1. Introduction
Due to the scarcity of land resources and the augmented economic growth demand for mineral resources, the deep-sea mining has become a hot research topic. However, the mineral resources are mainly distributed in the submarine sediments located thousands of meters deep in the ocean. The complexity and variability of the marine environment lead to the higher technical requirements for the detection and mining of mineral resources as well as environmental assessment [
1,
2,
3,
4]. During deep-sea mining, the subsidence, migration and transformation of submarine sediments can cause the migration of heavy metals, and the heavy metals are dissolved in water, which can lead to the marine pollution. Therefore, it is of momentous practical significance to model and simulate the migration and transformation of heavy metals for the marine environment evaluation.
In the last years, the migration and transformation of heavy metals pollutants in natural water and laboratory have been investigated. Generally, the heavy metals pollutants are enriched in sediment particles, which are thought to be the most essential carrier of heavy metals pollutants following water. Taking into account the effects of convection and diffusion on the concentration of heavy metals pollutants particle phase, Huang et al. [
5] established a mathematical model for the transport and transformation of heavy metals pollutants in fluvial rivers. In addition, the migration and transformation of heavy metals pollutants in a steady, uniform equilibrium sediment-laden flow was also simulated in [
6]. Considering the adsorption process of heavy metals on sediment, He et al. [
7] constructed an equilibrium model and a non-equilibrium model, respectively. By means of the hydrodynamic model and sediment transport model, Periáñez [
8] built a model considering the interaction of water, sand and metal to study the diffusion laws of heavy metals. Moreover, Wang et al. [
9] developed a coupled model based on environmental fluid dynamics code and applied it to Taihu Lake, and the results show that the transformation trend of the heavy metals pollutants is similar with that of sediment ones. Horvat et al. [
10] devolved a two-dimensional numerical model suiting approach to simulate the complex flow, sediment transport and heavy metals transport conditions in natural watercourses. By assessing the performance of the distributed hydrological model for simulating the transport of various heavy metals in rivers, Bouragba et al. [
11] utilized a hydrological model to numerically calculate the migration of multiple heavy metals, i.e. Pb, Hg, Cr, and Zn, in the Harrach Rivera. Zeng et al. [
12] studied the dissolution of heavy metals in the mining transportation process, and investigated its impact on seawater quality. Igoni et al. [
13] estimated the levels of heavy metals Mg, Cd, and Ni using a one-dimensional transport model in dry and rainy seasons in the Marine-Base waterfront. However, the dissolution and transportation of heavy metals pollutants are very complex due to the deep-sea environment. Therefore, it is necessary to establish an accurate model to describe the dissolution and transportation of heavy metals pollutants.
Lattice Boltzmann method (LBM) was proposed in the mid-1980s. Based on the kinetic theory, LBM has the clear physical background and distinct features, including program simplicity, easy implementation, and natural parallelism [
15]. In contrast with traditional methods, such as finite difference method, finite element method and spectral method, LBM is a mesoscopic numerical simulation method. It regards the motion of all particles as a whole, and their motion characteristics are expressed by distribution functions. So, LBM is used to study the mechanism of various complex phenomena, and it has been widely used in the engineering simulation, fluid mechanics, material engineering and environmental engineering [
16,
17,
18,
19]. LBM particularly is extensively concerned by scholars who research the mathematical and physical equations as convection-diffusion equations, diffusion equations, fractional Cahn-Hilliard equations [
20,
21,
22,
23]. Therefore, LBM can be adopted to solve the model for describing the dissolution and transportation of heavy metals pollutants during deep-sea mining.
In this work, the coupling model is established to describe the dissolution and transportation of heavy metals pollutants during deep-sea mining, and the LBM is adopted to solve the model simulation numerically. The paper is arranged as follows: the transfer-transformation model of heavy metals pollutants during deep-sea mining is constructed in
Section 2. In
Section 3, we will present the Lattice Boltzmann model to recover macroscopic equations by Chapman-Enskog analysis. Then
Section 4 focuses on the numerical simulation. Finally, the conclusions are given in
Section 5.
2. Transfer-Transformation Model of heavy metals Pollutants in Deep-Sea Mining
During deep-sea mining, heavy metals exist in seawater, suspended sediment and seabed sediment in a certain proportion. And the migration of heavy metals depends on the motion laws of the dissolved phase, suspended phase and sediment phase. The dissolution and transportation of heavy metals are affected by the convection-diffusion process, adsorption-desorption process and sediment settlement and resuspension.
2.1. Convection-diffusion model of heavy metals in water
According to the basic principle of water environment, the convective migration flux can be express as
where
fx and
fy are the convective migration flux of heavy metals in
x and
y directions, respectively.
ux and
uy are the velocity components of water in
x and
y directions, and
Cw stands for the concentration of heavy metals in water.
Based on the Fick 's first law, the mass flux of diffusion is proportional to the gradient of concentration,
where
Ix and
Iy represent the diffusive flux of heavy metals in
x and
y directions, respectively.
Ex and
Ey are the diffusion coefficients. Based on the conservation law of mass, the convection-diffusion equation is
2.2. Model of adsorption-desorption process
Most heavy metals are adsorbed by suspended sediment, and the desorption process is an inverse process of adsorption. The final trend of adsorption-desorption process is the decrease of heavy metals concentrations. The quantity of adsorption-desorption isotherm and kinetic models have been researched [
24,
25,
26]. The typical Langmuir kinetic model is adopted in this paper,
where
N is the adsorption content of heavy metals of unit weight of sediment.
k1 represents the coefficient of adsorption rate, and
k2 represents the coefficient of desorption rate.
b is the maximum absorption capacity of sediment.
2.3. Model of sediment settlement and resuspension
It is a natural purification process of natural water that the suspended particles suspended in water are deposited to form sediment due to gravity. However, due to the change of water environment, sediment may be suspended in water again, which causes secondary pollution. Therefore, it is of great significance to study the settlement and resuspension of suspended solids in environmental protection research. According to the principle of sediment engineering, the sedimentation process of heavy metals in suspension solids and the resuspension of sediment are described as follows [
27],
where
Hi is the average depth, and
u denotes the velocity of water.
