What this paper adds:
Chronic limb threatening ischemia (CLTI) is a serious medical condition that may have a wide range of severe consequences, and can lead to major amputation or death. This retrospective study evaluated the clinical outcomes of patients with CLTI and tissue loss treated with primary isolated femoral bifurcation endarterectomy (FBE) or FBE with bypass sugery. There is no significant difference between these two methods in terms of ulcer healing. Further prospective studies are needed.
1. Introduction
Chronic limb threatening ischemia (CLTI) with tissue loss is a serious medical condition that may have a wide range of severe consequences, and can lead to major amputation or death.1 CLTI is a clinical syndrome defined by the presence of the peripheral artery disease in combination with rest pain, gangrene, or a lower limb ulceration >2 weeks duration.2 The gold standard for the treatment of patients with CLTI due to lesions of the common femoral artery (CFA) and its bifurcation is femoral bifurcation endarterectomy (FBE) 3-8. In cases of CFA lesions combined with long superficial femoral artery (SFA) lesions, data regarding the best treatment are scarce.9 Some authors preferred primary isolated FBE10, whereas others advocated
FBE combined with bypass surgery.9 Now, the current guidelines recommend in-line flow via a targeted arterial pathway for optimal treatment of CLTI.2 This retrospective study aimed to evaluate the clinical outcomes of patients with CLTI and tissue loss treated with primary isolated FBE or FBE with bypass.
2. Material and Methods
2.1. Study Design
This retrospective study was conducted at a tertiary university-based care centre. A prospectively collected database of patients with CLTI and tissue loss who underwent primary isolated FBE (Group A) or primary FBE combined with bypass surgery (Group B) was analysed. The local ethics committee approved this study.
Patients with Global Limb Anatomic Staging System (GLASS)2 grade III and IV of femoropopliteal lesions who underwent primary isolated FBE or primary FBE combined with primary autologous vein surgical bypass (location below or above the knee) presented with intermediate or advanced limb-threatening conditions. The exclusion criteria were autologous bypass reconstruction combined with FBE to facilitate proximal anastomosis, composite or synthetic bypass grafts, additional intraoperative transluminal angioplasty, occluded deep femoral artery (DFA). We have excluded all DFA occlusions, which could not be treated endovascularly or with open surgery so that all patients included in our study had a patent DFA. Patients suffering from mixed or venous ulcers were also excluded.
The primary endpoint of this study was ulcer healing. The secondary endpoints were primary and secondary patency rates, limb salvage, and survival. All patients underwent preoperative peripheral vascular evaluation with physical examination, oscillography, and ankle brachial index (ABI) measurements. Vascular lesions were confirmed using duplex sonography (DS), computed tomography angiography (CTA), magnetic resonance angiography (MRA), and selective angiography. FBE alone or combined with bypass reconstruction was performed at the surgeon’s discretion. All procedures were performed under either general or epidural anaesthesia.
2.2. Definitions
Ulcer healing was defined as the complete epithelialisation of the tissue defect. Ulcers were considered non-healed if they failed to heal or in cases of major amputation. In patients who died before the lesion epithelialized, the ulcer was considered non-healed, with the date of death as the cut-off point.
Ulcers were categorised according to the Wound, Ischemia and foot Infection (WIfI) classification for peripheral arterial disease.2
Major amputation refers to amputation above the ankle. The limbs that required major amputation were not salvaged. Limbs that required minor amputation (toe, ray, or trans metatarsal) and healed were considered successful limb salvage.
Primary patency was defined as the period free from reintervention to maintain patency. Secondary patency was defined as the time from the first intervention to restoration of patency after occlusion. For hemodynamic improvement, ankle brachial index (ABI) should increase ≥ 0.10 or to an ABI ≥ 0.9.
Repeat target lesion reintervention (TLR) was measured by the frequency of the need to redo procedures due to a problem arising from the lesion, and repeat target extremity revascularization (TER) measured by the frequency of the need to redo procedures due to a problem arising remote from the lesion initially treated.
2.3. Lesion Anatomy
Lesions (occlusion or high-grade stenosis >70%) of the femoral bifurcation were divided into isolated CFA, combined CFA/proximal SFA, combined CFA/DFA, and combined CFA/DFA/proximal SFA.
2.4. Operative Technique
FBE and bypass procedures were performed under either general or regional anaesthesia. After preparation and exposure to CFA, 5000 IU of unfractionated heparin were administered. After clamping the femoral bifurcation, the CFA was incised, and endarterectomy was performed with Dacron or autologous patch graft angioplasty.
In cases of additional bypass surgery, veins were used as the graft material. The veins were evaluated using preoperative DS. Venous grafts included the greater saphenous, smaller saphenous, and arm veins. The grafts were used in reverse or non-reverse configurations after valve lysis.
After endarterectomy of the groin vessels, proximal anastomosis was performed with 6/0 Prolene using the continuous suture technique. Bypass were placed either anatomically or subcutaneously.
The distal anastomosis was reconstructed in an end-to-side or end-to-end fashion. Completion angiography was performed to ensure an adequate graft flow.
2.5. Follow Up
Patients were continually monitored during the ulcer healing process in an outpatient setting. Additionally, all patients were followed up at 1 week and, 1, 3, 6, and 12 months postoperatively. The attending surgeon evaluated the ulcer status and local management. Ulcer status and the time to complete healing were recorded. In cases of wound worsening or infection, antibiotic therapy was started or adapted, and the vessel status was re-evaluated by oscillography and DS and, if necessary, by CTA, MRA, or selective angiography.
