1. Introduction
Cardiogenic shock (CS) is characterized by a significant reduction in cardiac output, leading to inadequate end-organ perfusion resulting in multiorgan failure and consequently associated with extremely high mortality [
1,
2]. The predominant etiology of CS is acute myocardial infarction (AMI), which accounts for over 80% of cases and often precipitates critical dysfunction of the left, the right or both ventricles [
3].
While mechanical circulatory devices (MCS) are considered as last resort for maintaining circulation in CS, recent trials have not shown any benefit remaining with limited evidence concerning their indication [
4]. Data collected from randomized clinical trials regarding safety, efficacy and the optimal timing of MCS device delivery is scarce and the largest trials IABP-SHOCK II [
5] and ECLS shock [
6] failed to prove superiority of MCS use when considering survival rates [
5]. Further safety concerns were raised considering stroke, major bleeding complications and mortality [
7,
8].
Still, despite not having any clear evidence to support a beneficial impact on clinical outcomes, MCS is widely used in the treatment of CS [
9]. As the sole potential tool for maintaining systemic blood perfusion in patients with refractory CS, its utilization has generated conflicting data across various shock center registries. Nonetheless, this data does suggest that adhering to a standardized, multidisciplinary treatment algorithm for MCS implementation could lead to improved survival rates [
10,
11]. Still, the optimal timing for MCS initiation remains uncertain [
11].
While RCTs are the best tool to generate scientific evidence, registries have the potential to shed new light on real world application. Considering these aspects, the aim of our present analysis was to understand, in the context of real-world setting, whether the timing of the MCS implantation correlates with short-term outcome. Analysis was performed based on the PREPARE CS Registry [
12].
2. Materials and Methods
The PREPARE CS, a single-center prospective registry conducted from May 2019 to April 2023, enrolled all consecutive patients with cardiogenic shock as classified by SCAI stages C-E who were referred to the cardiac catheterization laboratory. Cardiogenic shock was identified based on criteria indicative of prolonged hypoperfusion and need for vasoactive medication for maintaining sufficient perfusion pressure [
2].
In this analysis we focused on a subset in whom CS was confirmed to be caused by myocardial infarction, indicating PCI. In conjunction with coronary revascularization, all patients in the present analysis received adjunct MCS. According to our centers practice, MCS devices incorporated into this study were either the Impella® CP Heart Pump (Impella; Abiomed Inc., Danvers, MA, USA) or a veno-arterial extracorporeal membrane oxygenator (VA-ECMO; Xenios AG, Heilbronn, BW, Germany). Patients were categorized into two groups according to the timing of MCS relative to PCI: the 'Upfront' group, receiving MCS prior to revascularization, and the 'Procedural' group, receiving MCS at any time after the PCI has been started. The analyses primary endpoint was in-hospital mortality.
Statistical Analysis
All analyses were performed with Prism GraphPad 9.0 (GraphPad Software Inc., California, US). Summary descriptive statistics are reported as mean ± SD or n (%), as appropriate. Normal distribution was tested by D’Agostino-Pearson omnibus normality test. Continuous variables were compared by Mann-Whitney tests or Kruskal-Wallis test and categorical variables were compared with Fisher’s exact or chi-square tests, as appropriate. A probability value of p<0.05 was considered as significant.
3. Results
Between May 2019 and April 2023 406 patients underwent percutaneous revascularization due to acute myocardial infarction associated CS (SCAI Classes C-E). Among these patients, any MCS was utilized in 71 (17%) cases.
In 33 (46%) of the cases, MCS was implanted before PCI (Upfront group), whereas in 38 (54%) of the patients, it was started at any point after the start of the procedure (Procedural group). In the Upfront group the mean age was 67 ± 10 years while in the Procedural group 62 ± 11 years (p=0.05). Both groups were mainly represented by male patients (76% vs 82%, respectively; p=0.57). Cardiovascular risk factors and baseline characteristics were found as comparable between the two groups.
Table 1.