Kw represents the comprehensive influence constant.
w0 means velocity of sedimentation, and
K stands for the resuspension coefficient.
m=4, and
n=2.
The suspended sediment is the carrier for the transport and transformation of heavy metals pollutants. The coupling model for transport and transformation of heavy metals is established by describing the transport of heavy metals with dissolved phase, suspended phase and sediment phase. For the dissolved and suspended phases affected by the migration of water, the adsorption and desorption of heavy metals are determined by suspended sediment and seabed sediment. For the sediment phase, the diffusion effect of heavy metals can be ignored, and the adsorption and desorption are considered. Based on [
6], the improved coupling model for the migration process of heavy metals during deep-sea mining can be expressed as,
where
Cw,
Cs,
Cd represent the concentrations of heavy metals in dissolved phase, suspended phase and sediment phase, respectively.
u=(
ux,
uy,
uz) is convection coefficient, and
v=(
vx,
vy,
vz) is diffusion coefficient. The suspended sediment concentration is denoted as
ss.
p is the porosity.
k1w and
k2w are expressed as adsorption rate coefficient and desorption rate coefficient of suspended solids.
k1b and
k2b represent adsorption rate coefficient and desorption rate coefficient of sediment.
bw and
bb are the saturation adsorption in suspended solids and sediment, respectively.
3. Lattice Boltzmann model for migration-transformation of heavy metals
In this section, the LBM is adapted to recover the macroscopic equations which are required in Eq. (6).
3.1. Lattice Boltzmann model
Applying the LBGK collision operator, the evolution equation with a source term can be written as
where
is the distribution functions in the
direction of the
equation.
is the equilibrium distribution function.
denotes the dimensionless relaxation time. And
satisfies the following conditions,
where
.
And the source term satisfies the following conditions,
3.2. The chapman-Enskog analysis
Applying the Taylor expansion to Eq. (7), the evolution equation can be expressed as,
Through the Chapman-Enskog analysis, the derivatives of time and space, and distribution function are expanded as,
denoting
and substituting (11) into (10), one can obtain
On the basis of (12), we can derive the following equation in order of
,