The postoperative ulcer status was defined as stable, healed, or worse. The stable wounds were continuously monitored and bandaged. The healed wounds were completely epithelialized and no further dressing was required. Deteriorating wounds require antibiotic treatment and further re-evaluation of circulation (see above).
2.6. Statistical Analysis
Data consistency was checked and the data were screened for outliers. Continuous variables were also tested for normality using the Kolmogorov–Smirnov test. Cross-tabulation tables with Pearson's chi-squared and Fisher’s exact tests were used to compare discrete variables, and t-tests were used for continuously distributed variables. Kaplan–Meier analyses with log-rank tests were used to compare the FBE and FBE + bypass groups. Pointwise 95% confidence intervals (CI) for the time-to-event curves were computed. All reported tests were two-sided, and p-values < 0.05 were considered statistically significant. All statistical analyses were performed using STATISTICA 13 (Hill, T. & Lewicki, P. Statistics: Methods and Applications. StatSoft, Tulsa, OK) and NCSS (NCSS 10, NCSS, LLC. Kaysville, UT).
3. Results
A total of 133 patients who underwent surgery between 01/2008 and 12/2019 were included in this study. FBE (group A) was performed in 73 patients, and FBE + bypass (group B) in 60 patients. All 133 patients received duplex ultrasound (100%) in both groups, 105 patients (79%) received