Introduction
Pulmonary tuberculosis (PTB) is a global disease and continues to be a health burden both in Africa as well as in South Africa, and the development of resistant strains of
Mycobacterium tuberculosis calls for the development of alternative treatment approaches[
1]. Furthermore, side-effects such as liver injuries and peripheral neuropathies associated with available tuberculosis medications fuel non-compliance and further development of resistant strains, and hence communities’ resort to medicinal plants as alternatives to manage the disease and these medicinal plants offer a still untapped source of medications with the potential to fight the TB scourge[
2]. In pulmonary tuberculosis infection, the obligatory cell that plays a major role in the inflammatory response that contributes to eradicating and eliminating
Mycobacterium tuberculosis is the alveolar macrophage[
3]. As the macrophages encounter
Mycobacterium tuberculosis, they can undergo polarization and switch between the two phenotypes (M1 and M2), depending on the microenvironment. This is usually driven by certain cytokines, as seen in patients with tuberculosis infection. In the overall process of polarization, Nitric Oxide (NO) serves as the signaling molecule produced by the macrophages and indicates the exact phenotype of the polarised macrophage[
4]. Its synthesis occurs intracellularly by the oxidation of L-arginine in a reaction catalyzed by an inducible NO synthase enzyme[
5]. The upstream regulation of iNOS genes by pro-inflammatory cytokines in inflammation results in NO synthase synthesis, which then drives the oxidation reaction to produce NO and L-citrulline. The M1/M2 phenotypes can thus be discriminated by using NO production as the indicator in macrophages stimulated by bacterial lipopolysaccharides (LPS)[
