1. Introduction
The severe acute respiratory syndrome of coronavirus 2 (SARS CoV-2) pandemic and the resulting unprecedented outbreak of coronavirus disease 2019 (COVID-19) have affected every aspects of life and after three years, despite the introduction of various vaccines, the virus still as present in the human population as it was in the year 2020 [
1]. In Puerto Rico, a Caribbean island of 3.2 millions people, the first confirmed case occurred in early March 2020 and at present day the island has recorded 1,101,469 confirmed cases and 5,823 deaths associated to this epidemic have been recorded (
https://coronavirus.jhu.edu/region/us/puerto-rico). Government measures were characterized by national lock downs together with tracing contact and isolation of diagnosed cases through passive surveillance of suspected cases [
2].
Since the beginning of the pandemic, numerous quantitative/qualitative serological assays were developed across the globe as a tool for detecting the presence of specific antibodies against SARS-CoV-2 [
3,
4,
5,
6]. As part of our contribution, our research group developed in-house serological assays to detect IgG antibodies in serum or plasma from individuals with COVID-19 in Puerto Rico [
7]. Although serological approaches based on detection of IgG cannot distinguish between acute and chronic infection, this type of test is useful for,
i) the identification of individuals that have developed an immune response and could serve as plasma donors,
ii) facilitate contact tracing,
iii) determining the immune response dynamic to natural SARS-CoV-2 infection or mRNA vaccination and,
iv) determining the capacity to induce neutralizing antibody against SARS-CoV2 after mRNA vaccination in populations with concomitant inflammatory diseases [
2,
7,
8,
9].
The antibody production is a hallmark of the adaptive immune response and plays essential roles in neutralizing or to eliminating microbial agents. Studies of the immune responses after a natural infection with SARS-CoV-2 have demonstrated that most of the symptomatic patients develop IgG antibodies within 2 weeks of symptoms onset and the levels of these antibodies are dependent on the severity of the disease [
10,
11]. However, it has been demonstrated that no-symptomatic patients also develop humoral response to SARS-CoV-2 [
12]. Most of the serological tests are based on the detection of IgM and/or IgG [
13], even though IgA antibodies also play an important role in mucosal immunity and have an earlier onset than IgM during SARS-CoV-2 infection [
13,
14]. IgG is undoubtedly the antibody most studied during SARS-CoV-2 infection and several recent studies have demonstrated that this immunoglobulin remains at detectable levels in convalescent patients after several months of a natural infection [
15,
16]. The naturally induced IgG antibodies play a crucial role in facilitating the natural recovery of the majority of patients [
17]. However, IgG exists in the form of four subclasses (IgG1, IgG2, IgG3 and IgG4) each with different properties [
18]. The IgG profile has been already analyzed in severe cases of COVID-19 [
19,
20]. IgG1 and IgG3 are the dominant antibody isotypes elicited specifically against the virus spike (S) protein and receptor-binding domain (RBD) [
21] during the acute phase of the infection. IgG1 has the ability to bind to Fc receptors on immune cells causing antibody dependent cytotoxicity (ADCC) and triggering complement dependent cytotoxicity (CDC) [
22]. IgG3 binds with high affinity to a variety of receptors (FcgRIIa, FcgRIIIa and FcgRIIIb) on the surface of neutrophils, macrophages, and natural killer (NK) cells, and regulates the activity of these effector cells [
23]. While it is reasonable to assume the presence of these isotypes in non-hospitalized subjects, whether asymptomatic or with mild symptoms, limited knowledge exists regarding the distribution of the four IgG subclasses (G1, G2, G3, G4) and their efficacy in neutralizing various variants of concern (VOC) within this particular population during the primary infection. Furthermore, it is acknowledged that upon the administration of two doses of SARS-CoV-2 mRNA vaccine, IgG1 and IgG3 are the predominant IgG subclasses [
24], the long-term development of all four IgG subclasses after the second dose, and particularly after repeated vaccine doses, has been poorly studied. In this context, the characterization of the four IgG subclasses in naturally infected subjects compared to those that have received two or more vaccinations seems to be important considering that a large number of re-infections have been reported in several countries [
25,
26] and the emergence of new SARS-CoV-2 variants, including Alpha Omicron (B.1.1.529) and subsequent lineage [
27], which may influence the retransmission, diagnosis and prevention of the infection.
The aim of the present study was to characterize the profile of the four anti-SARS-CoV-2 IgG subclasses (G1, G2, G3 & G4) produced by natural infection in a non-hospitalized COVID-19 Latino cohort with different time of convalescence, compare this profile with those showed by convalescent or naïve subjects that received repeated doses of the mRNA vaccines, Pfizer-BioNTech or Moderna (mRNA-1273), including the Bivalent vaccine (Original mRNA & Omicron BA0.5) and correlate these antibody responses with the ability to neutralize the virus. The longitudinal evolution of the four anti-SARS-CoV-2 IgG subclasses either in individuals with different immune states is highly relevant to estimate the long-term immune effects of vaccination or revaccination in the context of constant breakthrough variants infections.
2. Materials and Methods
2.1. Antigen and Reagents
We used commercially available recombinant SARS-CoV2 Spike-1-RBD from GenScript (No. Z03483-1), which can bind with Human ACE2 in functional ELISA. This protein is produced in human cells with a predicted molecular weight of 30kDa and >90% purity as analyzed by SDS-PAGE (GenScript, Piscataway, NJ, USA). The study also employed disposable, high-bind, clear, flat-bottomed, polystyrene 96-well plates (Costar, Corning MA No. 3361). As secondary antibody in the IgG ELISA we used a mouse anti-human IgG Fc conjugated with horseradish peroxidase (HRP) (GenScript, Catalog No. A01854), which is a mouse anti-human IgG Fc (50B4A9) monoclonal antibody that specifically react with human IgG and does not cross-react with IgG, IgA or IgY antibodies from other animal species. For detecting IgG subclasses of antibodies, we used an anti-human IgG1 Fc (Southern Biotech 364 9054-05), anti-human IgG2 Fc (Southern Biotech #9060-05), anti-human IgG3 hinge (Southern Biotech 9210-05), and anti-human IgG4 Fc (Southern Biotech 9200-05). Carbonate-bicarbonate buffer with sodium azide (Sigma-Aldrich catalog No. 08058), phosphate-citrate buffer (Catalog No. P4809) and o-phenylenediamine hydrochloride (tablets 10mg) (Sigma-Aldrich, catalog No. P8287) were used for coating and buffer substrate, respectively. In the neutralization assay we used SARS-CoV-2 Receptor binding domain (RBD) horseradish peroxidase (HRP) conjugate (RBD-HRP) from the following variants: wild-type (Genscript, Cat. No. Z03594), Alpha (B.1.1.7) (Genscript, Cat. No. Z03595), Delta (B.1.617.2) (Genscript, Cat. No. Z03614) and Omicron (B.1.1.529) (Genscript, Cat. No. Z03730).
2.2. Study cohort and ethical statements
The samples in this study were allotted into five different cohorts depending on the source:
Cohort-1: non-hospitalized-COVID-19 convalescent-not vaccinated subjects,
Cohort-2: previously infected subjects that received two doses of mRNA vaccine (Pfizer-BioNTech or Moderna-1273),
Cohort-3: not-previously infected subjects-that received multiple mRNA vaccinations (three doses plus bivalent),
Cohort-4: patients with inflammatory bowel disease (IBD) with no-previous SARS-CoV-2 infection that received three mRNA vaccine doses, and
Cohort-5: pre-pandemic samples. The cohort-1 (non-hospitalized COVID-19 convalescent-no vaccinated) consisted of 85 deidentified samples (31 sera and 54 plasma) that had been collected during the pandemic from December 2019 to May 2020 (
Table 1). Some of these samples were kindly donated by clinical laboratories or blood banks serving the University of Puerto Rico-Medical Sciences Campus (UPR-MSC) network, which received self-enrolled subjects for the purpose of donating plasma for the treatment of COVID-19 patients. Because the samples were not collected specifically for this study no personal identifiers were retained. Thus, prior to receiving the samples, they were stripped of all identifiers so that the information could not be traced back to the individuals. The only information gathered from these donors was that they had not been hospitalized; some did not develop any clinical symptoms and their RT-qPCR positive diagnosis for SARS-CoV-2 was incidental discovered during routine PCR testing of samples. Another retrieved information collected from 69 of 85 subjects was the date in which the confirmatory RT-qPCR was done and the dates in which subsequent samples were collected. Thus, these 69 COVID-19 samples were allotted into three categories: 1-30 days, 31-60 days and >60 days after infection, based on the time elapsed between the confirmatory RT-qPCR diagnosis and the date in which the subsequent sample was collected. Subjects were given the opportunity to ask questions to blood bank workers regarding their participation. Furthermore, collected samples were handled using the standard blood donors’ protocols, along with the blood bank’s signed consent form, which also detailed the possibility that samples would be used for research purposes.
The cohort-2 (previously SARSCoV-2 infected that received two doses of mRNA vaccine Pfizer-BioNTech or Modern-1273) consisted of serial samples from 12 subjects (7 female and 5 male) collected from October 2020 to September 2021. These individuals had been confirmed with SARS-CoV-2 infection between 30 to 150 days (median 75 days) prior to providing their baseline sample and receiving the first Pfizer-BioNTech or Moderna-1273 dose. From each of these subjects three samples were collected: sample-1 (baseline) was collected prior the first vaccine dose was administered, sample-2 was collected at a median of 21.5 days after the second Pfizer-BioNTech or Moderna-1273 dose and sample-3 was collected at a median of 96 days after the second Pfizer-BioNTech or Moderna-1273 dose (
Table 2). Cohort-3 (no-previous SARS-CoV-2 infection-multiple mRNA vaccinations) was allotted into two sub-cohorts. Cohort-3a comprised serial samples from four subjects (3 female and 1 male) that had not been infected with SARS-CoV-2, which was evidenced by the repeated absence of anti-SARS-CoV-2 IgG or IgM antibody testing and negative RT-qPCR. These subjects were followed-up for almost two years and received two doses of the Pfizer-BioNTech vaccine, a third dose (booster) and the bivalent vaccine. From each subject six samples were collected as follow: sample-1 was collected at baseline (prior to vaccination), sample-2 was collected at a median of 20 days after the second dose, sample-3 was collected at a median of 31 days after the third dose, sample-4 was collected at a median of 277.5 days (~9 months) after the third dose, and sample-5 and sample-6 were collected at a median of 30 and 180 days (6 months) after the bivalent vaccine, respectively. The cohort-3b included a single specimen from eight individuals (4 female and 4 male) that received multiple vaccinations. These subjects had no previous documented SARS-CoV-2 infection prior to receiving the Pfizer-BioNTech vaccine. Among these individuals four of these individuals had received three vaccine doses, two subjects had received four doses and two others had received a combination of three doses and the bivalent vaccine. Six of these subjects got infected between 90 to 365 days (median 240 days) following the third dose. The samples used in this study were collected between 351 to 723 days (median 634 days, (1.7 years) after receiving the last vaccination (
Table 2). Cohort-4 consisted of five individuals with inflammatory bowel disease (IBD) who had not been previously infected with SARS-CoV-2, a status confirmed through periodic RT-qPCR testing. These subjects were on treatment with immunomodulators and/or biologics and, at the time of samples collection they were in remission. Four samples from each individual were included in the present study: sample-1 was collected prior vaccination (baseline), sample-2 was collected at a median of 17 days after the second dose, sample-3: was collected 60 days after the third dose, and sample 4 was collected at a median of 180 days after the third dose (
Table 2).
The subjects from Cohorts-2 and 3 were all adults (>21 years-old) and were collected by the Virology Laboratory of the University of Puerto Rico-Medical Sciences Campus. These individuals were all volunteers participating in the IRB approved clinical protocol “Molecular Basis an Epidemiology of Viral Infections circulating in Puerto Rico”, Pro0004333, which was approved by Advarra IRB on April 21, 2020. The subjects from Cohort-4 were recruited at the University of Puerto Rico IBD Clinics and were all subjects diagnosed with Crohn’s disease (CD) or ulcerative colitis (UC) that were in remission and on treatment with biologic and/or immunomodulatory therapy. The samples were collected by the University of Puerto Rico Gastroenterology Unit between April-2021 to July-2022 and donated deidentified for the present study. An informed consent form and a study questionnaire also approved by IRB was administered to the volunteers.
The Cohort-5 (pre-pandemic samples) consisted of 125 serum samples from deidentified adult donors from which 78 were from healthy subjects and 47 from subjects with other common respiratory viral infection allergies affecting the Puerto Rican population (
Table 1). The samples from healthy subjects had been collected in 2012 and stored at the sample bank of the Immunology and Molecular Parasitology Laboratory of University of Puerto Rico-Medical Sciences Campus (UPR-MSC). Samples from individuals with respiratory allergies or viral infections were collected during 2019 and banked at the Virology laboratory of UPR-MSC or were kindly donated by the Center for Disease Control and Prevention (CDC) Dengue Branch, San Juan, PR. These samples included 13 specimens from subjects with a history of respiratory allergies, 5 from subjects with Zika-IgM positive diagnosis, 5 from subjects with Dengue virus-IgM positive diagnosis, 12 subjects with RT-qPCR Influenza A/B positive diagnosis, 6 from subjects with Respiratory Syncytial Virus (RSV)-IgM positive diagnosis, and 6 samples from individuals with positive diagnosis for Mycoplasma-IgM. All samples included in the present study were stored at -80
oC and the aliquots tested had not been thawed prior to testing.
2.3. Detection of anti-SARS-Cov-2 IgG antibodies.
Total IgG antibodies to SARS-CoV-2, were using a pre-established in-house ELISA that was optimized through checkerboard titration as previously described [
28]. Briefly, 96-well plates were coated overnight with 100µl/well of recombinant spike (S1)-RBD protein at a concentration of 2.5 µg/ml. The unbound spike-RBD was removed by washing thrice with 300 µl/well of phosphate buffered saline containing 0.05% Tween-20 (PBST). Non-specific binding was blocked by adding blocking buffer (5% skim milk in PBST) 300 µl/well and incubating for 30 min at 37
oC. After incubation blocking solution was removed by suction. The serum or plasma samples were diluted 1:100 in PBST and added in duplicate to the plate (100 µl/well). The blocking buffer was used as blank wells. Plates were washed three times after an incubation of 30 minutes at 37
oC. Then, the secondary antibody anti-human IgG antibody peroxidase (1:10,000) diluted in blocking solution was added to each well (100 µl/well) and incubated for 30 min at 37
oC. After another washing step, the peroxidase reaction was visualized by adding the substrate solution (100µl/well) 0.1M citrate phosphate buffer pH5.0, containing 20 mg
o-phenylenediamine hydrochloride and 30% H
2O
2. The reaction was incubated in the dark at room temperature for 15-20 minutes and stopped by adding 50µl/well of 1N hydrochloride acid. Absorbance at 492nm (OD
492) was measured with a spectrophotometer. The OD
492 of blanks were subtracted from the corresponding OD
492 values of each sample before data analysis. As positive and negative controls we used anti-SARS-CoV-2 IgG immunoglobulin purified by affinity chromatography from plasma donors with high titers of IgG as previously described [
7].
