1. Introduction
Tree diversity is a key element in ecological resilience and the provision of multiple ecosystem services in traditional silvopastoral systems [
1,
2,
3]. These systems, which integrate dispersed trees in pastures, are being recognized for their environmental [
4,
5,
6] and economic benefits [
7,
8,
9], highlighting the importance of tree diversity, which motivates an intense analysis along the Andean-Amazonian altitudinal gradient [
7,
10].
The presence of trees in pastures favors the improvement of biodiversity [
11,
12] and soil quality [
13,
14], while enhancing carbon sequestration [
7,
15] and the provision of other key ecosystem services [
14]. The capture and storage of atmospheric carbon is essential for climate change mitigation, and trees on pastures contribute significantly to this process [
4,
16]. Several studies have demonstrated that trees in silvopastoral systems act as carbon sinks and store large amounts of carbon in the tree biomass and in the soil [
12,
15,
17], not only reduces the concentration of carbon dioxide in the atmosphere, but also promotes the resilience of the systems to extreme climatic events by maintaining the stability of the microclimate [
18,
19].
On the other hand, trees generate a direct economic value, as a consequence of the production of fruits and wood of commercial interest [
20,
21]. The presence of fruit trees in silvopastoral systems not only diversifies the diet of livestock, but can also generate additional income for farmers [
12,
22]. Likewise, timber trees, such as those destined for the construction industry or furniture manufacturing, offer long-term sustainable economic opportunities [
23]. This multifunctionality of trees in silvopastoral systems underlines their value as key elements for resilience and sustainability in both environmental and economic terms [
2,
12,
15], emphasizing the need for a conscious management of tree diversity in forests [
24].
According to Montagnini & del Fierro [
25], the presence of tree species in silvopastoral systems can promote nutrient retention, reduce erosion and improve soil structure. The positive effect on soil health has direct implications for livestock productivity and the long-term sustainability of these systems. Therefore, the development of an exploratory study about tree diversity and its ecological importance value in silvopastoral systems is a priority step to optimize natural resource management and improve the resilience of agro-livestock activities in the current context of environmental and climatic challenges [
1,
2,
26].
In this context, this study conducted in an area widely recognized as a hotspot of biodiversity and endemism [
27,
28,
29], in the Ecuadorian Amazon Region (RAE) has the following objectives: (a) to know the tree diversity in grasslands with scattered trees; (b) to determine the abundance of tree species and their value of ecological importance; and (c) to analyze the density of trees and their conservation status in Ecuador and according to the International Union for Conservation of Nature (IUCN), in silvopastoral systems along the altitudinal gradient of the Sumaco Biosphere Reserve (BSR) in the Ecuadorian Amazon.
2. Materials and Methods
2.1.Á. rea de estudio
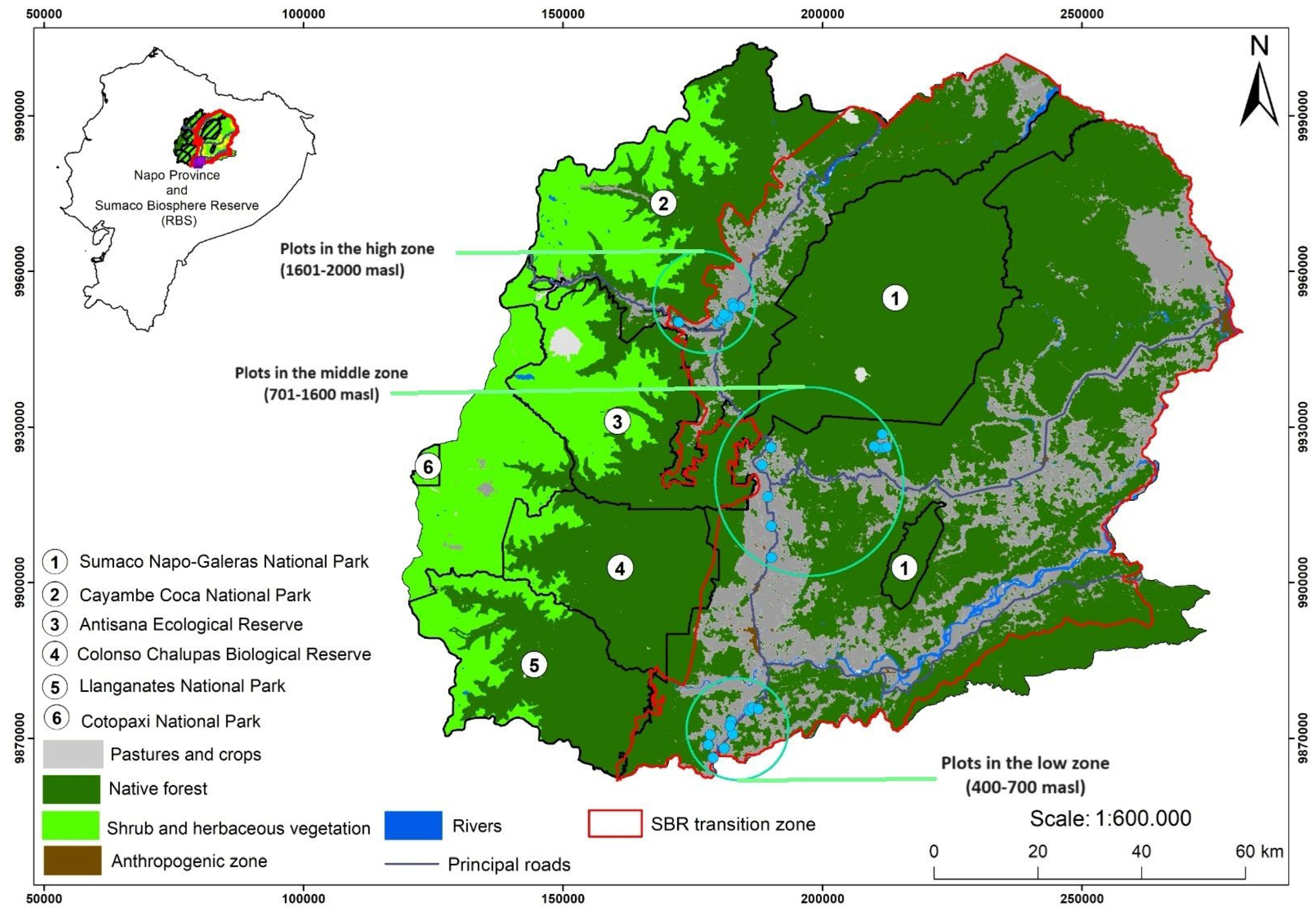
The study was conducted among households engaged in livestock-based livelihood strategies[
30] in an altitudinal gradient within the Sumaco Biosphere Reserve (SBR). The SBR is about one million ha in size [
31] and, according to the last multi-temporal assessment conducted in 2013, it had about 53% primary forest, 28% secondary forest and 9% grassland (81,693 ha) [
31]. Three Amazonian cantons located within the SBR were selected for this study: a) Arosemena Tola (low zone from 400 to 700 masl); b) Archidona (middle zone from 701-1600 masl); and c) Quijos (Amazon high zone from 1601-2000 masl) (
Figure 1). The whole study area is part of the world's biodiversity hotspots (Western Amazon Highlands) [
29,
32]. The predominant bioclimatic conditions vary along the altitudinal gradient studied, with a mean annual temperature of 35.67 °C and annual precipitation of 5209 mm in the low zone, 33.65 °C and 4728 mm in the middle zone; 26.70 °C and 2205 mm in the high zone [
7].
