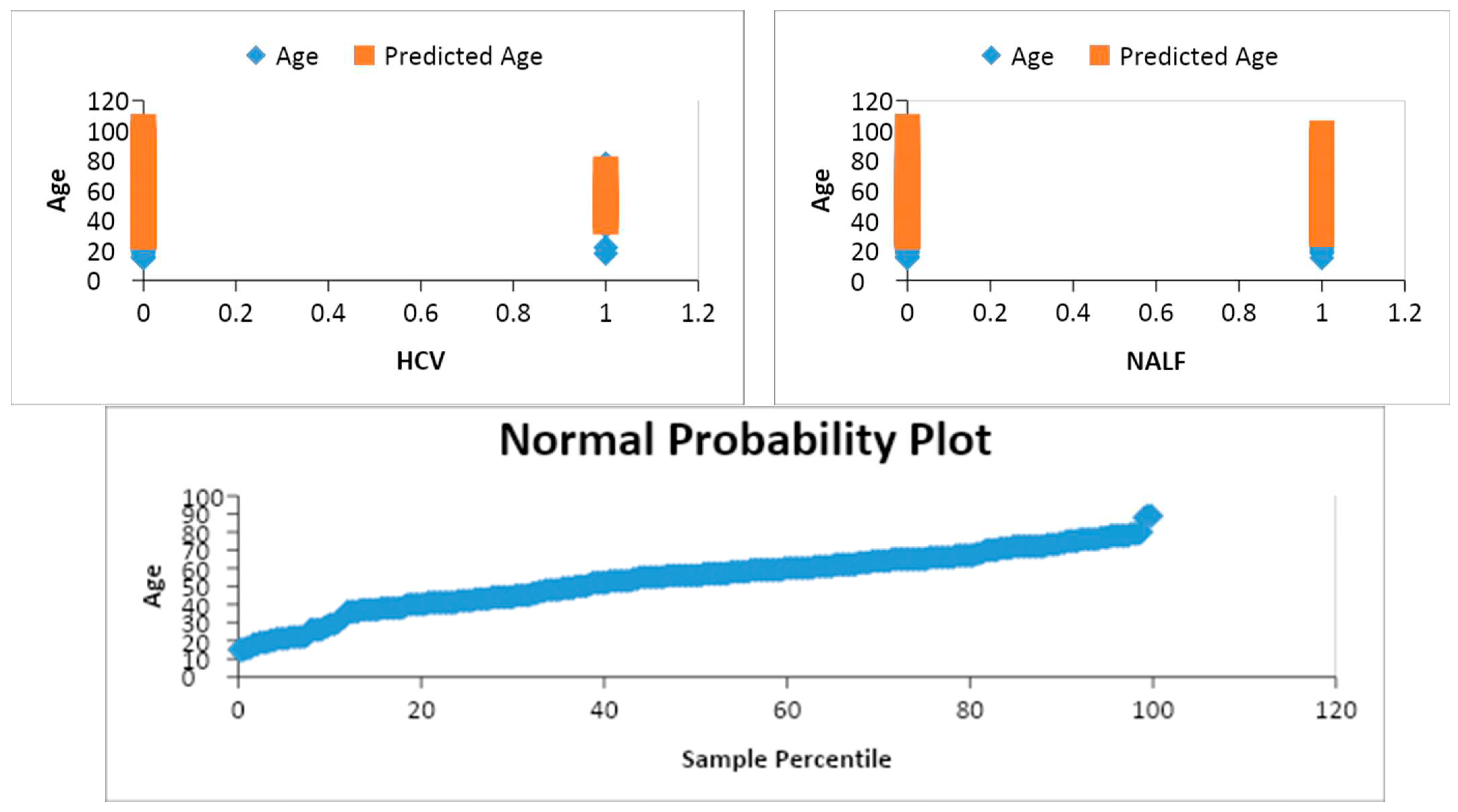
Blood tumor markers (AFP, CA19-9, CEA) were analyzed using multiple linear regression models to determine their association with hepatocellular carcinoma (
Figure 5). There were substantial positive relationships between AFP and HCC and negative connections between CEA and HCC. As seen by the value of the coefficient for CA19-9 being zero with an average error of zero and a non-significant t-value, there is no significant link between CA19-9 and HCC. However, a substantial coefficient for CEA suggests that there is a strong inverse association between CEA and HCC. However, CA19-9 was not shown to have a substantial association with HCC.