2.4. Detection of SARS-Cov-2 IgG subclasses.
For determining levels of IgG-subclasses we followed the same optimized protocol used for the in-house IgG ELISA described above replacing the secondary antibody for either anti-IgG1-, anti-IgG2-, anti-IgG3- or anti-IgG4-HRP conjugated diluted 1:3,000 in PBST as it was previously reported [
3], which were incubated for 30 minutes at 37
oC. The substrate solution was 0.1M citrate phosphate buffer pH5.0, containing 20 mg
o-phenylenediamine hydrochloride and 30% H
2O
2 and the readings were done at 492nm.
2.5. Neutralization Assay
For neutralizing activity, we used the cPass
TM SARS-CoV neutralization antibody detection kit (GenScript, Piscataway NJ) [
29], which correlates perfectly with the traditional PRNT [
7]. The assay was used to measure inhibitory capability based on the ability of the antibodies to target the interaction between the host ACE2 receptor and viral receptor-binding domain (RBD). Briefly, samples (serum or plasma) were diluted in the sample dilution buffer according to manufacturer’s instructions and incubated with either soluble SARS-CoV-2 RBD-HRP from Wild type strain or the RBD-HRP of three variants of concern: Alpha (B.1.1.7), Delta (B.1.617.2) and Omicron (B.1.1.529). The mixtures were incubated for 30 minutes at 37
oC, which permit the interaction and binding of antibodies with neutralizing capacity to the corresponding RBD-HRP conjugate. Following incubation, each reaction mixture is then added to a 96-well capture plate coated with human ACE-2 protein. Thus, RBD-HRP from each single variant complexed with antibodies cannot bind to the coated ACE-2 protein and are then removed in a subsequent wash step. The reaction is developed with tetramethylbenzidine (TMB) followed by a stop solution allowing the visualization of bound RBD-HRP to the ACE2. Since this is an inhibition assay, color intensity is inversely proportional to the number of neutralizing antibodies present in the samples. Data were interpreted by calculating the percent of inhibition of RBD-HRP binding calculated as follows: Percentage of inhibition = (1-OD value of sample / OD value of background) x 100%. Samples with percentage of inhibition ≥ 30% in a surrogate virus neutralization test (sVNT%) indicate an effective viral variant neutralization capacity.
2.6. Statistical analysis
All antibody determinations were done in duplicate and the results were reported as mean absorbance for each determination and each experiment was replicated twice. ROC (receiving operating characteristic) curve was generated to stablish the cut-off value of all assays using EpiTools epidemiological calculator (
http://epitools.ausvet.com.au). The area under curve (AUC) as a measure of how well our in-house ELISAs discriminate between positive and negatives was analyzed according to Hosmer et al. 2013 [
30] as follows: totally random (AUC = 0.5); poor (0.5 < AUC < 0.7); acceptable (0.7
AUC < 0.8); excellent (0.8
AUC < 0.9) and outstanding (AUC
0.9). Calculation of positive and negative percent agreement (PPA and NPA) and overall rate of agreement (ORA) between the in-house ELISA method and the RT-qPCR method was determined as reported by Obermier et al. 2016 [
31]. Deming regression analysis [
32] was performed on 10 paired plasma and serum specimens collected from the same individual to demonstrate the equivalence in the assays results for both specimens. For correlation between the absorbance values obtained for IgG-subclassess with the sVNT% obtained against SARS-CoV-2 variant of concern as well as the agreement between the sVNT% and the ELISA results was made with a Pearson correlation coefficient (with 95% CI) and the Cohen’s Kappa coefficient (κ) [
33,
34], respectively. The κappa values were considered as follows: slight agreement (κ = 0.01 to 0.2); fair agreement (κ = 0.21 to 0.40); moderate agreement (κ = 0.41 to 0.60); substantial agreement (κ = 0.61 to 0.80); almost perfect agreement (κ = 0.81 to 1.0) [
35]. All statistical analyses were performed in GraphPad Prism 9.
3. Results and Discussion
Immunity against SARS-CoV-2 is molded by various factors including the genetic background of the population [
36] and the manner in which our immune system responds to the evolving of new SARS-CoV-2 variants, each one with greater immune evasion capability. Therefore, the immunity against SARS-CoV-2 cannot be considered as a singular state. Nowadays a wide range of naïve, natural and hybrid immune states exist, which include unvaccinated individuals, those convalescing from a prior infection, those that recover from one or more prior infections, infected fully vaccinated individuals, naïve to infection vaccinated individuals with or without booster injections, and those that have received multiple vaccinations and that suffer of comorbidities. Although the humoral response does not drive the anti-viral response, the measurement of serum antibody levels, especially IgG, is globally accepted as a good marker for assessing post-COVID-19 immunity or vaccine-induced immunity [
37]. To study the longitudinal evolution of the antibody response in our Latino-population we developed an in-house ELISA, which was validated with a cohort of 85 specimens from convalescent subjects that were infected at the beginning of the pandemic. At present time, with more than 80% of the worldwide population vaccinated and/or exposed to constant breakthrough infections, it is a formidable challenge to configure a substantial Latino-cohorts that exclusively responds to just one of the immune states mentioned above. Despite this limitation we consider relevant the findings of this study, which are described below.
3.1. In-house IgG and IgG-isotypes ELISA performance assessment
In the in-house anti-SARS-CoV-2 IgG ELISA we used SARS-CoV-2 spike (S1) Receptor Binding Domain (RBD) as antigen. Previous studies have demonstrated that S1 domain is the most specific antigen for diagnosis of COVID-19 while the RBD exhibits greater sensitivity, particularly in diagnosing patients with mild infections [
38]. The N-protein of SARS-CoV-2 and S2 domain may not be the optimal targets for the diagnosing COVID-19 because of their high levels of cross-reactivity with the spike protein of SARS and MERS-CoV [
39]. Therefore, we tested the S1-RBD protein of SARS-CoV-2 as the target antigen for our in-house IgG ELISA. To establish the optimal positive cut-off for detecting the levels of anti-SARS-CoV-2 IgG antibody in serum/plasma of COVID-19 convalescent subjects we created an ROC curve. For the positive control group, we used a cohort of 85 specimens (cohort 1) from COVID-19 convalescent subjects that had been confirmed by RT-qPCR. These subjects had not been hospitalized, either because they had mild symptoms, were asymptomatic or had been diagnosed through the active contact-tracing program established by the Department of Health of the Commonwealth of Puerto Rico [
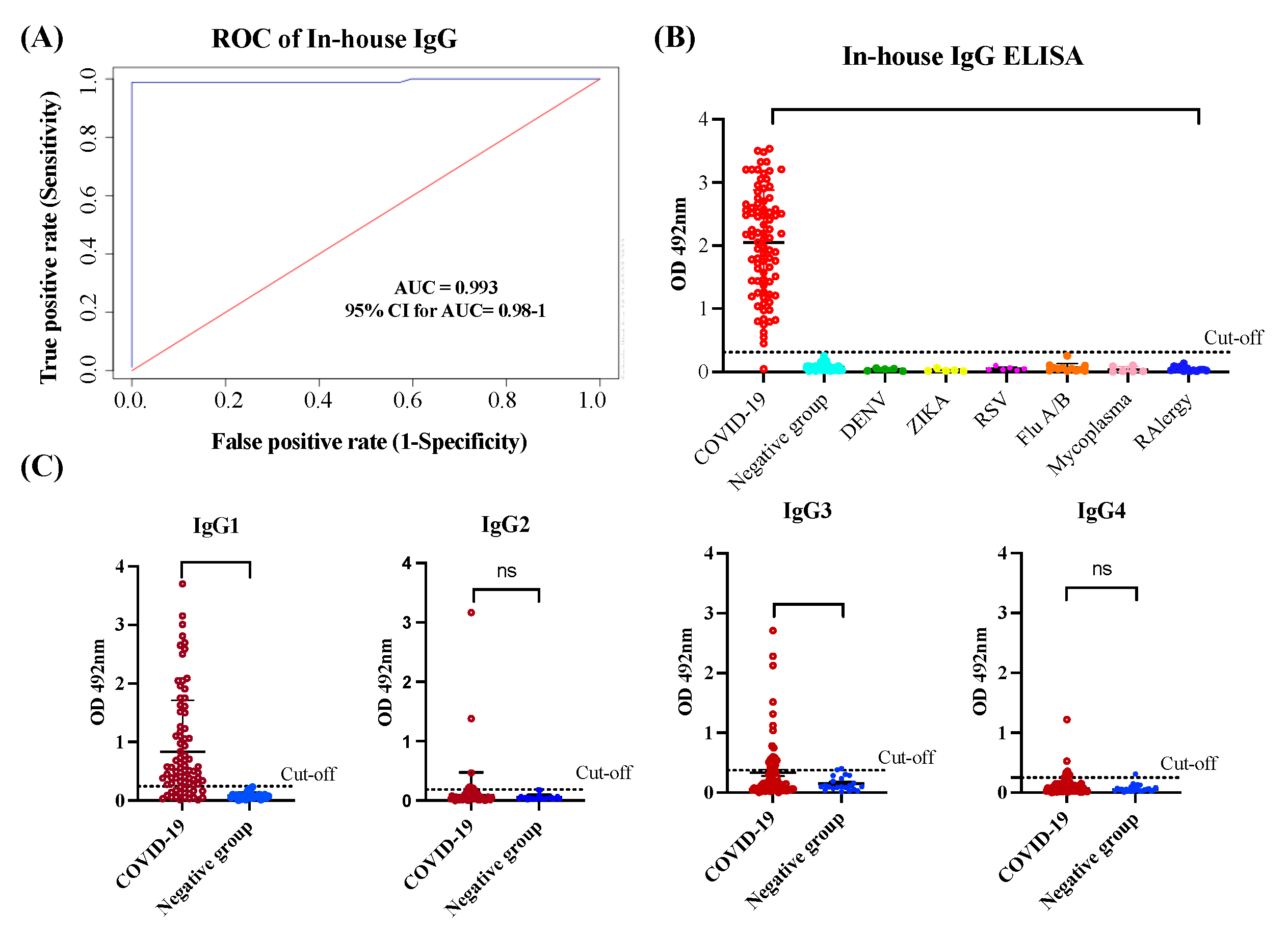
2]. For the negative control group, we used a cohort of 125 specimens from healthy subjects or subjects that had been diagnosed with other respiratory or viral infections collected several years before to the pandemic (Cohort 5). The ROC curve analysis revealed that the in-house IgG ELISA has outstanding diagnostic performance with an area under curve (AUC) value of 0.993 (95% confident interval (95% CI: 0.98-1) (
Figure 1A). The OD distribution for total IgG of validated samples is shown in a scatter plot with the cut-off lines
. We found that OD values 5-fold above the mean OD negative control (OD >0.312) could be used as a positive threshold. Based on this threshold 84 out of 85 (98.8%) RT-qPCR positive samples were identified as seropositive. Significant differences (
p<0.0001) were found between the mean OD values of the positive group and the negative group, and no cross-reactions were detected with samples from healthy subjects or carrying respiratory or other viral infections (
Figure 1B). These results correspond with a positive percentage agreement (PPA) between the RT-qPCR and the in-house IgG ELISA of 98.89%, a negative percentage agreement (NPA) of 100.00%, and an overall rate agreement (ORA) of 99.53%, (Cohen’s
=0.990). Moreover, Deming regression analysis revealed a lineal correlation between the results reported on serum and plasma specimens (
p<0.0001), demonstrating that the specimen type, whether serum or plasma, does not make a difference in the in-house IgG ELISA results (
Figure S1-AB).
We also assessed the reproducibility of the assay by calculating the coefficient of variation (CV) of three different assays, 30 repeats of controls and selected negative and positive samples. The intra-assay and inter-assay reproducibility values were all lower than 10% (
Figure 1S-C). Such an excellent diagnostic performance was further confirmed when the in-house IgG ELISA was validated with excellent results along other 27 in-house ELISAs, 13 multiplex assays and 12 different commercial serological methods developed across the globe as part of the serological science network (SeroNet) for COVID-19 established in October 2020 by the National Cancer Institute (NCI) [
40].