CTA and 28 patients (21%) received MRA respectively. Additionally, the selective arteriography was in 18 patients (13%) performed. Thirteen (21.6%) patients in group B recieved bypass above knee, and bypass below knee was in 47 (78.3%) patients performed. There were no significant differences between the groups in terms of demographic data, risk factors, comorbidities and preoperative ABI (
Table 1). Wound and foot infection grading in WIfI classification was equally distributed. There was also no significant difference between groups A and B regarding GLASS classification femoropopliteal or infrapopliteal, lesion anatomy of the femoral bifurcation (
Table 2). CFA occlusions were equally distributed between Group A and B (n = 28 vs n = 24, 38% vs 40%, p = 0.78) (
Table 2). There was no significant difference regarding mean length of CFA lesion, (2.65cm vs 2.52cm, p= 0.12 ) and no significant difference regarding mean diameter of treated CFA 6.48 vs 6.3mm p=0.41). The mean diameter od DFA was equal in both groups 5.75 vs 5.28mm (p=0.57).
The median follow-up time was 2.7 years (lower quartile: 1.38, upper quartile 4.56). It was completed by all patients, and none of them was lost to follow-up.
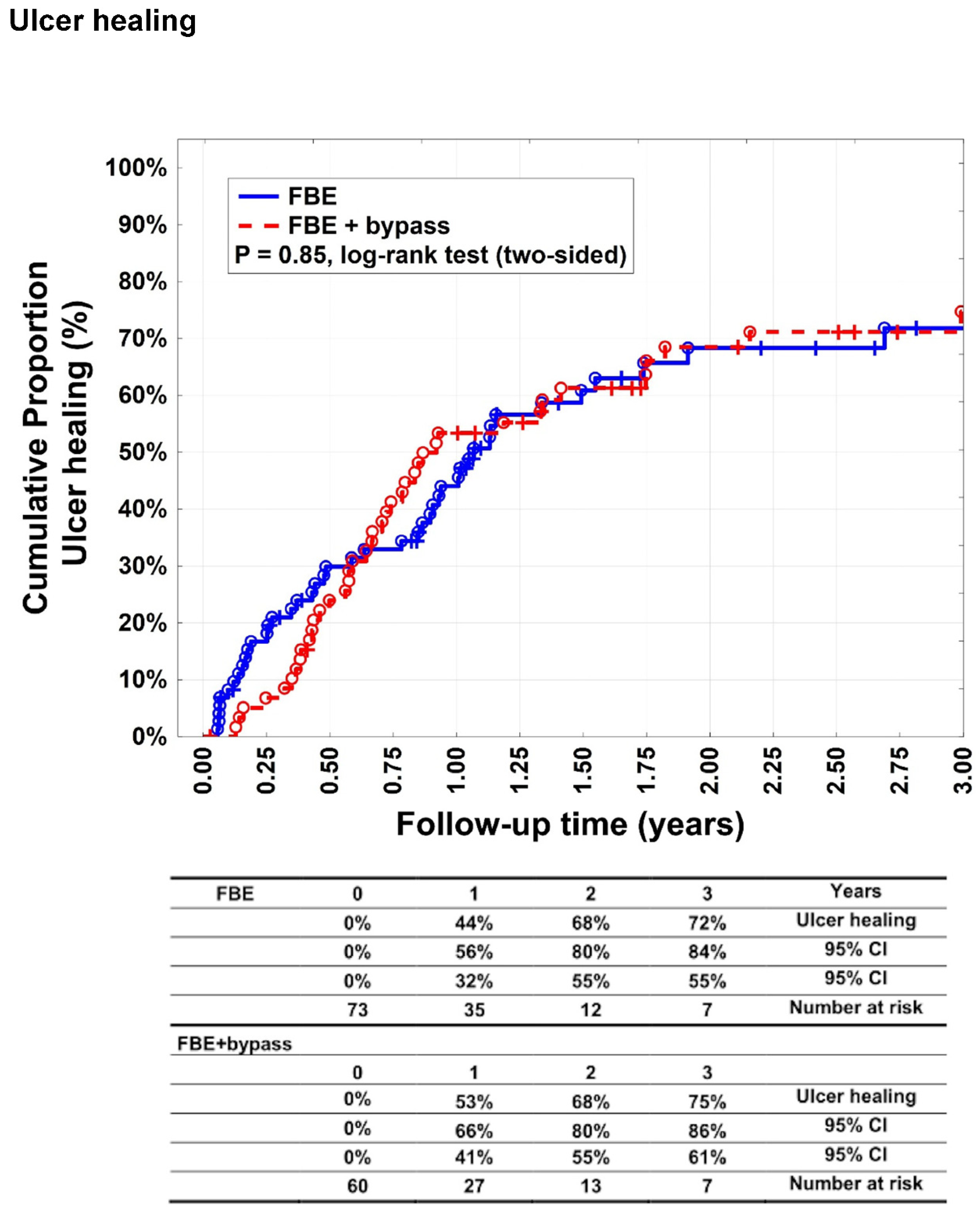
There was no statistically significant difference (p = 0.85) between groups A and B with respect to complete ulcer healing (
