Submitted:
08 January 2024
Posted:
10 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Participants
Placental Tissue Sampling and Gene Expression Analysis
Hormone Measurements
Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Azziz, R.; Woods, K.S.; Reyna, R.; Key, T.J.; Knochenhauer, E.S.; Yildiz, B.O. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004, 89, 2745-2749. [CrossRef]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.W.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010, 25, 544–551. [CrossRef]
- The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS). Hum Reprod. 2012, 27, 14–24.
- Wild, R.A.; Carmina, E.; Diamanti-Kandrarakis, E., et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010, 95, 2038–2049. [CrossRef]
- Yu, H.F.; Chen, H.S.; Rao, D.P.; Gong, J. Association between polycystic ovary syndrome and the risk of pregnancy complications: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2016, 95, e4863.
- Rassi, A.; Veras, A.B.; dos Reis, M., et al. Prevalence of psychiatric disorders in patients with polycystic ovary syndrome. Compr Psychiatry. 2010, 51, 599-602. [CrossRef]
- Raperport, C.; Homburg, R. The Source of Polycystic Ovarian Syndrome. Clin Med Insights Reprod Health. 2019, 13, 1179558119871467. [CrossRef]
- Crespo, R.P.; Bachega, T.A.; Mendonça, B.B.; Gomes, L.G. An Update of Genetic Basis of PCOS Pathogenesis. Arch Endocrinol Metab. 2018, 62, 352–361. [CrossRef]
- Palioura, E.; Diamanti-Kandarakis, E. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Rev Endocr Metab Disord. 2015, 16, 365-371. [CrossRef]
- Hakim, C.; Padmanabhan, V.; Vyas, A.K. Gestational Hyperandrogenism in Developmental Programming. Endocrinology. 2017, 158, 199-212. [CrossRef]
- Kelley, A.S.; Smith, Y.R.; Padmanabhan, V. A Narrative Review of Placental Contribution to Adverse Pregnancy Outcomes in Women With Polycystic Ovary Syndrome. Clin Endocrinol Metab. 2019, 104, 5299–5315. [CrossRef]
- Gorkem, U.; Togrul, C.; Arslan, E.; Sargin Oruc, A.; Buyukkayaci Duman, N. Is there a role for kisspeptin in pathogenesis of polycystic ovary syndrome? Gynecol Endocrinol. 2018, 34, 157-160.
- George, J.T.; Kakkar, R.; Marshall, J., et al. Neurokinin B Receptor Antagonism in Women With Polycystic Ovary Syndrome: A Randomized, Placebo-Controlled Trial. J Clin Endocrinol Metab. 2016, 101, 4313-4321. [CrossRef]
- Page, N.M. Neurokinin B and pre-eclampsia: a decade of discovery. Reprod Biol Endocrinol. 2010, 8, 4. [CrossRef]
- Szydełko-Gorzkowicz, M.; Poniedziałek-Czajkowska, E.; Mierzyński, R.; Sotowski, M.; Leszczyńska-Gorzelak, B. The Role of Kisspeptin in the Pathogenesis of Pregnancy Complications: A Narrative Review. Int J Mol Sci. 2022, 23, 6611. [CrossRef]
- The Rotterdam ESHRE/ASRM – Sponsored PCOS Consensus Workshop Group 2004. Revised 2003 consensus on the diagnostic criteria and long term health risks related to polycystic ovary syndrome. Fertil Steril. 2003, 81, 19-25.
- Panagodimou, E.; Koika, V.; Markatos, F.; Kaponis, A.; Adonakis, G.; Georgopoulos, N.A.; Markantes, G.K. Expression stability of ACTB, 18S, and GAPDH in human placental tissues from subjects with PCOS and controls: GAPDH expression is increased in PCOS. Hormones (Athens). 2022, 21, 329-333. [CrossRef]
- Cooper, H.E.; Spellacy, W.E.; Prem, K.A.; Cohen, W.D. Hereditary factors in Stein-Leventhal syndrome. Am J Obstet Gynecol. 1968, 100, 371–382. [CrossRef]
- Jones, M.R.; Goodarzi, M.O. Genetic Determinants of Polycystic Ovary Syndrome: Progress and Future Directions. Fertil Steril. 2016, 106, 25–32. [CrossRef]
- Barker, D.J. The fetal and infant origins of adult disease. Br Med J. 1990, 301, 1111. [CrossRef]
- Abbott, D.H.; Dumesic, D.A.; Levine, J.E.; Dunaif, A.; Padmanabhan, V. Animal models and fetal programming of PCOS. In Contemporary Endocrinology: Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders; Azziz, J.E., Nestler, J.E., Dewailly, D., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 259-272.