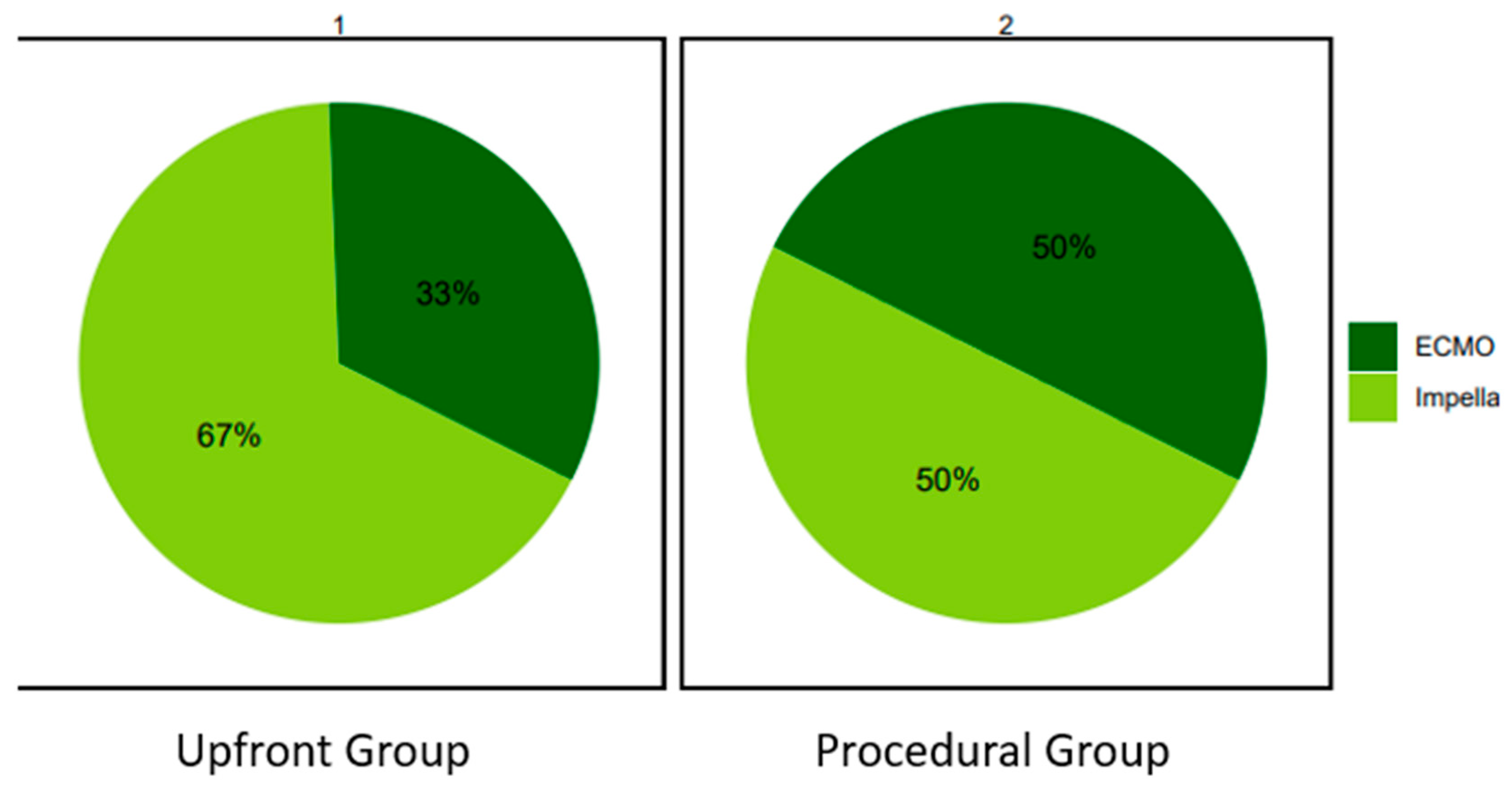
In the Upfront group an Impella was more frequently used compared to ECMO (67% vs 33%). In the Procedural group the proportion was similar (50% vs 50%).
Figure 1.
The vast majority of patients had multivessel coronary artery disease (MVD) in both groups (82% vs 84%, respectively; p=0.99). However, MVD-PCI was performed only in 45% and 42%, respectively (p=0.81). Accordingly, complete revascularization has been achieved in 52% and 34% of the cases, respectively (p=0.16).
Complex coronary artery interventions, defined by extensive calcification, bifurcation lesion, large volumes of contrast use or prolonged procedural time, were performed in the majority of cases in both groups. However, these were more frequent in the Upfront group (94% vs 71%, respectively; p=0.02). Detailed procedural aspects are presented in
Table 2.