6]. Inflammation is initiated by the immune system in response to various agents that could trigger immune system activation, in a process that controls the spread of diseases, and occurs upon injuries and in their healing[
7]. Exposure to certain chemicals, microorganisms, and pollutants may induce oxidative stress in tissues, and DNA damage that could trigger an inflammatory response[
8]. Therefore, this study aimed to investigate the
in vitro immunomodulatory effects of three medicinal plants that are used traditionally for the treatment of PTB in the Eastern Cape province of South Africa, the outcome which can contribute towards new interventions for management of tuberculosis.
Material and methods
RAW 264.7 mouse macrophages were purchased from Cellonex (South Africa). Lipopolysaccharide (LPS), Griess reagent, and aminoguanidine were purchased from Sigma-Aldrich (St Louis, MO, USA). RPMI1640 culture media and fetal bovine serum were purchased from GE Healthcare Life Science (Logan, UT, USA).
Preparation of concentrations
Ethanol extracts were solubilized in DMSO to make a stock solution of 100mg/ml. (50mg of the crude extract was dissolved in 500µl DMSO). Extracts were stored at 4ºC until used. To obtain the desired concentrations of 50, 100, and 200 µg/ml, 4µl of the solubilized extract was added to 996µl of culture medium to obtain a final volume of 1ml. Serial dilutions of 400µg/ml, 200µg/ml to 100µg/ml were prepared to obtain the final concentrations of 200, 100, and 50 µg/ml respectively. Amino guanidine (100µM) was used as a positive control to indicate anti-inflammatory activity, while LPS was used as a positive control to indicate the pro-inflammatory activity of plant samples.
Cytotoxicity screening on all test samples
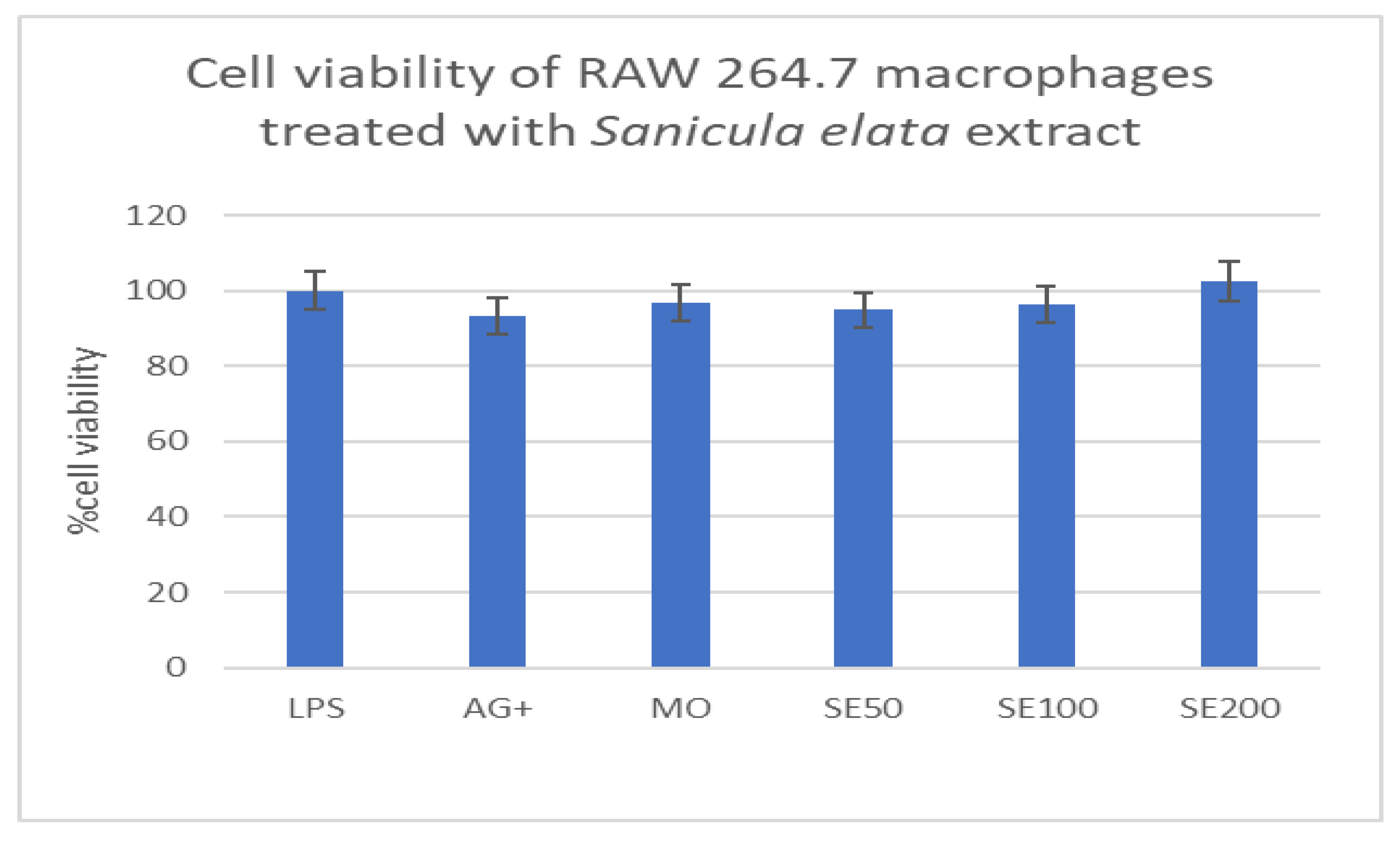
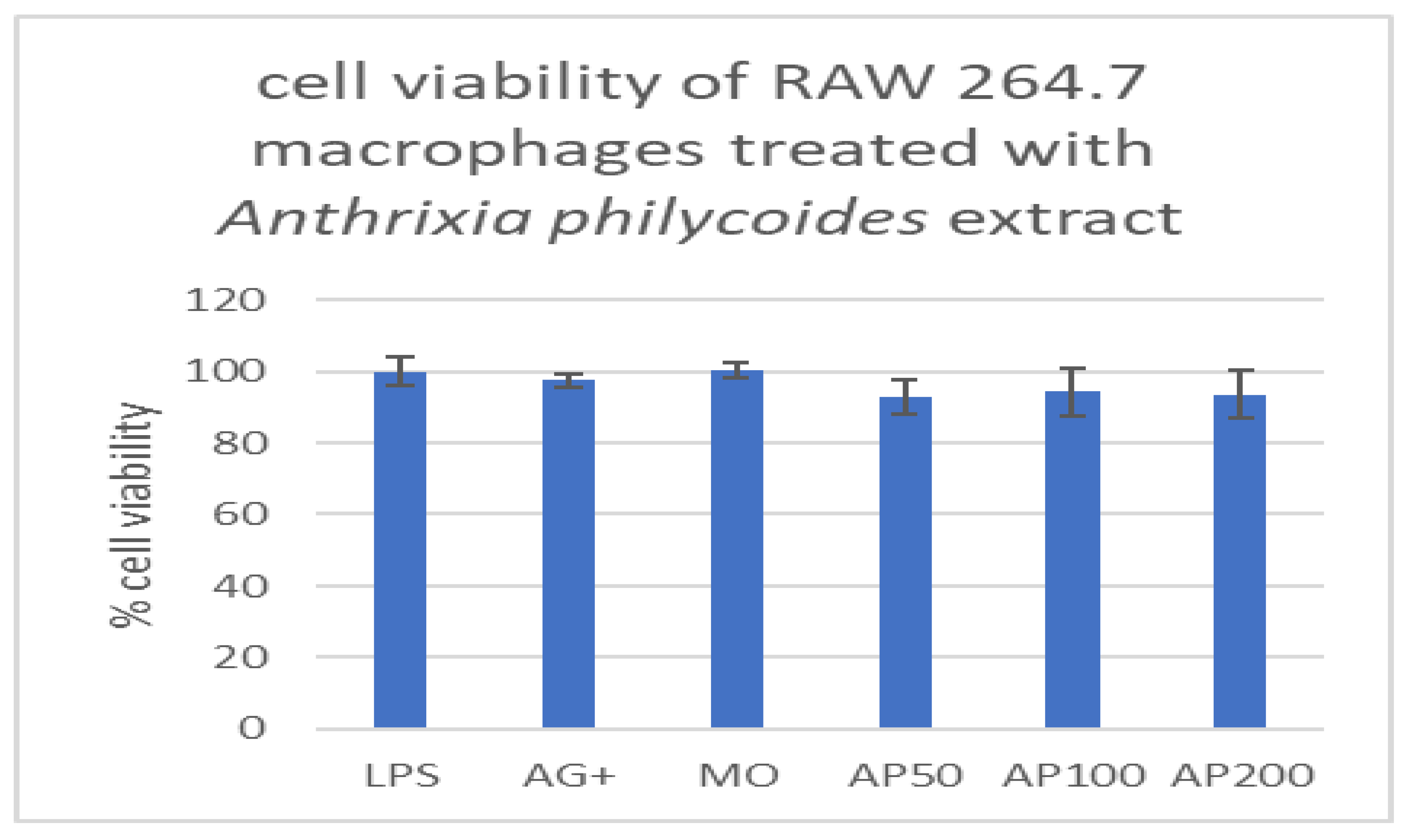
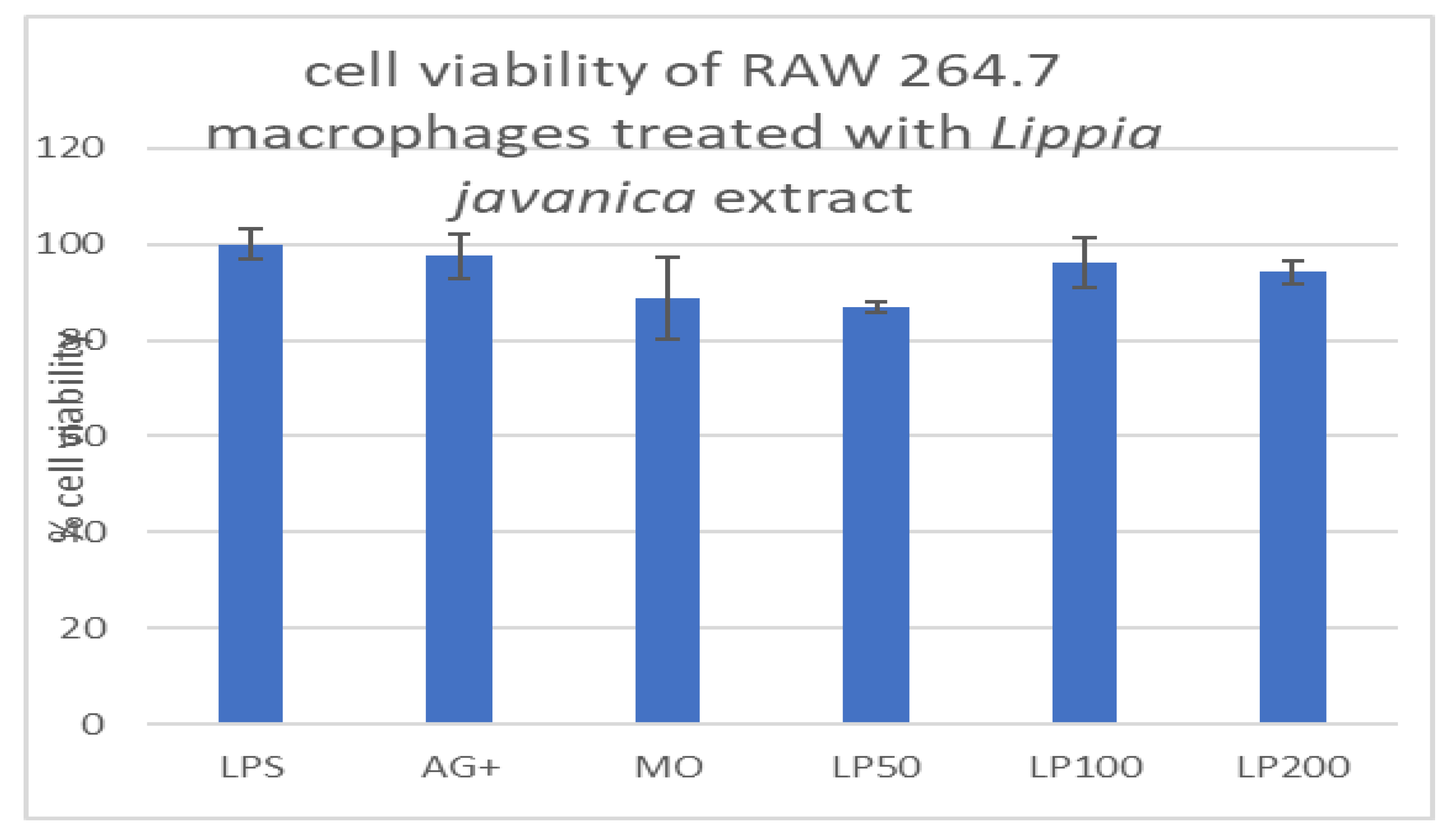
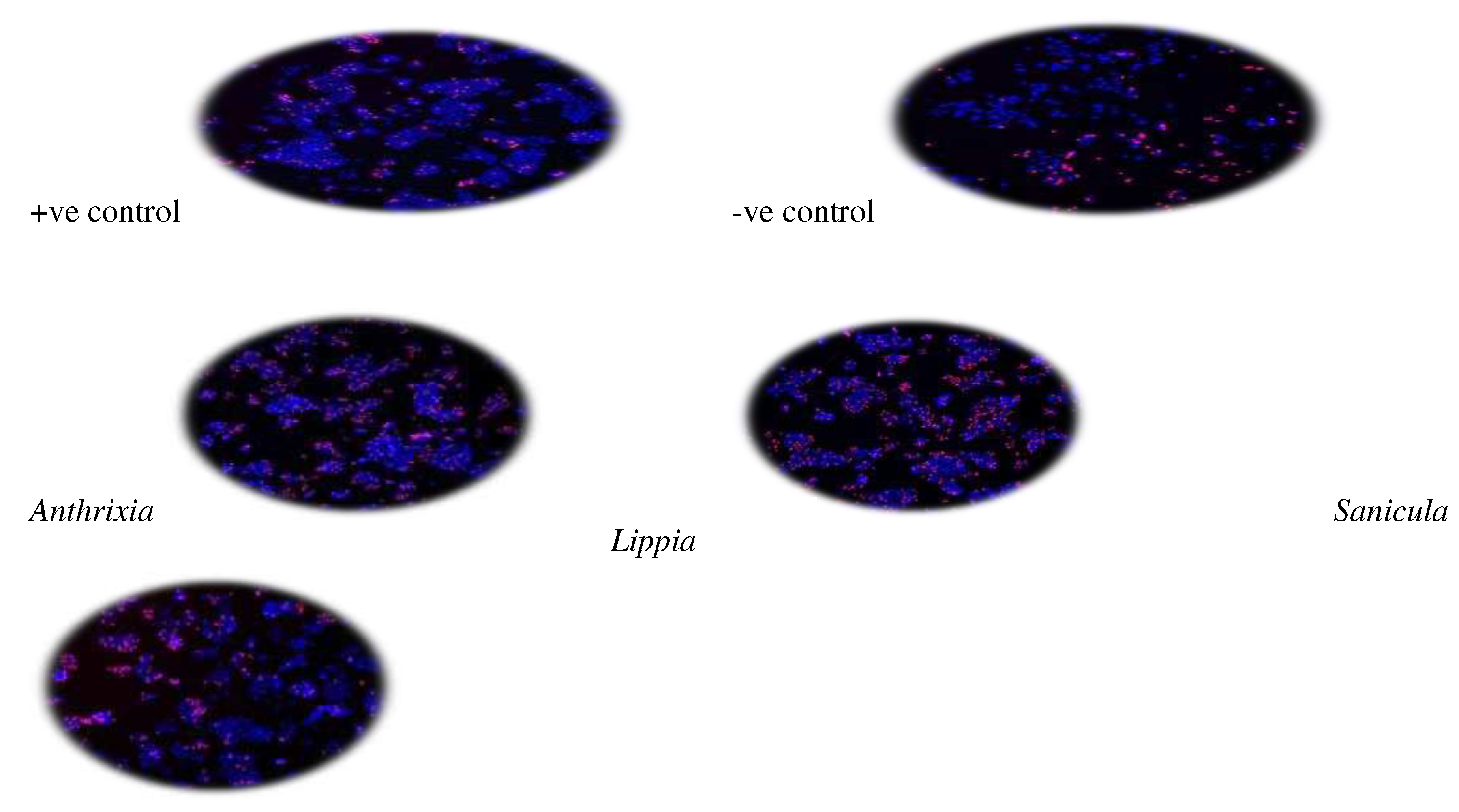