The in-house-ELISA for detecting IgG1, G2, G3 & G4 isotypes were developed on the same platform described above for total IgG. To establish the thresholds, we tested 85 samples from COVID-19 subjects and a subset of 20 samples selected at random from the cohort 5 that included 10 samples from healthy subjects and 10 from subjects carrying other viral infections. We found that OD values 4.56-fold above the mean OD negative control (OD > 0.242) could be used as a positive threshold for determining levels of IgG1, OD values 3.44-fold above the mean OD negative control (OD >0.186) could be used as a positive threshold for determining levels of IgG2, and OD values 3.31-fold above the mean OD negative control (OD >0.375) or 4.1-fold above the mean OD negative control (OD >0.250) could be used as a positive threshold for determining levels of IgG3 and IgG4, respectively (
Figure 1C
). Based on these thresholds, IgG1 was detected in 62 of the 85 samples (72.94%) of convalescent COVID-19 subjects, IgG3 was detected in 25 of the 85 samples (29.41%) whereas IgG2 and IgG4 were detected in only 7 (8.23%) and 8 specimens (9.41%) of the 85 samples, respectively. The IgG1 was not only the most frequent isotype detected but also the one with the highest levels, as indicated by the OD values, which ranged between 0.29 and 3.156 (mean OD=1.115
0.86). OD values for IgG3 ranged between 0.379 and 2.28 (mean OD= 0.881
0.63). Hence, the average OD for IgG1 was 1.27-fold higher than IgG3 and significant differences (
p<0.0001) were found between both isotypes. These results are consistent with the notion that IgG1 and IgG3 isotypes are typically elicited in response to viral infections [
18,
41] and can be considered prominent markers of late-stage post-infection after the primary SARS-CoV-2 infection. Therefore, IgG1 and IgG3 are the main contributors to the IgG titers commonly reported in the serological assays for COVID-19 (
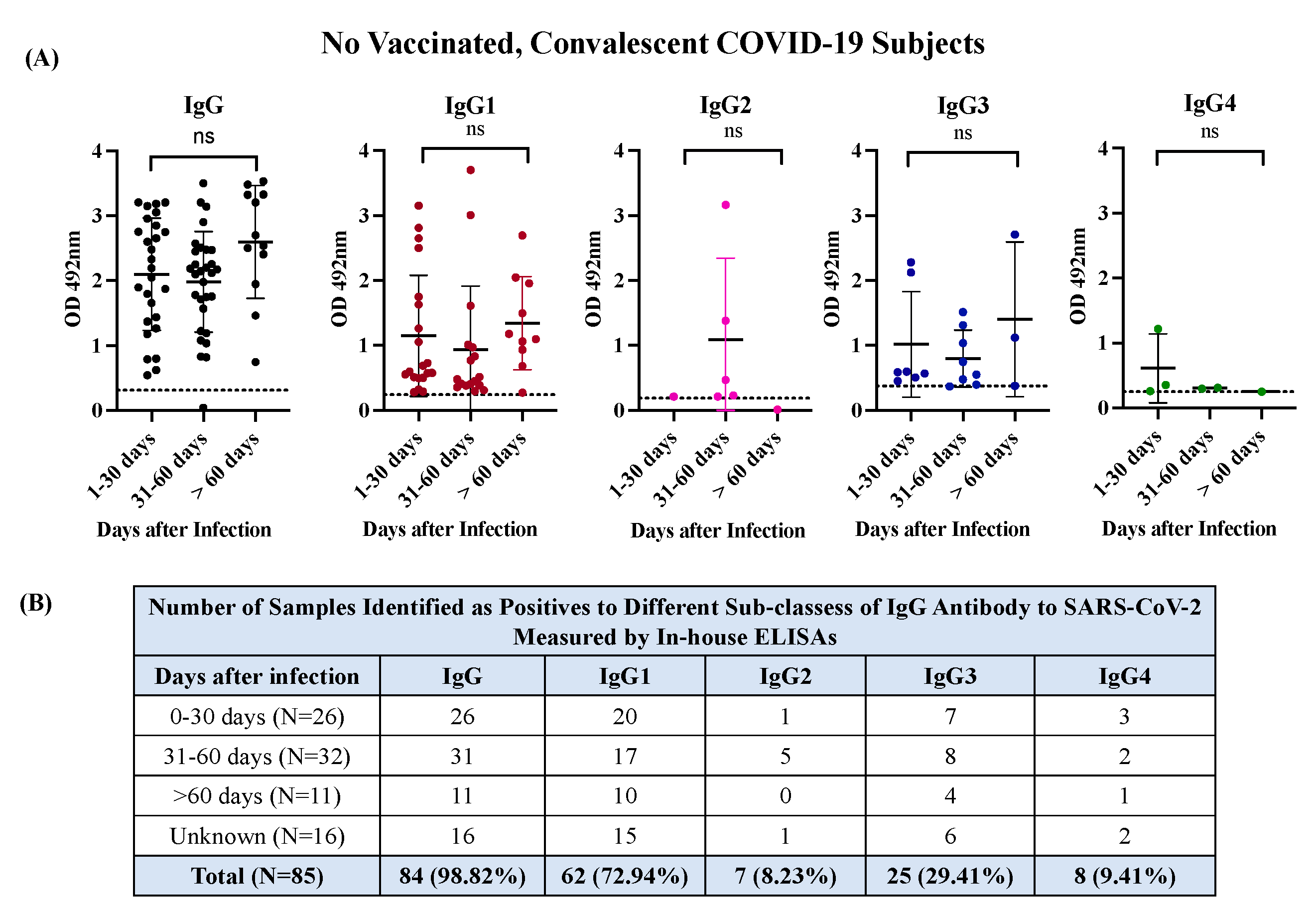
Table S1). Moreover, the observation that most specimens collected between 0 to 30 days of infection (79.6%) or at >60 days of infection (90.9%) resulted positive for IgG1 or IgG3 (
Figure 2) indicates that convalescent subjects develop high levels of these antibody isotypes early in the course of infection and remain at detectable levels for several months after recovery. This is an important finding since none of the subjects in this study required hospitalization and most existing knowledge regarding the antibody response against SARS-CoV-2 has been collected from hospitalized individuals that survived to various grades of COVID-19 disease severity, where the magnitude of the antibody response to a SARS-CoV-2 infection correlates with the severity of the disease [
42].
In contrast, aside from two samples that resulted highly immunoreactive for IgG2 (OD=1.383 and 3.166, respectively) the other five positive samples showed OD values very low, which ranged between 0.211 and 0.469 (mean OD =0.267
0.1). For IgG4, only one specimen had a high OD (OD=1.22), and the other seven seropositive had OD values barely over the threshold, which ranged between 0.253 and 0.542 (mean OD=0.336
0.0.09) (
Table S1). Thus, not only were IgG2 and IgG4 the less frequent antibody isotypes but also the less immunoreactive isotypes against S1-RBD of SARS-CoV-2, which is consistent with the reports of other authors [
41]. The IgG2 and IgG4 antibody isotypes are not commonly part of the antibody response to viral infections. IgG2 plays a relevant role in the response to bacterial infections whereas that IgG4 has a role in the response to allergens, helminth infections or therapeutically administered proteins [
43]. The IgG isotypes pattern observed in the present study seems to be slightly different from those reported for symptomatic patients who experienced different degrees of disease severity. In such patients, the presence of the four IgG isotypes was detected during the acute infection with IgG3 being the isotype most associated with the initial phase and severity of the disease, while IgG1 and IgG2 were the isotypes most associated with an upsurge in the latest stage of the disease and with the weakest correlation with disease severity [
42].
3.2. The antibody response in unvaccinated convalescent COVID-19 subjects is dominated by IgG1 and IgG3 isotypes, which neutralize the Wild-type strain and the VOC Alpha and Delta but are poorly effective against Omicron.
Once the IgG isotype profile in the cohort of convalescent unvaccinated COVID-19 subjects (Cohort-1) was characterized, we used a surrogate virus neutralization assay to determine the neutralizing activity of these specimens against the Wild-type SARS-CoV-2 and the VOC Alpha (lineage B.1.1.7), Delta (lineage B.1.1617.2), and Omicron (lineage B.1.1.529), which had broad circulation and predominance in the Puerto Rican population [
44]. We found that 83 of 84 seropositive specimens (98.8%) had virus neutralizing percentage (sVNT%) against the Wild-type (WT) strain that ranged between 33 to 97% (median = 82%). One seronegative specimen and another seropositive to IgG failed in showing neutralizing activity. The seronegative specimen, which also resulted negative to IgM (data not shown) and negative to other IgG isotypes, had been collected ~38 days after the positive RT-qPCR (
Table S1). Presumably, this subject harbored a low viral load leading to the immune system clearing the infection prior to mounting an appropriate immune response. This circumstance could account for the difficulties encountered in detecting IgG antibodies in this specimen. However, it is possible that the RT-qPCR result could be a false positive, since several molecular assays have been developed with high specificity and low limits of detections, suggesting that the test could have detected minimal amounts of the virus, leading to a false positive result [
45,
46,
47,
48]. Interestingly, 63 of 85 specimens (74.11%) showed detectable sVNT% against the Alpha variant in the range of 30 to 93% (median= 58.0%) and 73 of 85 samples (85.9%) showed sVNT% in the range of 32 to 90% (median =64%) against the Delta variant. In contrast, the sVNT% against Omicron was significantly low, being detected only in 3 of 85 specimens (3.53%) with sVNT% barely over the threshold (31 to 41%, median=31) (
Table S2). When the performance of the in-house IgG ELISA was compared with the neutralization assay in their capacity to detect seropositive specimens with detectable sVNT% a substantial agreement (98.87%,
appa value=0.6615) was found for the WT strain, a slight agreement was found for Alpha (75.29%,
appa=0.0659) and Delta (69.41%,
appa= 0.0498) variants and no agreement was found for the Omicron variant. We also found a slight agreement between sVNT% against the WT strain and the number of seropositive in the IgG1-ELISA (76.47%,
appa=0.0502), a fair agreement against Alpha (72.94%,
appa= 0.2608) and no-agreement against Delta or Omicron. Similarly, the IgG3-ELISA showed slight agreement with the sVNT% against the Wild-type strain (30.58%,
appa=0.0187), Alpha (48.23%,
appa=0.1515), or Delta (50.58%,
appa=0.1471) and has no agreement against Omicron (
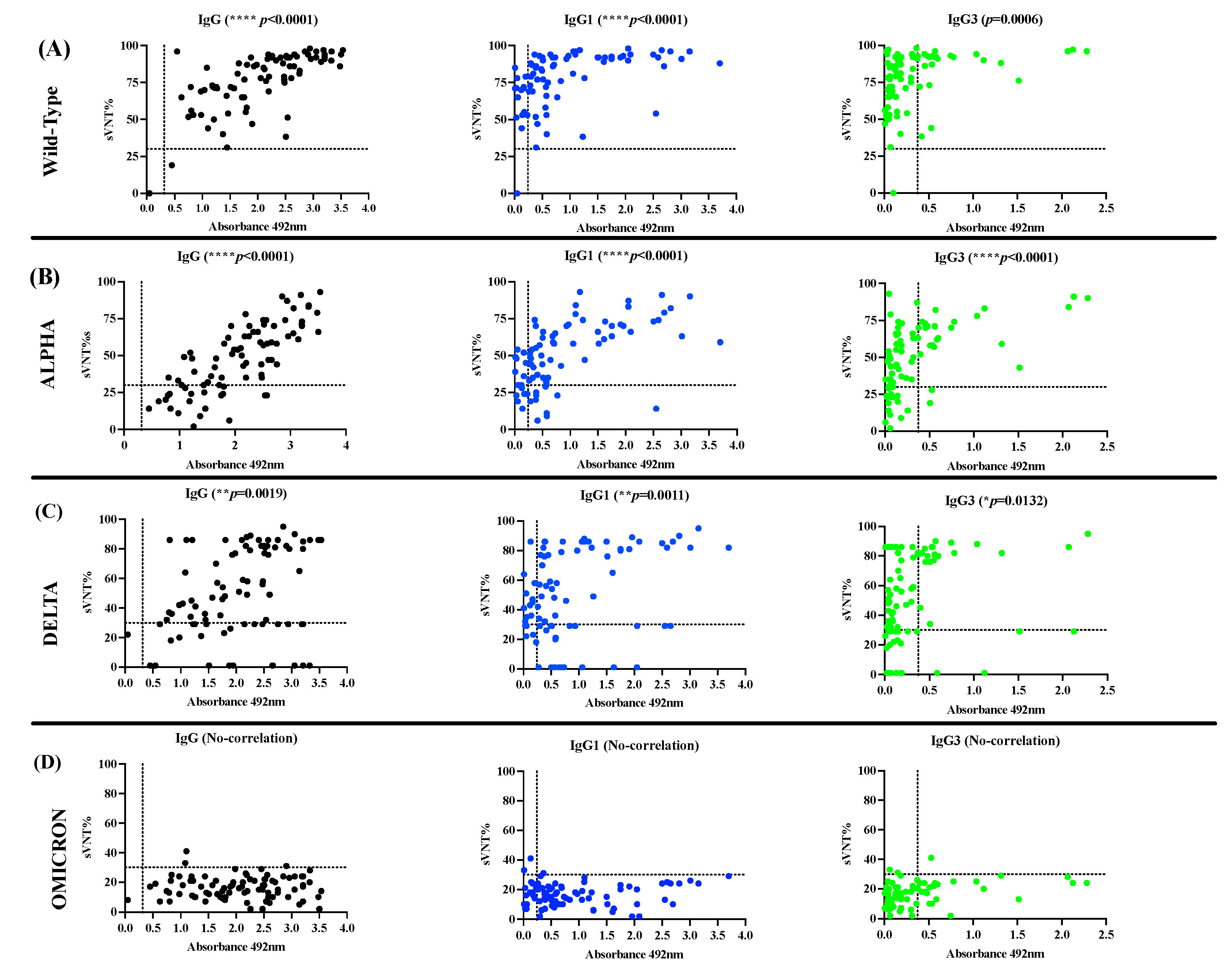
Table 3). Consistent with these results, the magnitude of total IgG, or IgG1 and IgG3 levels measured as average OD values above the established threshold for each isotype correlated positively with the percentages of neutralization (sVNT%) against WT (
p<0.001, IgG & IgG1,
p=0.0006 IgG3), Alpha (
p<0.0001, IgG, IgG1 & IgG3) and Delta variants (
p=0.0019, IgG,
p=0.011, IgG1,
p=0.0132, IgG3) (
Figure 3) and did not correlate with the number of seropositives or the levels of IgG2 or IgG4 for any of SARS-CoV-2 VOC analyzed.
The observation that most samples from COVID-19 convalescent subjects exhibited remarkably high neutralizing percentages against the WT strain was expected. This is attributed to the fact that these specimens were collected at the beginning of the pandemic at the time that a wide diversity of lineage B.1.x variants, emerged from the original Wuhan strain, were circulating in Puerto Rico [
44]. Due to the fact that VOC Alpha and Delta, which emerged and subsequently accumulated a relatively small number of mutations in their genome, especially in the spike protein, enhancing their fitness and pathogenicity [
49,
50], it was not surprising that the antibodies elicited against the original strain could still partially neutralize these variants as well. In our study, these antibodies showed a median of 54% effectiveness against Alpha and a median of 65% effectiveness against Delta. However, the antibodies elicited against the original strains became completely ineffective against Omicron, which is the heaviest mutated VOC [
51] that replaced Delta and any other variant in circulation.
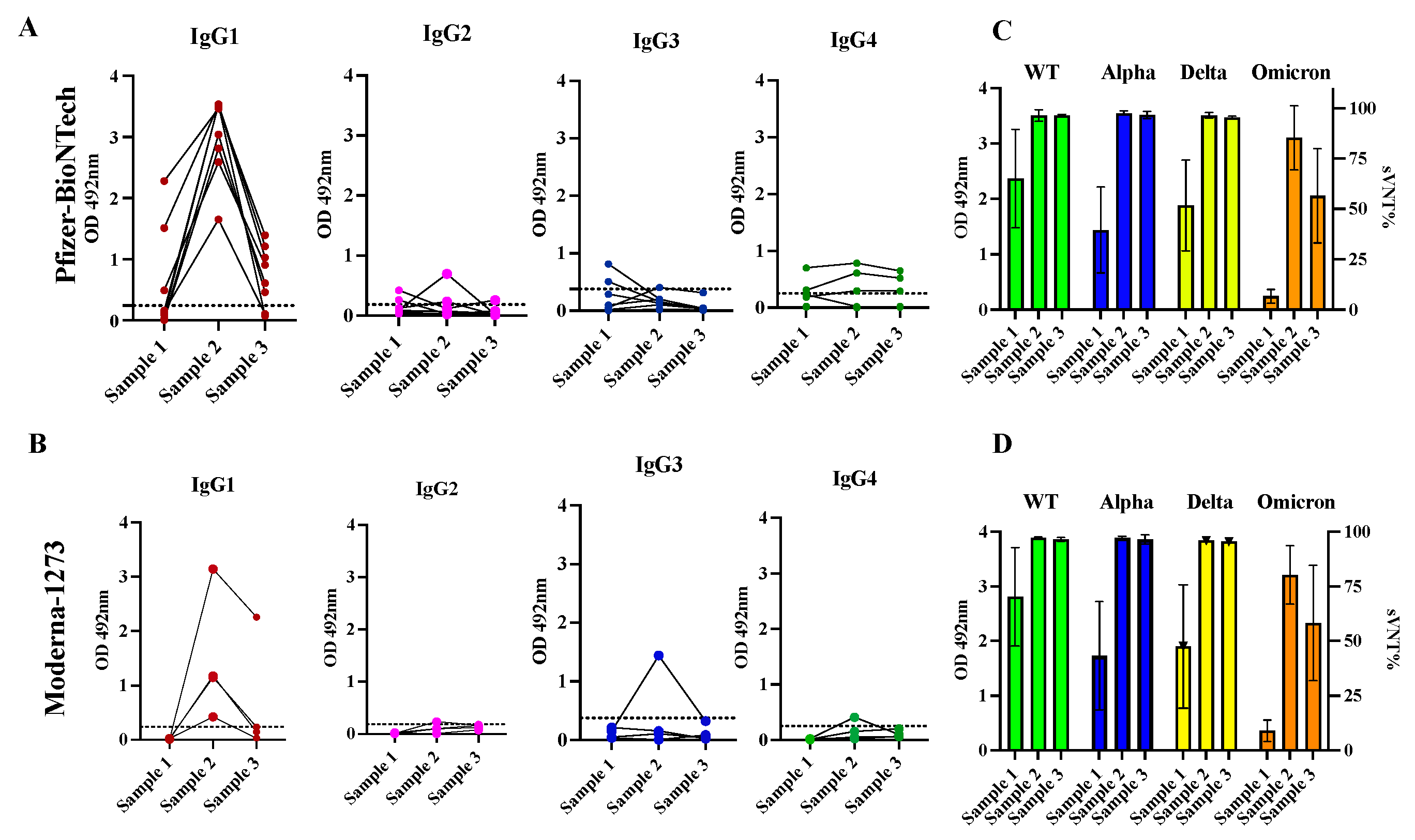
3.3. The antibody response in previously infected subjects that received two doses of Pfizer-BioNTech or Moderna-1273 is dominated by the IgG1 isotype that although declined fast, exhibited potent neutralizing activity against the VOC Alpha, Delta, and Omicron.