Figure 1). Complete ulcer healing was achieved in 44% (95% CI: 32–56%), 68% (95% CI: 55–80%), and 72 % (95% CI: 55–84%) of patients in Group A after 1, 2, and 3 years, respectively. In Group B, complete healing was observed in 53% (95% CI: 41–66%), 68% (95% CI: 55–80%), and 75% (95% CI: 61–86%) of patients after 1, 2, and 3 years, respectively.
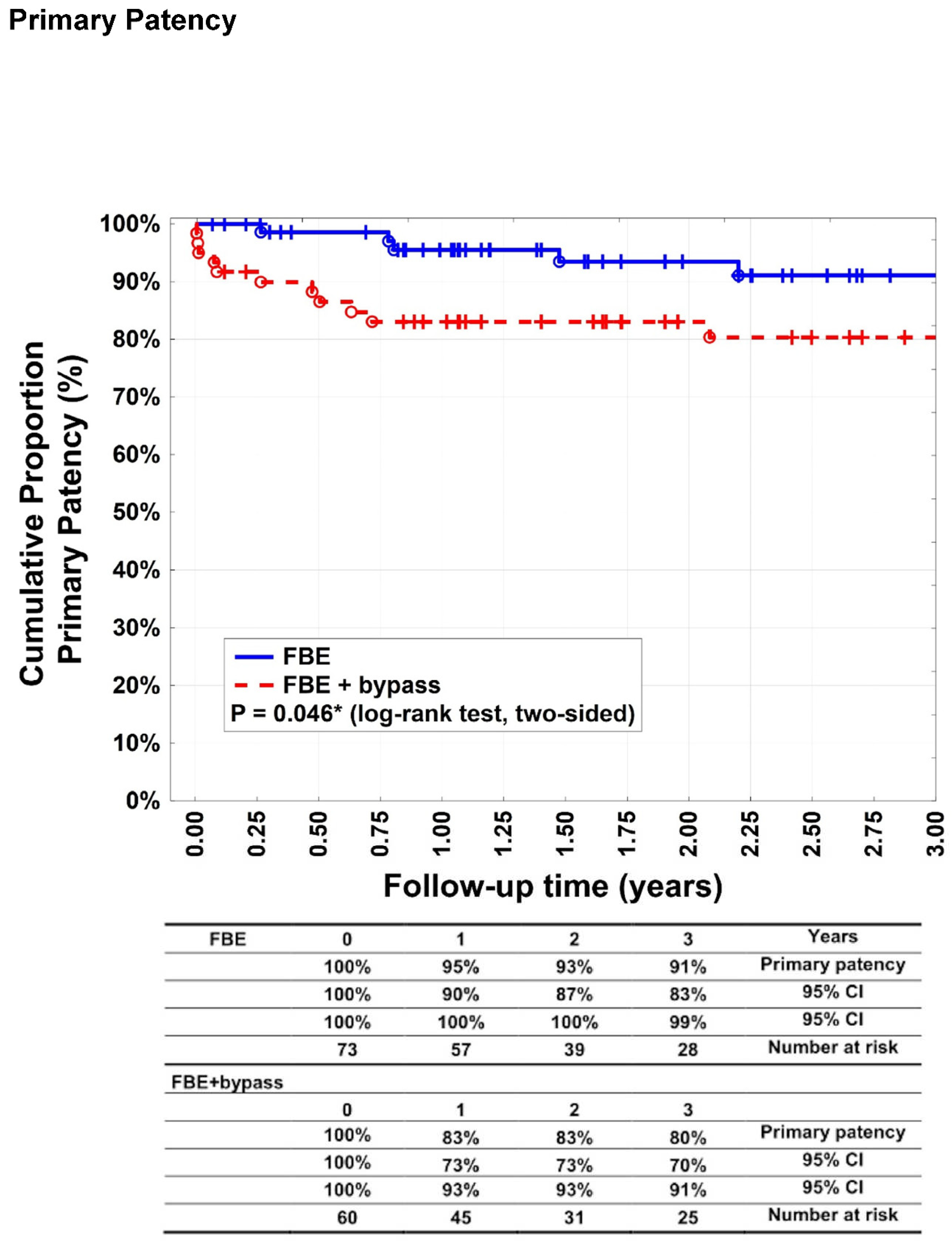
The primary patency rates (
Figure 2) after 1, 2, and 3 years were 95% (95% CI: 90–100%), 93% (95% CI: 87–100%), and 91% (95% CI: 83–99%), respectively, in group A patients. For group B, 83% (95% CI: 73–93%), 83% (95% CI: 73–93%), and 80% (95% CI: 70–91%), respectively (p= 0.046%). Secondary patency rates (
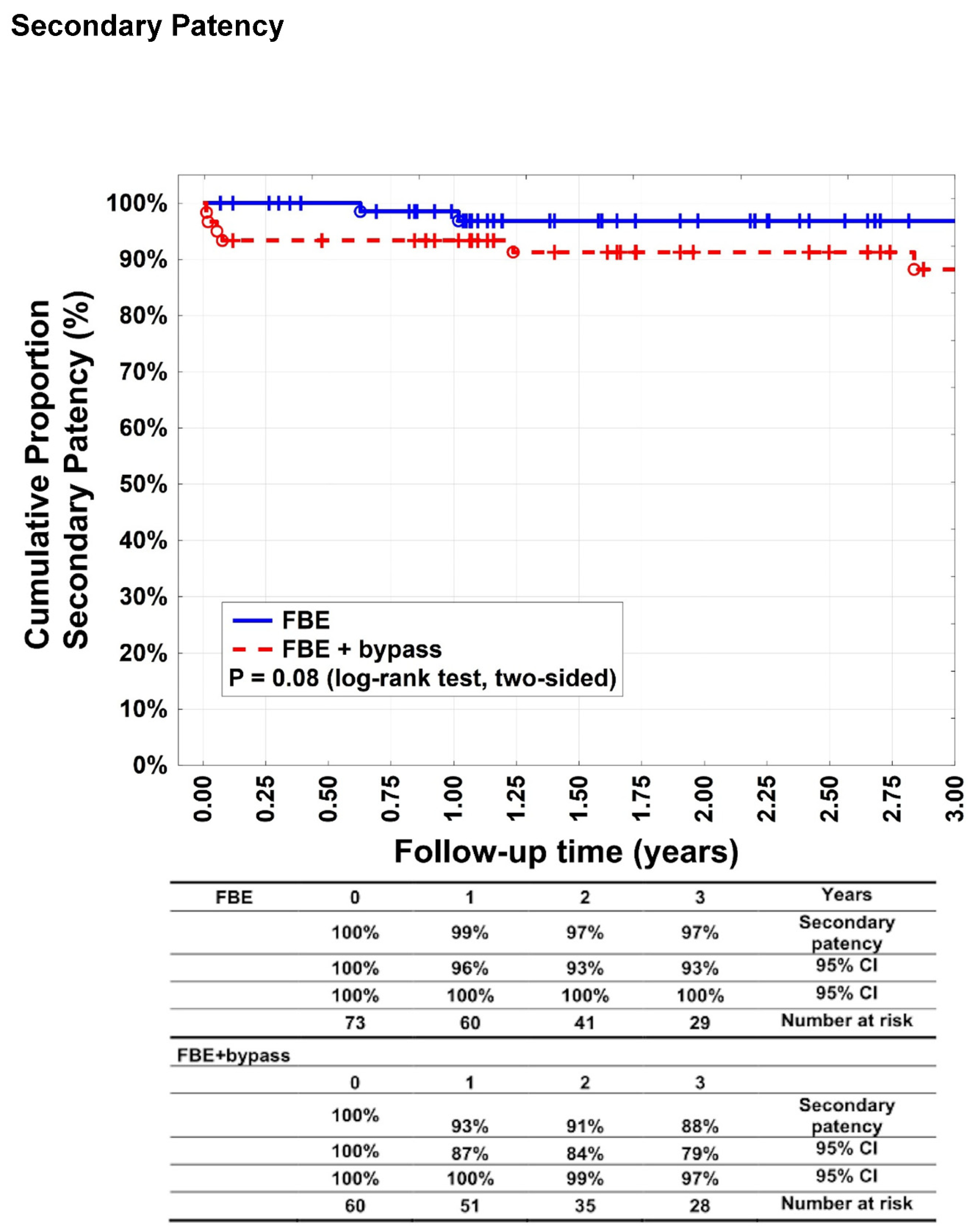
Figure 3) were 99% (95% CI: 96–100%), 97% (95% CI: 93–100%), and 97% (95% CI: 93–100%) after 1, 2, and 3 years, respectively, for patients in group A. For group B, 93% (95% CI: 87–100%), 91% (95% CI: 84–99%), and 88% (95% CI: 79–97%) after 1, 2, and 3 years, respectively (p = 0.08). Target lesion reinterventions were necessary in four patients (5.5%) in group A and 13 patients (21.6%) in group B (p = 0.0001).
Mean postoperative ABI was 0.71±0.33 in Group A and 0.82±0.2 in group B.This difference was statistically significant in interim analysis (p = 0.001).
We observed 11 (15%) surgical site infection (SSI) in Group A patients and 12 (20%) SSI in Group B. This was not statistically significant (p= 0.42). All SSI were minor and could be treated conservatively.