To confirm the absence of toxicity as a contributing factor across all samples, cell viability was assessed using tetrazolium salts assay (MTT) on RAW264.7 macrophages. To achieve that, the medium and treatments remaining in each well after incubation were removed and replaced with 100µl medium containing 0.5mg/ml MTT, and further incubation was allowed for 30 minutes at 37ºC. Thereafter, MTT was removed and 100µl DMSO was added to each well to solubilize the formazan crystals. Absorbance was then measured using a BioTekR Power Wave XS spectrophotometer (Winooski, VT, USA) at 540nm. All samples were tested in quadruplicate in both the inflammatory and MTT bioassays. Supplementing the colorimetric techniques, Hoechst33342 and Propidium iodide (PI) dyes were used to further evaluate the cytotoxicity of the crude extracts on hepatocarcinoma cells (C3A cells). Quantification of live and dead cells was performed using the ImageXpress Micro XLS Widefield Microscope (Molecular Devices), using a 10x Plan Fluor objective and DAPI and Texas Red filter cubes. Nine image sites were acquired per well, which is representative of roughly 75% of the surface area of each well. Acquired images were analyzed by the MetaXpress software and Multi-Wavelength Cell Scoring Application Module. Acquired data were transferred to an Excel spreadsheet and analyzed, processed, and presented as mean and standard deviation.
Pro/Anti-inflammatory screening
1ml vial of frozen cells was thawed in a 37ºC water bath. The seeding and sub-culturing were performed under sterile conditions in the laminar flow cabinets. The cells were seeded in RPMI1640 culture medium, supplemented with 10% FBS (RPMI complete medium) into 96-well plates at a density of 1x105 cells per well, and allowed to attach overnight. The following day the spent culture medium was aspirated