Combined with the conditions satisfied by the equilibrium distribution function, the macroscopic equations are accurately recovered. Additionally, the finite difference method (FDM) is applied to be compared with LBM.
and
are the space sizes and time sizes. Denote
,
and the difference scheme of Ref. [
6] can be derived as
where
4. Numerical simulations for the migration-transformation of heavy metals in deep-sea mining
In this section, the numerical simulations for the migration-transformation of heavy metals pollutants in the dissolved phase, suspended phase and sediment phase are presented, and the proposed method is compared with the FDM. In addition, the concentration variation of heavy metals as time and distances is analyzed.
4.1. Spatial-temporal distribution of heavy metals concentrations
In this research, the Cadmium (Cd) is selected as an example, and some physical parameters are shown in Table [
6].
Table 1.
The physical parameters.
Table 1.
The physical parameters.
| Physical quantities |
parameters |
Physical quantities |
parameters |
| ss
|
1.8378 kg/m3
|
k1w
|
0.0076 L/(mg·s) |
|
k1b
|
7.6e-4 L/(mg·s) |
k2w
|
0.00084 L/s |
|
k2b
|
8.4e-5 L/s |
bb |
5.34 g/m2
|
| K |
1.1e-6 |
bw |
0.534 mg/g |
| Kw |
9.0e-5 |
p |
0.5 |
In the simulations, and The relaxation time The boundary concentration which indicates the concentration of continuous discharge of polluted water flow. The diffusion and convection coefficients are and , respectively.
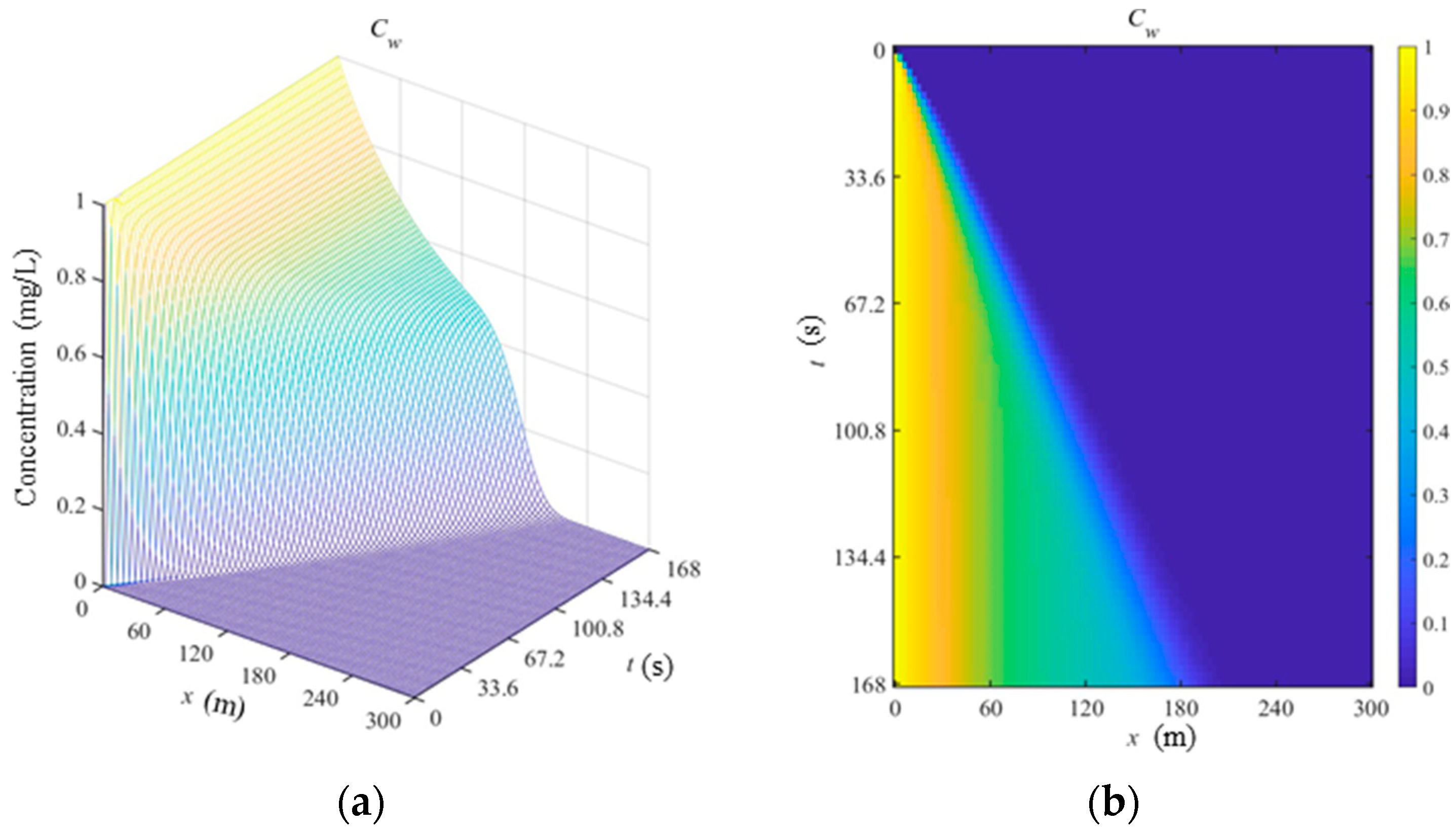
Based on the established coupling model and LBM, the simulated concentration distribution of Cadmium in the dissolved phase, suspended phase and sediment phase are shown in
Figure 1,
Figure 2 and
Figure 3. As shown in
Figure 1 (b), the concentration of Cadmium is decreased along x axis when the time is fixed. The pollution range is increased with time. The concentration of Cadmium rapidly increases to a certain value, and then gradually tend to balance value.
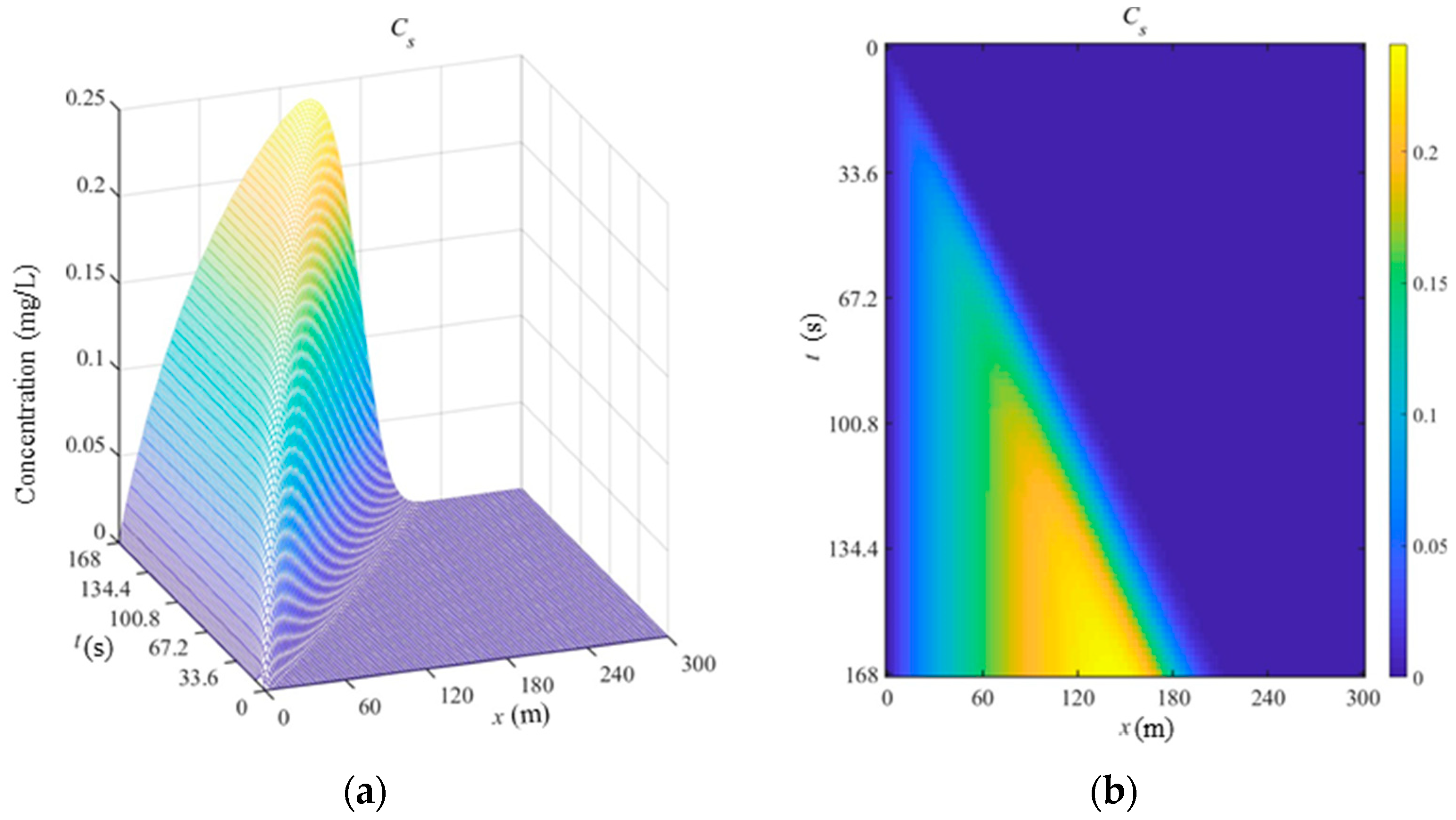
The spatial-temporal concentration distribution of Cadmium in the suspension phase is shown in
Figure 2 (a). As shown in
Figure 2(b), the concentration of Cadmium is firstly increased and then decreased when the time is fixed. At the same location, the change rule of concentration in the suspension is similar with that in the dissolved phase. However, the position where the concentration reaches the highest point is gradually moving forward due to the spread of polluted flow, and the concentration is also constantly augmented.