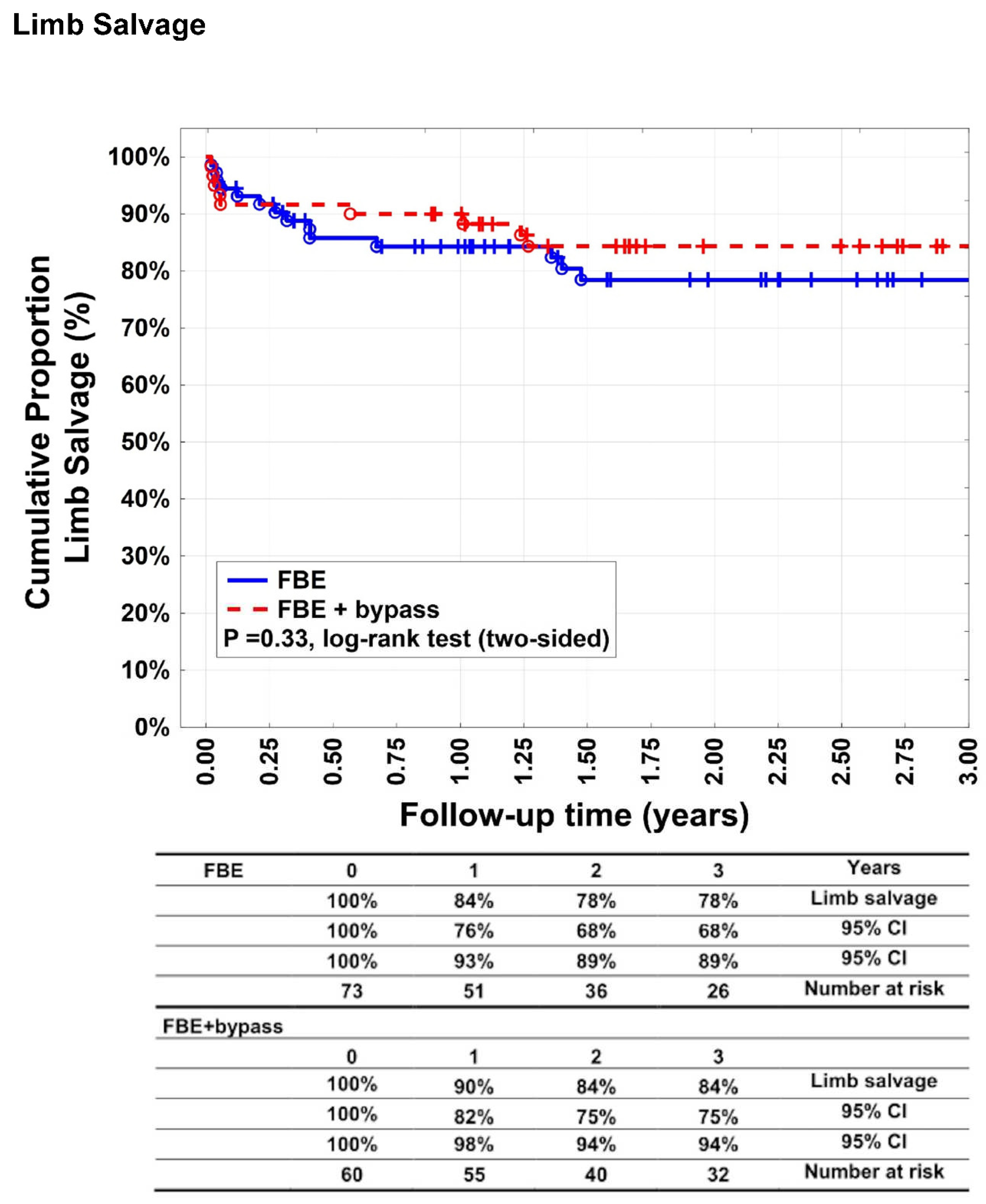
Target extremity revascularisation was indicated in 33 patients (45.2%) in group A and 23 patients (38.3%) in group B (p = 0.48). Twelve patients in group A (16.4%) of 33 which needed reintervention, needed an additional bypass surgery. Limb salvage rates (
Figure 4) after 1, 2, and 3 years were 84% (95% CI: 76–93%), 78% (95% CI: 68–89%), and 78% (95% CI: 68–89%) for group A patients and 90% (95% CI: 82–98%), 84% (95% CI: 75-94%) and 84% (95% CI: 75-94%) for group B patients (p=0.33). Fourteen patients (19.2%) underwent major amputation in group A versus nine patients (15%) in group B (p=0.64). Twenty patients (27.4%) underwent minor amputation in group A and 18 (30%) in group B (p = 0.84). Survival rates (
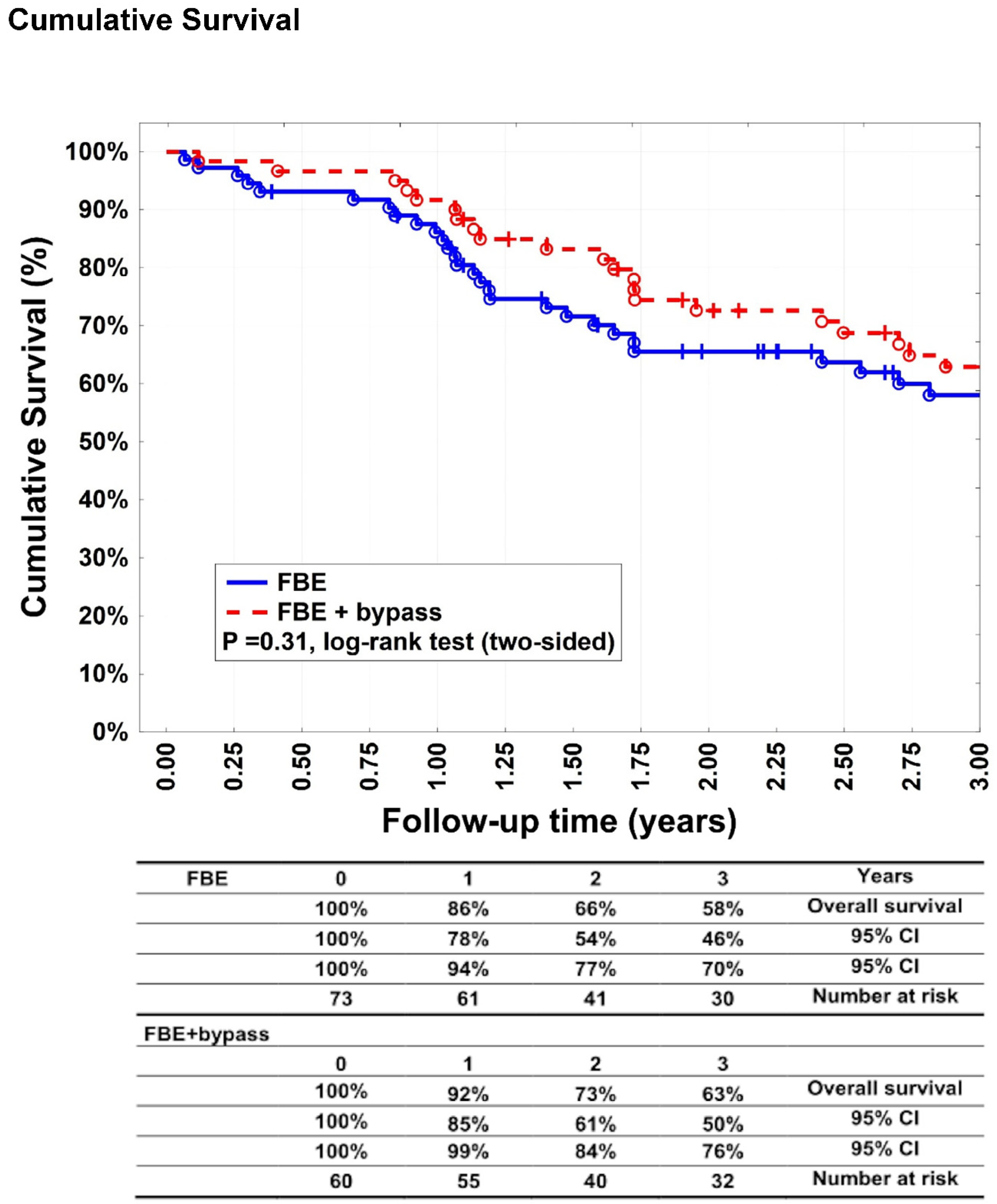
Figure 5) were 86% (95% CI: 78–94%), 66% (95% CI: 54–77%), and 58% (95% CI: 46–70%) after 1, 2, and 3 years, respectively, in group A. In group B, 92% (95% CI: 85–99%), 73% (95% CI: 61–84%), and 63% (95% CI: 50–76%) after 1, 2, and 3 years, respectively (p = 0.31). Most patients died of cardiovascular events.
4. Discussion
This retrospective study showed no differences in clinical outcomes (ulcer healing and major amputation rate); however, there was a significant difference in the primary patency rates and target lesion interventions in patients with tissue loss due to CLTI who underwent either primary isolated FBE or FBE combined with bypass surgery.
Currently, evidence regarding the preferential use of FBE alone or combined with bypass surgery in patients CFA lesions and long-segment SFA occlusion is lacking.9 Both methods are appropriate, and it is possible to choose one technique over another.
Malgor et al. compared isolated FBE and FBE combined with bypass surgery. In their retrospective study, which included Rutherford 3–6 patients with TASC A–D lesions, the authors found that patients with tissue loss and TASC D lesions profited from additional bypass procedures and that a low runoff score resulted in poor clinical outcomes.11 However, the populations in the