Pfizer and Moderna vaccines contain synthetic mRNA molecules with the coding sequence necessary to build the SARS-CoV-2 Spike protein encased in a lipidic nanoparticle that allow the vaccine delivery of mRNA to cells. Thus, this protein can be synthesized inside the host cell, mimicking a natural infection with SARS-CoV-2 [
52]. To determine if the administration of two doses of mRNA vaccines could modify the IgG subclass profile established by a previous natural infection, we selected a cohort of 12 convalescent subjects that received a full course of two doses of Pfizer-BioNTech (n=8) or Moderna-1273 (n=4) vaccine (cohort 2). The two doses were given at an interval of three to four weeks. From each subject we analyzed a sample collected at baseline (sample 1) and two subsequent samples collected after a mean of 21.5 days (sample 2) and 96 days (sample 3) after the second dose, respectively. At baseline, all specimens but one had detectable levels of IgG antibody levels. Considering that all these subjects had a single documented previous infection at a mean of 150 days (5 months) prior to receiving the first dose of the vaccine, it is plausible to suggest that the antibody response to the natural infection was long-lasting and did not wane as fast as has been reported by other researchers [
53,
54]. At baseline, a total of 3 specimens (25.0%) had detectable levels of IgG1, and two specimens had IgG3 (16.6%) that ranged between 0.49 and 2.281 (mean OD=1.427
0.733) and between 0.509 and 0.813 (mean OD=0.661
0.152), respectively. The IgG2 and IgG4 were only detected in a single specimen at very low levels. It was interesting to find that the antibody response detected after the second dose was exclusively dominated by the IgG1 isotype in all specimens with OD values ranging between 1.651 and 3.536 (mean OD=3.017
0.619) in those vaccinated with Pfizer-BioNTech and between 0.426 and 3.144 (average OD=1.475
1.00) in the subjects vaccinated with Moderna-1273 vaccine. A single subject who received the Pfizer vaccine had detectable levels of IgG2, while another subject who received the Moderna vaccine had detectable levels of IgG3. However, at a mean of 96 days following the second dose (sample 3) the levels of IgG1 were undetectable in two subjects who received Pfizer-BioNTech vaccine and in three subjects who received Moderna-1273 vaccine. In the other subjects the levels of IgG1 dropped to an average of OD=0.723
0.460 or OD=0.669,
0.92 for those vaccinated with Pfizer or Moderna, respectively. Levels of IgG4 were markedly low or undetectable during the entire follow-up course (
Figure 4 and
Table S3).
As we reported before, most specimens (91.66%) collected at baseline from these 12 subjects showed detectable neutralization percentages (sVNT%) against WT (average of 66.75%
23.96), 58.33% showed detectable sVNT% against Alpha (42.33%
22.27), 83.3% against Delta (50.33%
24.69) and no specimens had detectable sVNT% against Omicron. After receiving the second dose of vaccine, the longitudinal duration of neutralizing activity was similar for subjects that received Pfizer or Moderna. In both groups the sVNT% increased notably at >95% against all variants tested including Omicron. However, about 96 days following the second dose, these levels significantly declined against Omicron (average 57.58%
23.44) in the subjects that received Pfizer or Moderna vaccine (
Figure 4). No subjects reported having breakthrough infections during the follow-up period. It is possible that the natural immunity boosted by two doses of mRNA vaccines contributed to preventing reinfections, although the antibodies declined over the same period, which is consistent with other studies [
55]. A case-control study performed in Qatar found that the natural infection was 90.2% effective (95% CI 60.2-97.6) in preventing reinfections with the Alpha variant, 85.7% effective against the Beta (95% CI 75.8%-91.7), 92% effective against Delta (95% CI 87.9-94.7), but was notably reduced against Omicron (56%, 95% CI 50.6-60.9) [
56]. The observation that in some subjects the levels of antibodies declined faster than the neutralizing activity (
Figure 4), reinforces the notion that the function of the antibodies measured as their capacity to binding to the RBD-Spike protein and preventing the entry of the SARS-CoV-2 virus to the cells is more reliable than the quantity of antibodies produced (measured as average of OD) to monitor the antibody response against infections and vaccinations [
7]. The observation that the durability of neutralizing capacity induced by both vaccines against the Omicron decline faster is also consistent with other studies [
57]. Pfizer-BioNTech and Moderna-1273 were designed against the original SARS-CoV-2 strain and these vaccines produce antibodies with a substantially reduced ability to recognize and block the entry of the Omicron spike protein which harbors numerous mutations [
58].
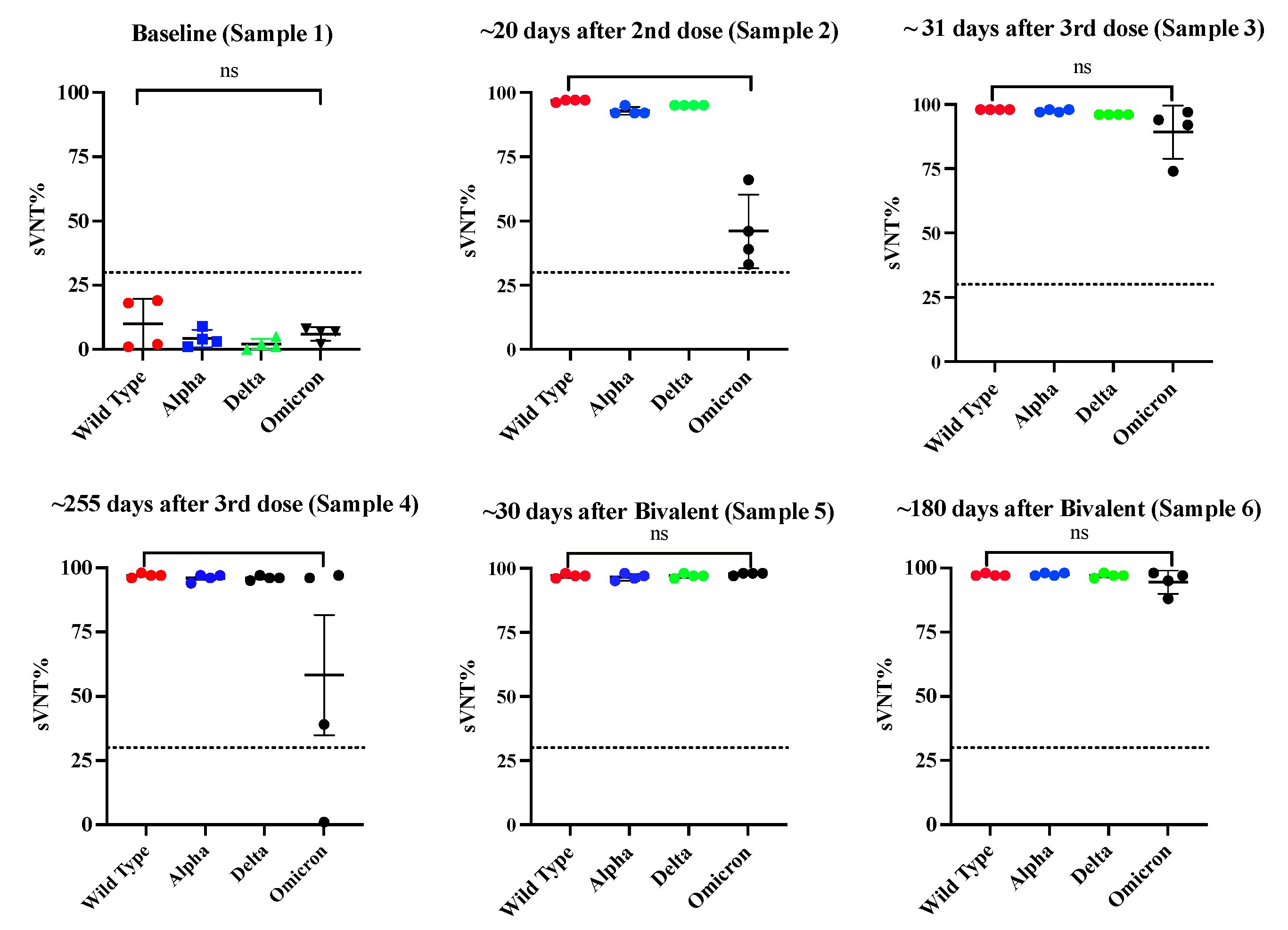
3.4. Class switch toward IgG4 occurs in subjects that receive multiple doses of Pfizer-BioNTech vaccine, which is sustained over the time.
Because the sterilizing protection offered by the existing immunization schedule against the Omicron lineage is minimal [
59], the Pfizer-BioNTech and Moderna-1273 vaccines were updated to include a monovalent (single) component that corresponds to the Omicron variant XBB.1.5. Furthermore, the U.S. Food and Drug Administration (FDA) authorized Moderna and Pfizer-BioNTech the use of a single Bivalent vaccine as a booster dose, which contains two mRNA components of SARS-CoV-2 virus, one from the original strain and the another from a common component found in the BA.4 and BA.5 lineages of the Omicron variant of SARS-CoV-2 (
https://www.fda.gov/about-fda/about-website/fdagov-archive). To determine whether the administration of multiple vaccinations could modify the profile of antibody isotypes induced by the initial two doses of Pfizer-BioNTech vaccine we followed up a small group of four subjects (Cohort 3a) for almost two years and collected samples at different time points following the second dose and the third dose and the administration of bivalent vaccines. Before receiving the first dose, these subjects had not been previously exposed to SARS-CoV-2. As it was expected, at baseline, every subject had detectable anti-SARS-CoV-2 antibodies (Sample 1). The initial two vaccinations were administered with a three-week interval and the third vaccination (booster dose) was administered about eight months after the second dose. About 12 months after the booster dose, all subjects received the bivalent vaccine. As described before, IgG1 (average OD= 3.26
0.135) and IgG3 (average OD = 2.97
0.889) were the dominant isotypes shortly after the second dose (Sample 2) with an absence of IgG2 or IgG4 (
Figure 5A,
Table S4). Our results are consistent with reports from others, indicating that IgG1 and IgG3 specific to the spike protein of SARS-CoV-2 and its receptor binding domain (RBD) emerge as the predominant antibodies subclasses [
21] in individuals who are both infected and vaccinated. Pfizer and Moderna vaccines contain synthetic mRNA molecules with the coding sequence necessary to build the SARS-CoV-2 Spike protein encased in a lipidic nanoparticle that is delivered to the cells. This allows the the protein to be synthesized within the host cell, mimicking a natural infection with SARS-CoV-2 [
52]. Both, IgG1 and IgG3 are typically elicited in response to viral infections [
18].
Of note, after the second vaccination all samples showed very high sVNT% (>95%) against the WT, Alpha, and Delta variants and reduced sVNT% (46.0%) against Omicron (
Figure 6). However, an intriguing observation was that the specimens of these subjects had 1.85-fold less neutralization capacity against Omicron than specimens from Cohort 2 (previously infected, fully vaccinated with Pfizer), which at a similar time point after the second dose (~20 days) had an average sVNT% of 85.25%, and these differences were found significant (
p=0.0028). Moreover, the observation that the subjects of cohort 2 at a mean of 96 days after the second dose still had 1.24-fold more sVNT% (average 52.2%) against Omicron than the Cohort 3a at 20 days indicates that the protection induced by the Pfizer-BioNTech vaccine wanes faster than the protection induced by a combination of natural infection plus vaccination. Interestingly, at a mean of 31 days after receiving the third dose (sample 3), IgG1 and IgG3 were no longer the dominant antibody isotypes. The IgG1 levels declined to an average of OD= 1.35
0.422, which are 2.41-fold lower than those detected after the second dose, whereas IgG3 was undetectable in most of the subjects. The IgG1 levels continued declining and at a mean of 277.5 days (~9 months) after the third dose (sample 4) they were barely detected in two subjects at very low levels (average OD = 0.565
0.433). Intriguingly, after administering the third dose (a mean of 34 days), the IgG4 subclass, considered as an anti-inflammatory antibody [
60] reached levels notably higher than the lower limit of quantification in the sera of all vaccinees (average OD= 3.615
0.445) and remained at almost identical levels (average OD= 3.574
0.506) at the subsequent sampling that occurred about 9 months later (
Figure 5A). The third dose also elicited detectable levels of IgG2 in three subjects (75%) at low levels (average OD =0.356
0.216) around 30 days after being administered, but these levels became undetectable thereafter. The third dose also boosted the sVNT% against Omicron at a range of 74 and 97% (median 93%), but these levels dropped again to an average of 67.5% around 9 months after this third dose (
Figure 6). It was interesting the observation that during the time elapsed between the second and the third dose none of the subjects reported being naturally infected. However, about 3 to 4 months after receiving the third dose, two subjects reported having a breakthrough SARS-CoV-2 variant infection. This infection did not appear to have any effect on IgG4 levels as judged by the lack of fluctuations in OD values for IgG4 of these subjects. To determine if a subsequent immunization with the bivalent vaccine could modify the magnitude of IgG4, two additional samples of 30 and 180 days following the bivalent vaccination were analyzed. As our results shows, IgG4 levels were boosted in only one subject after the administration of the bivalent vaccine, while on the other subjects, the IgG4 levels remained unaltered. In turn, the bivalent injection increased the levels of IgG1 in all subjects (average OD= 1.615
0.239), as well as the IgG2 levels in most of individuals (75%) (average OD= 1.636
0.782) (sample 5), although both isotypes dropped to background levels or were undetectable during the subsequent follow-up course (sample 6). The IgG3 isotype was not detected in any of specimens after the third dose or bivalent vaccine. After the administration of the bivalent vaccine the neutralizing percentages against all VOC including Omicron were also boosted; however, in contrast to the IgG1 levels, the neutralizing percentages remained high at a range of 88 and 98% (median 96%) by 180 days after bivalent vaccine (
Figure 6).