2.2. Sampling and data collection
The selection criteria for farm selection were pasture area ≥ 0.5 ha, with at least one pasture plot with scattered trees, at a canopy cover ≥ 10% [4, 6, 7, 10]. Thus, 26 circular temporary plots of 2826 m
2 were installed, in pasture with scattered trees, distributed among the three zones (
Figure 2 and
Table 1).
2.3. Data analysis
Shannon's diversity index (H) and the Equity (E) of each species were determined to consider both the abundance and the variety of species present in each zone along the studied gradient. Rarefaction curves were used to statistically represent the accumulation of species in relation to the number of samples, which is useful in several sampling approaches [
33].
The Importance Value Index (IVI) was calculated for each species [
34] and
[IVI = relative abundance + relative dominance + relative frequency]
Where:
Relative abundance = number of individuals ha-1
Relative dominance = basal area (m2 ha-1)
Frequency = percentage of plots in which a species is present
SPSS 22.0 for Windows software was used to perform the statistical analyses. Each sample collected was considered a priori as a discrete group. Prior to the statistical analyses, the normality of the data distribution was evaluated using the Kolmogorov-Smirnov test (with the Lilliefords correction). For those variables that did not show a normal distribution, the Bartlett test was applied to assess that the data had equal variances. Quantitative variables (original and adjusted) were analyzed by means of a one-way analysis of variance (ANOVA) qualitative variables were compared with the Kruskal–Wallis test [
35].
On the other hand, regarding the uses of the analyzed species, a verification of the main uses (edible, medicinal, handicraft, material) was carried out in the useful plants of Ecuador published by De la Torre et al.[
36]. Finally, with respect to the IUCN categories, the "iucn_summary" function of the taxize package was used [
37] developed for the R programming language environment.
3. Results and Discussion
3.1. Richness and floristic composition of silvopastoral system
The results of the 26 temporary plots of 2826 m
2 show a significant variation in species and family richness along the altitudinal gradient in the BS. In the
lower zone (lower altitude), the highest species richness was recorded, with an average of 10.17 ± 3.21 species per hectare, followed by the
middle zone with 6.63 ± 2.72 and the
Amazon high zone with 5.53 ± 2.51 (
Table 2). These differences are highly significant (p < 0.01) and show a decrease in species and family diversity with increasing altitude. This variation may be due to the fact that species distribution patterns are the result of multiple ecological processes [
38], influenced by geographic differences and environmental factors such as climate and soil [
39], but on the other hand, the dispersed trees in these pasture systems could be established and managed by populations of mestizo settlers who came at different times and with different cultural backgrounds and different inheritance of the ecosystem, according to Torres et al. [
40]. The first livestock settlements in the SBR occurred in the
Amazon highland zone about 70 years ago, then in the
lower zone about 45 years ago, and finally in the
middle zone about 35 years ago, which is in agreement with Lei and Zhouping reports [
41], that different stages of succession showed different species composition in natural pastures studied in China.
The Shannon index, which measures species diversity, shows no significant differences between the three altitudinal zones, indicating a similar relative distribution of species in all of them. Similarly, Simpson's index and Equity index do not show significant differences. Regarding tree density, it is observed that the low zone shows the highest density with an average of 193 ± 97.23 trees per hectare, followed by the high zone with 101 ± 41.54 and the medium zone with 83.25 ± 38.33. These differences are significant (p < 0.01) between the low zone compared to the medium and high zones. In contrast, basal area (m2), average diameter at breast height (DBH) and maximum DBH did not show significant differences between the three altitudinal zones studied.
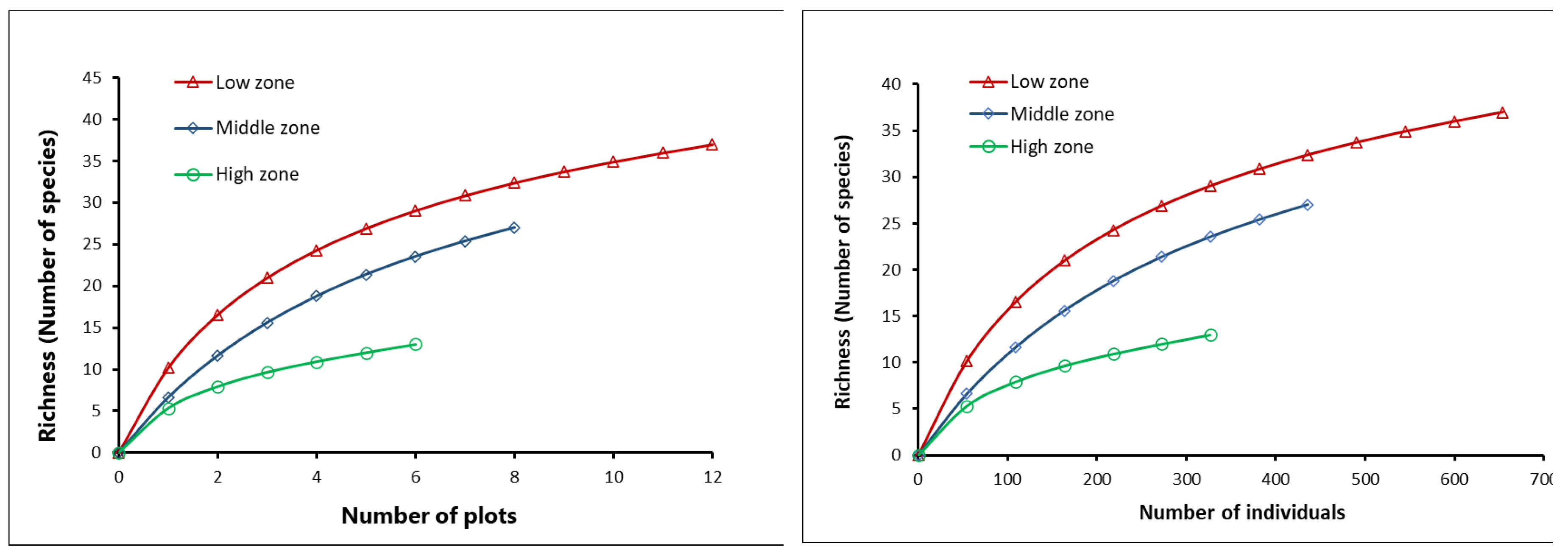
Tree species rarefaction curves indicate lower tree species richness in grasslands in the
high zone compared to the
low and
middle zones. These differences in species richness follow the same pattern both in the analysis by number of plots and by number of individuals. (
Figure 3).