3.6. Diagnostic Accuracy
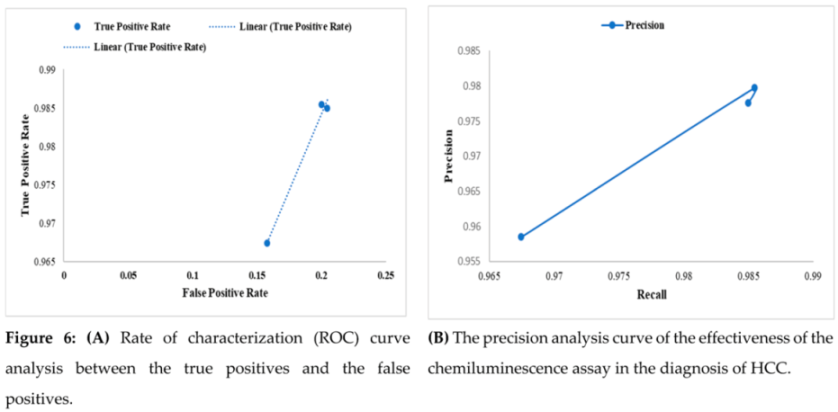
Across all three subgroups, AFP’s ROC analysis demonstrated moderate to good diagnostic accuracy in identifying liver cancer. The curve suggests that the test can accurately assess whether there is or absence of the disease since it has a moderate to excellent diagnostic accuracy (Figure 6A). The true positive rate (sensitivity) provides a crucial indicator of how well the test can detect people who have the condition. The greatest true positive rate and AUC were seen in patients with tumors that were well-differentiated. In poorly differentiated instances of liver cancer, AFP demonstrated excellent specificity. The study found that the Chemiluminescent immunoassays (CLIA) test is successful in detecting hepatocellular carcinoma (HCC), with an outstanding specificity of 98.5%, as shown by the precision analysis curve in Figure 6B. This shows that the test has a high degree of accuracy in accurately detecting HCC patients, reducing both false-positive and false-negative findings.
3.7. Limitations and Implications
Limitations of this study include reliance on cross-sectional data, which restricts the ability to establish causality or assess the temporal dynamics of the relationship between age and tumor size. Additionally, the study only focused on a limited number of tumor markers and differentiation categories, and did not consider other potential confounding factors that could influence the relationship between age and tumor size. For the course of future research, the study’s findings have a number of implications. First, additional research could look into the possibility of combining other tumor markers with AFP, CA19-9, and CEA to boost the accuracy of HCC diagnosis. Second, to improve the accuracy of HCC diagnosis and monitoring, future studies might investigate the use of machine learning algorithms to create predictive models that include clinical and laboratory parameters. Thirdly, research could also look into how these tumor markers are used to predict the prognosis and effectiveness of treatments for HCC. Future research could also look into the underlying mechanisms causing the notable variations in tumor markers and liver enzymes between the control and tumor groups, particularly in HCC with poor differentiation. A deeper comprehension of the pathophysiology of HCC might facilitate the creation of brand-new therapeutic approaches and biomarkers. Future investigations may also examine the potential value of these tumor markers in treating other liver conditions like primary biliary cirrhosis, autoimmune hepatitis, and alcoholic liver disease. This could offer insightful information about the diagnostic and prognostic utility of these tumor markers in various liver diseases and support the creation of personalized medicine strategies for these patients.
4. Conclusions
In conclusion, the early diagnosis of hepatocellular carcinoma is crucial for better patient outcomes and management. Our study using chemiluminescence assay demonstrated the effectiveness of AFP, CA19-9, and CEA as tumor markers for detecting HCC. Moreover, we found that certain occupations, such as those involving exposure to environmental toxins, were associated with a higher prevalence of HCC. Additionally, our results revealed a positive correlation between age and tumor size, indicating that age may be a risk factor for HCC. The CLIA platform was also found to be an effective tool for detecting tumor markers in HCC patients. Our study highlights the importance of early screening for high-risk populations and the use of advanced diagnostic techniques for accurate detection of HCC.