Because our cohort of multiple vaccinated subjects was small and it was not possible to prolong the follow-up of these subjects for a longer period, we decided to analyze an independent cohort to examine the contribution of IgG4 antibodies to the long-lived antibody pool after the third immunization. The new cohort comprised eight volunteers (cohort-3b) that had received the primary two doses of Pfizer-BioNTech vaccine and the booster dose. A single specimen from each of these subjects was collected between 12 to 24 months after receiving the third dose or bivalent vaccine (
Table 2). None of these individuals had been exposed to SARS-CoV-2 prior receiving the initial two Pfizer doses; however, six of them had, at least, a documented breakthrough infection occurring, in all cases, between 30 to 60 days following the third dose. Intriguingly, despite the specimens from these subjects had been collected long after the booster dose or bivalent vaccine, 7 of 8 specimens had high levels of IgG4 with OD values ranging from 2.709 to 3.975 (average OD= 3.557
0.510). Low to moderate levels of IgG1 that ranged from 0.286 to 0.738 (average OD=0.58
0.208) were also detected in three of the subjects. The IgG2 isotype was detected in two subjects only (average OD=3.153 and 0.482, respectively) (
Figure 5B). When the data of all specimens from Cohort-3a and Cohort 3b were collectively analyzed, it was confirmed that all subjects with multiple vaccinations had significantly more IgG4 levels than IgG1, IgG2 or IgG3 after ~30 days (
p=0.0003) and at a mean of 18 months (
p<0.0001) following the booster dose (
Figure 5C), which confirms that a class switch toward IgG4 was induced after the third dose of Pfizer-BioNTech vaccine and remained at high levels for a long-term. No statistical differences found between the IgG4 values of subjects that had breakthrough infections after the third dose compared to those did not have documented infections with SARS-CoV-2. A group of researchers found that although a switch class toward IgG4 occurring some months after the second dose in approximately 0.04% of the subjects, IgG4 became the most steadily isotype of all vaccinees after the third dose [
61]. Interestingly, the samples of all the subjects in our study, also showed very high sVNT% against all VOC including Omicron that ranged between 95 and 98%, which was concurrent with the high levels of IgG4 detected in the same samples. The presence of IgG4 in these samples is atypical because it is not considered an anti-viral sub-class and it is not expected that IgG4 plays a role in preventing the entry of virus into cells. Thus, it is possible to speculate that the observed neutralizing activity in these samples can be attributed to the IgG1 isotype, which was found in high values following every booster, or attributed to IgA [
62] or IgM [
63], which can also have a role in neutralizing the virus, but were not measured in the present study.
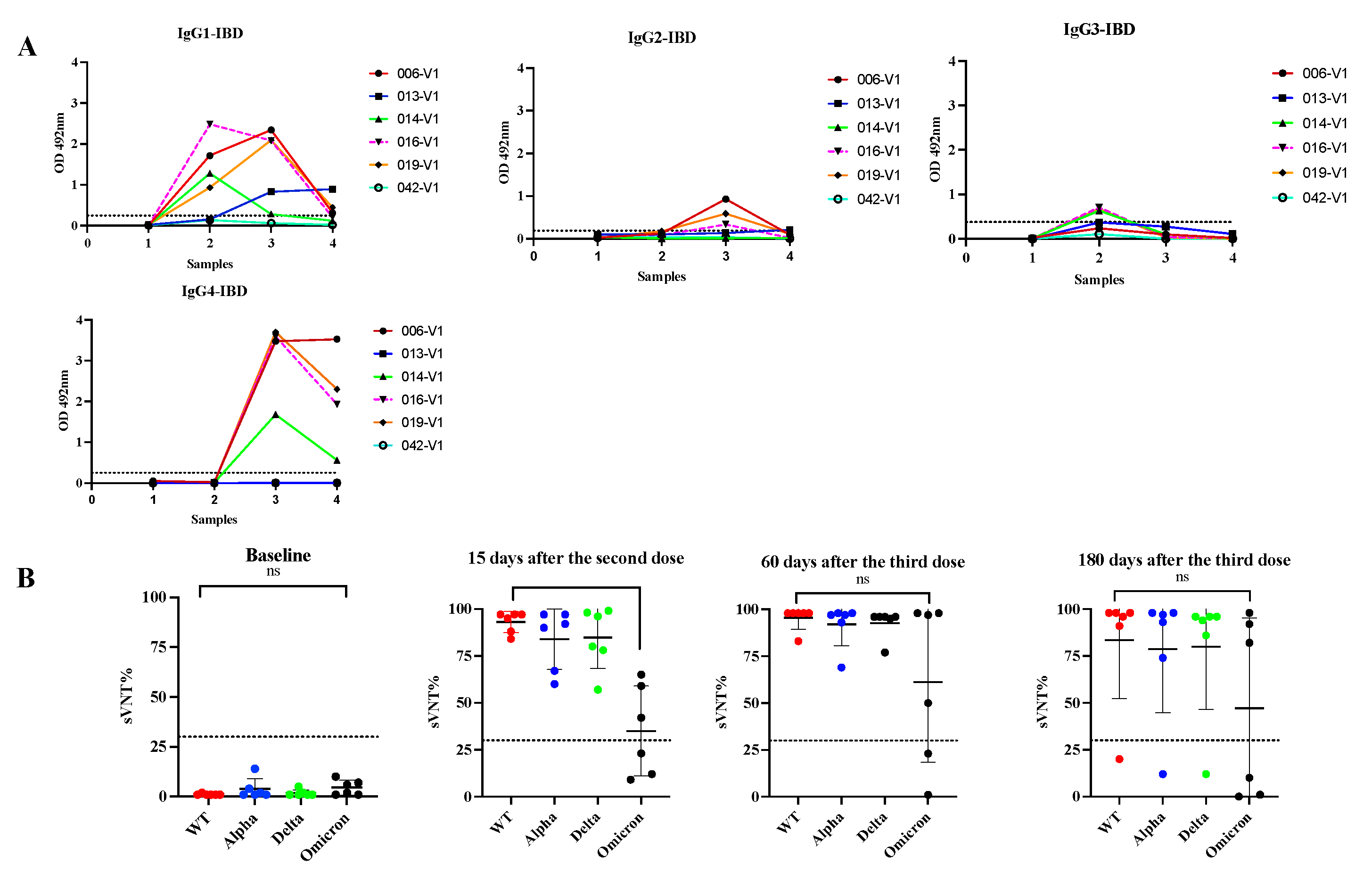
3.5. Switch class toward IgG4 is also observed in subjects with Inflammatory Bowel Diseases with multiple vaccinations.
Treatment with immunomodulators and/or biologic agents is standard in the management of moderate to severe IBD for achieving disease control and maintaining remission. These medications impact the immune system and make them vulnerable to several microbial infections that require inflammatory responses for protection [
64]. These treatments also reduce the protective immune response to vaccines like hepatitis A/B, pneumococcal and influenza [
65]. A previous study with IBD patients of Puerto Rico revealed that although two doses of the COVID-19 mRNA vaccines induce seroconversion and neutralizing activity against several VOCs these responses are mostly ineffective against Omicron, a status that does not seem to change after administering a booster shot [
8]. In the present study we tested serial samples from five IBD subjects that received multiple Pfizer-BioNTech doses (Cohort 4) to ascertain if in these subjects the above-described antibody class switch of antibody to IgG4 after the third dose also occur. The booster injection was given to these individuals about 6 months after the second injection. As our results showed, 15 days following the second dose of Pfizer vaccine four subjects (80.0%) elicited antibody responses to the vaccine similar to that observed in immunocompetent subjects with an average IgG1 subclass OD= 1.599
0.579, and undetectable levels of IgG2, IgG3 or IgG4 in all specimens. After the third dose (30 days), IgG4 became the most prevalent antibody isotype (average OD=3.11
0.828) and remained at high levels about 180 days after the booster shot (average OD= 2.078
1.056) (
Figure 7). The only difference between the IBD group compared to those immunocompetent is that at 180 days following the booster shot, the IBD group had average IgG4 levels 1.45-fold lower than those observed at 30-days after the third dose, suggesting a tendence to decline faster. In contrast, the IBD cohort showed low sVNT% (average 56.4%) against Omicron like subjects of Cohort 3a, indicating that the immune responses elicited after the booster dose are inefficient against breakthrough infections in both groups.
The finding of a class switch toward IgG4 either in immunocompetent or immunocompromised subjects occurring after the third dose was the most striking finding of this study. It is not clear whether the consistent switch to this antibody isotype could be beneficious or detrimental for the Latino-population that received multiple vaccinations. Interestingly, studies have reported that mRNA vaccines, and not those based on adenovirus like the AztraZeneca vaccine, are the ones inducing long-lasting IgG4 responses [
61], although the reasons for this are still not very well understood. IgG4 is considered an unusual antibody because it exhibits defective ability to destroy infected cells through the activation of the complement system [
66,
67].
IgG4 is typically found during helminth infections and allergic diseases where this antibody subclass plays a protective role [
61]. However, in other circumstances, high levels of IgG4 on serum is considered pathogenic because it could trigger autoimmune disease [
68], cancer [
69,
70] and many other illnesses such as lymphadenopathy [
71], interstitial pneumonitis [
72] and aortic aneurism [
73]. Hence, it has been proposed that instead of being beneficious, repeated vaccinations tend to induce immunological tolerance. This could occur because the amount of spike protein produced in response to repeated mRNA administration is too high and lasts too long in the body. The continuous exposure of T-cells to such a large amount of spike protein could desensitize the CD4+ and CD8+ cells, making them lose their capacity to proliferate, consequently, losing their capacity to respond appropriately to re-infections with SARS-CoV-2 variants [
74]. If this happens, the immune system could become exhausted and cause autoimmunity [
74]. Therefore, it is not unlikely that subjects who have received booster injections and who are re-exposed to the virus may suffer of more severe COVID-19 disease. Particularly, subjects with IBD could concurrently suffer of exacerbated intestinal inflammation. The induction of immunological tolerance by repeated vaccinations could perhaps explain the large number of deaths occurred in vaccinated people who received the third dose compared with those unvaccinated in some countries of Europe [
75,
76,
77]. These negative outcomes may be accumulative and debut several years later. Likely, aging people or individuals with immunodeficiency can be the most affected, which paradoxically, are the more vulnerable population to SARS-CoV-2 and constantly encouraged to get vaccinated periodically.
4. Limitations of the study
The main limitation of the present study was the small size of most of the cohorts, especially those comprised of vaccinated cohorts with or without booster injections, which restricts reaching conclusions statistically validated by a power analysis. Considering that at present time most people worldwide, and especially in Puerto Rico, have received at least a single dose vaccine, have been infected or both, the possibility of recruiting volunteers that belong to a single immune state is almost impossible. Other limitations were the lack of samples from Cohort 3b, which corresponds to baseline or shorter time points before receiving the third dose. This did not allow us to determine the exact moment in which the IgG4 levels began to rise in these subjects. We also could not add a group of people who had not been vaccinated and who had suffered from repeated infections. It would be interesting to find out if repeated infections with several variants or subvariants could also induce the class switch to IgG4.
5. Conclusions
This is the first study that describes the longitudinal evolution of the four IgG antibody subclasses in a Latin-population by using cohorts with different immune status: infected, infected-vaccinated, non-infected-vaccinated with one or more booster injections, and immunocompromised non-infected-vaccinated with a booster dose. The most notable findings from this study can be summarized as follows: 1) Convalescent subjects that were not hospitalized developed high and long-lasting antibody response. 2) IgG1 and IgG3 are the most prevalent antibody subclasses more prevalent in the SARS-CoV-2 infected population whereas IgG1 and not IgG3 is the most prevalent antibody subclass after vaccination. 3) Despite either the natural infection or a full course of mRNA vaccination having limited capacity to neutralize Omicron, those individuals who first had the infection and later received two doses of mRNA vaccines exhibited more robust neutralizing capacity than those that were not infected and prior receiving only two doses of Pfizer-BioNTech vaccines, which reinforces the advantage of natural immunity combined with induced immunity. 4) A class switch towards the “anti-inflammatory” antibody isotype IgG4 is induced a few weeks after the booster dose. The levels of IgG4 rise and peak abruptly and remain at high levels for a long period and these levels seems not to be affected either by a subsequent booster dose (e.g. bivalent vaccine) or by breakthrough infections. These high levels of IgG4 are concurrent with high neutralizing percentages against various VOC including Omicron. 5) Subjects with an immunocompromised immune system such as those with IBDs, who receive treatments with immunomodulators, also develop IgG4 after the third dose, although these antibody levels have limited effect on the neutralizing capacity.
Further studies are needed to elucidate the mechanisms that induce this class switch and determine its potential negative effect on people with comorbidities, immunosuppression, or aging population. At present time, recognizing that the mRNA vaccines do not prevent reinfections [
59], all Omicron subvariants that are dominant worldwide have been shown to be less pathogenic [
78,
79], and being already aware of the potential negative effects that multiple boosters could have on the immune system, revising the continuous booster vaccination guidelines should be considered.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Deming analysis for serum/plasma equivalence and reproducibility of the in-house IgG ELISA.; Table S1: Mean absorbance values obtained by in-house IgG ELISA for detection of IgG, and IgG1, IgG2, IgG3 and IgG4 antibody isotypes in samples (serum/plasma) collected from 85 no-hospitalized COVID-19 convalescent subjects; Table-S2: Neutralization activity measured as surrogate virus neutralization percentage (sVNT%) against the Wild Type, Alpha (B.1.1.7), Delta (B.1.617.2), and Omicron (B.1.1.529) variant of concern were determined by C-Pass method in samples (serum/plasma) collected from 85 no-hospitalized COVID-19 convalescent subjects; Table-S3: Levels of IgG antibody subclasses to SARS-CoV-2 measured by in-house ELISAs and neutralizing percentages (sVNT%) measured by C-Pass surrogate virus neutralization test in serial samples collected from 12 COVID-19 convalescent subjects that received two doses of the mRNA vaccine (Pfizer-BioNTech or Moderna-1273); Table-4S: Levels of IgG antibody subclasses to SARS-CoV-2 measured by in-house ELISAs and neutralizing activity measured by C-Pass a surrogate virus neutralization assay (sVNT%) in serial samples collected from 14 subjects without previous SARS-CoV-2 infection that received multiple mRNA vaccinations (Pfizer-BioNTech or Moderna-mRNA-1273).
Author Contributions
Conceptualization, A.M.E. and C.A.S; methodology, A.M.E., C.A.S., E.A.T., P.L.M. and E.R.B.; validation, A.M.E. and C.A.S.; formal analysis, A.M.E.; C.A.S., E.A.T. and P.L.M., investigation, A.M.E., C.A.S., A.A.R, L.A., C.O. and R.R.; resources, C.A.S. and A.M.E.; data curation, A.M.E; writing—original draft preparation, A.M.E.; writing—review and editing, C.A.S, R., A.A.R, L.A., C.O., R.R., E.A.T., P.L.M., E.R.B.; supervision, A.M.E, C.A.S.; funding acquisition, C.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by 1U01CA260541-01 to CAS (NCI/NIAID). The Puerto Rico Science, Technology and Research Trust also supported the research reported in this work under agreement number 2020-00272 to CAS and AME. The IBD study was supported by NIGMS award U54GM133807.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the University of Puerto Rico Medical Sciences Campus IRB (protocol: #1250121). Volunteers in the control group participated in the Advarra IRB-approved clinical protocol “Molecular Basis and Epidemiology of Viral Infections Circulating in Puerto Rico” (Pro0004333). Continuing review approval March 10, 2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. 2022. Available online: https://covid19.who.int/ (accessed on 29 March 2022).