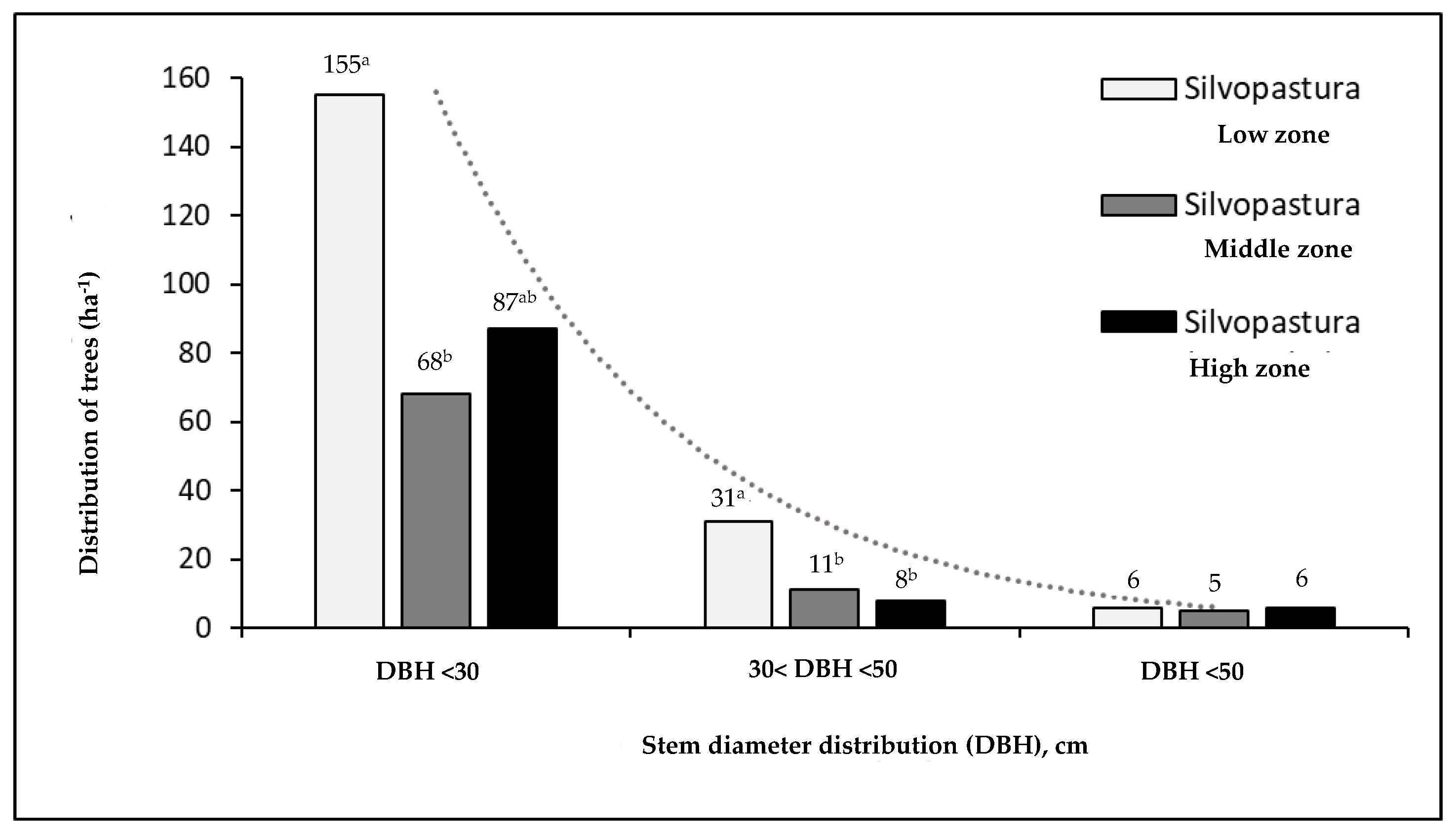
Figure 4 shows the distribution of trees at the diameter class level (DBH ≥10 cm) in the three altitudinal gradients. In the
lower zone, there was a greater presence of trees with diameters less than 30 cm (DBH), with an average of 155 individuals /ha
-1 in this range. In addition, 31 individuals were recorded /ha
-1 with diameters between 30 and 50 cm (DBH), while only 6 individuals were recorded /ha
-1 with diameters greater than 50 cm (DBH). In contrast, in the
middle zone, trees with a DBH of less than 30 cm predominated, with an average of 68 individuals /ha
-1. Trees with DBH between 30 and 50 cm were an average of 11 individuals /ha
-1, and those with an average DBH of 50 cm were 39 individuals /ha
-1. Finally, in the high zone, 87 individuals were identified /ha
-1 with DBH less than 30 cm. The presence of trees with DBH between 30 and 50 cm was an average of 8 individuals /ha
-1, and with an average of 6 individuals with a DBH greater than 50 cm /ha
-1.
3.2. Abundance of tree species and value of ecological importance
Among the most important shade tree species in the lower zone,
Cordia alliodora (Cordiaceae) stands out with a high relative abundance (RA) of 27.37% and a relative density (RD) of 16.97%. In the middle zone,
Cordia alliodora continues to be important, but there is an increase in the dominance of
Jacaranda copaia (Bignoniaceae) with a RD of 21.12%. In addition,
Piptocoma discolor (Asteraceae) has a high relative frequency (RF) of 11.32%. In the high zone,
Ficus sp. (Moraceae) emerges as the most relevant species, with a RA of 5.85% and a RD of 53.51%.
Heliocarpus americanus (Malvaceae) also stands out with an AR of 25.15% and a RD of 21.41% (
Table 3).
The importance value indices (IVI) reveal the most influential species in each altitudinal zone. In the low zone,
Cordia alliodora, Jacaranda copaia and
Psidium guajava have high IVIs of 17.24, 12.96 and 9.91 respectively. Meanwhile, in the middle zone,
Cordia alliodora and
Piptocoma discolor are dominant with IVIs of 14.76 and 11.97, respectively. Finally, in the high zone, Ficus sp. and
Heliocarpus americanus stand out with IVIs of 23.95 and 21.77, respectively. The IVI made it possible to identify the 10 tree species with the greatest ecological importance in the pasture systems with dispersed trees along the altitudinal gradient studied (
Table 3). The abundance of species in the three zones should be studied further to determine associated factors such as seed production in the zone or the ease of propagation through natural regeneration as reported by Villanueva et al. [
42] and Esquivel [
43] in cattle ranches in Costa Rica.
