Submitted:
10 January 2024
Posted:
10 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Meat samples
2.2. Heat processing
2.3. Chemical analysis
2.4. Calculation indices
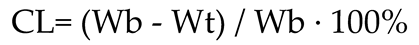
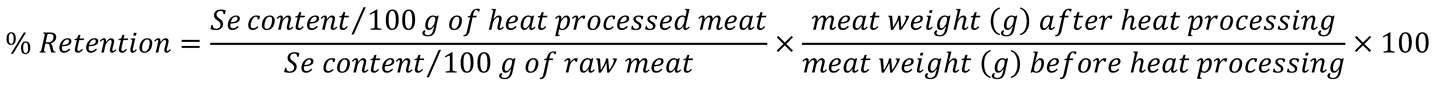
2.5. Statistical Analysis
3. Results and Discussion
3.1. Cooking loss
3.2. Moisture content
3.3. Ash content
3.4. Ash retention
3.5. Selenium content and retention
3.6. Coverage of selenium intake standards
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kieliszek, M. Selenium in the Prevention of SARS-CoV-2 and Other Viruses. Biol Trace Elem Res 2023, 201, 655–662. [CrossRef]
- Alshammari, M.K.; Fatima, W.; Alraya, R.A.; Khuzaim Alzahrani, A.; Kamal, M.; Alshammari, R.S.; Alshammari, S.A.; Alharbi, L.M.; Alsubaie, N.S.; Alosaimi, R.B.; et al. Selenium and COVID-19: A Spotlight on the Clinical Trials, Inventive Compositions, and Patent Literature. J Infect Public Health 2022, 15, 1225–1233. [CrossRef]
- Méplan, C.; Hughes, D.J. The Role of Selenium in Health and Disease: Emerging and Recurring Trends. Nutrients 2020, 12, 10–13. [CrossRef]
- dos Reis, A.R.; El-Ramady, H.; Santos, E.F.; Gratão, P.L.; Schomburg, L. Overview of Selenium Deficiency and Toxicity Worldwide: Affected Areas, Selenium-Related Health Issues, and Case Studies. In Selenium in plants: Molecular, Physiological, Ecological and Evolutionary Aspects; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Springer International Publishing: Cham, 2017; pp. 209–230 ISBN 978-3-319-56249-0.
- Kieliszek, M.; Błażejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms : A Review. Molecules 2016, 21, 1–16. [CrossRef]
- El Sabry, M.I.; Almasri, O. Global Waterfowl Production: Stocking Rate Is a Key Factor for Improving Productivity and Well-Being-a Review. Tropical animal health and production 2023, 55, 419. [CrossRef]
- FAOSTAT Crops and Livestock Products Available online: https://www.fao.org/faostat/en/?#data/QCL/visualize (accessed on 12 December 2023).
- Pathare, P.B.; Roskilly, A.P. Quality and Energy Evaluation in Meat Cooking. Food Engineering Reviews 2016, 8, 435–447. [CrossRef]
- Wereńska, M. Comparative Study on the Effects of Sous-Vide, Microwave Cooking, and Stewing on Functional Properties and Sensory Quality of Goose Meat. Poult Sci 2023, 102, 103064. [CrossRef]
- Tornberg, E. Effects of Heat on Meat Proteins – Implications on Structure and Quality of Meat Products. Meat Sci 2005, 70, 493–508. [CrossRef]
- Goluch, Z.; Pilarczyk, B. Goose Meat As a Nutritional Source of Dietary Selenium. Journal of Elementology 2022, 27, 521–531. [CrossRef]
- van Boekel, M.; Fogliano, V.; Pellegrini, N.; Stanton, C.; Scholz, G.; Lalljie, S.; Somoza, V.; Knorr, D.; Jasti, P.R.; Eisenbrand, G. A Review on the Beneficial Aspects of Food Processing. Molecular Nutrition and Food Research 2010, 54, 1215–1247. [CrossRef]
- Thippareddi, H.; Sanchez, M. Thermal Processing of Meat Products. In Thermal Food Processing. New technologies and quality issues.; Da-Wen Sun, Ed.; Taylor & Francis Group, LLC: Boca Raton, 2006; pp. 155–196 ISBN 1-57444-628-2.