- Franks, S. Animal models and the developmental origins of polycystic ovary syndrome: increasing evidence for the role of androgens in programming reproductive and metabolic dysfunction. Endocrinology. 2012, 153, 2536-2538. [CrossRef]
- Padmanabhan, V.; Veiga-Lopez, A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013, 373, 8-20. [CrossRef]
- Cernea, M.; Padmanabhan, V.; Goodman, R.L.; Coolen, L.M.; Lehman, M.N. Prenatal testosterone treatment leads to changes in the morphology of KNDy neurons, their inputs, and projections to GnRH cells in female sheep. Endocrinology. 2015, 156, 3277–3291. [CrossRef]
- Kondo, M.; Osuka, S.; Iwase, A.; Nakahara, T.; Saito, A.; Bayasula; Nakamura, T.; Goto, M.; Kotani, T.; Kikkawa, F. Increase of kisspeptin-positive cells in the hypothalamus of a rat model of polycystic ovary syndrome. Metab Brain Dis. 2016, 31, 673–681. [CrossRef]
- Dumesic, D.A.; Abbott, D.H.; Eisner, J.R.; Goy, R.W. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997, 67, 155–163. [CrossRef]
- Foecking, E.M.; Szabo, M.; Schwartz, N.B.; Levine, J.E. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005, 72, 1475–1483. [CrossRef]
- Padmanabhan, V.; Veiga-Lopez, A.; Abbott, D.H.; Recabarren, S.E.; Herkimer, C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. 2010, 151, 595–605. [CrossRef]
- Lu, C.; Cardoso, R.C.; Puttabyatappa, M.; Padmanabhan, V. Developmental programming: prenatal testosterone excess and insulin signaling disruptions in female sheep. Biol Reprod. 2016, 94, 113. [CrossRef]
- Eisner, J.R.; Dumesic, D.A.; Kemnitz, J.W.; Colman, R.J.; Abbott, D.H. Increased adiposity in female rhesus monkeys exposed to androgen excess during early gestation. Obes Res. 2003, 11, 279–286. [CrossRef]
- Sir-Petermann, T.; Codner, E.; Pérez, V.; Echiburú, B.; Maliqueo, M.; Ladrón de Guevara, A.; Preisler, J.; Crisosto, N.; Sánchez, F.; Cassorla, F.; Bhasin, S. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009, 94, 1923–1930. [CrossRef]
- Hague, W.M.; Adams, J.; Rodda, C.; Brook, C.G.; de Bruyn, R.; Grant, D.B.; Jacobs, H.S. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin Endocrinol (Oxf). 1990, 33, 501–510. [CrossRef]
- Boomsma, C.M.; Eijkemans, M.J.; Hughes, E.G.; Visser, G.H.; Fauser, B.C.; Macklon, N.S. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006, 12, 673–683. [CrossRef]
- Recabarren, S.E.; Smith, R.; Rios, R.; Maliqueo, M.; Echiburú, B.; Codner, E.; Cassorla, F.; Rojas, P.; Sir-Petermann, T. Metabolic profile in sons of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008, 93, 1820–1826. [CrossRef]
- Speiser, P.W.; Serrat, J.; New, M.I.; Gertner, J.M. Insulin insensitivity in adrenal hyperplasia due to nonclassical steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1992, 75, 1421–1424. [CrossRef]
- de Zegher, F.; Reinehr, T.; Malpique, R., et al. Reduced Prenatal Weight Gain and/or Augmented Postnatal Weight Gain Precedes Polycystic Ovary Syndrome in Adolescent Girls. Obesity (Silver Spring). 2017, 25, 1486-1489. [CrossRef]