latter study were not equally distributed (claudicants were more common in the FBE group). According to Zlatanovic et al, the femoropopliteal GLASS IV grade is similar to that of TASC D.12 In contrast, our study revealed no significant difference in patient characteristics or risk factors between groups A and B. Additionally, only patients with GLASS grade III and IV were observed. These patients can usually recommended to undergo open surgery.2,13
A study performed by Soden et al. compared the 1-year outcomes of patients who underwent bypass surgery and additional FBE in lower-extremity bypass. In their retrospective study, the authors compared patients who underwent lower-extremity bypass with FBE with those who underwent bypass alone. They demonstrated that patients who underwent an additional FBE with bypass had improved 1-year freedom from major amputation compared with those who underwent bypass only (91% vs. 87%). Their results confirmed that additional FBE improves limb perfusion through the DFA and its collaterals, which is the cause for a better outcome.9
The primary endpoint of our study was ulcer healing, which did not differ significantly between groups A and B (p = 0.85). After 3 years, ulcers completely healed in more than 70% of patients in groups A and B, and there was no statistical difference in major amputations (p = 0.64) or minor amputations (p = 0.84). Furuyama et al. reported a median ulcer healing time of 90 days after bypass revascularisation or endovascular treatment. In their retrospective study, they described the prognostic factors after arterial infrainguinal revascularisation for Rutherford class 5 critical limb ischaemia and assessed the efficacy of cilostazol therapy. Complete ulcer healing was achieved in 74% of patients, which is similar to our results.14 In their study, only patients with Rutherford class 5 were included, whereas we included patients with all ulcer categories according to WIfI classification.2,15 In our study, isolated FBE was as successful as FBE combined with bypass, in terms of ulcer healing.