3.3. Tree density, conservation status in Ecuador and IUCN
Analysis of tree species density in the different altitudinal zones studied reveals notable patterns of distribution and abundance. In the
lower zone (400-700 masl),
Jacaranda copaia leads with the highest density, averaging 84 individuals per hectare (ind/ha). It is followed by Psidium guajava, with a density of 48 ind/ha, and
Cordia alliodora with 18 ind/ha. In contrast, in the
middle zone (701-1600 masl),
Piptocoma discolor stands out as the dominant species, with an average density of 26 ind/ha. It is followed by
Cordia alliodora with 18 ind/ha and
Cedrela odorata with 12 ind/ha. These findings show on the one hand the knowledge of the timber markets on the part of the producers in these areas where, according to Torres et al. [
44] and Mejía et al. [
45] wood is still harvested as part of their livelihoods, both formally and informally [
46]. Finally, in the
high zone (1601-2000 masl),
Sterculia tessmannii emerges as the dominant species, with an average density of 37 ind/ha, while
Psidium guajava has 28 ind/ha and
Inga spp. reports 16 ind/ha (
Table 4). Regarding the species identified, their use in silvopastoral systems in the Ecuadorian Amazon is frequent [
7] due to their high commercial value in terms of timber quality, such as
Jacaranda copaia, Cordia alliodora, Cedrela odorata, Sterculia tessmannii, Cordia alliodora and
Cedrela odorata [
47,
48,
49], in addition to providing shade for livestock [
50] and storing atmospheric carbon [
12]. Meanwhile,
Psidium guajava and
Inga ssp. stand out for their importance in the production of fruits for both human and livestock consumption. [
7,
51,
52]. In addition, several authors [
53,
54] suggest that these species contribute to the improvement of soil quality by means of nitrogen fixation, as in the case of
Inga spp.
Regarding biological distribution, it was observed that of the 51 species analyzed in the three different altitudinal zones, 49 were native and 2 were non-native:
Citrus limon and
Citrus sinensis (Rutaceae). In relation to the number of native species, this may be related to the fact that the cattle ranchers of the SRB still conserve up to 40% of the area of forest around the pastures on their farms [
10]. Meanwhile, as far as non-native species are concerned, the use of
C. limon and
C. sinensis in pastures is frequent, due to their benefits as a source of dietary supplementation for dairy cattle for their antioxidant, antimicrobial, anti-stress and anti-inflammatory properties [
55,
56].
From the 51 species analyzed, according to the IUCN conservation categories, the findings indicate that a total of 33 species (64.7%) could currently be classified as "Least Concern" (LC). In addition, 2 species (3.9%) were identified as "Vulnerable" (Vu):
Cedrela odorata (in the three zones) and
Cedrela Montana (in the high zone). It was an important finding, given that in the Ecuadorian Amazon 7 species of genus
Cedrela have been reported in primary forests [
57,
58] from which two are found in pastures with dispersed trees, contributing to their conservation.
Regarding the percentage of trees classified as LC, this is similar to that reported by López-Tobar et al. [
48], who analyzed 214 of the most commercialized timber species in the Amazon and found that 67.6% (142 spp.) are currently classified as LC. Likewise, 16 taxa (31.4%) were recorded as being in the "Not Evaluated" (NE) category according to the latest IUCN update. Of these, 7 have been taxonomically identified down to the species:
Apeiba membranaceae, Bactris gasipaes, Croton lechleri, Ceiba samauma, Ficus cuatrecasana, Citrus limon, Citrus sinensis. This suggests the need for further research efforts to catalog unassessed species, which according to our results corresponds to 31.4%.
In addition, 9 of the taxa are described at the genus level:
Annona spp.,
Rollinia spp.,
Inga spp.,
Nectandra spp.,
Ocotea spp.,
Miconia spp.,
Ficus spp. This pattern of non-evaluated species is similar to that reported by Guevara et al. [
59], who reported that 89% of the lowland tree species in the RAE have not been evaluated by the IUCN. Similarly, López-Tobar et al. [
48] suggest that 28% (60 spp.) of the most traded timber species in the last 10 years (2012-2021) do not have a current conservation category according to the latest IUCN red list update.
In addition, the wood density for the 51 species reported in the three altitudinal zones of the SBR is presented in
Table 4. In general terms, the species with the highest density was Pouteria caimito with an average density of 0.81 g/cm
3. In addition, species with notable density were identified as
Piptadenia pteroclada with 0.76 g/cm
3, followed by
Psidium guajava, Citrus limon and
Citrus sinensis, all with the same average density of 0.71 g/cm
3. The values for species with higher density were obtained from the results obtained by Ketterings et al. [
60]. On the other hand, the presence of these species in pastures may be related to the fact that their weight and hardness make them highly desirable in the production of materials used for the manufacture of doors and floors in the construction and fine cabinetmaking industries. [
49,
61].
3.4. Main reported uses of tree and palm species
4. Conclusions
The tree characterization across altitudinal zones showed significant differences. Factors such as zone use, age, production system and pasture management influence tree diameter distribution, density, and floristic composition. The low and middle zones of the altitudinal gradient showed a greater number of individuals and floristic richness in the silvopastoral systems; this positive relationship was associated with a positive regeneration of tree species and their abundance, given that in these zones there is a greater amount of remnant forest surrounding the pastures. In these areas, species of high commercial value such as Cedrela odorata, Jacaranda copaia, Piptocoma discolor and Sterculia tessmannii predominate, which indicates the knowledge of the timber markets by the producers in these areas.
The use of the IVI helped to identify that only the 10 most important tree species in the pastures with trees dispersed along the altitudinal gradient studied represented approximately more than 70% of the IVI in the low and middle zones, and up to 96% in the high zone. This suggests further studies focused on the factors associated with the abundance of these species, as well as for the design of strategies for greater species diversification in pastures as measures to promote valuable species in an area of high diversity and endemism such as the SBR.
In this study, a total of 51 tree species were recorded, of which 49 are of native origin and 2 are exotic species. Among the most frequent species throughout the altitudinal range were Jacaranda copaia, Psidium guajava, Cordia alliodora, Piptocoma discolor and Sterculia tessmannii, which are widely known for their use in terms of timber and fruit for human consumption and livestock. In addition, the presence of Citrus limon and Citrus sinensis was identified, which are frequently used due to their nutritional and biological benefits for livestock. On the other hand, it was found that Cordia alliodora reports the highest density recorded in the study area. Regarding the conservation categories of the International Union for Conservation of Nature (IUCN), 64.7% of the species analyzed were classified as "Least Concern" (LC), followed by 31.4% as "Not Evaluated" (NE) and 3.9% as "Vulnerable" (VU).