- Zhang, Z.H.; Wang, L.H.; Zeng, X.A.; Han, Z.; Brennan, C.S. Non-Thermal Technologies and Its Current and Future Application in the Food Industry: A Review. International Journal of Food Science and Technology 2019, 54, 1–13. [CrossRef]
- Stangierski, J.; Lesnierowski, G. Nutritional and Health-Promoting Aspects of Poultry Meat and Its Processed Products. World’s Poultry Science Journal 2015, 71, 71–82. [CrossRef]
- Goluch, Z.; Barbara, K.; Haraf, G.; Wołoszyn, J.; Okruszek, A.; Wereńska, M. Impact of Various Types of Heat Processing on the Energy and Nutritional Values of Goose Breast Meat. Poult Sci 2021, 100, 101473. [CrossRef]
- Association of Official Analysis Chemists International Official Methods of Analysis of AOAC International; Latimer, G.W.Jr., Ed.; 20th ed.; Association of Official Analysis Chemists International: Rockville MD, 2016; ISBN 9780935584875.
- Grzebuła, S.; Witkowski, P. The Determination of Selenium Trace Levels in Biological Materials with Fluorometric Method. Selenium Determination in Tissues and Bodily Fluids (in Polish). Pol. Arch. Weter 1977, 20.
- Wołoszyn, J.; Wereńska, M.; Goluch, Z.; Haraf, G.; Okruszek, A.; Teleszko, M.; Król, B. The Selected Goose Meat Quality Traits in Relation to Various Types of Heat Treatment. Poult Sci 2020, 99, 7214–7224. [CrossRef]
- Bognár, A.; Piekarski, J. Guidelines for Recipe Information and Calculation of Nutrient Composition of Prepared Foods (Dishes). Journal of Food Composition and Analysis 2000, 13, 391–410. [CrossRef]
- StatSoft Inc Statistica (Data Analysis Software System) 2022.
- Hassoun, A.; Aït-Kaddour, A.; Sahar, A.; Cozzolino, D. Monitoring Thermal Treatments Applied to Meat Using Traditional Methods and Spectroscopic Techniques: A Review of Advances over the Last Decade. Food and Bioprocess Technology 2021, 14, 195–208. [CrossRef]
- Wereńska, M.; Haraf, G.; Okruszek, A.; Marcinkowska, W.; Wołoszyn, J. The Effects of Sous Vide, Microwave Cooking, and Stewing on Some Quality Criteria of Goose Meat. Foods 2023, 12. [CrossRef]
- Belinsky, D.L.; Kuhnlein, H. V. Macronutrient, Mineral, and Fatty Acid Composition of Canada Goose (Branta Canadensis): An Important Traditional Food Resource of the Eastern James Bay Cree of Quebec. Journal of Food Composition and Analysis 2000, 13, 101–115. [CrossRef]
- Oz, F.; Celik, T. Proximate Composition, Color and Nutritional Profile of Raw and Cooked Goose Meat with Different Methods. J Food Process Preserv 2015, 39, 2442–2454. [CrossRef]
- Satpute, M.; Annapure, U.; Marg, P.; -, M. Approaches for Delivery of Heat Sensitive Nutrients through Food Systems for Selection of Appropriate Processing Techniques: A Review. Journal of Hygienic Engineering and Design 2013, 4, 71–88.
- Geldenhuys, G.; Hoffman, L.C.; Muller, N. The Fatty Acid, Amino Acid, and Mineral Composition of Egyptian Goose Meat as Affected by Season, Gender, and Portion. Poult Sci 2015, 94, 1075–1087. [CrossRef]
- Baowei, W.; Guoqing, H.; Qiaoli, W.; Bin, Y. Effects of Yeast Selenium Supplementation on the Growth Performance, Meat Quality, Immunity, and Antioxidant Capacity of Goose. J Anim Physiol Anim Nutr (Berl) 2011, 95, 440–448. [CrossRef]
- Horak, K.; Chipman, R.; Murphy, L.; Johnston, J. Environmental Contaminant Concentrations in Canada Goose (Branta Canadensis) Muscle: Probabilistic Risk Assessment for Human Consumers. J Food Prot 2014, 77, 1634–1641. [CrossRef]
- Łukaszewicz, E.; Kowalczyk, A.; Jerysz, A. Effect of Dietary Selenium and Vitamin E on Chemical and Fatty Acid Composition of Goose Meat and Liver. Anim Sci Pap Rep 2016, 34, 181–194.