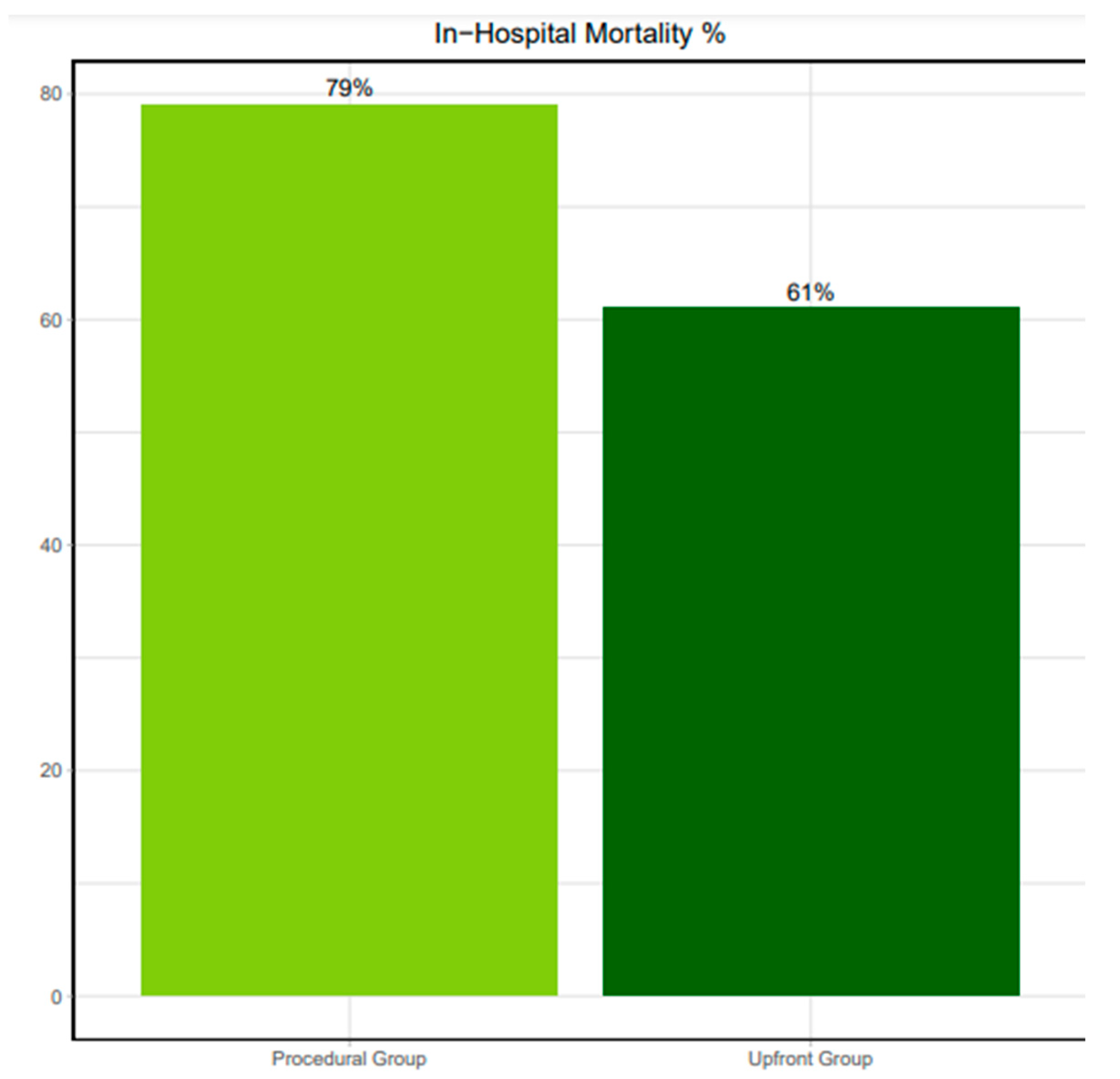
Comparing patients with Upfront- versus Procedural MCS, periprocedural CPR was significantly more frequent in the latter (45% vs 79%, p<0.05). Still, in-hospital mortality remained similar in both groups (61% vs 79%, respectively; hazard ratio 1.55 [0.93 to 2.46]; p=0.12).
Figure 2.
4. Discussion
Present data, including similar proportion of patients, who received the device either before or after the start of the PCI, suggest that timing of MCS implantation has no impact on in-hospital survival of patients with myocardial infarction-related CS.