- Khan, G.H.; Galazis, N.; Docheva, N.; Layfield, R.; Atiomo, W. Overlap of proteomics biomarkers between women with pre-eclampsia and PCOS: a systematic review and biomarker database integration. Hum Reprod. 2015, 30, 133-148. [CrossRef]
- Palomba, S.; Russo, T.; Falbo, A., et al. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Hum Reprod. 2013, 28, 2838-2847. [CrossRef]
- Koster, M.P.; de Wilde, M.A.; Veltman-Verhulst, S.M.; Houben, M.L.; Nikkels, P.G.; van Rijn, B.B.; Fauser, B.C. Placental characteristics in women with polycystic ovary syndrome. Hum Reprod. 2015, 30, 2829-2837. [CrossRef]
- Hochberg, A.; Mills, G.; Volodarsky-Perel, A.; Nu, T.N.T.; Machado-Gedeon, A.; Cui, Y.; Shaul, J.; Dahan, M.H. The impact of polycystic ovary syndrome on placental histopathology patterns in in-vitro fertilization singleton live births. Placenta. 2023, 139, 12-18. [CrossRef]
- Maliqueo, M.; Lara, H.E.; Sánchez, F.; Echiburú, B.; Crisosto, N.; Sir-Petermann, T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013, 166, 151-155. [CrossRef]
- Sir-Petermann, T.; Maliqueo, M.; Angel, B.; Lara, H.E.; Pérez-Bravo, F.; Recabarren, S.E. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002, 17, 2573–2579. [CrossRef]
- Glintborg, D.; Jensen, R.C.; Bentsen, K.; Schmedes, A.V.; Brandslund, I.; Kyhl, H.B.; Bilenberg, N.; Andersen, M.S. Testosterone levels in third trimester in polycystic ovary syndrome. Odense Child Cohort. J Clin Endocrinol Metab. 2018, 103, 3819–3827. [CrossRef]
- Sun, M.; Sun, B.; Qiao, S.; Feng, X.; Li, Y.; Zhang, S.; Lin, Y.; Hou, L. Elevated maternal androgen is associated with dysfunctional placenta and lipid disorder in newborns of mothers with polycystic ovary syndrome. Fertil Steril. 2020, 113, 1275-1285.e2. [CrossRef]
- Palomba, S.; Marotta, R.; Di Cello, A., et al. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol. 2012, 77, 898-904. [CrossRef]
- Hsu, T.Y.; Lan, K.C.; Tsai, C.C.; Ou, C.Y.; Cheng, B.H.; Tsai, M.Y.; Kang, H.Y.; Tung, Y.H.; Wong, Y.H.; Huang, K.E. Expression of androgen receptor in human placentas from normal and preeclamptic pregnancies. Taiwan J Obstet Gynecol. 2009, 48, 262–267. [CrossRef]
- Sathishkumar, K.; Elkins, R.; Chinnathambi, V.; Gao, H.; Hankins, G.D.; Yallampalli, C. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol. 2011, 9, 110. [CrossRef]
- Cleys, E.R.; Halleran, J.L.; Enriquez, V.A.; da Silveira, J.C.; West, R.C.; Winger, Q.A.; Anthony, R.V.; Bruemmer, J.E.; Clay, C.M.; Bouma, G.J. Androgen receptor and histone lysine demethylases in ovine placenta. PLoS One. 2015, 10, e0117472. [CrossRef]
- Sun, M.; Maliqueo, M.; Benrick, A.; Johansson, J.; Shao, R.; Hou, L.; Jansson, T.; Wu, X.; Stener-Victorin, E. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012, 303, E1373–E1385. [CrossRef]
- Gopalakrishnan, K.; Mishra, J.S.; Chinnathambi, V.; Vincent, K.L.; Patrikeev, I.; Motamedi, M.; Saade, G.R.; Hankins, G.D.; Sathishkumar, K. Elevated testosterone reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant rats. Hypertension. 2016, 67, 630–639. [CrossRef]
- Chinnathambi, V.; Blesson, C.S.; Vincent, K.L.; Saade, G.R.; Hankins, G.D.; Yallampalli, C.; Sathishkumar, K. Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension. 2014, 64, 405–414.