- Gerber, N.; Scheeder, M.R.L.; Wenk, C. The Influence of Cooking and Fat Trimming on the Actual Nutrient Intake from Meat. Meat science 2009, 81, 148—154. [CrossRef]
- Geldenhuys, G.; Hoffman, L.C.; Muller, N. Aspects of the Nutritional Value of Cooked Egyptian Goose (Alopochen Aegyptiacus) Meat Compared with Other Well-Known Fowl Species. Poult Sci 2013, 92, 3050–3059. [CrossRef]
- SobolevА.; Gutyj, B.; Grynevych, N.; Bilkevych, V.; Mashkin, Y. Enrichment of Meat Products with Selenium by Its Introduction to Mixed Feed Compounds for Birds. Regul Mech Biosyst 2017, 8, 417–422. [CrossRef]
- Gómez, I.; Janardhanan, R.; Ibañez, F.C.; Beriain, M.J. The Effects of Processing and Preservation Technologies on Meat Quality: Sensory and Nutritional Aspects; 2020; Vol. 9; ISBN 3494816913.
- National Academies of Sciences, Engineering, and M.H. and M.D.F. and N.B.C. to R. the D.R.I. for S. and P. Dietary Reference Intakes for Sodium and Potassium.
- Chen, S.S.; Lin, Y.W.; Kao, Y.M.; Shih, Y.C. Trace Elements and Heavy Metals in Poultry and Livestock Meat in Taiwan. Food Additives and Contaminants: Part B Surveillance 2013, 6, 231–236. [CrossRef]
- Wideman, N.; O’bryan, C.A.; Crandall, P.G. Factors Affecting Poultry Meat Colour and Consumer Preferences - A Review. World’s Poultry Science Journal 2016, 72, 353–366. [CrossRef]
- Fairweather-Tait, S.; Hurrel, R.F. Bioavailability of Minerals and Trace Elements. Nutrition Research Reviews 1996, 9, 295–324.
- Grosicka-Maciąg, E.; Szumiło, M.; Kurpios-Piec, D.; Rahden-Staroń, I. Biomedical Effects of Selenium in a Human Organism. Journal of Elementology 2017, 22, 1269–1284. [CrossRef]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Official Journal of the European Union 2011, 54, 18–41.
- Lewis, J. Codex Nutrient Reference Values.; Food and Agriculture Organisation of the United Nations and World Health Organisation: Rome. Italy, 2019; ISBN 9781626239777.
| Item | Meat | Raw | Heat processing | SEM | Level of significance | |||||||
| Water bath cooking (WBC) |
Grilled (G) |
Oven convection roasting (OCR) |
Pan-fried (PF) |
Total | ||||||||
| Meat (M) |
Heat processing (HP) |
M x HP | ||||||||||
| Cooking loss (%) | without skin | - | 27.2b | 42.5a | 40.6a | 34.9b | 36.3Y | 1.84 | 0.001 | 0.001 | 0.032 | |
| with skin | - | 40.4b | 51.7a | 43.6ab | 45.7ab | 45.4X | 1.52 | |||||
| Total | - | 33.8B | 47.1A | 42.1A | 40.3A | 40.8 | 1.50 | |||||
| SEM | 3.43 | 2.15 | 0.82 | 2.50 | ||||||||
| Moisture (%) | without skin | 73.3 | 65.3 | 54.0 | 56.9 | 58.0 | 61.5 X | 1.88 | 0.018 | 0.001 | 0.084 | |
| with skin | 64.5 | 62.5 | 56.2 | 55.1 | 54.5 | 58.6 Y | 1.41 | |||||
| Total | 68.9A | 63.9A | 55.1B | 56.0B | 56.2B | 60.0 | 1.19 | |||||
| SEM | 2.66 | 1.30 | 0.68 | 0.78 | 1.21 | |||||||
| Ash (%) | without skin | 1.41 | 1.34 | 1.63 | 2.45 | 1.56 | 1.48 | 0.19 | 0.102 | 0.001 | 0.727 | |