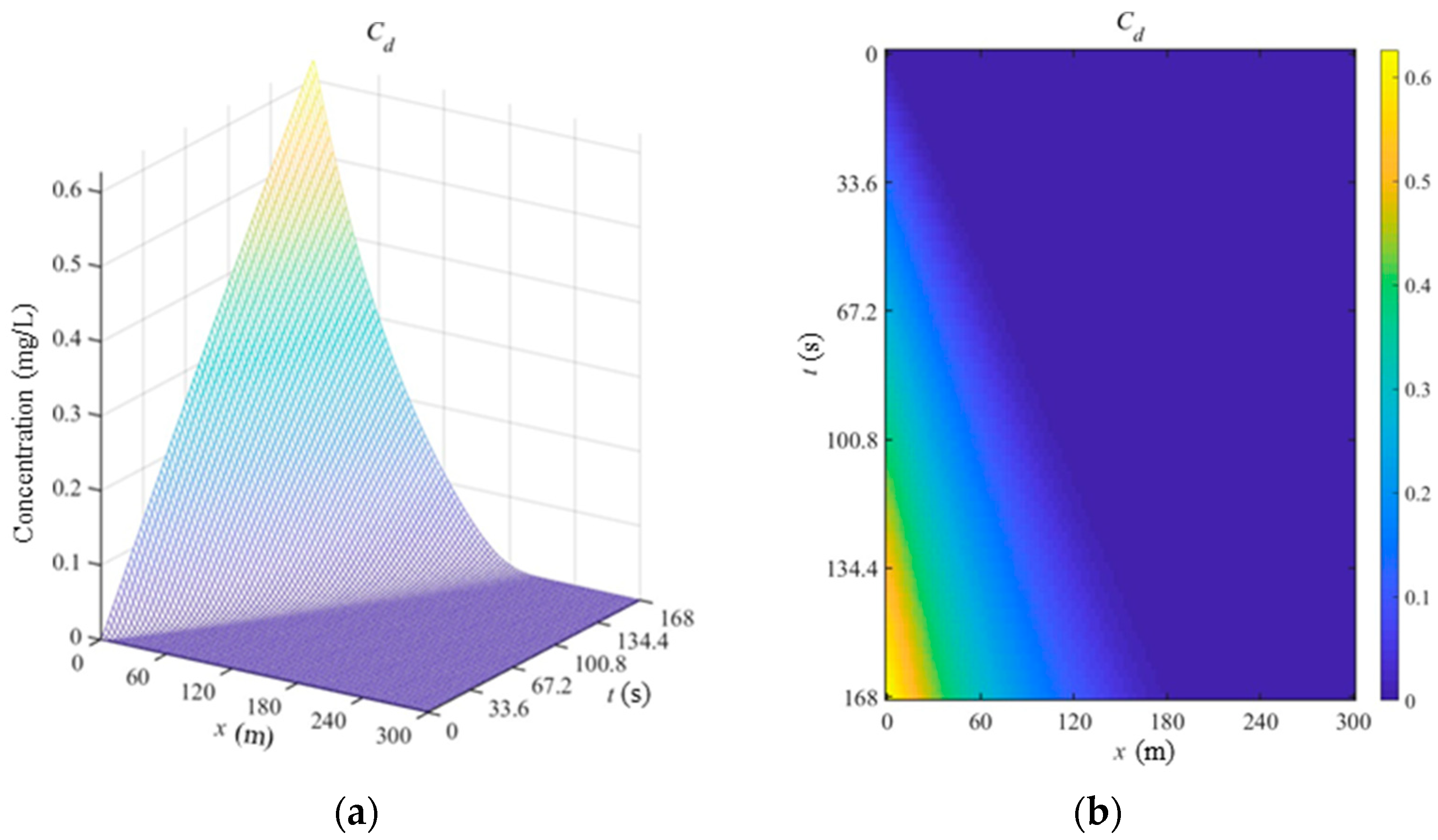
The spatial-temporal concentration distribution of Cadmium in sediment phase is shown in
Figure 3. Obviously, the change rule of concentration is simple. The concentration of Cadmium is decreased along x axis when the time is fixed, but the concentration of Cadmium is increased at the same location with more time for the continuous spread of polluted flow.