- Hu, M.; Richard, J.E.; Maliqueo, M.; Kokosar, M.; Fornes, R.; Benrick, A.; Jansson, T.; Ohlsson, C.; Wu, X.; Skibicka, K.P.; Stener-Victorin, E. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc Natl Acad Sci USA. 2015, 112, 14348–14353. [CrossRef]
- Abbott, D.H.; Cristin, R.; Bruns, C.R.; Barnett, D.K.; Dunaif, A.; Theodore, L.; Goodfriend, T.L.; Daniel, A.; Tarantal, D.; Tarantal, A. Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring. Am J Physiol Endocrinol Metab. 2010, 299, E741–E751. [CrossRef]
- Maliqueo, M.; Sundström Poromaa, I.; Vanky, E.; Fornes, R.; Benrick, A.; Åkerud, H.; Stridsklev, S.; Labrie, F.; Jansson, T.; Stener-Victorin, E. Placental STAT3 signaling is activated in women with polycystic ovary syndrome. Hum Reprod. 2015, 30, 692-700. [CrossRef]
- Fornes, R.; Maliqueo, M.; Hu, M.; Hadi, L.; Jimenez-Andrade, J.M.; Ebefors, K.; Nyström, J.; Labrie, F.; Jansson, T.; Benrick, A.; Stener-Victorin, E. The effect of androgen excess on maternal metabolism, placental function and fetal growth in obese dams. Sci Rep. 2017, 7, 8066. [CrossRef]
- Mayama, R.; Izawa, T.; Sakai, K.; Suciu, N.; Iwashita, M. Improvement of insulin sensitivity promotes extravillous trophoblast cell migration stimulated by insulin-like growth factor-I. Endocr J. 2013, 60, 359–368. [CrossRef]
- Zhang, Y.; Zhao, W.; Xu, H.; Hu, M.; Guo, X.; Jia, W.; Liu, G.; Li, J.; Cui, P.; Lager, S.; Sferruzzi-Perri, A.N.; Li, W.; Wu, X.K.; Han, Y.; Brännström, M.; Shao, L.R.; Billig, H. Hyperandrogenism and insulin resistance-induced fetal loss: evidence for placental mitochondrial abnormalities and elevated reactive oxygen species production in pregnant rats that mimic the clinical features of polycystic ovary syndrome. J Physiol. 2019, 597, 3927-3950. [CrossRef]
- Tata, B.; Mimouni, N.E.H.; Barbotin, A.L.; Malone, S.A.; Loyens, A.; Pigny, P.; Dewailly, D.; Catteau-Jonard, S.; Sundström-Poromaa, I.; Piltonen, T.T.; Dal Bello, F.; Medana, C.; Prevot, V.; Clasadonte, J.; Giacobini, P. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018, 24, 834-846. [CrossRef]
- Kapustin, R.V.; Drobintseva, A.O.; Alekseenkova, E.N.; Onopriychuk, A.R.; Arzhanova, O.N.; Polyakova, V.O.; Kvetnoy, I.M. Placental protein expression of kisspeptin-1 (KISS1) and the kisspeptin-1 receptor (KISS1R) in pregnancy complicated by diabetes mellitus or preeclampsia. Arch Gynecol Obstet. 2020, 301, 437–445. [CrossRef]