- Soto-Canetti G, Garcia L, Julia AE, Gordian EI, Bartolomei JA, Camareno N, Rodriguez JF, Montoya M. 2022. Developing a Case Investigation and Contact-Tracing System in Puerto Rico, 2020. Am J Public Health 112:223-226. [CrossRef]
- Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, Jiang K, Arunkumar GA, Jurczyszak D, Polanco J, Bermudez-Gonzalez M, Kleiner G, Aydillo T, Miorin L, Fierer DS, Lugo LA, Kojic EM, Stoever J, Liu STH, Cunningham-Rundles C, Felgner PL, Moran T, Garcia-Sastre A, Caplivski D, Cheng AC, Kedzierska K, Vapalahti O, Hepojoki JM, Simon V, Krammer F. 2020. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 26:1033-1036. [CrossRef]
- Lisboa Bastos M, Tavaziva G, Abidi SK, Campbell JR, Haraoui LP, Johnston JC, Lan Z, Law S, MacLean E, Trajman A, Menzies D, Benedetti A, Ahmad Khan F. 2020. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ 370:m2516. [CrossRef]
- Ramirez-Reveco A, Velasquez G, Aros C, Navarrete G, Villarroel-Espindola F, Navarrete M, Fica A, Plaza A, Castro N, Verdugo C, Acosta-Jamett G, Verdugo CC. 2023. Performance estimation of two in-house ELISA assays for COVID-19 surveillance through the combined detection of anti-SARS-CoV-2 IgA, IgM, and IgG immunoglobulin isotypes. PLoS One 18:e0270388. eCollection 2023. [CrossRef]
- Rungrojcharoenkit K, Suthangkornkul R, Utennam D, Buddhari D, Pinpaiboon S, Mongkolsirichaikul D, Fernandez S, Jones AR, Cotrone TS, Hunsawong T. 2023. Standardization of in-house anti-IgG and IgA ELISAs for the detection of COVID-19. PLoS One 18:e0287107. eCollection 2023. [CrossRef]
- Sariol CAA, Pantoja P, Serrano-Collazo C, Rosa-Arocho T, Armina-Rodriguez A, Cruz L, Stone ETT, Arana T, Climent C, Latoni G, Atehortua D, Pabon-Carrero C, Pinto AKK, Brien JDD, Espino AMM. 2021. Function Is More Reliable than Quantity to Follow Up the Humoral Response to the Receptor-Binding Domain of SARS-CoV-2-Spike Protein after Natural Infection or COVID-19 Vaccination. Viruses 13 (10):1972. [CrossRef]
- Lopez-Marte P, Soto-Gonzalez A, Ramos-Tollinchi L, Torres-Jorge S, Ferre M, Rodriguez-Martino E, Torres EA, Sariol CA. 2022. Inefficient Induction of Neutralizing Antibodies against SARS-CoV-2 Variants in Patients with Inflammatory Bowel Disease on Anti-Tumor Necrosis Factor Therapy after Receiving a Third mRNA Vaccine Dose. Vaccines (Basel) 10(8):1301. [CrossRef]
- Sariol CA, Serrano-Collazo C, Ortiz EJ, Pantoja P, Cruz L, Arana T, Atehortua D, Pabon-Carrero C, Espino AM. 2021. Limited Impact of Delta Variant's Mutations on the Effectiveness of Neutralization Conferred by Natural Infection or COVID-19 Vaccines in a Latino Population. Viruses 13(12):2405. [CrossRef]
- Li K, Huang B, Wu M, Zhong A, Li L, Cai Y, Wang Z, Wu L, Zhu M, Li J, Wang Z, Wu W, Li W, Bosco B, Gan Z, Qiao Q, Wu J, Wang Q, Wang S, Xia X. 2020. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat Commun 11(1):6044. [CrossRef]
- Hashem AM, Algaissi A, Almahboub SA, Alfaleh MA, Abujamel TS, Alamri SS, Alluhaybi KA, Hobani HI, AlHarbi RH, Alsulaiman RM, ElAssouli MZ, Hala S, Alharbi NK, Alhabbab RY, AlSaieedi AA, Abdulaal WH, Bukhari A, Al-Somali AA, Alofi FS, Khogeer AA, Pain A, Alkayyal AA, Almontashiri NAM, Ahmad BM, Li X. 2020. Early Humoral Response Correlates with Disease Severity and Outcomes in COVID-19 Patients. Viruses 12(12):1390. [CrossRef]
- Trieu MC, Bansal A, Madsen A, Zhou F, Saevik M, Vahokoski J, Brokstad KA, Krammer F, Tondel C, Mohn KGI, Blomberg B, Langeland N, Cox RJ, Bergen C-RG. 2021. SARS-CoV-2-Specific Neutralizing Antibody Responses in Norwegian Health Care Workers After the First Wave of COVID-19 Pandemic: A Prospective Cohort Study. J Infect Dis 223:589-599. [CrossRef]
- Wang, P. 2021. Significance of IgA antibody testing for early detection of SARS-CoV-2. J Med Virol 93:1888-1889. Epub 2020 Dec 17. [CrossRef]
- Yu HQ, Sun BQ, Fang ZF, Zhao JC, Liu XY, Li YM, Sun XZ, Liang HF, Zhong B, Huang ZF, Zheng PY, Tian LF, Qu HQ, Liu DC, Wang EY, Xiao XJ, Li SY, Ye F, Guan L, Hu DS, Hakonarson H, Liu ZG, Zhong NS. 2020. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J 56(2):2001526. Print 2020 Aug. [CrossRef]
- Xiang T, Liang B, Fang Y, Lu S, Li S, Wang H, Li H, Yang X, Shen S, Zhu B, Wang B, Wu J, Liu J, Lu M, Yang D, Dittmer U, Trilling M, Deng F, Zheng X. 2021. Declining Levels of Neutralizing Antibodies Against SARS-CoV-2 in Convalescent COVID-19 Patients One Year Post Symptom Onset. Front Immunol 12:708523. eCollection 2021. [CrossRef]
- Zhu L, Xu X, Zhu B, Guo X, Xu K, Song C, Fu J, Yu H, Kong X, Peng J, Huang H, Zou X, Ding Y, Bao C, Zhu F, Hu Z, Wu M, Shen H. 2021. Kinetics of SARS-CoV-2 Specific and Neutralizing Antibodies over Seven Months after Symptom Onset in COVID-19 Patients. Microbiol Spectr 9:e0059021. Epub 2021 Sep 22. [CrossRef]
- Hantz, S. 2020. [Biological diagnosis of Sars-CoV-2 infection: strategies and interpretation of results]. Rev Francoph Lab 2020:48-56. Epub 2020 Oct 31. [CrossRef]
- Vidarsson G, Dekkers G, Rispens T. 2014. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5:520. eCollection 2014. [CrossRef]
- Husain-Syed F, Vadasz I, Wilhelm J, Walmrath HD, Seeger W, Birk HW, Jennert B, Dietrich H, Herold S, Trauth J, Tello K, Sander M, Morty RE, Slanina H, Schuttler CG, Ziebuhr J, Kassoumeh S, Ronco C, Ferrari F, Warnatz K, Stahl K, Seeliger B, Hoeper MM, Welte T, David S. 2021. Immunoglobulin deficiency as an indicator of disease severity in patients with COVID-19. Am J Physiol Lung Cell Mol Physiol 320:L590-L599. Epub 2020 Nov 25. [CrossRef]
- Patil HP, Rane PS, Shrivastava S, Palkar S, Lalwani S, Mishra AC, Arankalle VA. 2021. Antibody (IgA, IgG, and IgG Subtype) Responses to SARS-CoV-2 in Severe and Nonsevere COVID-19 Patients. Viral Immunol 34:201-209. Epub 2021 Mar 3. [CrossRef]
- Yates JL, Ehrbar DJ, Hunt DT, Girardin RC, Dupuis AP, 2nd, Payne AF, Sowizral M, Varney S, Kulas KE, Demarest VL, Howard KM, Carson K, Hales M, Ejemel M, Li Q, Wang Y, Peredo-Wende R, Ramani A, Singh G, Strle K, Mantis NJ, McDonough KA, Lee WT. 2021. Serological analysis reveals an imbalanced IgG subclass composition associated with COVID-19 disease severity. Cell Rep Med 2:100329. Epub 2021 Jun 15. [CrossRef]
- Kellner C, Otte A., Cappuzzello E., Klausz K., Peipp M.. 2017. Modulating cytotoxic effector functions by Fc engineering to improve cancer tharpy. Transfus Med Hemother 44:327-336. Epub 2017 Sep 8. [CrossRef]
- Damelang T, Rogerson S.J., Kent S.J., Chung A.W.. 2019. Role of IgG3 in infections diseases. Trends Immunol 40:197-211. Epub 2019 Feb 10. [CrossRef]
- Tejedor Vaquero S, de Campos-Mata L, Ramada JM, Diaz P, Navarro-Barriuso J, Ribas-Llaurado C, Rodrigo Melero N, Carolis C, Cerutti A, Gimeno R, Magri G. 2021. The mRNA-1273 Vaccine Induces Cross-Variant Antibody Responses to SARS-CoV-2 With Distinct Profiles in Individuals With or Without Pre-Existing Immunity. Front Immunol 12:737083. eCollection 2021. [CrossRef]
- Rahman S, Rahman MM, Miah M, Begum MN, Sarmin M, Mahfuz M, Hossain ME, Rahman MZ, Chisti MJ, Ahmed T, Arifeen SE, Rahman M. 2022. COVID-19 reinfections among naturally infected and vaccinated individuals. Sci Rep 12:1438. [CrossRef]
- Ren X, Zhou J, Guo J, Hao C, Zheng M, Zhang R, Huang Q, Yao X, Li R, Jin Y. 2022. Reinfection in patients with COVID-19: a systematic review. Glob Health Res Policy 7(1):12. [CrossRef]
- Fernandes Q, Inchakalody VP, Merhi M, Mestiri S, Taib N, Moustafa Abo El-Ella D, Bedhiafi T, Raza A, Al-Zaidan L, Mohsen MO, Yousuf Al-Nesf MA, Hssain AA, Yassine HM, Bachmann MF, Uddin S, Dermime S. 2022. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med 54(1):524-540. [CrossRef]
- Espino AM, Pantoja P., Sariol C.A. 2021. Validation and performance of a quantitative IgG assay for the screening of SARS-CoV-2 antibodies. bioRxv https: doi.org/10.1101/2020.06.11.146332.
- Taylor SC, Hurst B, Charlton CL, Bailey A, Kanji JN, McCarthy MK, Morrison TE, Huey L, Annen K, DomBourian MG, Knight V. 2021. A New SARS-CoV-2 Dual-Purpose Serology Test: Highly Accurate Infection Tracing and Neutralizing Antibody Response Detection. J Clin Microbiol 59(4): e02438-20. Print 2021 Mar 19. [CrossRef]
- Hosmer Jr DW, Lemeshow S., Sturdivant, R.X. 2013. Applied Logistic Regression. John Wiley & Sons.
- Obermeier P, Muehlhans S, Hoppe C, Karsch K, Tief F, Seeber L, Chen X, Conrad T, Boettcher S, Diedrich S, Rath B. 2016. Enabling Precision Medicine With Digital Case Classification at the Point-of-Care. EBioMedicine 4:191-6. eCollection 2016 Feb. [CrossRef]
- Linnet, K. 1993. Evaluation of regression procedures for methods comparison studies. Clin Chem 39:424-32.
- J. C. 1960. A coefficient of agreement for nominal scales. Educational and Psycological Measurement 20:37-46.
- Wieckowska B, Kubiak KB, Jozwiak P, Moryson W, Stawinska-Witoszynska B. 2022. Cohen's Kappa Coefficient as a Measure to Assess Classification Improvement following the Addition of a New Marker to a Regression Model. Int J Environ Res Public Health 19(16):10213. [CrossRef]
- Landis JR, Koch GG. 1977. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 33:363-74.