4.2. Compare with finite difference schemes
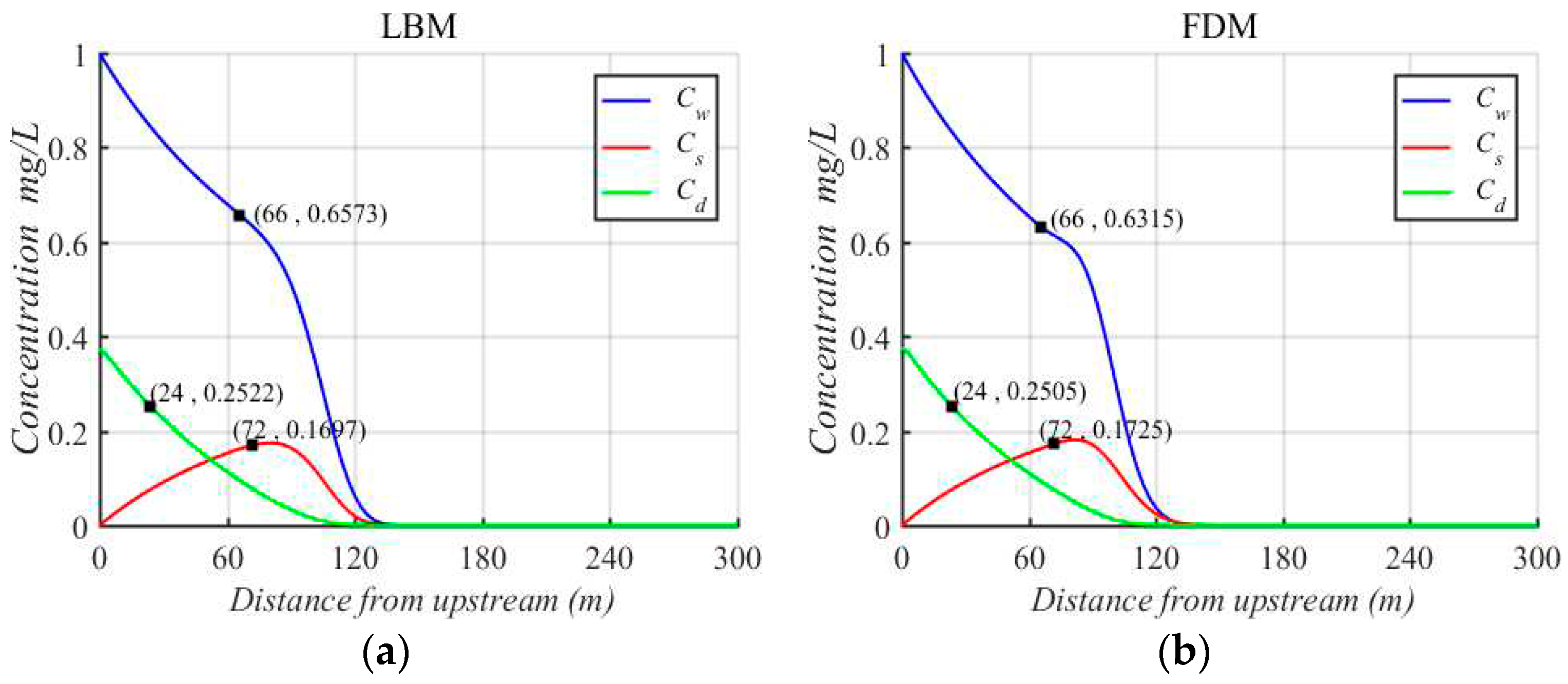
The concentration variations of Cadmium in different phases simulated by LBM are compared with those simulated by FDM. From
Figure 4, the concentration variations of Cadmium by LBM and FDM are nearly consistent. In addition, three location spots of the dissolved phase (66 m), suspended phase (72 m) and sediment phase (24 m) are selected to compare the concentrations of Cadmium between LBM and FDM, and the errors are very small. However, the time consumption of LBM is less than that of FDM. This is because that the time step
of FDM is smaller to satisfy the stability conditions.
4.3. Analysis for the concentration variation of heavy metals
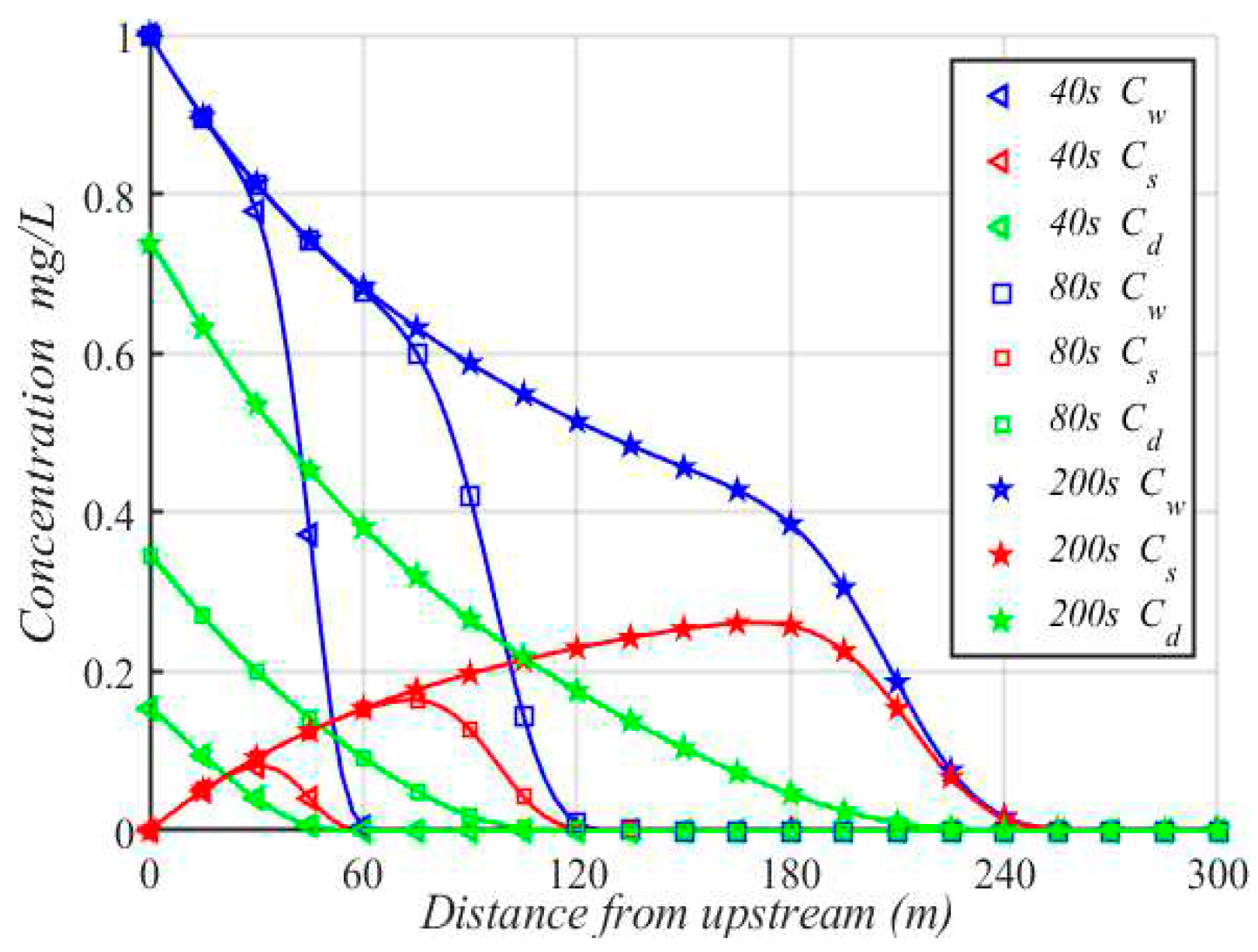
The concentration variations of Cadmium vs. distance are shown in
Figure 5. Obviously, the concentration of Cadmium is decreased with increases of distance in the dissolved phase. However, the decrease trend becomes slower near the peak, which is caused by rapidly moving and slower settling of dissolved phase. The concentration of Cadmium initially increases and then gradually decreases in the suspended phase, and the concentration of Cadmium near the peak is always at the highest point. This is because that the suspended sediment moves along with the flow, and the heavy metals have enough time to be absorbed by the suspended sediment. In the sediment phase, the sediment particles nearly do not move with the flow, so the concentration of sediment deposited at the entrance is the highest, and the concentration decreases with the increase of distance from the upstream.