- Bowe, J.E.; Hill, T.G.; Hunt, K.F.; Smith, L.I.; Simpson, S.J.; Amiel, S.A.; Jones, P.M. A role for placental kisspeptin in β cell adaptation to pregnancy. JCI Insight 2019, 4, e124540. [CrossRef]
- Fang, L.; Gao, Y.; Wang, Z.; Li, Y.; Yan, Y.; Wu, Z.; Cheng, J.-C.; Sun, Y.-P. EGF stimulates human trophoblast cell invasion by downregulating ID3-mediated KISS1 expression. Cell Commun Signal. 2021, 19, 101. [CrossRef]
- Adali, E.; Kurdoglu, Z.; Kurdoglu, M.; Kamaci, M.; Kolusari, A.; Yildizhan, R. Metastin levels in pregnancies complicated by pre-eclampsia and their relation with disease severity. J Matern Fetal Neonatal Med. 2012, 25, 2671–2675. [CrossRef]
- Qiao, C.; Wang, C.; Zhao, J.; Liu, C.; Shang, T. Elevated expression of KiSS-1 in placenta of Chinese women with early-onset preeclampsia. PLoS ONE 2012, 7, e48937. [CrossRef]
- Vazquez-Alaniz, F.; Galaviz-Hernandez, C.; Marchat, L.A.; Salas-Pacheco, J.M.; Chairez-Hernandez, I.; Guijarro-Bustillos, J.J.;Mireles-Ordaz, A. Comparative expression profiles for KiSS-1 and REN genes in preeclamptic and healthy placental tissues. Eur J Obstet Gynecol Reprod Biol. 2011, 159, 67–71. [CrossRef]
- Smets, E.M.L.; Deurloo, K.L.; Go, A.T.J.I.; van Vugt, J.M.G.; Blankenstein, M.A.; Oudejans, C.B.M. Decreased plasma levels of metastin in early pregnancy are associated with small for gestational age neonates. Prenat Diagn. 2008, 28, 299–303. [CrossRef]
- Santos, B.R.; Cordeiro, J.M.D.A.; Santos, L.C.; Santana, L.D.S.; Nascimento, A.E.J.; Silva, J.F. Kisspeptin Suppresses Inflammasome-NLRP3 Activation and Pyroptosis Caused by Hypothyroidism at the Maternal-Fetal Interface of Rats. Int J Mol Sci. 2023, 24, 6820. [CrossRef]
- Liu, Y.; Chen, X.; Chen, H. Placental and umbilical cord levels of neurokinin B and neurokinin B receptor in pre-eclampsia. Int J Gynaecol Obstet. 2009, 107, 58-59. [CrossRef]
- Yang, J.; Dhawan, V.; Morrish, D.W.; Kaufman, S. Bimodal effects of chronically administered neurokinin B (NKB) on in vivo and in vitro cardiovascular responses in female rats. Regul Pept. 2007, 143, 136-142. [CrossRef]