| with skin | 1.38 | 1.06 | 1.63 | 1.96 | 1.23 | 1.25 | 0.16 | |||||
| Total | 1.39C | 1.20B | 1.63AB | 2.21A | 1.39B | 1.36 | 0.12 | |||||
| SEM | 0.07 | 0.18 | 0.07 | 0.20 | 0.13 | |||||||
| Ash retention (%) | without skin | - | 108.4 | 113.8 | 147.7 | 118.4 | 122.1 | 8.20 | 0.046 | 0.205 | 0.410 | |
| with skin | - | 112.1 | 96.5 | 111.2 | 81.4 | 100.3 | 5.96 | |||||
| Total | - | 110.2 | 105.1 | 129.4 | 99.9 | 111.2 | 5.45 | |||||
| SEM | 11.87 | 7.89 | 11.5 | 10.6 | ||||||||
| Item | Meat | Raw | Heat processing | SEM | Level of significance | |||||||
| Water bath cooking (WBC) |
Grilled (G) |
Oven convection roasting (OCR) |
Pan-fried (PF) |
Total | ||||||||
| Meat (M) |
Heat processing (HP) |
M x HP | ||||||||||
| Se (μg/100g FM) |
without skin | 17.4Bb | 20.2 | 24.3a | 26.7A | 24.9a | 21.8 | 1.01 | 0.559 | 0.001 | 0.001 | |
| with skin | 13.2B | 23.3A | 26.9A | 24.9A | 23.7A | 20.9 | 1.46 | |||||
| Total | 15.3B | 21.8A | 25.6A | 25.8A | 24.3A | 21.3 | 0.89 | |||||
| SEM | 0.99 | 1.22 | 1.29 | 0.96 | 1.12 | |||||||
| Se retention (%) |
without skin | - | 85.1 | 79.9 | 91.1 | 93.0 | 87.3 | 3.28 | 0.082 | 0.087 | 0.913 | |
| with skin | - | 113.4 | 101.5 | 111.4 | 99.2 | 106.4 | 8.61 | |||||
| Total | - | 99.3 | 90.7 | 101.3 | 96.1 | 96.8 | 4.92 | |||||
| SEM | - | 15.4 | 7.78 | 10.7 | 4.78 | |||||||
| Meat | Se [μg /100g] |
DACH (2015) AI (μg) |
EFSA (2014) AI (μg) |
HCNL (2014) NCM (2014) RI (μg) |
WHO/FAO (2004) RI (μg ) |
NIPH-NIH (2020) IOM (2000) EAR (μg) |
NIPH-NIH (2020) IOM (2000) RDA (μg ) |
NRV-R (μg) |
|||
| 60♀ | 70♂ | 70 ♀♂ | 50♀ | 60♂ | 25-26♀ | 33-34♂ | 45♀♂ | 55♀♂ | 60♀♂ | ||
| Raw meat without skin | 17.4 | 29.0 | 24.9 | 24.9 | 34.8 | 29.0 | 69.6-66.9 | 52.7-51.2 | 38.7 | 31.6 | 29.0 |
| Raw meat with skin | 13.2 | 22.0 | 18.9 | 18.9 | 26.4 | 22.0 | 52.8-50.8 | 40.0-38.8 | 29.3 | 24.0 | 22.0 |
| Water bath cooking without skin | 20.0 | 33.3 | 28.6 | 28.6 | 40.0 | 33.3 | 80.0-76.9 | 60.6-58,8 | 44.4 | 36.4 | 33.3 |
| Water bath cooking with skin | 23.3 | 38.8 | 33.3 | 33.3 | 46.6 | 38.8 | 93.2-89.6 | 70.6-68.5 | 51.8 | 51,8 | 38.3 |
| Grilled without skin | 24.3 | 40.5 | 34.7 | 34.7 | 48.6 | 40.5 | 97.2-93.5 | 73.6-71.5 | 54.0 | 44.2 | 40.5 |
| Grilled with skin | 26.9 | 44.8 | 38.4 | 38.4 | 53.8 | 44.8 | 107.6-103,5 | 81.5-79.1 | 59.8 | 48.9 | 44.8 |
| Oven convection roasting without skin | 26.7 | 44.5 | 38.1 | 38.1 | 53.4 | 44.5 | 106.8-102.7 | 80.9-78.5 | 59.3 | 48.5 | 44.5 |
| Oven convection roasting with skin | 24.9 | 41.5 | 35.6 | 35.6 | 49.8 | 41.5 | 99.6-95.8 | 75.5-73.2 | 55.3 | 45.3 | 41.5 |
| Pan-fried without skin | 24.9 | 41.5 | 35.6 | 35.6 | 49.8 | 41.5 | 99.6-95.8 | 75.5-73.2 | 55.3 | 45.3 | 41.5 |
| Pan-fried with skin | 23.7 | 39.5 | 33.9 | 33.9 | 47.4 | 39.5 | 94.8-91.1 | 71.8-69.7 | 52.7 | 43.1 | 39.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