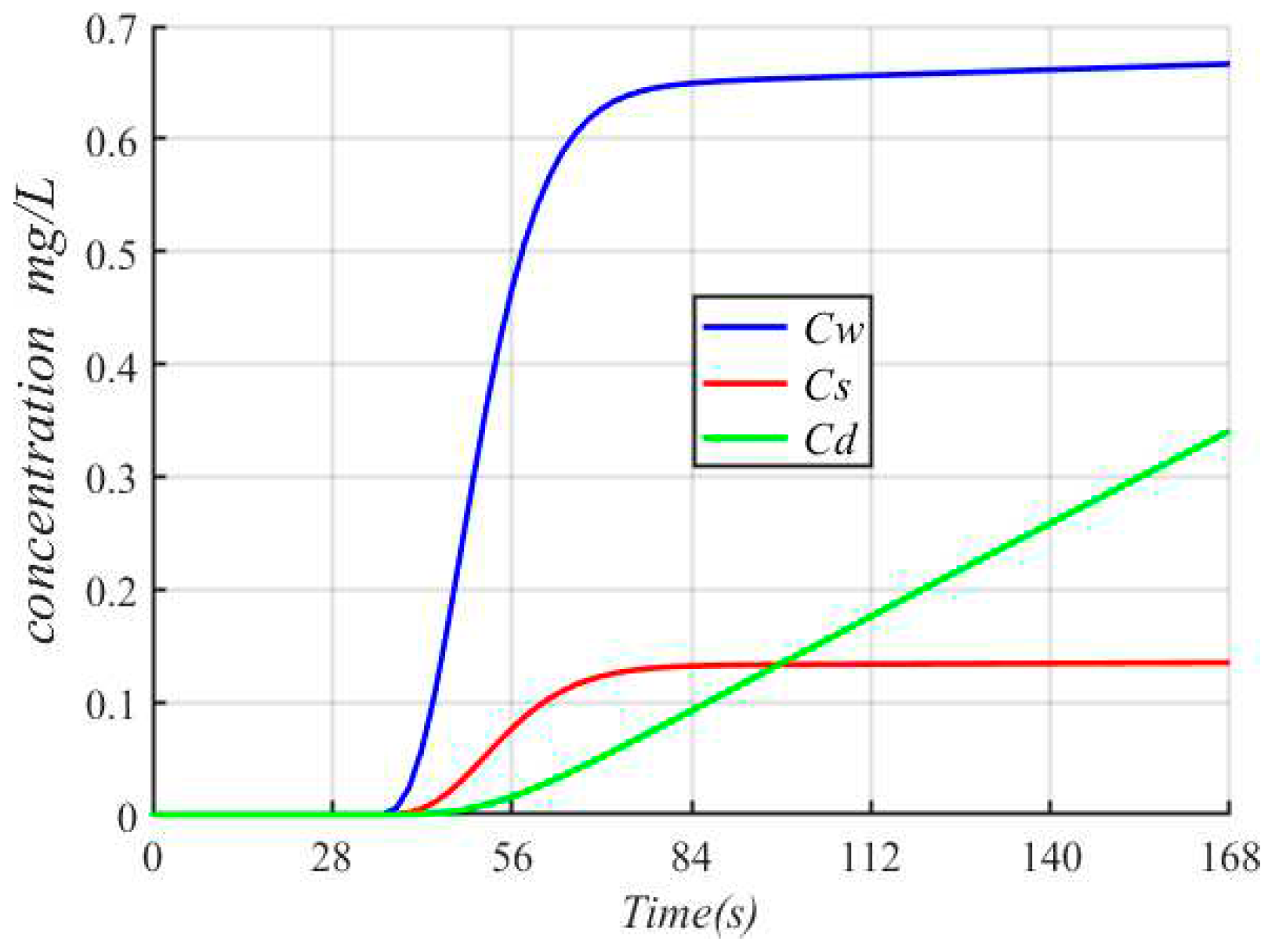
The concentration variations of Cadmium vs. time is shown in
Figure 6, and the observation location is 20 m away from upstream. The concentration of Cadmium is 0 mg/L, when the polluted water has not spread to this position. The concentration of Cadmium is increased as the polluted flow spreads over time. The concentration of Cadmium in the dissolved phase is gradually increased and then reaches equilibrium, which is the similar with that in the suspension phase, but the concentration of Cadmium in the suspension phase is slowly increased. This is because that the polluted water flows continuously, and the heavy metals absorbed in the suspended phase migrates follow polluted water. In addition, the concentration of Cadmium in the sediment phase is increased, because of the low migration rate of sediment and the little influence of convective diffusion.