- D'Anna, R.; Baviera, G.; Corrado, F.; Crisafulli, A.; Ientile, R.; Buemi, M.; Squadrito, F. Neurokinin B and nitric oxide plasma levels in pre-eclampsia and isolated intrauterine growth restriction. BJOG. 2004, 111, 1046-1050. [CrossRef]
- Torricelli, M.; Giovannelli, A.; Leucci, E.; Florio, P.; De Falco, G.; Torres, P.B.; Reis, F.M.; Leoncini, L.; Petraglia, F. Placental neurokinin B mRNA expression increases at preterm labor. Placenta. 2007, 28, 1020-1023. [CrossRef]
- Blasco, V.; Pinto, F.M.; Fernández-Atucha, A.; Prados, N.; Tena-Sempere, M.; Fernández-Sánchez, M.; Candenas, L. Altered expression of the kisspeptin/KISS1R and neurokinin B/NK3R systems in mural granulosa and cumulus cells of patients with polycystic ovarian syndrome. J Assist Reprod Genet. 2019, 36, 113-120. [CrossRef]
- Beckett, E.M.; Astapova, O.; Steckler, T.L.; Veiga-Lopez, A.; Padmanabhan, V. Developmental programing: impact of testosterone on placental differentiation. Reproduction. 2014, 148, 199–209. [CrossRef]
- Oride, A.; Kanasaki, H.; Mijiddorj, T.; Sukhbaatar, U.; Ishihara, T.; Kyo, S. Regulation of kisspeptin and gonadotropin-releasing hormone expression in rat placenta: study using primary cultures of rat placental cells. Reprod Biol Endocrinol. 2015, 13, 90. [CrossRef]
- Sawicki, G.; Dakour, J.; Morrish, D.W. Functional proteomics of neurokinin B in the placenta indicates a novel role in regulating cytotrophoblast antioxidant defences. Proteomics. 2003, 3, 2044-2051. [CrossRef]
| PCOS (n=31) | Controls (n=37) | p Value | |
|---|---|---|---|
| Age (years) | 31.52±5.32 | 32.06±5.78 | 0.677 |
| BMI at 1st visit (kg/m2) | 26.81±5.06 | 25.27±4.44 | 0.191 |
| BMI at delivery (kg/m2) | 31.98±5.49 | 29.84±4.44 | 0.105 |
| Gestational diabetes | 4 (12.9%) | 4 (10.8%) | 0.790 |
| Delivery week | 39 (2) | 39 (2) | 0.785 |
| Mode of delivery (VD/CS) | 15 (48.4%) / 16 (51.6%) | 19 (51.4%) / 18 (48.6%) | 0.808 |
| Offspring gender (M/F) | 14 (45.2%) / 17 (54.8%) | 21 (56.8%) / 16 (43.2%) | 0.274 |
| Offspring weight (g) | 3161.33±555.14 | 3330.27±462.90 | 0.179 |
| Offspring length (cm) | 49.75±2.44 | 50.88±2.27 | 0.139 |
| Maternal Serum | PCOS (n=31) | Controls (n=37) | p Value |
|---|---|---|---|
| Total testosterone (ng/dL) | 88.29 (98.77) | 91.27 (70.99) | 0.538 |
| SHBG (nmol/L) | 415.99±135.61 | 478.20±120.47 | 0.049 |
| FAI | 0.68 (0.40) | 0.56 (0.67) | 0.048 |
| Androstenedione (ng/mL) | 2.19 (2.10) | 1.64 (2.12) | 0.387 |