from the wells. About 100µl RPMI medium was then added to the cells to give final concentrations of 50, 100, and 200µg/ml. To assess the anti-inflammatory/pro-inflammatory activity of the 3 extracts, 100µl of LPS (final concentration 500ng/ml)-containing medium was added to the corresponding wells, and aminoguanidine at 100µM was used as the positive control for anti-inflammatory effects and the LPS as the positive control for the pro-inflammatory. The cells were incubated for a further 24 hrs. To quantify NO production, 50µl of spent culture medium was transferred to a new 96-well plate after 24 hrs incubation, and 50µl Griess reagent was added. Absorbances of four wells per concentration were measured using a Bio TekR Power Wave XS spectrophotometer (Winooski, VT, USA) at 540nm, and the data was exported to an Excel sheet for calculation of means and standard deviations for all concentrations. To determine the concentration of NO in each sample, a standard curve of sodium nitrite dissolved in culture media was used.
Discussion and Conclusion
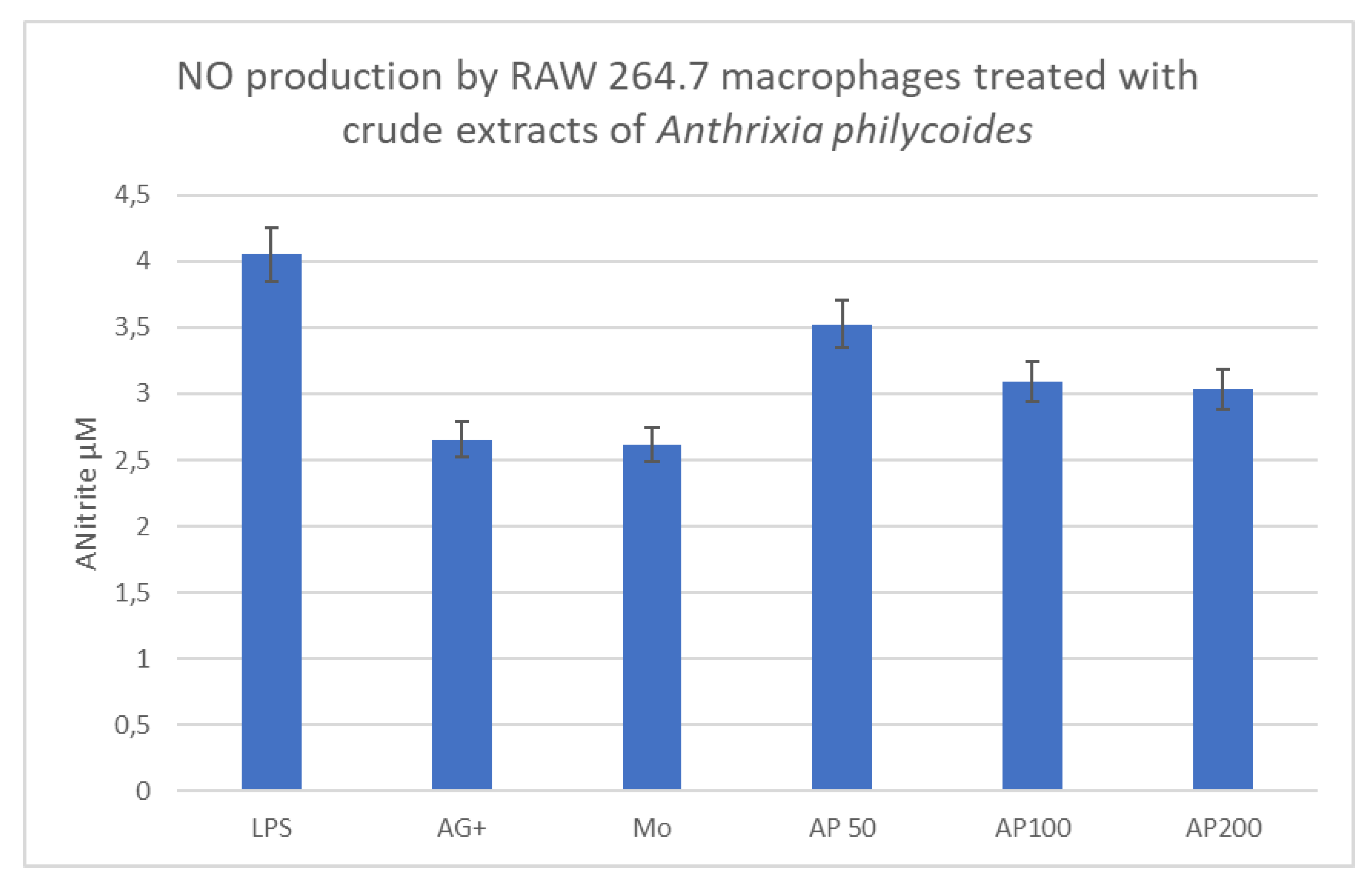
Anthrixia philycoides DC, is a medicinal plant indigenous to South Africa, commonly known as bush tea and as Icholocholo in Isixhosa. It has been used traditionally by South Africans history to treat boils, headaches, cuts, and wounds, it is also used for blood purification and, above all, for the treatment of coughs and colds[
9]. The antiproliferative activity of this plant has been investigated using HeLa cells and the cell colds proliferation in that study was shown to be 55% at the concentration of 100mg/g [