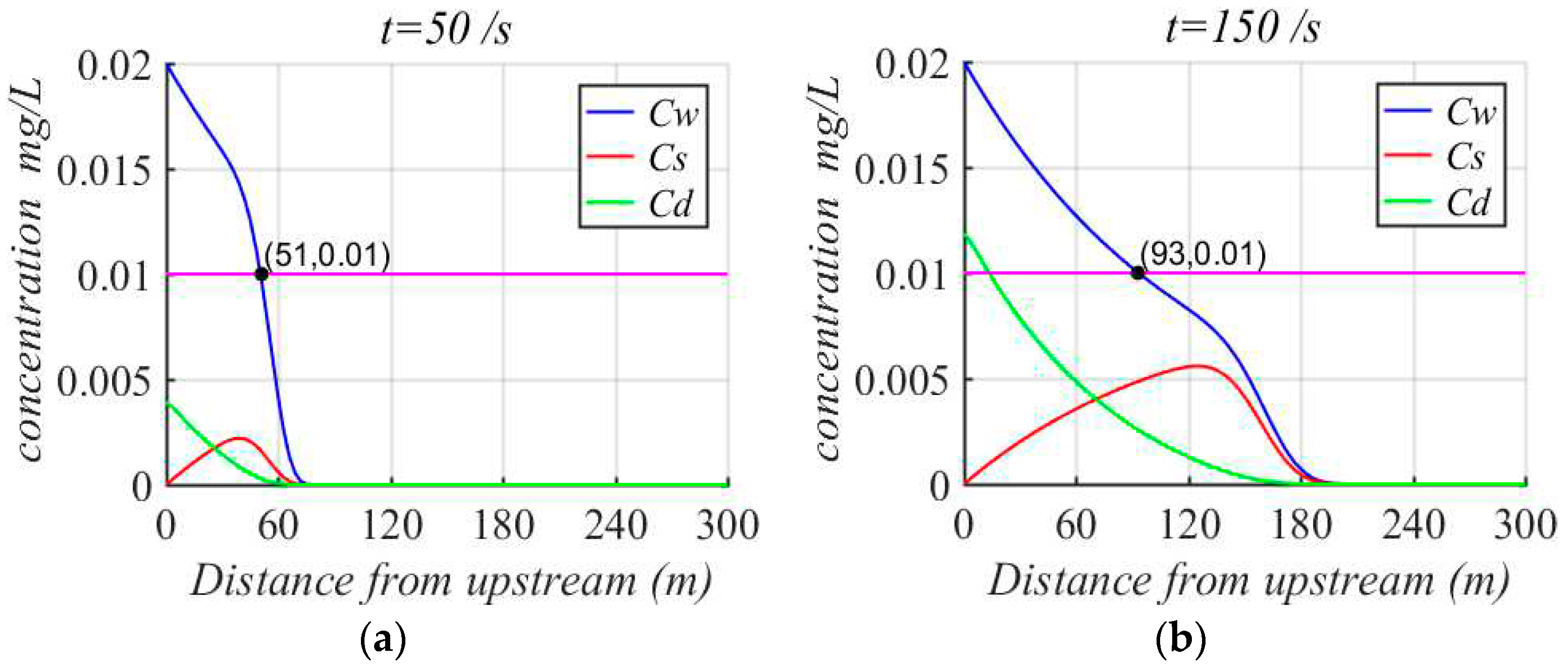
The effect of heavy metals on the water quality is simply investigated by the proposed model. Clearly, the pollution range in the dissolved phase is expanded with time. When the concentration of Cadmium at the entrance is 0.02 mg/L, the concentration of Cadmium in the dissolved phase is less than 0.01 mg/L at the location beyond 51m at 50s, as shown in
Figure 7 (a). The concentration of Cadmium in the suspension phase is not exceeding 0.01mg/L within 150 s, as shown in
Figure 7(b). Although the concentration of Cadmium is more than 0.01 mg/L in the sediment phase, there is almost no diffusion of Cadmium in sediment. So the concentration of Cadmium at the location beyond 93 m in the sea can satisfy the water quality standard. Therefore, the pollution of heavy metals should be controlled according to the mining time and the initial concentration of heavy metals, which can guarantee the water quality to meet the standards within a certain range during deep-sea mining.
5. Conclusion
In this paper, the convection-diffusion, adsorption-desorption, and the sedimentation-resuspension of heavy metals are analyzed during deep-sea mining, and the coupling model is established to simulate the migration of heavy metals in the dissolved phase, suspended phase and sediment phase. The LBM is used to numerically solve the coupling model. Compared with FDM, the LBM takes less time, and the errors between LBM and FDM are very small. The spatial/temporal distribution and movement regularity of Cadmium are investigated by the developed model. The concentration variations of Cadmium with time and distance are discussed and analyzed in detail, which can provide the reasonable bases for the evaluation of Cadmium pollution. In addition, the developed model can be used to describe the migration of other heavy metals pollutants during deep-sea mining.
Author Contributions
Conceptualization, L.Y. and D.C.; Resources, C.D. and H.D.; Writing— Original draft preparation, L.Y., Y.Y. and X.W.; Data curation, Y.Y. and H.D.; Writing-Reviewing and Editing, D.C., H.D. and J.Z.; Supervision, J.Z. and H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Project of China (2016YFC0304105); Natural Science Foundation of Hunan Province(2021JJ30548); Graduate Research Innovation Project of Jishou University(JGY202140).