Given these findings, coupled with the absence of definitive evidence or clear guidelines for MCS in AMI-related CS [
13], several questions naturally arise that could influence decision-making in everyday practice. Firstly, the impact of MCS intervention on the acute phase of CS and its subsequent effects on prognosis and mortality remains uncertain. Secondly, the optimal timing for MCS deployment is still undetermined, lacking clear guidance from existing literature. Lastly, choice of the best available device [
14,
15] remains unclear. In light of these uncertainties, MCS currently holds a Class IIa recommendation in the European Guidelines [
16].
Multiple RCTs have addressed the question of a potential benefit for the use of MCS devices in AMI patients [
5,
6,
17,
18]. Landmark ECLS-SHOCK trial, which addressed the impact of extracorporeal life support on mortality in patients presenting with MI complicated by CS, failed to demonstrate a benefit of MCS compared to medical therapy alone in terms of 30-days survival [
6].
On the other hand, smaller studies suggest an association between early utilization of MCS and improved early hemodynamics, survival rates and prognosis in patients presenting with AMI-CS [
19,
20]. A further retrospective study conducted on 64 AMI-CS patients, randomized to IABP and Impella, showed that patients receiving Impella pumps before the PCI procedure, experienced a reduction in infarct size as well as an improved myocardial recovery at 6 months follow-up [
21]. The same study highlighted the importance of an early MCS strategy in reducing reperfusion injury and left ventricular wall stress post-AMI, while reducing the need for high-dose inotropes. These advantages were particularly evident when accompanied by a lower rate of MCS-related complications.
The literature suggest that an early phase delivery of these devices was associated with improved clinical outcomes [
22,
23,
24]. Moreover, reduction in mortality was reported by Basir et al [
19] when MCS was initiated within 90 minutes after the onset of the cardiogenic shock. For STEMI patients presenting with CS, early diagnosis of the condition with a short time to coronary reperfusion, the so called “door to support” time, similar to the ‘time-is-muscle’ paradigm [
25], is key for improving survival and became routine in the management workup [
26]. Since a reduction in all-cause mortality was observed by implementation of MCS devices pre-PCI or even before initiation of vascular resuscitation, actual studies support the concept of door to support time in ACS-CS patients [
27].
5. Limitations
An important limitation to our study was the relatively small number of patients. Given the non-randomized nature, device selection, as well as the timing of MCS delivery were at the discretion of the operators, resulting in certain treatment biases.
6. Conclusions
Real-life dataset did not show statistical benefit in in-hospital mortality, when MCS is introduced prior coronary intervention.
Author Contributions
Conceptualization, Dan Prunea, Lukas Herold, Dirk Von Lewinski and Gabor Toth-Gayor; Data curation, Dan Prunea, Eva Bachl and Dirk Von Lewinski; Formal analysis, Dan Prunea, Eva Bachl, Lukas Herold, Andreas Praschk and Gabor Toth-Gayor; Investigation, Dan Prunea, Eva Bachl, Lukas Herold, Sadeek Kanoun Schnur, Dirk Von Lewinski and Gabor Toth-Gayor; Methodology, Dan Prunea, Lukas Herold, Sascha Pätzold, Andreas Praschk, Dirk Von Lewinski and Gabor Toth-Gayor; Resources, Eva Bachl, Lukas Herold, Dirk Von Lewinski and Gabor Toth-Gayor; Software, Andreas Praschk and Gabor Toth-Gayor; Supervision, Dirk Von Lewinski and Gabor Toth-Gayor; Validation, Dan Prunea, Lukas Herold, Sadeek Kanoun Schnur, Sascha Pätzold, Dirk Von Lewinski and Gabor Toth-Gayor; Visualization, Dan Prunea, Eva Bachl, Lukas Herold, Sadeek Kanoun Schnur and Dirk Von Lewinski; Writing – original draft, Dan Prunea; Writing – review & editing, Dan Prunea, Eva Bachl, Lukas Herold, Sadeek Kanoun Schnur, Sascha Pätzold, Siegfried Altmanninger-Sock, Gudrun Sommer, Theresa Glantschnig, Ewald Kolesnik, Markus Wallner, Klemens Ablasser, Heiko Bugger, Eva Buschmann, Andreas Praschk, Friedrich M. Fruhwald, Albrecht Schmidt, Dirk Von Lewinski and Gabor Toth-Gayor. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee).
Conflicts of Interest
Conflict of interest: GT receives consultancy fees and unrestricted research support from Abbott, Abiomed, Biotronik, Medtronic and Terumo. No other authors report any COI.