Our study showed a significant difference between groups A and B in terms of primary patency rates after 1, 2, and 3 years (p = 0.046), whereas the secondary patency rates were equal. Primary and secondary patency rates > 90% in patients undergoing FBE have been previously reported and are consistent with our results.16,17. Surprisingly, secondary patency rates did not differ significantly between the groups and reached 88% in the bypass group, whereas the literature revealed that secondary patency rates of isolated autologous bypasses were worse,11,18,19 It is possible that obligatory FBE can enhance the bypass patency. Long-term patency rates after FBE with additional bypass revascularisation have rarely been reported.
El-Bakr et al. presented in their abstract at the IAVS & NIVASC Joint Annual Meeting in 2015 that for patients with TASC D lesions FBE and profundoplasty were sufficient to maintain adequate limb perfusion. In their study, high-risk patients presenting with ASA grade ≥ 3 with femoropopliteal TASC-D lesions were considered for limited FBE endarterectomy with profundoplasty without further revascularisation. They found that limb perfusion through the DFA is effective and could be a safe option in patients with a poor condition.20
In our study, patients in group B underwent significantly more target lesion reinterventions than those in group A patients (23% vs. 5.5%, p= 0.0001). Balotta et al. described freedom from revascularization after FBE of the ipsilateral extremity as 80% after 7 years.16 Kim et al. described endovascular femoropopliteal bypass reintervention rates of up to 21% after 1-year. 21 In another retrospective study, the reintervention rate for bypass was 30% during a 5-year follow-up period.12 The target extremity reinterventions were not significantly different between the groups in our study (p = 0.48).
According to the recent literature, it is unclear whether a patent DFA combined with an occluded SFA is sufficient for adequate ulcer healing in patients with CLTI. De Athayde Soares et al. showed that PTA of the DFA alone shows a similar limb salvage rate, patency rates, and survival, as patients undergoing PTA of the DFA and SFA.22 Our study showed that a patent DFA combined with SFA lesions was sufficient for adequate limb vascularisation.
Manenti et al. explained in their correspondence article of “the pathophysiology of the profunda femoris artery in chronic lower limb ischemia” that the DFA forms the main collateral pathway for the perfusion of the distal limb. According to these studies, in cases of chronic limb ischaemia, the DFA can progressively double in size because it is an elastic blood vessel. This may have been responsible for the formation of new collaterals in the DFA and its branches.23
Studies evaluating the revascularisation effect before and after FBE are limited. The extent to which collaterals that enable sufficient healing of the ulcer are built after FBE when the DFA is patent and SFA is occluded. Measuring and counting collaterals before and after FBE using angiography may be useful in prospective randomised studies.
This study is limited by its retrospective design. The choice of revascularisation type was operator dependent. Randomised trials are required to determine the best approach for treating CLTI with tissue loss.
5. Conclusion
FBE is a less invasive option for surgical repair of GLASS III and IV grade in patients with CLTI with tissue loss. It is a feasible option with good short- and mid-term results in patients with patent DFA. With the exception of primary patency, there was no significant difference in secondary patency, limb salvage, or survival rates between FBE and FBE with additional bypass. Our study suggests that FBE alone may be sufficient to achieve ulcer healing in a typically frail, elderly population and avoids the potential morbidity, necessity for venous conduit and prolonged surgery associated with distal bypass. As a less invasive procedure, FBE should be considered first for patients in a poor general condition.
Conflicts of Interest
The authors declare that there is no conflict of interests.
References
- Aitken SJ, Randall DA, Noguchi N, Blyth FM, Naganathan V. Multiple peri-operative complications are associated with reduced long term amputation free survival following revascularisation for lower limb peripheral artery disease: A population based linked data study. Eur J Vasc Endovasc Surg. 2020;59:437-445.
- Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur J Vasc Endovasc Surg. 2019 Jul;58(1S):S1-S109.e33. doi: 10.1016/j.ejvs.2019.05.006. Epub 2019 Jun 8. Erratum in: Eur J Vasc Endovasc Surg. 2020 Mar;59(3):492-493. Erratum in: Eur J Vasc Endovasc Surg. 2020 Jul;60(1):158-159. PMID: 31182334; PMCID: PMC8369495.
- Elsherif M, Tawfick W, Elsharkawi M, Campell R, Hynes N, Sultan S. Common femoral artery endarterectomy in the age of endovascular therapy. Vascular. 2018;26:581-590.