10]. In our study, the antiproliferative or cytotoxic activity was determined on macrophages (RAW 264.7) and on hepatocarcinoma cells (C3A), using concentrations of 50, 100, and 200µg/ml. Our study shows that cell viability or proliferation of macrophages was above the 80% mark, which was set to be the determinant cut-off limit for cell death. For the C3A cells, the inhibitory effect of the medicinal plant correlates with increasing concentrations. The lowest concentration of 50µg/ml did not have any antiproliferative or toxic effect on the liver cells. Because of the limitations of cell culture experiments, in which the cells have no means of excreting secondary metabolites, the apparent toxicity of the plants may be higher in such systems than in animal models. The toxicological assessment of
Anthrixia philycoides conducted by Chellan
et al (2008) on Wistar rats documented histopathologically that the medicinal plant is not toxic to the liver, kidney, or gastrointestinal tract [
11]. In animal studies, the excretion of secondary metabolites is well taken care of by the excretory system whereas, in the case of cell cultures, it does not exist. In view of Mathivha’s results in cell cultures on HeLa cells and Chellan’s results in animals, and our results on C3A cells, we fully agree that
Anthrixia phylocoides is not toxic. With respect to Mathivha’s findings on HeLa cells and our findings in C3A cells, this may suggest how different types of cells exercise different physiological mechanisms to handle drug metabolism. Additionally, the study by Mokwena
et. al. (2021), conducted on Wistar rats showed that
Anthrixa has anti-obesity and anti-inflammatory properties, and the treated rats showed improved glucose uptake and reduced insulin resistance [
12]. Our study also confirmed the anti-inflammatory activity of
Anthrixia: the NO production by cells treated with the medicinal plant correlated well with the positive anti-inflammatory (AG+) control, which indicates the anti-inflammatory properties of
Anthrixia as reported by Mokwena
et al in their investigations.
Lippia jananica (Burm. F.)