The results support the importance of adapting sectoral policies to different altitudes in silvopastoral systems, especially focused on the protection of vulnerable species such as C. odorata and C. montana, which are crucial for the conservation of forest biodiversity in pastures. The significant presence of species not evaluated by the IUCN, which constitute 31% of the identified forest species, reveals a vital opportunity for their inclusion in future evaluations and deepening of their knowledge. This approach is crucial not only to understand the ecology and distribution of these species in silvopastoral systems, but also to identify those at risk and develop appropriate conservation strategies, thus ensuring the protection and sustainable management of both species already identified as vulnerable, particularly Cedrela species, as well as those that remain to be evaluated in these dynamic and essential ecosystems.
Author Contributions
Conceptualization, B.T. and A.G.; methodology, B.T. and A.T.; software, B.T.; validation, R.H-F.; C.B., and A.T.; formal analysis, B.T.; investigation, B.T., C.B., A.T.; data curation B.T. and R.H-F.; writing—original draft preparation, B.T., R.H.F. C.B., A.T., A.G.; writing—review and editing, A.G., B.T., A.T. and R.H.F.; supervision B.T. and A.G. All authors have been involved in developing, writing, commenting, editing, and reviewing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Pontificia Universidad Católica del Ecuador Sede Ibarra, on 4 April 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
This is not applicable as the data are not in any data repository of public access, however if editorial committee needs access, we will happily provide them, please use this email:
btorres@uea.edu.ec.
Acknowledgments
This work is part of the results of a joint research agreement between the Amazon State University (UEA) and Rainforest Alliance Inc. The authors thank Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ), through the REDD + Early Movers Program (REM) and Rainforest Alliance Inc., for all support. We also thank the MAG, MAATE, UEA, UTEQ and ECONGEST AGR267 Group at Cordoba University for their support during the fieldwork stage, as well as the households in the three zones that shared valuable information about their livestock activities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lőrincz, Á.; Hábenczyus, A.A.; Kelemen, A.; Ratkai, B.; Tölgyesi, C.; Lőrinczi, G.; Bátori, Z.; Maák, I.E. Wood-Pastures Promote Environmental and Ecological Heterogeneity on a Small Spatial Scale. Sci. Total Environ. 2023, 167510. [Google Scholar] [CrossRef]
- Álvarez, F.; Casanoves, F.; Suárez, J.C.; Rusch, G.M.; Ngo Bieng, M.A. An Assessment of Silvopastoral Systems Condition and Their Capacity to Generate Ecosystem Services in the Colombian Amazon. Ecosyst. People 2023, 19, 2213784. [Google Scholar] [CrossRef]
- Aryal, D.R.; Gómez-González, R.R.; Hernández-Nuriasmú, R.; Morales-Ruiz, D.E. Carbon Stocks and Tree Diversity in Scattered Tree Silvopastoral Systems in Chiapas, Mexico. Agrofor. Syst. 2019, 93, 213–227. [Google Scholar] [CrossRef]
- Brook, R.; Forster, E.; Styles, D.; Mazzetto, A.M.; Arndt, C.; Esquivel, M.J.; Chadwick, D. Silvopastoral Systems for Offsetting Livestock Emissions in the Tropics: A Case Study of a Dairy Farm in Costa Rica. Agron. Sustain. Dev. 2022, 42. [Google Scholar] [CrossRef] [PubMed]
- Flores-Coello, G.; Hernández-Medrano, J.H.; Ku-Vera, J.; Diaz, D.; Solorio-Sánchez, F.J.; Sarabia-Salgado, L.; Galindo, F. Intensive Silvopastoral Systems Mitigate Enteric Methane Emissions from Cattle. Atmosphere (Basel). 2023, 14, 863. [Google Scholar] [CrossRef]
- Ortiz, J.; Neira, P.; Panichini, M.; Curaqueo, G.; Stolpe, N.B.; Zagal, E.; Dube, F.; Gupta, S.R. Silvopastoral Systems on Degraded Lands for Soil Carbon Sequestration and Climate Change Mitigation. Agrofor. Sustain. Intensif. Agric. Asia Africa 2023, 207–242. [Google Scholar] [CrossRef]
- Torres, B.; Bravo, C.; Torres, A.; Tipán-Torres, C.; Vargas, J.C.; Herrera-Feijoo, R.J.; Heredia-R, M.; Barba, C.; García, A. Carbon Stock Assessment in Silvopastoral Systems along an Elevational Gradient: A Study from Cattle Producers in the Sumaco Biosphere Reserve, Ecuadorian Amazon. Sustainability 2023, 15. [Google Scholar] [CrossRef]
- Sandoval, D.F.; Florez, J.F.; Valencia, K.J.E.; Cabrera, M.E.S.; Stefan, B. Economic-Environmental Assessment of Silvo-Pastoral Systems in Colombia: An Ecosystem Service Perspective. Heliyon 2023, 9. [Google Scholar] [CrossRef]
- Alvarado Sandino, C.O.; Barnes, A.P.; Sepúlveda, I.; Garratt, M.P.D.; Thompson, J.; Escobar-Tello, M.P. Examining Factors for the Adoption of Silvopastoral Agroforestry in the Colombian Amazon. Sci. Rep. 2023, 13, 12252. [Google Scholar] [CrossRef]
- Torres, B.; Andrade, V.; Heredia-R, M.; Toulkeridis, T.; Estupiñán, K.; Luna, M.; Bravo, C.; García, A. Productive Livestock Characterization and Recommendations for Good Practices Focused on the Achievement of the SDGs in the Ecuadorian Amazon. Sustainability 2022, 14, 10738. [Google Scholar] [CrossRef]
- Mauricio, R.M.; Ribeiro, R.S.; Paciullo, D.S.C.; Cangussú, M.A.; Murgueitio, E.; Chará, J.; Estrada, M.X.F. Silvopastoral Systems in Latin America for Biodiversity, Environmental, and Socioeconomic Improvements. In Agroecosystem diversity; Elsevier, 2019; pp. 287–297. [Google Scholar] [CrossRef]
- Torres, B.; Herrera-Feijoo, R.; Torres, Y.; García, A. Global Evolution of Research on Silvopastoral Systems through Bibliometric Analysis: Insights from Ecuador. Agronomy 2023, 13, 479. [Google Scholar] [CrossRef]
- Bravo, C.; Goyes-Vera, F.; Arteaga-Crespo, Y.; García-Quintana, Y.; Changoluisa, D. A Soil Quality Index for Seven Productive Landscapes in the Andean-Amazonian Foothills of Ecuador. L. Degrad. Dev. 2021, 32, 2226–2241. [Google Scholar] [CrossRef]
- Bravo, C.; Torres, B.; Alemán, R.; Changoluisa, D.; Marín, H.; Reyes, H.; Navarrete, H. Soil Structure and Carbon Sequestration as Ecosytem Services under Different Land Uses in the Ecuadorian Amazon Region. In Proceedings of the Int. Conf. Ser. Multidiscip. Sci; 2017; Vol. 3, pp. 1–8.