- Valdes-Fernandez BN, Duconge J, Espino AM, Ruano G. 2021. Personalized health and the coronavirus vaccines-Do individual genetics matter? Bioessays 43:e2100087. Epub 2021 Jul 26. [CrossRef]
- Isho B, Abe KT, Zuo M, Jamal AJ, Rathod B, Wang JH, Li Z, Chao G, Rojas OL, Bang YM, Pu A, Christie-Holmes N, Gervais C, Ceccarelli D, Samavarchi-Tehrani P, Guvenc F, Budylowski P, Li A, Paterson A, Yue FY, Marin LM, Caldwell L, Wrana JL, Colwill K, Sicheri F, Mubareka S, Gray-Owen SD, Drews SJ, Siqueira WL, Barrios-Rodiles M, Ostrowski M, Rini JM, Durocher Y, McGeer AJ, Gommerman JL, Gingras AC. 2020. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol 5(52): eabe5511. [CrossRef]
- Chia WN, Tan CW, Foo R, Kang AEZ, Peng Y, Sivalingam V, Tiu C, Ong XM, Zhu F, Young BE, Chen MI, Tan YJ, Lye DC, Anderson DE, Wang LF. 2020. Serological differentiation between COVID-19 and SARS infections. Emerg Microbes Infect 9:1497-1505. [CrossRef]
- Oliveira BA, Oliveira LC, Sabino EC, Okay TS. 2020. SARS-CoV-2 and the COVID-19 disease: a mini review on diagnostic methods. Rev Inst Med Trop Sao Paulo 62:e44. eCollection 2020. [CrossRef]
- Karger AB, Brien JD, Christen JM, Dhakal S, Kemp TJ, Klein SL, Pinto LA, Premkumar L, Roback JD, Binder RA, Boehme KW, Boppana S, Cordon-Cardo C, Crawford JM, Daiss JL, Dupuis AP, 2nd, Espino AM, Firpo-Betancourt A, Forconi C, Forrest JC, Girardin RC, Granger DA, Granger SW, Haddad NS, Heaney CD, Hunt DT, Kennedy JL, King CL, Krammer F, Kruczynski K, LaBaer J, Lee FE, Lee WT, Liu SL, Lozanski G, Lucas T, Mendu DR, Moormann AM, Murugan V, Okoye NC, Pantoja P, Payne AF, Park J, Pinninti S, Pinto AK, Pisanic N, Qiu J, Sariol CA, Simon V, Song L, et al. 2022. The Serological Sciences Network (SeroNet) for COVID-19: Depth and Breadth of Serology Assays and Plans for Assay Harmonization. mSphere 7:e0019322. Epub 2022 Jun 15. [CrossRef]
- Luo H, Jia T, Chen J, Zeng S, Qiu Z, Wu S, Li X, Lei Y, Wang X, Wu W, Zhang R, Zou X, Feng T, Ding R, Zhang Y, Chen YQ, Sun C, Wang T, Fang S, Shu Y. 2021. The Characterization of Disease Severity Associated IgG Subclasses Response in COVID-19 Patients. Front Immunol 12:632814. eCollection 2021. [CrossRef]
- Korobova ZR, Zueva EV, Arsentieva NA, Batsunov OK, Liubimova NE, Khamitova IV, Kuznetsova RN, Rubinstein AA, Savin TV, Stanevich OV, Kulikov AN, Pevtsov DE, Totolian AA. 2022. Changes in Anti-SARS-CoV-2 IgG Subclasses over Time and in Association with Disease Severity. Viruses 14(5):941. [CrossRef]
- Rispens T, Huijbers MG. 2023. The unique properties of IgG4 and its roles in health and disease. [CrossRef]
- Santiago GA, Flores B, Gonzalez GL, Charriez KN, Huertas LC, Volkman HR, Van Belleghem SM, Rivera-Amill V, Adams LE, Marzan M, Hernandez L, Cardona I, O'Neill E, Paz-Bailey G, Papa R, Munoz-Jordan JL. 2022. Genomic surveillance of SARS-CoV-2 in Puerto Rico enabled early detection and tracking of variants. Commun Med (Lond) 2:100. eCollection 2022. [CrossRef]
- Arevalo-Rodriguez I B-GD, Simancas-Racines D, Zambrano-Achig P, del Campo R, Ciapponi A, Sued O, Martinez-Garcia L, Rutjes A, Low N, Perez-Molina JA, Zamora J. 2020. FALSE-NEGATIVE RESULTS OF INITIAL RT-PCR ASSAYS FOR COVID-19: A SYSTEMATIC REVIEW. [CrossRef]
- Chan JF, Yip CC, To KK, Tang TH, Wong SC, Leung KH, Fung AY, Ng AC, Zou Z, Tsoi HW, Choi GK, Tam AR, Cheng VC, Chan KH, Tsang OT, Yuen KY. 2020. Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated In Vitro and with Clinical Specimens. J Clin Microbiol 58(5): e00310-20. Print 2020 Apr 23. [CrossRef]
- Loeffelholz MJ, Tang YW. 2020. Laboratory diagnosis of emerging human coronavirus infections - the state of the art. Emerg Microbes Infect 9:747-756. [CrossRef]
- Tang YW, Schmitz JE, Persing DH, Stratton CW. 2020. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J Clin Microbiol 58(6):e00512-20. Print 2020 May 26. [CrossRef]
- Giovanetti M, Benedetti F, Campisi G, Ciccozzi A, Fabris S, Ceccarelli G, Tambone V, Caruso A, Angeletti S, Zella D, Ciccozzi M. 2021. Evolution patterns of SARS-CoV-2: Snapshot on its genome variants. Biochem Biophys Res Commun 538:88-91. Epub 2020 Nov 6. [CrossRef]
- Roy, U. 2022. Comparative structural analyses of selected spike protein-RBD mutations in SARS-CoV-2 lineages. Immunol Res 70:143-151. Epub 2021 Nov 16. [CrossRef]
- Chakraborty C, Bhattacharya M, Sharma AR, Mallik B. 2022. Omicron (B.1.1.529) - A new heavily mutated variant: Mapped location and probable properties of its mutations with an emphasis on S-glycoprotein. Int J Biol Macromol 219:980-997. Epub 2022 Aug 8. [CrossRef]
- Verbeke R, Lentacker I, De Smedt SC, Dewitte H. 2021. The dawn of mRNA vaccines: The COVID-19 case. J Control Release 333:511-520. Epub 2021 Mar 30. [CrossRef]
- De Greef J, Scohy A, Zech F, Aboubakar F, Pilette C, Gerard L, Pothen L, Yildiz H, Belkhir L, Yombi JC. 2021. Determinants of IgG antibodies kinetics after severe and critical COVID-19. J Med Virol 93:5416-5424. Epub 2021 May 12. [CrossRef]
- Wei J, Stoesser N, Matthews PC, Ayoubkhani D, Studley R, Bell I, Bell JI, Newton JN, Farrar J, Diamond I, Rourke E, Howarth A, Marsden BD, Hoosdally S, Jones EY, Stuart DI, Crook DW, Peto TEA, Pouwels KB, Eyre DW, Walker AS, team C-IS. 2021. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol 6:1140-1149. Epub 2021 Jul 21. [CrossRef]
- Choe PG, Hong J, Park J, Chang E, Kang CK, Kim NJ, Lee CH, Park WB, Oh MD. 2022. Persistent Antibody Responses Up to 18 Months After Mild Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Infect Dis 226:1224-1230. [CrossRef]
- Altarawneh HN, Chemaitelly H, Hasan MR, Ayoub HH, Qassim S, AlMukdad S, Coyle P, Yassine HM, Al-Khatib HA, Benslimane FM, Al-Kanaani Z, Al-Kuwari E, Jeremijenko A, Kaleeckal AH, Latif AN, Shaik RM, Abdul-Rahim HF, Nasrallah GK, Al-Kuwari MG, Butt AA, Al-Romaihi HE, Al-Thani MH, Al-Khal A, Bertollini R, Tang P, Abu-Raddad LJ. 2022. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. N Engl J Med 386:1288-1290. Epub 2022 Feb 9. [CrossRef]
- Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, Miller J, Schrag SJ, Verani JR. 2022. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 327:639-651. [CrossRef]
- Hoffmann M, Kruger N, Schulz S, Cossmann A, Rocha C, Kempf A, Nehlmeier I, Graichen L, Moldenhauer AS, Winkler MS, Lier M, Dopfer-Jablonka A, Jack HM, Behrens GMN, Pohlmann S. 2022. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 185:447-456 e11. Epub 2021 Dec 24. [CrossRef]
- Subramanian SV, Kumar A. 2021. Increases in COVID-19 are unrelated to levels of vaccination across 68 countries and 2947 counties in the United States. Eur J Epidemiol 36:1237-1240. Epub 2021 Sep 30. [CrossRef]
- Maslinska M, Dmowska-Chalaba J, Jakubaszek M. 2021. The Role of IgG4 in Autoimmunity and Rheumatic Diseases. Front Immunol 12:787422. eCollection 2021. [CrossRef]
- Irrgang P, Gerling, J., Kocher, K., Lapuente D., Steininger P., Habenicht, K., Wytopil, M., Beileke, S., Schafer S., Zhong J. 2022. Class switch towards non-inflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci Immunol 8:eade2798. Epub 2023 Jan 27. [CrossRef]
- Fernandes-Siqueira LO, Sousa BG, Cleto CE, Wermelinger LS, Caetano BLL, Pacheco AR, Costa SM, Almeida FCL, Ferreira GC, Salmon D, Alves AMB, Da Poian AT. 2022. IgA quantification as a good predictor of the neutralizing antibodies levels after vaccination against SARS-CoV-2. J Clin Virol Plus 2:100121. Epub 2022 Nov 4. [CrossRef]
- Hale M, Netland J, Chen Y, Thouvenel CD, Smith KN, Rich LM, Vanderwall ER, Miranda MC, Eggenberger J, Hao L, Watson MJ, Mundorff CC, Rodda LB, King NP, Guttman M, Gale M, Abraham J, Debley JS, Pepper M, Rawlings DJ. 2022. IgM antibodies derived from memory B cells are potent cross-variant neutralizers of SARS-CoV-2. J Exp Med 219 (9):e20220849. Epub 2022 Aug 8. [CrossRef]
- Kim KO, Jang BI. 2022. Management of inflammatory bowel disease in the COVID-19 era. Intest Res 20(1):3-10. Epub 2021 Feb 3. [CrossRef]
- Alexander JL, Kennedy NA, Ibraheim H, Anandabaskaran S, Saifuddin A, Castro Seoane R, Liu Z, Nice R, Bewshea C, D'Mello A, Constable L, Jones GR, Balarajah S, Fiorentino F, Sebastian S, Irving PM, Hicks LC, Williams HRT, Kent AJ, Linger R, Parkes M, Kok K, Patel KV, Teare JP, Altmann DM, Boyton RJ, Goodhand JR, Hart AL, Lees CW, Ahmad T, Powell N, investigators VIPs. 2022. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepatol 7(4):342-352. Epub 2022 Feb 4. [CrossRef]
- Aalberse RC, Schuurman J. 2002. IgG4 breaking the rules. Immunology 105(1):9-19. [CrossRef]
- Aalberse RC, Stapel SO, Schuurman J, Rispens T. 2009. Immunoglobulin G4: an odd antibody. Clin Exp Allergy 39(4):469-77. [CrossRef]
- Huijbers MG, Plomp JJ, van der Maarel SM, Verschuuren JJ. 2018. IgG4-mediated autoimmune diseases: a niche of antibody-mediated disorders. Ann N Y Acad Sci 1413(1):92-103. Epub 2018 Jan 28. [CrossRef]
- Karagiannis P, Gilbert AE, Josephs DH, Ali N, Dodev T, Saul L, Correa I, Roberts L, Beddowes E, Koers A, Hobbs C, Ferreira S, Geh JL, Healy C, Harries M, Acland KM, Blower PJ, Mitchell T, Fear DJ, Spicer JF, Lacy KE, Nestle FO, Karagiannis SN. 2013. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest 123(4):1457-74. [CrossRef]
- Karagiannis P, Gilbert AE, Nestle FO, Karagiannis SN. 2013. IgG4 antibodies and cancer-associated inflammation: Insights into a novel mechanism of immune escape. Oncoimmunology 2:e24889. Epub 2013 May 7. [CrossRef]
- Sato Y, Kojima M, Takata K, Morito T, Asaoku H, Takeuchi T, Mizobuchi K, Fujihara M, Kuraoka K, Nakai T, Ichimura K, Tanaka T, Tamura M, Nishikawa Y, Yoshino T. 2009. Systemic IgG4-related lymphadenopathy: a clinical and pathologic comparison to multicentric Castleman's disease. Mod Pathol 22(4):589-99. Epub 2009 Mar 6. [CrossRef]
- Zen Y, Inoue D, Kitao A, Onodera M, Abo H, Miyayama S, Gabata T, Matsui O, Nakanuma Y. 2009. IgG4-related lung and pleural disease: a clinicopathologic study of 21 cases. Am J Surg Pathol 33(12):1886-93. [CrossRef]
- Stone JH, Khosroshahi A, Hilgenberg A, Spooner A, Isselbacher EM, Stone JR. 2009. IgG4-related systemic disease and lymphoplasmacytic aortitis. Arthritis Rheum 60(10):3139-45. [CrossRef]
- Billeskov R, Beikzadeh B, Berzofsky JA. 2019. The effect of antigen dose on T cell-targeting vaccine outcome. Hum Vaccin Immunother 15(2):407-411. Epub 2018 Oct 25. [CrossRef]
- Deaths by Vaccination Status in England. Office for National Statistics. Available online: https://wwwonsgovuk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsbyvaccinationstatusengland.
- Aarstad JK, O.A. 2023. Is There a Link between the 2021 COVID-19 Vaccination Uptake in Europe and 2022 Excess All-Cause Mortality? Asian Pac J Health Sci 10:25-31.
- Uversky VN, Redwan EM, Makis W, Rubio-Casillas A. 2023. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines (Basel) 11 (5):991. [CrossRef]
- Shuai H, Chan JF, Hu B, Chai Y, Yuen TT, Yin F, Huang X, Yoon C, Hu JC, Liu H, Shi J, Liu Y, Zhu T, Zhang J, Hou Y, Wang Y, Lu L, Cai JP, Zhang AJ, Zhou J, Yuan S, Brindley MA, Zhang BZ, Huang JD, To KK, Yuen KY, Chu H. 2022. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature 603(7902):693-699. Epub 2022 Jan 21. [CrossRef]
- Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, Amoako DG, Everatt J, Bhiman JN, Scheepers C, Tebeila N, Chiwandire N, du Plessis M, Govender N, Ismail A, Glass A, Mlisana K, Stevens W, Treurnicht FK, Makatini Z, Hsiao NY, Parboosing R, Wadula J, Hussey H, Davies MA, Boulle A, von Gottberg A, Cohen C. 2022. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 399(10323):437-446. Epub 2022 Jan 19. [CrossRef]
Figure 1.
Receiver Operating Characteristic (ROC) Curve and OD distribution. ROC curve analysis using in-house anti-SARS-CoV-2 IgG ELISA (Panel A) including the area under curve (AUC) and 95% confidence interval. OD distribution of sera/plasma from COVID-19 subjects, negative controls and samples from individuals carrying other respiratory and viral infections (Panel B). OD distribution of COVID-19 and negative control samples using in-house anti-SARS-CoV-2 IgG subclasses ELISA (Panel C). Black dashed line represents positive cut-off for each assay.
Figure 1.
Receiver Operating Characteristic (ROC) Curve and OD distribution. ROC curve analysis using in-house anti-SARS-CoV-2 IgG ELISA (Panel A) including the area under curve (AUC) and 95% confidence interval. OD distribution of sera/plasma from COVID-19 subjects, negative controls and samples from individuals carrying other respiratory and viral infections (Panel B). OD distribution of COVID-19 and negative control samples using in-house anti-SARS-CoV-2 IgG subclasses ELISA (Panel C). Black dashed line represents positive cut-off for each assay.
Figure 2.
OD distribution of specimens and number of samples positives from no-vaccinated COVID-19 convalescent subjects. OD distribution of samples from no-vaccinated, convalescent COVID-19 subjects tested by in-house IgG ELISA and IgG subclasses ELISAs, which were alloted into three groups (1-30 days, 31-60 days and > 60 days) based on “the time after infection” (time elapsed betwen the date of confirmatory SARS-CoV-2 RT-PCR SARS-CoV-2 testing) (Panel A). Number of seropositive for each assay is showed in Panel B.
Figure 2.
OD distribution of specimens and number of samples positives from no-vaccinated COVID-19 convalescent subjects. OD distribution of samples from no-vaccinated, convalescent COVID-19 subjects tested by in-house IgG ELISA and IgG subclasses ELISAs, which were alloted into three groups (1-30 days, 31-60 days and > 60 days) based on “the time after infection” (time elapsed betwen the date of confirmatory SARS-CoV-2 RT-PCR SARS-CoV-2 testing) (Panel A). Number of seropositive for each assay is showed in Panel B.
Figure 3.
Correlation between the levels of IgG subclasses and the neutralizing activity. The OD values obtained for each IgG subclass was correlated with the surrogate virus neutralization percentage (sVNT%) obtained agaisnt the the wild type strain (Panel A), and the VOCs Alpha (Panel B), Delta (Panel C) and Omicron.(Panel D).
Figure 3.
Correlation between the levels of IgG subclasses and the neutralizing activity. The OD values obtained for each IgG subclass was correlated with the surrogate virus neutralization percentage (sVNT%) obtained agaisnt the the wild type strain (Panel A), and the VOCs Alpha (Panel B), Delta (Panel C) and Omicron.(Panel D).
Figure 4.