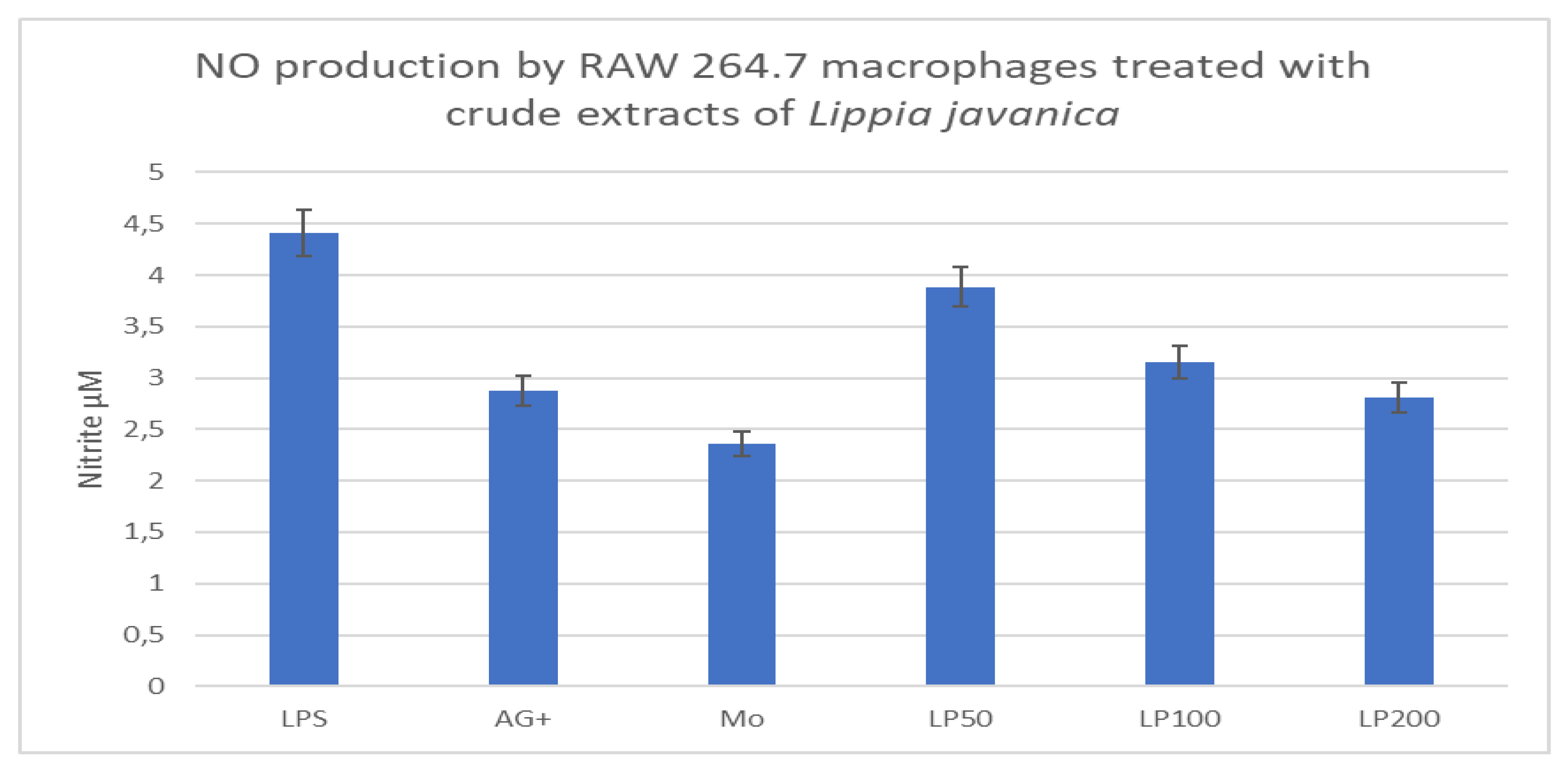
Lippia javanica is indigenous to Southern Africa and is commonly referred to as a fever tree and called Umsuzwane in Isixhosa. The pharmacological studies conducted on this medicinal plant found many effects, including anti-plasmodial, anti-diabetic, antioxidant, anti-cancer, and pesticide effects [
13]. In the current study, the plant was investigated for immunomodulatory properties on macrophages. Traditionally, the medicinal plant is used for colds, coughs, asthma, fevers, tuberculosis, bronchitis, chest pains, and malaria infections [
14]. In our study, we assessed the immunomodulatory effect of the medicinal plant, and found an anti-inflammatory effect of
Lippia on macrophages, while it remained non-toxic to the tested macrophages and to hepatocarcinoma cells. The anti-inflammatory findings from this study correlate well with the results obtained in previous studies conducted on Wistar rats, which demonstrated that the medicinal plant possesses neuroprotective effects as it reduced Pb-induced brain oxidative stress, inflammation, and neuronal damage [
15], and again
Lippia inhibited the Th
2 -mediated immune response in asthmatic Wistar rats, with the reduction in serum IgE, and inflammatory cytokines and NO [
16].
Sanicula elata Buch.HAM. ex D.Don
Sanicula elata plant is indigenous to South Africa and Ethiopia. In South Africa, it is commonly known as
Umvuthuza in Isixhosa [
17], and in Ethiopia is commonly called
Gororsa [
18]. There is very little published data from scientific investigations on this medicinal plant to date, including investigation of any immunomodulatory effects. The only available data is from the study by Kebede
et al on the anti-bacterial effect of the crude extracts of
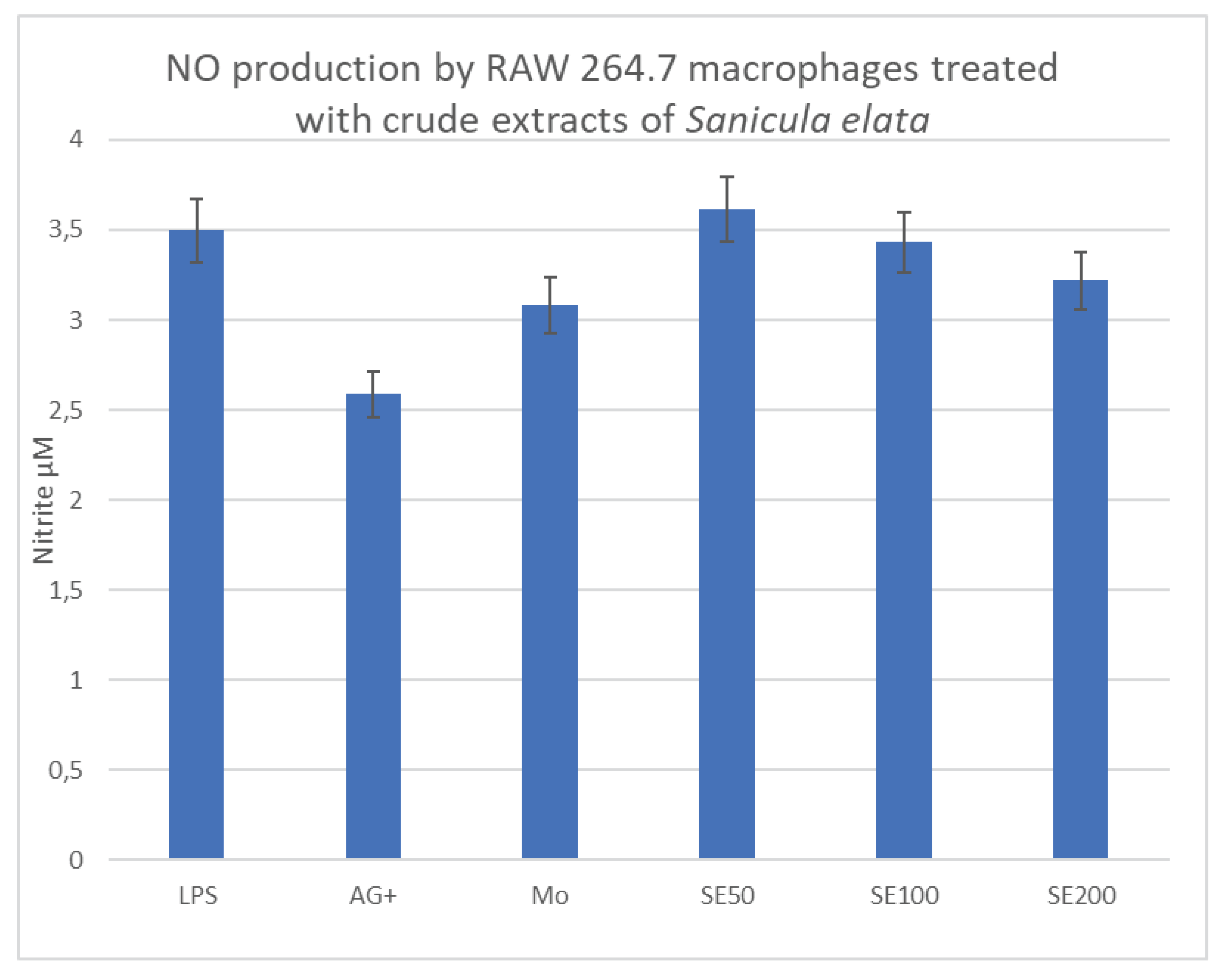
Sanicula elata on streptococcus mutants isolated from dental decay, a study conducted in Addis Ababa, Ethiopia in 2021. Our study investigated the immunomodulatory effect of the plant on macrophages and the findings of this study, revealed a pro-inflammatory effect of the plant on macrophages, as shown by the increased amount of NO produced by cells that were activated by the bacterial proteins (LPS) in the presence of the plant. While this plant exhibited such good results, it remained non-toxic to both macrophages and C3A liver cells across all the tested concentrations.
Alveolar macrophages are activated upon infection with
Mycobacterium tuberculosis in the lung tissue. The activation is driven by the microenvironment in which the cells find themselves, resulting in their polarization into a pro-inflammatory phenotype (M1) that can degrade the invading pathogen [
19]. As the disease progresses, this activation is manipulated by the pathogen to suit its own interest at the expense of macrophages [
20], as seen in TB, and the cells are diverted to the M2 phenotype which favors the intracellular multiplication of the pathogen. As this multiplication progresses, the priming of the specific immune response to produce antibodies against the pathogen is halted [
21], resulting in the formation of granulomas within the lung parenchyma in TB patients [
22]. Current TB treatment has no effect on macrophages, instead, it specifically targets the different pathways and organelles, and enzymes in
Mycobacterium [