- McGroddy, M.E.; Lerner, A.M.; Burbano, D. V; Schneider, L.C.; Rudel, T.K. Carbon Stocks in Silvopastoral Systems: A Study from Four Communities in Southeastern Ecuador. Biotropica 2015, 47, 407–415. [Google Scholar] [CrossRef]
- Sandoval-Pelcastre, A.A.; Ramírez-Mella, M.; Rodríguez-Ávila, N.L.; Candelaria-Martínez, B. Tropical Trees and Shrubs with Potential to Reduce the Production of Methane in Ruminants [Árboles y Arbustos Tropicales Con Potencial Para Disminuir La Producción de Metano En Rumiantes]. Trop. Subtrop. Agroecosystems 2020, 23. [Google Scholar] [CrossRef]
- Montagnini, F.; Ibrahim, M.; Murgueitio Restrepo, E. Silvopastoral Systems and Climate Change Mitigation in Latin America. Bois Forets des Trop. 2013, 67, 3–16. [Google Scholar] [CrossRef]
- Schinato, F.; Munka, M.C.; Olmos, V.M.; Bussoni, A.T. Microclimate, Forage Production and Carbon Storage in a Eucalypt-Based Silvopastoral System. Agric. Ecosyst. Environ. 2023, 344, 108290. [Google Scholar] [CrossRef]
- Schmitt Filho, A.L.; Kretzer, S.G.; Farley, J.; Kazama, D.C.; Sinisgalli, P.A.; Deniz, M. Applied Nucleation under High Biodiversity Silvopastoral System as an Adaptive Strategy against Microclimate Extremes in Pasture Areas. Int. J. Biometeorol. 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ortiz Timoteo, J.; Kainer, K.A.; Luna Cavazos, M.; García Moya, E.; Sánchez Sánchez, O.; Vibrans, H. Trees in Pastures: Local Knowledge, Management, and Motives in Tropical Veracruz, Mexico. Agrofor. Syst. 2023, 97, 687–698. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, R.; Kumar, A.; Dhyani, S.K. Integration of Fruit Trees in Agroforestry for Sustainability and Profitability of Farming Systems in Arid and Semi-Arid Regions. Indian J. Agrofor. 2019, 21, 95–99. [Google Scholar]
- Paut, R.; Dufils, A.; Derbez, F.; Dossin, A.-L.; Penvern, S. Orchard Grazing in France: Multiple Forms of Fruit Tree–Livestock Integration in Line with Farmers’ Objectives and Constraints. Forests 2021, 12. [Google Scholar] [CrossRef]
- Lugo, A.E. The Apparent Paradox of Reestablishing Species Richness on Degraded Lands with Tree Monocultures. For. Ecol. Manage. 1997, 99, 9–19. [Google Scholar] [CrossRef]
- Trujillo-Miranda, A.L.; Toledo-Aceves, T.; López-Barrera, F.; Gerez-Fernández, P. Active versus Passive Restoration: Recovery of Cloud Forest Structure, Diversity and Soil Condition in Abandoned Pastures. Ecol. Eng. 2018, 117, 50–61. [Google Scholar] [CrossRef]
- Montagnini, F.; del Fierro, S. Functions of Agroforestry Systems as Biodiversity Islands in Productive Landscapes. In Biodiversity Islands: Strategies for Conservation in Human-Dominated Environments; Springer, 2022; pp. 89–116. [Google Scholar] [CrossRef]
- Chará, J.; Rivera, J.; Barahona, R.; Murgueitio R, E.; Deblitz, C.; Reyes, E.; Mauricio, R.M.; Molina, J.J.; Flores, M.; Zuluaga, A. Intensive Silvopastoral Systems: Economics and Contribution to Climate Change Mitigation and Public Policies. Integr. landscapes Agrofor. Biodivers. Conserv. food sovereignty 2017, 395–416. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, R.A.; Myers, N.; Thomsen, J.B.; da Fonseca, G.A.B.; Olivieri, S. Biodiversity Hotspots and Major Tropical Wilderness Areas: Approaches to Setting Conservation Priorities. Conserv. Biol. 1998, 12, 516–520. [Google Scholar] [CrossRef]
- Myers, N. Threatened Biotas:" Hot Spots" in Tropical Forests. Environmentalist 1988, 8, 187–208. [Google Scholar] [CrossRef]
- Torres, B.; Günter, S.; Acevedo-Cabra, R.; Knoke, T. Livelihood Strategies, Ethnicity and Rural Income: The Case of Migrant Settlers and Indigenous Populations in the Ecuadorian Amazon. For. Policy Econ. 2018, 86, 22–34. [Google Scholar] [CrossRef]
- MAATE Sistema Nacional de Indicadores Ambientales y Sostenibilidad (SINIAS). Available online: http://sinias.ambiente.gob.ec:8099/proyecto-sinias-web/estadisticasAmbientales.jsf?menu=01.
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Longino, J.T.; Coddington, J.; Colwell, R.K. The Ant Fauna of a Tropical Rain Forest: Estimating Species Richness Three Different Ways. Ecology 2002, 83, 689–702. [Google Scholar] [CrossRef]
- Curtis, J.T.; McIntosh, R.P. An Upland Forest Continuum in the Prairie-Forest Border Region of Wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]
- Gil, J.A. Estadística e Informática (Spss) En La Investigación Descriptiva e Inferencial.(Versión Actualizada Spss24) 2017.
- De la Torre, L.; Navarrete, H.; Muriel, P.; Macía, M.J.; Balslev, H. Enciclopedia de Las Plantas Útiles Del Ecuador (Con Extracto de Datos); Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia …, 2008; ISBN 9978771352.
- Chamberlain, S.A.; Szöcs, E. Taxize: Taxonomic Search and Retrieval in R. F1000Research 2013, 2. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Willis, K.J.; Field, R. Scale and Species Richness: Towards a General, Hierarchical Theory of Species Diversity. J. Biogeogr. 2001, 28, 453–470. [Google Scholar] [CrossRef]
- Willis, K.J.; Whittaker, R.J. Species Diversity--Scale Matters. Science (80-. ). 2002, 295, 1245–1248. [Google Scholar] [CrossRef]
- Torres, B.; Bravo, C.; Torres, A.; Tipán-Torres, C.; Vargas, J.C.; Herrera-Feijoo, R.J.; Heredia-R, M.; Barba, C.; García, A. Carbon Stock Assessment in Silvopastoral Systems along an Elevational Gradient: A Study from Cattle Producers in the Sumaco Biosphere Reserve, Ecuadorian Amazon. Sustainability 2022, 15, 449. [Google Scholar] [CrossRef]
- Deng, L.; Shangguan, Z. Species Composition, Richness and Aboveground Biomass of Natural Grassland in Hilly-Gully Regions of the Loess Plateau, China. J. Integr. Agric. 2014, 13, 2527–2536. [Google Scholar] [CrossRef]
- Villanueva, C.; Tobar López, D.; Ibrahim, M.A.; Casasola Coto, F.; Barrantes, J.; Arguedas, R. Árboles Dispersos En Potreros En Fincas Ganaderas Del Pacífico Central de Costa Rica. Agroforestería en las Américas, número 45 2013.