Levels of IgG subclasses and neutralizing capacity detected in serial samples from previously infected subjects that received two doses of mRNA vaccine. The OD distribution of serial samples collected from previously infected subjects that received two doses of Pfizer-BioNTech (n=6) or Moderna1273 (n=6) vaccines are showed in Panel A & B, respectively. Sample-1 was collected before vaccination (baseline), sample-2 was collected at a median of 21.5 days after the second dose and sample 3 was collected at a median of 96 days after the second dose. Black dashed line represent the positive cut-off value for each in-house IgG subclass ELISA. The average OD values for each sample from subjects that received the Pfizer-BioNTech or Moderna-1273 vaccine associated to the corresponding average surrogate virus neutralization percentage (sVNT%) are showed in Panel-C and Panel D, respectively.
Figure 4.
Levels of IgG subclasses and neutralizing capacity detected in serial samples from previously infected subjects that received two doses of mRNA vaccine. The OD distribution of serial samples collected from previously infected subjects that received two doses of Pfizer-BioNTech (n=6) or Moderna1273 (n=6) vaccines are showed in Panel A & B, respectively. Sample-1 was collected before vaccination (baseline), sample-2 was collected at a median of 21.5 days after the second dose and sample 3 was collected at a median of 96 days after the second dose. Black dashed line represent the positive cut-off value for each in-house IgG subclass ELISA. The average OD values for each sample from subjects that received the Pfizer-BioNTech or Moderna-1273 vaccine associated to the corresponding average surrogate virus neutralization percentage (sVNT%) are showed in Panel-C and Panel D, respectively.
Figure 5.
Kinetic of IgG subclassess in naive subjects that received multiple vaccinations with the Pfizer-BioNTech vaccine show a switch toward the IgG4 subclass. Six samples were collected from four (n=4) naive subjects that received multiple vaccinations with the Pfizer-BioNTech vaccine (Cohort-3a). Sample-1 was collected at baseline, sample-2 was collected at a median of 20 days after the second dose, sample-3 was collected at a median of 31 days after the third dose, sample 4 was collected at a median of 255 days after the third dose, sample-5 was collected at a median of 30 days after the bivalent vaccine and sample-6 was collected at a median of 180 days after the bivalent vaccine. Red arrow indicate the time in which the third dose or bivalent vaccine was administered to each subject (Panel A). A single sample from eight (n=8) subjects (Cohort-3b), which received three doses of Pfizer-BioNTech was collected at a median of 277.5 days (~9 months) following the third Pfizer-BioNTech dose and tested for IgG subclassess (Panel B). OD distribution of each IgG subclass in the samples of the Cohort-3b clearly demonstrate the dominance and durability of IgG4 in subjects that received multiple doses of the Pfizer-BioNTech vaccine. ****p<0.0001 (Panel C).
Figure 5.
Kinetic of IgG subclassess in naive subjects that received multiple vaccinations with the Pfizer-BioNTech vaccine show a switch toward the IgG4 subclass. Six samples were collected from four (n=4) naive subjects that received multiple vaccinations with the Pfizer-BioNTech vaccine (Cohort-3a). Sample-1 was collected at baseline, sample-2 was collected at a median of 20 days after the second dose, sample-3 was collected at a median of 31 days after the third dose, sample 4 was collected at a median of 255 days after the third dose, sample-5 was collected at a median of 30 days after the bivalent vaccine and sample-6 was collected at a median of 180 days after the bivalent vaccine. Red arrow indicate the time in which the third dose or bivalent vaccine was administered to each subject (Panel A). A single sample from eight (n=8) subjects (Cohort-3b), which received three doses of Pfizer-BioNTech was collected at a median of 277.5 days (~9 months) following the third Pfizer-BioNTech dose and tested for IgG subclassess (Panel B). OD distribution of each IgG subclass in the samples of the Cohort-3b clearly demonstrate the dominance and durability of IgG4 in subjects that received multiple doses of the Pfizer-BioNTech vaccine. ****p<0.0001 (Panel C).
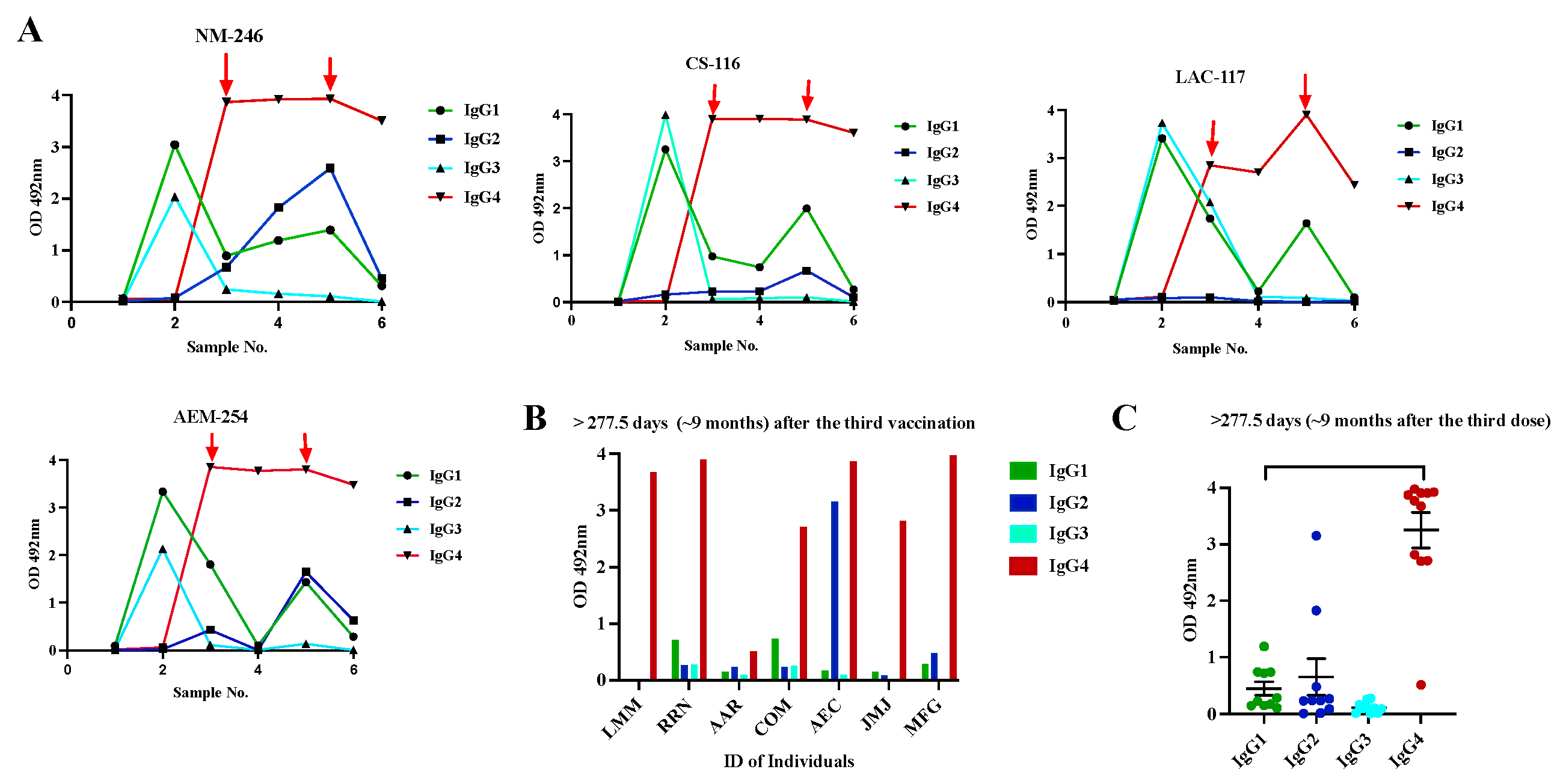
Figure 6.
Kinetic of the neutralizing capacity in specimens from naive subjects that received multiple vaccinations with the Pfizer-BioNTech vaccine. Serial samples were collected from four naive subjects (n=4) that received multiple vaccinations were tested by a surrogate neutralization assay to quantify the viral neutralization percentage (sVNT%) against the wild-type strain and the variant of concern (VOC) Alpha, Delta and Omicron. Sample-1 was collected at baseline, sample-2 was collected at a median of 20 days after the second dose, sample-3 was collected at a median of 31 days after the third dose, sample-4 was collected at a median of 255 days after the third dose, sample-5 was collected at a median of 30 days after the bivalent vaccine and sample-6 was collected at a median of 180 days after the bivalent vaccine. Black dashed line the positive cut-off for the sVNT% (>30%). *** p=0.0002, ****p=0.0001.
Figure 6.
Kinetic of the neutralizing capacity in specimens from naive subjects that received multiple vaccinations with the Pfizer-BioNTech vaccine. Serial samples were collected from four naive subjects (n=4) that received multiple vaccinations were tested by a surrogate neutralization assay to quantify the viral neutralization percentage (sVNT%) against the wild-type strain and the variant of concern (VOC) Alpha, Delta and Omicron. Sample-1 was collected at baseline, sample-2 was collected at a median of 20 days after the second dose, sample-3 was collected at a median of 31 days after the third dose, sample-4 was collected at a median of 255 days after the third dose, sample-5 was collected at a median of 30 days after the bivalent vaccine and sample-6 was collected at a median of 180 days after the bivalent vaccine. Black dashed line the positive cut-off for the sVNT% (>30%). *** p=0.0002, ****p=0.0001.
Figure 7.
Kinetic of IgG subclasses in immunocompromised subjects that received multiple vaccinations with the Pfizer-BioNTech vaccine. Four samples were collected from six subjects (n=6) with inflammatory bowel disease (IBDs), which received standard treatment with immunomodulators or biologic. Sample-1 was collected at baseline, sample-2 was collected at a median of 15 days after second dose, sample-3 was collected at a median of 60 days after the third dose, and sample-4 was collected at a median of 180 days after the third dose (Panel A). The neutralizing capacity of these samples measured as surrogate virus neutralization percentages (sVNT%) against wild type strain (WT) and the VOCs Alpha, Delta and Omicron is showed in Panel B. *** p=0.0002.
Figure 7.
Kinetic of IgG subclasses in immunocompromised subjects that received multiple vaccinations with the Pfizer-BioNTech vaccine. Four samples were collected from six subjects (n=6) with inflammatory bowel disease (IBDs), which received standard treatment with immunomodulators or biologic. Sample-1 was collected at baseline, sample-2 was collected at a median of 15 days after second dose, sample-3 was collected at a median of 60 days after the third dose, and sample-4 was collected at a median of 180 days after the third dose (Panel A). The neutralizing capacity of these samples measured as surrogate virus neutralization percentages (sVNT%) against wild type strain (WT) and the VOCs Alpha, Delta and Omicron is showed in Panel B. *** p=0.0002.
Table 1.
Characteristics of no-hospitalized COVID-19 Convalescent-No vaccinated and Pre-pandemic specimens used in the study.
Table 1.
Characteristics of no-hospitalized COVID-19 Convalescent-No vaccinated and Pre-pandemic specimens used in the study.
| COVID-19 Convalescent No-Vaccinated (Cohort-1) |
|
|
04/26/2020 to 06/05/2020 |
|
85 (31 sera and 54 plasma) |
| Days since RT-qPCR confirmation test |
|
|
0 to 139 days |
|
35.5 days |
|
Median Median Median
|
26 22 days 32 37.5 days 11 84 days 16 |
| Pre-Pandemic Samples (Cohort-5) |
|
| Healthy subjects |
|
|
2012 |
|
78 |
| Other respiratory / viral infections |
|
|
2018-2019 |
|
47 |
Table 2.
Characteristics of specimens from Cohorts-2 to 4 used in the study.
Table 2.
Characteristics of specimens from Cohorts-2 to 4 used in the study.
| Cohort-2: Serial samples from previously SARS-CoV-2 infected subjects that received two doses of mRNA vaccine (Pfizer-BioNTech or Moderna-1273) |
|---|
| Number of subjects |
12 |
|
7 female and 5 males |
|
10/27/2020 to 09/20/2021 |
|
36 |
| Sample 1 (Baseline) |
Days after infection |
|
|
|
|
| Sample 2 |
Days after the 2nd dose |
|
15 to 32 days |
|
21.5 |
| Sample 3 |
Days after the 2nd dose |
|
74 to 169 days |
|
96 |
| Cohort-3a: Serial samples from no previous SARS-CoV-2 infected subjects that received multiple Pfizer-BioNTech vaccinations. |
|
4 |
|
3 female and 1 male |
|
08/10/2020 to 08/10/2023 |
|
26 |
| Sample 1 (baseline) |
Previous 1st dose |
| Sample 2 |
Days after the 2nd dose |
|
19 to 35 days |
|
20 |
| Sample 3 |
Days after the 3rd dose |
|
31 to 43 days |
|
31 |
| Sample 4 |
Days after the 3rd dose |
|
180 to 420 days |
|
255 |
| Sample 5 |
Days after bivalent vaccine |
|
30 days |
| Sample 6 |
Days after bivalent vaccine |
|
90-180 days |
| Median |
180 |
| Cohort-3b: Single sample from no previous SARS-CoV-2 infection multiple Pfizer-BioNTech vaccinations, collected ~ 2 years after the last dose. |
|
8 |
|
4 female and 4 males |
|
8 |
|
08/03/2023 to 10/23/2023 |
|
09/30/2021 to 12/28/2021 |
| Sample 1 |
Days after the 3rd dose |
|
351 to 723 days |
|
634 |
| Cohort-4: Serial samples from no-previous SARS-CoV-2 infected subjects with inflammatory bowel disease (IBD) that received three doses of mRNA vaccine (Pfizer). |
|
6 |
|
3 female and 3 males. |
|
04/14/2021 to 07/22/2022 |
|
24 |
| Sample 1 (baseline) |
Days after 2nd dose |
|
15 to 28 days |
|
17 |
| Sample 2 |
Days after 3rd dose |
|
60 |
| Sample 3 |
Days after 3rd dose |
|
180 |
Table 3.
Cohen’s Kappa analysis between antibody subclasses and their neutralizing activity.
Table 3.
Cohen’s Kappa analysis between antibody subclasses and their neutralizing activity.
| Antibody class/subclass |
Neutralization Test |
| Total IgG |
WT |
Alpha |
Delta |
Omicron |
|
98.87 |
75.29 |
69.41 |
3.529 |
|
0.661 |
0.056 |
0.049 |
0.00057 |
|
Substantial agreement |
Slight agreement |
Slight agreement |
No agreement |
| IgG1 |
|
|
|
|
|
76.47 |
72.94 |
57.64 |
24.70 |
|
0.050 |
0.268 |
-0.038 |
-0.016 |
|
Slight agreement |
Fair agreement |
No agreement |
No agreement |
| IgG2 |
|
|
|
|
|
9.41 |
31.76 |
34.88 |
-- |
|
0.002 |
0.032 |
-0.014 |
No computed |
|
No agreement |
Slight agreement |
No agreement |
-- |
| IgG3 |
|
|
|
|
|
30.58 |
48.23 |
50.58 |
69.23 |
|
0.018 |
0.151 |
0.147 |
0.006 |
|
Slight agreement |
Slight agreement |
Slight agreement |
No agreement |
| IgG4 |
|
|
|
|
|
14.11 |
30.588 |
35.29 |
-- |
|
0.0064 |
0.011 |
-0.026 |
No computed |
|
No agreement |
Slight agreement |
No agreement |
-- |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).