References
- Tehrani BN, Truesdell AG, Psotka MA, et al. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC Heart Fail. 2020;8(11):879-891. [CrossRef]
- Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015;36(20):1223-1230. [CrossRef]
- Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40(32):2671-2683. [CrossRef]
- Levy B, Bastien O, Bendjelid K, et al. Experts’ recommendations for the management of adult patients with cardiogenic shock. Ann Intensive Care. 2015;5(1):17. [CrossRef]
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. New England Journal of Medicine. 2012;367(14):1287-1296. [CrossRef]
- Thiele H, Zeymer U, Akin I, et al. Extracorporeal Life Support in Infarct-Related Cardiogenic Shock. New England Journal of Medicine. 2023;389(14):1286-1297. [CrossRef]
- Amin AP, Spertus JA, Curtis JP, et al. The Evolving Landscape of Impella Use in the United States Among Patients Undergoing Percutaneous Coronary Intervention With Mechanical Circulatory Support. Circulation. 2020;141(4):273-284. [CrossRef]
- Dhruva SS, Ross JS, Mortazavi BJ, et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump With In-Hospital Mortality and Major Bleeding Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA. 2020;323(8):734. [CrossRef]
- Thiele H, Desch S, de Waha S. Mechanical circulatory support: the last resort in cardiogenic shock? EuroIntervention. 2018;13(18):2099-2101.
- Taleb I, Koliopoulou AG, Tandar A, et al. Shock Team Approach in Refractory Cardiogenic Shock Requiring Short-Term Mechanical Circulatory Support. Circulation. 2019;140(1):98-100. [CrossRef]
- van Diepen S, Katz JN, Albert NM, et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017;136(16).
- von Lewinski D, Herold L, Stoffel C, et al. PRospective REgistry of PAtients in REfractory cardiogenic shock—The PREPARE CardShock registry. Catheterization and Cardiovascular Interventions. 2022;100(3):319-327. [CrossRef]
- Helgestad OKL, Josiassen J, Hassager C, et al. Contemporary trends in use of mechanical circulatory support in patients with acute MI and cardiogenic shock. Open Heart. 2020;7(1):e001214. [CrossRef]
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: Executive Summary. J Am Coll Cardiol. 2013;61(4):485-510. [CrossRef]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37(27):2129-2200. [CrossRef]
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726.
- Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2017;69(3):278-287. [CrossRef]
- Seyfarth M, Sibbing D, Bauer I, et al. A Randomized Clinical Trial to Evaluate the Safety and Efficacy of a Percutaneous Left Ventricular Assist Device Versus Intra-Aortic Balloon Pumping for Treatment of Cardiogenic Shock Caused by Myocardial Infarction. J Am Coll Cardiol. 2008;52(19):1584-1588. [CrossRef]
- Basir MB, Schreiber TL, Grines CL, et al. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am J Cardiol. 2017;119(6):845-851. [CrossRef]
- Basir MB, Lemor A, Gorgis S, et al. Early Utilization of Mechanical Circulatory Support in Acute Myocardial Infarction Complicated by Cardiogenic Shock: The National Cardiogenic Shock Initiative. J Am Heart Assoc. Published online November 28, 2023. [CrossRef]
- Pieri M, Sorrentino T, Oppizzi M, et al. The role of different mechanical circulatory support devices and their timing of implantation on myocardial damage and mid-term recovery in acute myocardial infarction related cardiogenic shock. J Interv Cardiol. 2018;31(6):717-724. [CrossRef]
- O’Neill WW, Grines C, Schreiber T, et al. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J. 2018;202:33-38. [CrossRef]
- Kapur NK, Paruchuri V, Urbano-Morales JA, et al. Mechanically Unloading the Left Ventricle Before Coronary Reperfusion Reduces Left Ventricular Wall Stress and Myocardial Infarct Size. Circulation. 2013;128(4):328-336. [CrossRef]
- Meyns B, Stolinski J, Leunens V, Verbeken E, Flameng W. Left ventricular support by Catheter-Mountedaxial flow pump reduces infarct size. J Am Coll Cardiol. 2003;41(7):1087-1095. [CrossRef]
- Antman EM. Time Is Muscle. J Am Coll Cardiol. 2008;52(15):1216-1221.
- Pieri M, Contri R, Winterton D, et al. The contemporary role of Impella in a comprehensive mechanical circulatory support program: a single institutional experience. BMC Cardiovasc Disord. 2015;15(1):126. [CrossRef]
- Esposito ML, Kapur NK. Acute mechanical circulatory support for cardiogenic shock: the “door to support” time. F1000Res. 2017;6:737.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).