- Esquivel, M.J.; Harvey, C.A.; Finegan, B.; Casanoves, F.; Skarpe, C.; Nieuwenhuyse, A. Regeneración Natural de Árboles y Arbustos En Potreros Activos de Nicaragua. Agroforestería en las Américas 2009, 47, 76–84. [Google Scholar]
- Torres, B.; Günter, S.; Acevedo-Cabra, R.; Knoke, T. Livelihood Strategies, Ethnicity and Rural Income: The Case of Migrant Settlers and Indigenous Populations in the Ecuadorian Amazon. For. Policy Econ. 2018, 86, 22–34. [Google Scholar] [CrossRef]
- Mejía, E.; Pacheco, P.; Muzo, A.; Torres, B. Smallholders and Timber Extraction in the Ecuadorian Amazon: Amidst Market Opportunities and Regulatory Constraints. Int. For. Rev. 2015, 17, 38–50. [Google Scholar] [CrossRef]
- Vasco, C.; Torres, B.; Pacheco, P.; Griess, V. The Socioeconomic Determinants of Legal and Illegal Smallholder Logging: Evidence from the Ecuadorian Amazon. For. Policy Econ. 2017, 78, 133–140. [Google Scholar] [CrossRef]
- Argote, K.; Rodríguez-Sánchez, B.; Quintero, M.; Francesconi, W. One Tree at a Time: Restoring Landscape Connectivity through Silvopastoral Systems in Transformed Amazon Landscapes. Diversity 2022, 14. [Google Scholar] [CrossRef]
- López-Tobar, R.; Herrera-Feijoo, R.J.; Mateo, R.G.; García-Robredo, F.; Torres, B. Botanical Collection Patterns and Conservation Categories of the Most Traded Timber Species from the Ecuadorian Amazon: The Role of Protected Areas. Plants 2023, 12. [Google Scholar] [CrossRef]
- Mejía, E.; Pacheco, P. Forest Use and Timber Markets in the Ecuadorian Amazon; CIFOR, 2014; Vol. 111; ISBN 6021504143.
- Giro, A.; Pezzopane, J.R.M.; Barioni Junior, W.; Pedroso, A.D.F.; Lemes, A.P.; Botta, D.; Romanello, N.; Barreto, A.D.N.; Garcia, A.R. Behavior and Body Surface Temperature of Beef Cattle in Integrated Crop-Livestock Systems with or without Tree Shading. Sci. Total Environ. 2019, 684, 587–596. [Google Scholar] [CrossRef]
- Congo Yépez, C.; Velástegui Lara, F.; Caicedo Vargas, C.; Rodríguez Iturralde, L.; Vera Zambrano, A.; Montero Cruz, O. Árboles Dispersos y Su Efecto En La Productividad de Los Potreros En La Amazonía Ecuatoriana. LA GRANJA. Rev. Ciencias la Vida 2018, 27, 64–76. [Google Scholar] [CrossRef]
- Chará-Serna, A.M.; Chará, J. Effect of Silvopastoral Systems on Biodiversity and the Provision of Ecosystem Services in Tropical Agro-Landscapes [Efecto de Los Sistemas Silvopastoriles Sobre La Biodiversidad y La Provisión de Servicios Ecosistémicos En Agropaisajes Tropicales]. Livest. Res. Rural Dev. 2020, 32. [Google Scholar]
- Lojka, B.; Dumas, L.; Preininger, D.; Polesny, Z.; Banout, J. The Use and Integration of Inga Edulis in Agroforestry Systems in the Amazon-Review Article. Agric. Trop. Subtrop. 2010, 43, 352–359. [Google Scholar]
- Barron, A.R.; Purves, D.W.; Hedin, L.O. Facultative Nitrogen Fixation by Canopy Legumes in a Lowland Tropical Forest. Oecologia 2011, 165, 511–520. [Google Scholar] [CrossRef]
- Yu, S.; Li, L.; Zhao, H.; Zhang, S.; Tu, Y.; Liu, M.; Zhao, Y.; Jiang, L. Dietary Citrus Flavonoid Extract Improves Lactational Performance through Modulating Rumen Microbiome and Metabolites in Dairy Cows. Food Funct. 2023, 14, 94–111. [Google Scholar] [CrossRef]
- Yu, S.; Li, L.; Zhao, H.; Liu, M.; Jiang, L.; Zhao, Y. Citrus Flavonoid Extracts Alter the Profiling of Rumen Antibiotic Resistance Genes and Virulence Factors of Dairy Cows. Front. Microbiol. 2023, 14, 1201262. [Google Scholar] [CrossRef]
- PALACIOS, W.A.; TORRES, M.D.E.L.; QUINTANA, M.A.; ASADOBAY, P.; IGLESIAS, J.; QUILLUPANGUI, R.; ROJAS, E.; SANTIANA, J.; SOLA, A.; RIVAS-TORRES, G. A New Species and a New Record for Cedrela (Meliaceae, Sapindales) in Ecuador: Morphological, Molecular, and Distribution Evidence. Phytotaxa 2023, 595, 127–138. [Google Scholar] [CrossRef]
- Llerena, S.A.; Salinas, N.; Oliveira, O.L.; Jadán-Guerrero, M.; Segovia-Salcedo, C. DISTRIBUTION OF THE GENUS CEDRELA IN ECUADOR. Rudn J. Ecol. Life Saf. 2018, 26, 125–133. [Google Scholar] [CrossRef]
- Guevara Andino, J.E.; Pitman, N.C.A.; Ulloa Ulloa, C.; Romoleroux, K.; Fernández-Fernández, D.; Ceron, C.; Palacios, W.; Neill, D.A.; Oleas, N.; Altamirano, P. Trees of Amazonian Ecuador: A Taxonomically Verified Species List with Data on Abundance and Distribution 2019.
- Ketterings, Q.M.; Coe, R.; van Noordwijk, M.; Palm, C.A. Reducing Uncertainty in the Use of Allometric Biomass Equations for Predicting Above-Ground Tree Biomass in Mixed Secondary Forests. For. Ecol. Manage. 2001, 146, 199–209. [Google Scholar] [CrossRef]
- Carrasco, A.; Terán, C.; Crespo, E.; Mejía, E. Domestic Timber Market. For. use timber Mark. Ecuadorian Amaz. Occas. Pap. 2014, 111, 28–44. [Google Scholar]
Figure 1.