23]. The aim of this study was to investigate means of immunomodulating the macrophages by medicinal plant extracts to help sustain the M1 phenotype which is pro-inflammatory and capable of destroying the pathogen. Two of the tested medicinal plants (
Anthrixia and
Lippia) have specifically demonstrated anti-inflammatory properties, as shown by the low NO production in treated macrophages, compared with the LPS control, on the other hand,
Sanicula elata has demonstrated excellent results in producing NO in large amounts, especially the lowest concentration (50µg/ml). The NO produced by macrophages is satisfactory evidence of macrophage polarization towards the M1 phenotype. NO is a by-product of the reaction that is catalyzed by inducible nitric oxide synthase within the infected macrophages [
24]. This finding demonstrates that
Sanicula elata extract can induce the expression of this enzyme upon activation by LPS, resulting in the maintenance of the M1 phenotype, which is shown by the large amounts of NO produced. Our study, therefore, reports strong evidence that
Sanicula elata has immunomodulatory properties effective on macrophages, and further studies involving animal disease models are warranted for this medicinal plant.
Conclusion
Through the findings obtained from this study, we can conclude that Sanicula elata has an immunomodulatory effect on macrophages in cell culture systems. This study has provided preliminary data that will afford an opportunity for further studies to explore the compound composition of this medicinal plant towards novel drug discovery.
Author Contributions
M.S was a major contributor in data production as well as writing the manuscript. The rest of the authors read and approved the final manuscript.
Funding
This study was funded by the institutional grant at Walter Sisulu University, through Research Innovation & Development (UCDG Research Grant Award, 2021).
Availability of Data Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Mr M.Mtokwana, the herbalist who provided the medicinal plants that were investigated. Mr Z. Cawe, for identifying and authenticating the medicinal plants. Walter Sisulu University Research & Innovation for the financial support that made this study possible. Dr Miranda Thomas, International Centre for Genetic Engineering and Biotechnology, Trieste Italy for her dedicated time in peer reviewing this manuscript.
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
This study was approved by the Research Ethics Committee, Faculty of Health Sciences. Walter Sisulu University. Protocol no005/2020.
References
- Krishna SD, Rohan RP, Heena BC, Aramash ZM, Vaishali RU, Priyanka M et.al. Herbal approach for tuberculosis management: A systematic review. World Journal of Advanced Research and Reviews. 2022;14(2).
- Laghari M, Talpur BA, Sulaiman SAS, Khan AH, Bhatti Z. Adverse drug reactions of anti-tuberculosis treatment among children with tuberculosis. Int J Mycobacteriol. 2020;9(3). [CrossRef]
- Ke Z, Lu J, Zhu J, Yang Z, Jin Z, Yuan L. Down-regulation of lincRNA-EPS regulates apoptosis and autophagy in BCG-infected RAW264.7 macrophages via JNK/MAPK signaling pathway. Infection, Genetics and Evolution [Internet]. 2020;77:104077. Available from: https://www.sciencedirect.com/science/article/pii/S156713481930303X.