Conflicts of Interest
The authors declare no conflicts of interest
References
- Jankowski, J.A.; Malcherek, A.; Zielke, W. Numerical modeling of suspended sediment due to deep-sea mining. J. Geophys. Res-Oceans 1996, 101, 3545–3560. [CrossRef]
- Tran-Duc, T; Phan-Thien, N; Khoo, B.C. A three-dimensional smoothed particle hydrodynamics dispersion simulation of polydispersed sediment on the seafloor using a message passing interface algorithm. Phys. Fluids 2019, vol. 31, no. 4, pp. 043301. [CrossRef]
- Ma, W; Schott, D; van Rhee, C. Numerical calculations of environmental impacts for deep sea mining activities. Sci. Total Environ 2019, vol. 652, pp. 996-1012. [CrossRef]
- Ma, W; van Rhee C; Schott, D. A numerical calculation method of environmental impacts for the deep sea mining industry a review. Environ. Sci-Proc, 2018, vol. 20, no. 3, pp. 454-468. [CrossRef]
- Huang, S.L; Wan, Z.H; Smith, P. Numerical modeling of heavy metal pollutant transport-transformation in fluvial rivers. J. Hydraul. Res 2007, vol. 45, no. 4, pp. 451-461. [CrossRef]
- Huang, S.L. Equations and their physical interpretation in numerical modeling of heavy metals in fluvial rivers. Sci. China Technol. Sc 2010, vol. 53, no. 2, pp. 548-557. [CrossRef]
- He, Y; Li, Y.T. Study of the model of heavy metal pollutants transport. Adv. Water Sci 2004, vol. 15, no. 5, pp. 576-583.
- Periáñez, R. Environmental modelling in the Gulf of Cadiz: heavy metal distributions in water and sediments. Sci. Total. Environ 2009, vol. 407, no. 10, pp. 3392-3406. [CrossRef]
- Wang, C; Shen, C; Wang, P.F; Qian, J; Hou, J; Liu, J.J. Modeling of sediment and heavy metal transport in Taihu Lake, China. J. Hydrodyn 2013, vol. 25, no. 3, pp. 379-387. [CrossRef]
- Horvat, Z; Horvat, M. Two dimensional heavy metal transport model for natural watercourses. River Res. Appl 2016, vol. 32, no. 6, pp. 1327-1341. [CrossRef]
- Bouragba, S; Komai, K; Nakayama, K. Assessment of distributed hydrological model performance for simulation of multi-heavy-metal transport in Harrach River, Algeria. Water Sci. Technol 2019, vol. 80, no. 1, pp. 11-24. [CrossRef]
- Zeng, J; Zhao, Q.L; Yu, K.P; Xiao, G.G; Chen, C. Impact of deep-sea mining of manganese nodules on seawater quality. Min. Metall. Eng 2019, vol. 39, no. 6, pp. 78-80+84.
- Igoni, A.H; Tegu, T.B; Okparanma, R.N. Estimation of Mg, Cd, and Ni levels in urban waterfront using one-dimensional transport model. Int. J. Water Resour. Environ. Eng 2020, vol. 12, no. 4, pp. 71-80.
- Safdari Shadloo, M. Numerical simulation of compressible flows by lattice Boltzmann method. Numer. Heat Tr. A-Appl 2019, vol. 75, no. 3, pp. 167-1829. [CrossRef]
- Mohamad, A.A. Lattice Boltzmann Method. Springer, London 2011.
- Wei, Y.K; Hu, X.Q. Two-dimensional simulations of turbulent flow past a row of cylinders using lattice Boltzmann method. Int J Comp Meth-Sing 2017, vol. 14, no. 1, pp. 1750002. [CrossRef]
- Dai, H.P; Chen, D.D; Zheng, Z.S. Modelling the sintering neck growth process of metal fibers under the surface diffusion mechanism using the Lattice Boltzmann method. Metals-Basel 2019, vol. 9, no. 5, pp. 614. [CrossRef]
- Zhu, Y; Tian, F.B; Young, J; Liao, J.C; Lai, J. A numerical study of fish adaption behaviors in complex environments with a deep reinforcement learning and immersed boundary lattice Boltzmann method. Sci. Rep-UK 2021, vol. 11, no. 1, pp. 1-20. [CrossRef]
- Jiang, F; Matsumura, K; Liao, K.P; Ohgi, J; Chen, X. Simulation of fluid-structure interaction problems with thin elastic plate via the coupling of finite element and lattice Boltzmann methods. Int J Comp Meth-Sing 2020, vol. 17, no. 10, pp. 2050013. [CrossRef]
- Du, R; Liu, Z.X. A lattice Boltzmann model for the fractional advection-diffusion equation coupled with incompressible Navier-Stokes equation. Appl. Math. Lett 2020, vol. 101, pp. 106074. [CrossRef]
- Dai, H.P; Zheng, Z.S; Tan, W. Lattice Boltzmann model for the Riesz space fractional reaction-diffusion. Therm. Sci 2018, vol. 22, no. 4, pp. 1831-1843. [CrossRef]
- Du, R; Sun, D.K; Shi, B.C; Chai, Z.H. Lattice Boltzmann model for time sub-diffusion equation in Caputo sense. Appl. Math. Comput 2019, vol. 358, pp. 80-90. [CrossRef]
- Liang, H; Zhang, C.H; Du, R; Wei, Y.K. Lattice Boltzmann method for fractional Cahn-Hilliard equation. Commun. Nonlinear Sci 2020, vol. 91, pp. 105443. [CrossRef]
- Huang, S.L; Wan, Z.H; Wang, L.X. Study on the effects of concentrations of heavy metals in sediment and initially in water phase on their adsorption. Acta Sci. Circumstantiae 1995, vol. 15, no. 1, pp. 66-76.
- Huang, S.L. Adsorption of cadmium ions onto the yellow river sediment. Water Qual. Res. J. Can 2003, vol. 38, no. 2, pp. 413-432. [CrossRef]
- Bai, B; Rao, D.Y; Chang, T; Guo, Z.G. A nonlinear attachment-detachment model with adsorption hysteresis for suspension-colloidal transport in porous media. J. Hydrol 2019, vol. 578, pp. 124080. [CrossRef]
- Dou, M; Ma, J.X; Xie, P; Li, G.Q. Numerical simulation for the transformation process of heavy metal contamination transport in rivers. Water Resources and Power 2007, vol. 25, no. 3, pp. 22-25.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).