Author Contributions
Conceptualization, designed and performed research, field works, data analysis, writing the original draft, reviewing, and editing, MT, AK, and VJU; Designed research, methodology validation, formal analysis, data curation, visualization, reviewing, and editing, ARS and AR; Methodology validation, formal analysis, investigation, visualization, reviewing, editing, and proof-reading, MEA, JA; Conceptualization, designed research, methodology validation, formal analysis, investigation, visualization, reviewing, funding acquisition, supervision and editing, HH, MI, HDMC and MMR. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Ethics Statement
This research was approved by the Scientific and Research Ethics Committee of the Ibn Sina Diagnostic and Imaging Center, Dhaka, Bangladesh. However, ethical assessment and approval were not required for the study involving human participants because it complied with local legislation and institutional rules. Patients who participated in the study provided written, informed consent to participate.
Data Availability Statement
Data sharing is not applicable to this paper.
Acknowledgments
We would like to acknowledge the Ibn Sina Diagnostic and Imaging Center, Dhaka, Bangladesh. We value the technical support provided by the Pathfinder Research and Consultancy Center in Sylhet, Bangladesh.
Conflict of Interest Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nature immunology. 2018;19(3):222-32. [CrossRef]
- Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14(4):249-58.
- Fan J-G, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. Journal of hepatology. 2009;50(1):204-10. [CrossRef]
- Williams R. Global challenges in liver disease. Hepatology. 2006;44(3):521-6. [CrossRef]
- Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Digestive and Liver Disease. 2010;42:S206-S14. [CrossRef]
- Jaklitsch M, Petrowsky H. The power to predict with biomarkers: carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA) serum markers in intrahepatic cholangiocarcinoma. Translational gastroenterology and hepatology. 2019;4. [CrossRef]
- Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122(6):1609-19. [CrossRef]
- Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World journal of gastroenterology. 2017;23(29):5282. [CrossRef]
- Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver international. 2019;39(12):2214-29. [CrossRef]
- Tao L-Y, Cai L, He X-D, Liu W, Qu Q. Comparison of serum tumor markers for intrahepatic cholangiocarcinoma and hepatocellular carcinoma. The American Surgeon. 2010;76(11):1210-3. [CrossRef]
- Huang C, Zhan T, Liu Y, Li Q, Wu H, Ji D, et al. Glycomic profiling of carcinoembryonic antigen isolated from human tumor tissue. Clinical proteomics. 2015;12(1):1-7. [CrossRef]
- Zhao S, Long M, Zhang X, Lei S, Dou W, Hu J, et al. The diagnostic value of the combination of Golgi protein 73, glypican-3 and alpha-fetoprotein in hepatocellular carcinoma: a diagnostic meta-analysis. Annals of Translational Medicine. 2020;8(8). [CrossRef]
- Tufael, Hasan SE, Jubayer A, Akter K, Akter A, Akter F, Shiam SAA, Sunny AR. Effects of Nigella Sativa and Syzygium Cumini Seed Extracts on Blood Glucose Levels in Swiss Albino Mice. Journal of Knowledge Learning and Science Technology ISSN: 2959-6386 (online), 2023, 2(3), 53-62. [CrossRef]
- Bertino G, Ardiri A, Malaguarnera M, Malaguarnera G, Bertino N, Calvagno GS, editors. Hepatocellualar carcinoma serum markers. Seminars in oncology; 2012: Elsevier. [CrossRef]
- Li D, Mallory T, Satomura S. AFP-L3: a new generation of tumor marker for hepatocellular carcinoma. Clinica chimica acta. 2001;313(1-2):15-9. [CrossRef]
- Wee A, Sampatanukul P, Jhala N. Cytohistology of focal liver lesions: Cambridge University Press; 2014.