- Kuo TT, Chen PL, Huang CY, Lee CY, Shih CC, Chen IM. Outcome of drug-eluting balloon angioplasty versus endarterectomy in common femoral artery occlusive disease. J Vasc Surg. 2019;69:141-147.
- Setacci C, de Donato G, Teraa M, Moll FL, Ricco JB, Becker F, et al. Chapter IV: Treatment of critical limb ischaemia. Eur J Vasc Endovasc Surg. 2011;42 Suppl 2:S43-59.
- Kuma S, Tanaka K, Ohmine T, Morisaki K, Kodama A, Guntani A, et al. Clinical outcome of surgical endarterectomy for common femoral artery occlusive disease. Circ J. 2016;80:964-969.
- Nishibe T, Maruno K, Iwahori A, Fujiyoshi T, Suzuki S, Takahashi S, Ogino H, Nishibe M. The Role of Common Femoral Artery Endarterectomy in the Endovascular Era. Ann Vasc Surg. 2015 Nov;29(8):1501-7. doi: 10.1016/j.avsg.2015.05.005. Epub 2015 Jul 4. PMID: 26148640.
- Langenberg JCM, Te Slaa A, de Groot HGW, Ho GH, Veen EJ, Buimer TMG, van der Laan L. Infection Risk Following Common Femoral Artery Endarterectomy Versus a Hybrid Procedure. Ann Vasc Surg. 2018 Nov;53:148-153. doi: 10.1016/j.avsg.2018.03.046. Epub 2018 Jun 8. PMID: 29890219.
- Soden PA, Zettervall SL, Shean KE, Deery SE, Kalish JA, Healey CT, et al. Effect of adjunct femoral endarterectomy in lower extremity bypass on perioperative and 1-year outcomes. J Vasc Surg. 2017;65:711-719.e1.
- Peters AS, Meisenbacher K, Weber D, Bisdas T, Torsello G, Böckler D, et al. Isolated femoral artery revascularisation with or without iliac inflow improvement - a less invasive surgical option in critical limb ischemia. Vasa. 2021;50:217-223.
- Malgor RD, Ricotta JJ 2nd, Bower TC, Oderich GS, Kalra M, Duncan AA, et al. Common femoral artery endarterectomy for lower-extremity ischemia: evaluating the need for additional distal limb revascularization. Ann Vasc Surg. 2012;26:946-956.
- Zlatanovic P, Mahmoud AA, Cinara I, Cvetic V, Lukic B, Davidovic L. Comparison of long term outcomes after endovascular treatment versus bypass surgery in chronic limb threatening ischaemia patients with long femoropopliteal lesions. Eur J Vasc Endovasc Surg. 2021;61:258-269.
- Jaff MR, White CJ, Hiatt WR, Fowkes GR, Dormandy J, Razavi M, et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: A supplement to the inter-society consensus for the management of peripheral arterial disease (TASC II): The TASC steering committee. Catheter Cardiovasc Interv. 2015;86:611-625.
- Furuyama T, Onohara T, Yamashita S, Yoshiga R, Yoshiya K, Inoue K, et al. Prognostic factors of ulcer healing and amputation-free survival in patients with critical limb ischemia. Vascular. 2018;26:626-633.
- Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517-538.. Erratum in: J Vasc Surg 2001;33:805.
- Ballotta E, Gruppo M, Mazzalai F, Da Giau G. Common femoral artery endarterectomy for occlusive disease: an 8-year single-center prospective study. Surgery. 2010;147:268-274.
- Kang JL, Patel VI, Conrad MF, Lamuraglia GM, Chung TK, Cambria RP. Common femoral artery occlusive disease: contemporary results following surgical endarterectomy. J Vasc Surg. 2008;48:872-877.
- Chang H, Veith FJ, Rockman CB, Cayne NS, Jacobowitz GR, Garg K. Non-reversed and reversed great saphenous vein graft configurations offer comparable early outcomes in patients undergoing infrainguinal bypass. Eur J Vasc Endovasc Surg. 2022;63:864-873.
- Nierlich P, Enzmann FK, Metzger P, Dabernig W, Aspalter M, Akhavan F, et al. Alternative venous conduits for below knee bypass in the absence of ipsilateral great saphenous vein. Eur J Vasc Endovasc Surg. 2020;60:403-409.
- El-Bakr A, Tawfick Wael, Tubassam M. Limited common femoral endarterectomy, & profundoplasty as an effective option in limb threatening ischaemia. A minimalistic approach in high-risk patients. Eur J Vasc Endovasc Surg. 2015;50:e19.
- Kim TI, Zhang Y, Cardella JA, Guzman RJ, Ochoa Chaar CI. Outcomes of bypass and endovascular interventions for advanced femoropopliteal disease in patients with premature peripheral artery disease. J Vasc Surg. 2021;74:1968-1977.e3.
- De Athayde Soares R, Matielo MF, Brochado Neto FC, Martins Cury MV, Matoso Chacon AC, Nakamura ET, et al. The importance of the superficial and profunda femoris arteries in limb salvage following endovascular treatment of chronic aortoiliac occlusive disease. J Vasc Surg. 2018;68:1422-1429.
- Manenti A, Roncati L, Manco G, Zizzo M, Farinetti A. Pathophysiology of the profunda femoris artery in chronic lower limb ischemia. Ann Vasc Surg. 2021;77:e2-e3.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).