| DHEAS (μg/dL) | 105.97±54.46 | 120.99±72.82 | 0.347 |
| AMH (pmol/L) | 7.23 (5.41) | 3.84 (6.07) | 0.012 |
| Estradiol (pg/mL) | 8860 (16301) | 6698 (15510) | 0.310 |
| Umbilical cord blood | |||
| Female Offspring | PCOS (n=17) | Controls (n=16) | |
| Total testosterone (ng/dL) | 142.54±56.71 | 130.97±37.27 | 0.501 |
| SHBG (nmol/L) | 32.16 (18.15) | 32.50 (23.31) | 0.624 |
| FAI | 14.57 (9.37) | 12.69 (11.52) | 0.468 |
| Androstenedione (ng/mL) | 0.52±0.12 | 0.44±0.11 | 0.185 |
| DHEAS (μg/dL) | 425.58±194.81 | 353.89±151.84 | 0.255 |
| AMH (pmol/L) | 1.50 (2.03) | 1.19 (1.56) | 0.624 |
| Estradiol (pg/mL) | 2577.19±929.94 | 2959.82±1095.36 | 0.295 |
| Male Offspring | PCOS (n=14) | Controls (n=21) | |
| Total testosterone (ng/dL) | 168.76±69.34 | 166.62±56.62 | 0.923 |
| SHBG (nmol/L) | 33.54 (9.02) | 37.06 (12.43) | 0.255 |
| FAI | 17.14 (9.13) | 16.12 (6.73) | 0.893 |
| Androstenedione (ng/mL) | 0.56±0.24 | 0.48±0.17 | 0.341 |
| DHEAS (μg/dL) | 394.01±166.72 | 349.44±161.11 | 0.449 |
| AMH (pmol/L) | 165.84 (80.60) | 180.10 (90.90) | 0.439 |
| Estradiol (pg/mL) | 3266.23±1375.43 | 2730.19±1769.12 | 0.363 |
| All Samples | PCOS (n=31) | Controls (n=37) | p value |
|---|---|---|---|
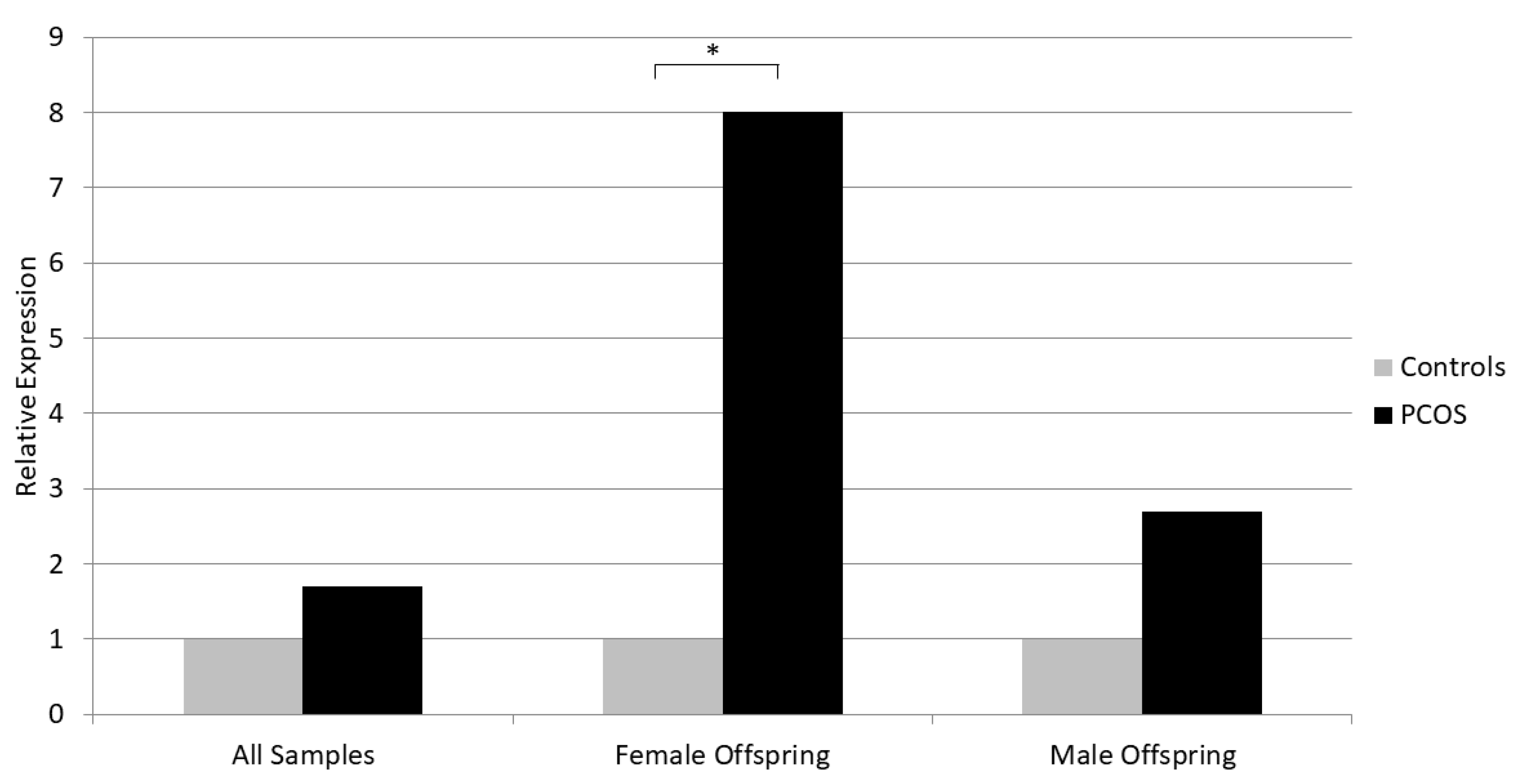
| NKB | 0.0017 (0.04) | 0.0010 (0.02) | 0.160 |
| NK1R | 2.58x10-5 (5.3x10-5) | 2.44x10-5 (9.0x10-5) | 0.514 |
| NK2R | 3.66x10-6 (9x10-6) | 4.14 x10-6 (14x10-6) | 0.298 |
| NK3R | 5.41x10-5 (18.8x10-5) | 2.80x10-5 (7.4x10-5) | 0.394 |
| KISS1 | 0.0160 (0.07) | 0.0079 (0.07) | 0.120 |
| Female Offspring | PCOS (n=17) | Controls (n=16) | |
| NKB | 0.0008 (0.03) | 0.0001 (0.0001) | 0.021 |
| NK1R | 1.51x10-5 (2.9x10-5) | 2.43x10-5 (7.0x10-5) | 0.762 |
| NK2R | 0.77x10-6 (24x10-6) | 1.57 x10-6 (4x10-6) | 0.579 |
| NK3R | 1.66x10-5 (37.0x10-5) | 1.42 x10-5 (6.3x10-5) | 0.631 |
| KISS1 | 0.0136 (0.03) | 0.0022 (0.03) | 0.315 |
| Male Offspring | PCOS (n=14) | Controls (n=21) | |
| NKB | 0.0135 (0.07) | 0.0050 (0.02) | 0.586 |
| NK1R | 5.62x10-5 (9.4x10-5) | 3.56x10-5 (31.4x10-5) | 0.867 |
| NK2R | 4.63x10-6 (12x10-6) | 2.97x10-6 (5x10-6) | 0.660 |
| NK3R | 8.40x10-5 (18.7x10-5) | 2.20x10-5 (6.8x10-5) | 0.363 |
| KISS1 | 0.0435 (0.26) | 0.0089 (0.08) | 0.135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