- Loosen SH, Roderburg C, Kauertz KL, Koch A, Vucur M, Schneider AT, et al. CEA but not CA19-9 is an independent prognostic factor in patients undergoing resection of cholangiocarcinoma. Scientific reports. 2017;7(1):16975. [CrossRef]
- El-Abd NE, Fawzy NA, El-Sheikh SM, Soliman ME. Circulating miRNA-122, miRNA-199a, and miRNA-16 as biomarkers for early detection of hepatocellular carcinoma in Egyptian patients with chronic hepatitis C virus infection. Molecular diagnosis & therapy. 2015;19:213-20. [CrossRef]
- Li Z, Song W, Rubinstein M, Liu D. Recent updates in cancer immunotherapy: a comprehensive review and perspective of the 2018 China Cancer Immunotherapy Workshop in Beijing. Journal of Hematology & Oncology. 2018;11(1):1-15. [CrossRef]
- Song Q, Hawkins GA, Wudel L, Chou PC, Forbes E, Pullikuth AK, et al. Dissecting intratumoral myeloid cell plasticity by single cell RNA-seq. Cancer medicine. 2019;8(6):3072-85. [CrossRef]
- Wang X, Wang N, Zhong L, Wang S, Zheng Y, Yang B, et al. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: a systematic review and meta-analysis of 282,203 patients. Molecular psychiatry. 2020;25(12):3186-97. [CrossRef]
- Sinha SR, Prakash P, Singh RK, Sinha DK. Assessment of tumor markers CA 19-9, CEA, CA 125, and CA 242 for the early diagnosis and prognosis prediction of gallbladder cancer. World Journal of Gastrointestinal Surgery. 2022;14(11):1272. [CrossRef]
- Kiran BR. A study of acute renal failure in patients associated with acute liver dysfunction at vims combined hospital: Rajiv Gandhi University of Health Sciences (India); 2013.
- Mirambo MM, Mkumbo E, Selega H, Msemwa B, Mushi MF, Silago V, et al. Hepatitis B virus infections among health professional students in Mwanza city, Tanzania in 2016. Archives of Public Health. 2020;78(1):1-5. [CrossRef]
- Garg R, Kaur S, Aseri R, Aggarwal S, Singh JP, Mann S, et al. Hepatitis B & C Among Farmers–A Seroprevalence Study. Journal of Clinical and Diagnostic Research: JCDR. 2014;8(11):MC07. [CrossRef]
- Chatsirisupachai K, Lagger C, de Magalhães JP. Age-associated differences in the cancer molecular landscape. Trends in cancer. 2022. [CrossRef]
- Suzuki C, Blomqvist L, Sundin A, Jacobsson H, Byström P, Berglund Å, et al. The initial change in tumor size predicts response and survival in patients with metastatic colorectal cancer treated with combination chemotherapy. Annals of oncology. 2012;23(4):948-54. [CrossRef]
- Kwak M-S, Chung G-E, Yang JI, Yim JY. Long-term outcomes of HBsAg/anti-HBs double-positive versus HBsAg single-positive patients with chronic hepatitis B. Scientific Reports. 2019;9(1):19417. [CrossRef]
- Edoo MIA, Chutturghoon VK, Wusu-Ansah GK, Hai Z, Zhen TY, Xie HY, et al. Serum biomarkers AFP, CEA and CA19-9 combined detection for early diagnosis of hepatocellular carcinoma. Iranian journal of public health. 2019;48(2):314. [CrossRef]
- Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor markers for hepatocellular carcinoma: simple and significant predictors of outcome in patients with HCC. Liver cancer. 2015;4(2):126-36. [CrossRef]
- Bari KF, Salam MT, Hasan SE, Sunny AR. Serum zinc and calcium level in patients with psoriasis. Journal of Knowledge Learning and Science Technology ISSN: 2959-6386 (online). 2023;2(3):7-14. [CrossRef]
- Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World journal of gastroenterology: WJG. 2006;12(8):1175. [CrossRef]
- Hernandez LM, Blazer DG. Genetics and Health. Genes, Behavior, and the Social Environment: Moving Beyond the Nature/Nurture Debate: National Academies Press (US); 2006.
- Bialecki ES, Di Bisceglie AM. Diagnosis of hepatocellular carcinoma. Hpb. 2005;7(1):26-. [CrossRef]
- Motlagh A, Elmi F, Yamrali M, Ranjbar M, Azmin M, Moshiri F, et al. Routine COVID-19 testing may not be necessary for most cancer patients. Scientific Reports. 2021;11(1):23294. [CrossRef]
- Huang K, Dong Z, Cai H, Huang M, Peng Z, Xu L, et al. Imaging biomarkers for well and moderate hepatocellular carcinoma: preoperative magnetic resonance image and histopathological correlation. BMC cancer. 2019;19:1-10. [CrossRef]
- Gurakar A, Hamilton JP, Koteish A, Li Z, Mezey E. Hepatocellular carcinoma (liver cancer): introduction. ASCO, Alexandria. 2001.
- Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer imaging: the official publication of the International Cancer Imaging Society. 2013;12:530-47. [CrossRef]
- Tang Z-Y. Hepatocellular carcinoma-cause, treatment and metastasis. World journal of gastroenterology. 2001;7(4):445. [CrossRef]
- Castaldi F, Marino M, Beneduce L, Belluco C, De Marchi F, Mammano E, et al. Detection of circulating CEA-IgM complexes in early stage colorectal cancer. The International journal of biological markers. 2005;20(4):204-8. [CrossRef]
- Zhang J, Chen G, Zhang P, Zhang J, Li X, Gan Dn, et al. The threshold of alpha-fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. PLoS One. 2020;15(2):e0228857. [CrossRef]
- Yoshikawa M, Morine Y, Ikemoto T, Imura S, Higashijima J, Iwahashi S, et al. Elevated Preoperative Serum CEA Level Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma Through the Epithelial–Mesenchymal Transition. Anticancer research. 2017;37(3):1169-75. [CrossRef]
- Hadi H, Wan Shuaib WMA, Raja Ali RA, Othman H. Utility of PIVKA-II and AFP in Differentiating Hepatocellular Carcinoma from Non-Malignant High-Risk Patients. Medicina. 2022;58(8):1015. [CrossRef]
- Agaram N, Shia J, Klimstra D. Liver & Pancreas.
- Parra NS, Ross HM, Khan A, Wu M, Goldberg R, Shah L, et al. Advancements in the Diagnosis of Hepatocellular Carcinoma. International Journal of Translational Medicine. 2023;3(1):51-65. [CrossRef]
- Giannini EG, Cucchetti A, Erroi V, Garuti F, Odaldi F, Trevisani F. Surveillance for early diagnosis of hepatocellular carcinoma: how best to do it? World journal of gastroenterology: WJG. 2013;19(47):8808. [CrossRef]
- Song MJ, Jung CK, Park CH, Hur W, Choi JE, Bae SH, et al. RPL36 as a prognostic marker in hepatocellular carcinoma. Pathology international. 2011;61(11):638-44. [CrossRef]
- Singal AK, Singh A, Jaganmohan S, Guturu P, Mummadi R, Kuo YF, et al. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus–related cirrhosis. Clinical Gastroenterology and Hepatology. 2010;8(2):192-9. [CrossRef]
- Kew M, Hodkinson J, Paterson A, Song E. Hepatitis-B virus infection in black children with hepatocellular carcinoma. Journal of Medical Virology. 1982;9(3):201-7. [CrossRef]
- AlSalloom AAM. An update of biochemical markers of hepatocellular carcinoma. International journal of health sciences. 2016;10(1):121. [CrossRef]
- Song H-J, Xue Y-L, Xu Y-H, Qiu Z-L, Luo Q-Y. Rare metastases of differentiated thyroid carcinoma: pictorial review. Endocrine-related cancer. 2011;18(5):R165-R74. [CrossRef]
- Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. The Lancet Oncology. 2018;19(7):940-52. [CrossRef]
- He C-Z, Zhang K-H, Li Q, Liu X-H, Hong Y, Lv N-H. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC gastroenterology. 2013;13(1):1-5. [CrossRef]
- Barrios CH. Global challenges in breast cancer detection and treatment. The Breast. 2022;62:S3-S6. [CrossRef]
- Ogunwobi OO, Harricharran T, Huaman J, Galuza A, Odumuwagun O, Tan Y, et al. Mechanisms of hepatocellular carcinoma progression. World journal of gastroenterology. 2019;25(19):2279. [CrossRef]
- Wang J, Chen G, Christie P, Zhang M, Luo Y, Teng Y. Occurrence and risk assessment of phthalate esters (PAEs) in vegetables and soils of suburban plastic film greenhouses. Science of the Total Environment. 2015;523:129-37. [CrossRef]
- Pedroso TMA, Benvindo-Souza M, de Araújo Nascimento F, Woch J, Dos Reis FG, de Melo e Silva D. Cancer and occupational exposure to pesticides: a bibliometric study of the past 10 years. Environmental Science and Pollution Research. 2022:1-12. [CrossRef]
- Sazzad SA, Billah M, Sunny AR, Anowar S, Pavel JH, Rakhi MS, Rahman GM, Ahmed KT, Haider KM, Rahman MZ, Al-Mamun MA. Sketching Livelihoods and Coping Strategies of Climate Vulnerable Fishers. Egyptian Journal of Aquatic Biology & Fisheries. 2023, 1;27(4). [CrossRef]
- .