- Wang L, Shang X, Qi X, Ba D, Lv J, Zhou X, et al. Clinical Significance of M1/M2 Macrophages and Related Cytokines in Patients with Spinal Tuberculosis. Dis Markers. 2020;2020.
- Lisi F, Zelikin AN, Chandrawati R. Nitric Oxide to Fight Viral Infections. Advanced Science. 2021;8(7). [CrossRef]
- Pringle NA, Koekemoer TC, Holzer A, Young C, Venables L, Van De Venter M. Potential Therapeutic Benefits of Green and Fermented Rooibos (Aspalathus linearis) in Dermal Wound Healing. Planta Med. 2018;84(9–10). [CrossRef]
- Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89. [CrossRef]
- Yu Z, Wu XQ, Zheng LJ, Dai ZY, Wu LF. Effect of acute exposure to ammonia and BFT alterations on Rhynchocypris lagowski: Digestive enzyme, inflammation response, oxidative stress and immunological parameters. Environ Toxicol Pharmacol. 2020;78. [CrossRef]
- Mudau FN, Araya HT, Du Toit ES, Soundy P, Olivier J. Bush Tea (Athrixia phylicoides DC.) as an Alternative Herbal and Medicinal Plant in Southern Africa:Opportunity for Commercialization. Med Aromat Plant Sci Biotechnol. 2007;1(Pietta 2000).
- Mathivha LP, Thibane VS, Mudau FN. Anti-diabetic and anti-proliferative activities of herbal teas, Athrixia phylicoides DC and Monsonia burkeana Planch. ex Harv, indigenous to South Africa. British Food Journal. 2019;121(4). [CrossRef]
- Chellan N, De Beer D, Muller C, Joubert E, Louw J. A toxicological assessment of Athrixia phylicoides aqueous extract following sub-chronic ingestion in a rat model. Hum Exp Toxicol. 2008;27(11). [CrossRef]
- Mokwena MAM, Engwa GA, Nkeh-Chungag BN, Sewani-Rusike CR. Athrixia phylicoides tea infusion (bushman tea) improves adipokine balance, glucose homeostasis and lipid parameters in a diet-induced metabolic syndrome rat model. BMC Complement Med Ther. 2021;21(1). [CrossRef]
- Maharaj VJ, Naidoo-Maharaj D, Fouche G, Mianda SM. Are scientists barking up the wrong tree to “scientifically validate” traditional medicines? Vol. 126, South African Journal of Botany. 2019.
- Maroyi, A. Lippia javanica (Burm.f.) Spreng.: Traditional and Commercial Uses and Phytochemical and Pharmacological Significance in the African and Indian Subcontinent. Vol. 2017, Evidence-based Complementary and Alternative Medicine. 2017. [CrossRef]
- Suleman Z, Engwa GA, Shauli M, Musarurwa HT, Katuruza NA, Sewani-Rusike CR. Neuroprotective effects of Lippia javanica (Burm.F.) Spreng. Herbal tea infusion on Lead-induced oxidative brain damage in Wistar rats. BMC Complement Med Ther. 2022;22(1). [CrossRef]
- Mfengu MOM, Shauli M, Engwa GA, Musarurwa HT, Sewani-Rusike CR. Lippia javanica (Zumbani) herbal tea infusion attenuates allergic airway inflammation via inhibition of Th2 cell activation and suppression of oxidative stress. BMC Complement Med Ther. 2021;21(1). [CrossRef]
- Dold AP, Cocks ML. Preliminary list of Xhosa plant names from Eastern Cape, South Africa. Bothalia. 1999;29(2). [CrossRef]
- Kebede M, Dejene M, Abatenh E. Antibacterial Activity of Sanicula Elata Crude Extracts Invitro Against on Streptococcus Mutans Isolated from Dental Decays Ethiopian Cactus Fruit Peel Waste: The potential phytoagents for the green synthesis of metal nanoparticles for designated applications View project 4 PUBLICATIONS 12 CITATIONS SEE PROFILE Antibacterial Activity of Sanicula Elata Crude Extracts Invitro Against on Streptococcus Mutans Isolated from Dental Decays. International Journal of Bioprocess & Biotechnological Advancements IJBBA [Internet]. 7(3):321–5. Available from: www.scitcentral.com.
- Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: Different gene signatures in M1(Lps+) vs. Classically and M2(LPS-) vs. Alternatively activated macrophages. Vol. 10, Frontiers in Immunology. 2019. [CrossRef]
- Upadhyay S, Mittal E, Philips JA. Tuberculosis and the art of macrophage manipulation. Vol. 76, Pathogens and Disease. 2018. [CrossRef]
- Dabla A, Liang YC, Rajabalee N, Irwin C, Moonen CGJ, Willis J V., et al. TREM2 Promotes Immune Evasion by Mycobacterium tuberculosis in Human Macrophages. mBio. 2022;13(4). [CrossRef]
- Müller A, Krause B, Kerstein-Stähle A, Comdühr S, Klapa S, Ullrich S, et al. Granulomatous inflammation in anca-associated vasculitis. Vol. 22, International Journal of Molecular Sciences. 2021. [CrossRef]
- Saxena AK, Singh A. Mycobacterial tuberculosis Enzyme Targets and their Inhibitors. Curr Top Med Chem. 2019;19(5). [CrossRef]
- Somasundaram V, Gilmore AC, Basudhar D, Palmieri EM, Scheiblin DA, Heinz WF, et al. Inducible nitric oxide synthase-derived extracellular nitric oxide flux regulates proinflammatory responses at the single cell level. Redox Biol. 2020;28. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).