- Kuddus MA, Sunny AR, Sazzad SA, Hossain M, Rahman M, Mithun MH, Hasan SE, Ahmed KJ, Zandonadi RP, Han H, Ariza-Montes A. Sense and Manner of WASH and Their Coalition With Disease and Nutritional Status of Under-five Children in Rural Bangladesh: A Cross-Sectional Study. Frontiers in Public Health. 2022, 17;10:890293. [CrossRef]
- .
- Hasan MR, Hossain MM, Islam MS, Sunny AR., Ferdous J, Chowdhury MZA, Maria AM, Sarder SAA, Sultana A. 2023. Seasonal Variation of Quality and the Total Viable Count of Lean and Fatty Fish. Egyptian Journal of Aquatic Biology & Fisheries, 2023, 27(5).
- .
- Gómez-Barroso D, García-Pérez J, López-Abente G, Tamayo-Uria I, Morales-Piga A, Pardo Romaguera E, et al. Agricultural crop exposure and risk of childhood cancer: new findings from a case–control study in Spain. International journal of health geographics. 2016;15:1-11. [CrossRef]
- Kang Y, Li L, Chen W, Zhang F, Du Y, Wu T. Rapid in situ SERS analysis of pesticide residues on plant surfaces based on micelle extraction of targets and stabilization of Ag nanoparticle aggregates. Food Analytical Methods. 2018;11:3161-9. [CrossRef]
- Jin H, Chen Y, Fu Q, Qu Q. Occupational risk factors of contracting COVID-19 among health workers: A systematic review. Work. 2021;69(3):721-34. [CrossRef]
- Kisling LA, Das JM. Prevention strategies. StatPearls [internet]: StatPearls Publishing; 2021.
- McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clinics in liver disease. 2015;19(2):223-38. [CrossRef]
- Liu A, Galoosian A, Kaswala D, Li AA, Gadiparthi C, Cholankeril G, et al. Nonalcoholic fatty liver disease: epidemiology, liver transplantation trends and outcomes, and risk of recurrent disease in the graft. Journal of Clinical and Translational Hepatology. 2018;6(4):420. [CrossRef]
- Zhu RX, Seto W-K, Lai C-L, Yuen M-F. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut and liver. 2016;10(3):332. [CrossRef]
- Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharmaceutical research. 2008;25:2097-116. [CrossRef]
- Allahverdi N, Yassin M, Ibrahim M. Environmental Factors, Lifestyle Risk Factors, and Host Characteristics Associated With Philadelphia Negative Myeloproliferative Neoplasm: A Systematic Review. Cancer Control. 2021;28:10732748211046802. [CrossRef]
- Faruk O, Hasan SE, Jubayer A, Akter K, Al Shiam SA, Rahman K, Ali MY. Microbial Isolates from Urinary Tract Infection and their Antibiotic Resistance Pattern in Dhaka city of Bangladesh. Journal of Knowledge Learning and Science Technology ISSN: 2959-6386 (online). 2023 Dec 21;2(3):76-87. [CrossRef]
- Ferdous J, Sunny AR, Khan RS, Rahman K, Chowdhury R, Mia MT, Al Shiam A, Mithun MH. Impact of Varying Synthetic Hormone on Mystus cavasius (Hamilton):: Fertilization, Hatching, and Survival Rates. Journal of Knowledge Learning and Science Technology ISSN: 2959-6386 (online). 2023 Dec 21;2(3):88-105. [CrossRef]
- Mithun MH, Sunny AR, Billah M, Sazzad SA, Salehin S, Jahan N, Rahman K, Al Shiam A, Chowdhury R, Arafat J, Baten A. Assessing Impact of Microplastics on Aquatic Food System and Human Health.
- Alam K, Chowdhury MZ, Jahan N, Rahman K, Chowdhury R, Mia MT, Mithun MH. Relationship between Brand Awareness and Customer Loyalty in Bangladesh: A Case Study of Fish Feed Company. Journal of Knowledge Learning and Science Technology ISSN: 2959-6386 (online). 2023 Dec 21;2(3):212-22. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).