Study area location and temporary plots established along the altitudinal gradient.
Figure 1.
Study area location and temporary plots established along the altitudinal gradient.
Figure 2.
Grassland system with scattered trees: a) Amazonian high zone, b) middle zone, c) low zone, d) team in the Amazonian high zone.
Figure 2.
Grassland system with scattered trees: a) Amazonian high zone, b) middle zone, c) low zone, d) team in the Amazonian high zone.
Figure 3.
Rarefaction curves: a) Tree species richness based on temporary plots of 2826 m2 and b) Tree species richness based on individuals (trees with DBH ≥10 cm).
Figure 3.
Rarefaction curves: a) Tree species richness based on temporary plots of 2826 m2 and b) Tree species richness based on individuals (trees with DBH ≥10 cm).
Figure 4.
Stem diameter distribution (DBH ≥10 cm) at three altitude levels in silvopastoral systems. Letters in superscript denote significant differences between altitudinal gradients (p < 0.01).
Figure 4.
Stem diameter distribution (DBH ≥10 cm) at three altitude levels in silvopastoral systems. Letters in superscript denote significant differences between altitudinal gradients (p < 0.01).
Table 1.
Characteristics of livestock producers along the altitudinal gradient, Sumaco Biosphere Reserve, Napo Province, Ecuadorian Amazon.
Table 1.
Characteristics of livestock producers along the altitudinal gradient, Sumaco Biosphere Reserve, Napo Province, Ecuadorian Amazon.
| Variable |
Amazon Altitudinal gradient (zone) |
p-Valor 1
|
| Low |
Middle |
High |
| Elevation range (masl) |
400 – 700 |
701 – 1600 |
1601 – 2000 |
- |
| Average elevation (masl) |
543.1a
|
1114.1 b
|
1778.0 c
|
*** |
| Year of settlement |
1975 |
1984 |
1952 |
n.s |
| Ethnicity of head of household (% Kichwa) |
0.0 a
|
56.1 b
|
0.0 a
|
*** |
Table 2.
Averages and standard deviations of floristic composition, diversity index and structural parameters in plots (2826 m2) along the altitudinal gradient, Napo, Sumaco Biosphere Reserve, Ecuadorian Amazon.
Table 2.
Averages and standard deviations of floristic composition, diversity index and structural parameters in plots (2826 m2) along the altitudinal gradient, Napo, Sumaco Biosphere Reserve, Ecuadorian Amazon.
Variable |
Amazon Altitudinal gradient (zone) |
Average |
p-Value 1
|
Low
n=12 |
Middle
n=8 |
High
n=6 |
|
|
| Richness (species) |
10.17 ±3.21a
|
6.63 ±2.72ab
|
5.53 ±2.51b
|
7.96 ± 3.49 |
*** |
| Richness (family) |
8.50 ±2.23a
|
6.13 ±2.58ab
|
4.67 ±1.63b
|
6.88 ± 2.68 |
*** |
| Shannon |
1.63 ±0.45 |
1.50 ±0.52 |
1.25 ±0.44 |
1.50 ± 0.47 |
n/s |
| Simpson |
0.7 ±0.14 |
0.68 ±0.16 |
0.62 ±0.19 |
0.67 ± 0.15 |
n/s |
| Equity |
0.57 ±0.19 |
0.73 ±0.09 |
0.71 ±0.12 |
0.65 ± 0.16 |
n/s |
| Ha-1 |
|
|
|
|
|
| Tree density |
193 ±97.23a
|
83.25 ±38.33b
|
101 ±41.54b
|
138 ± 87.50 |
*** |
| Basal area (m2) |
8.67 ±4.23 |
4.19 ±3.65 |
6.03 ±4.97 |
6.68 ± 4.53 |
n/s |
| Average DBH (cm) |
20.32 ±5.39 |
22.37 ±11.82 |
22.85 ±12.69 |
21.53 ± 9.24 |
n/s |
| Maximum DBH (cm) |
27.78 |
40.67 |
41.54 |
41.54 |
|
Table 3.
List of the most important shade tree species with their relative abundance (R.A.), relative frequency (R.F.), relative dominance (R.D.) and important value indices (IVI) in 26 plots (2826 m2) along the studied gradient, Napo, SBR.
Table 3.
List of the most important shade tree species with their relative abundance (R.A.), relative frequency (R.F.), relative dominance (R.D.) and important value indices (IVI) in 26 plots (2826 m2) along the studied gradient, Napo, SBR.
| Familia |
Species |
R .A. (%) |
R .F. (%) |
R.D. (%) |
IVIs |
| Silvopasture low zone (400-700 masl) |
| Cordiaceae |
Cordia alliodora |
27,37 |
7,38 |
16,97 |
17,24 |
| Bignoniaceae |
Jacaranda copaia |
12,84 |
4,92 |
21,12 |
12,96 |
| Myrtaceae |
Psidium guajava |
17,58 |
8,20 |
3,94 |
9,91 |
| Vochysiaceae |
Vochysia braceliniae |
4,43 |
4,92 |
10,92 |
6,76 |
| Meliaceae |
Cedrela odorata |
3,21 |
5,74 |
4,68 |
4,54 |
| Fabaceae |
Piptadenia pteroclada |
1,53 |
4,92 |
5,44 |
3,96 |
| Myristicaceae |
Virola flexuosa |
2,60 |
1,64 |
6,99 |
3,74 |
| Lauraceae |
Ocotea spp. |
1,99 |
5,74 |
3,16 |
3,63 |
| Lauraceae |
Nectandra spp. |
2,45 |
4,10 |
3,23 |
3,26 |
| Asteraceae |
Piptocoma discolor |
2,75 |
5,74 |
0,93 |
3,14 |
| Subtotal |
|
76,76 |
53,28 |
77,37 |
69,14 |
| Silvopasture middle zone (701-1600 masl) |
| Cordiaceae |
Cordia alliodora |
12,23 |
5,66 |
26,39 |
14,76 |
| Asteraceae |
Piptocoma discolor |
17,02 |
5,66 |
13,21 |
11,97 |
| Meliaceae |
Cedrela odorata |
15,96 |
7,55 |
4,28 |
9,26 |
| Fabaceae |
Inga spp. |
5,85 |
11,32 |
6,99 |
8,06 |
| Myrtaceae |
Psidium guajava |
10,64 |
5,66 |
1,78 |
6,03 |
| Fabaceae |
Inga edulis |
3,19 |
5,66 |
5,72 |
4,86 |
| Lecythidaceae |
Grias neuberthii |
1,60 |
5,66 |
6,08 |
4,44 |
| Burseraceae |
Dacryodes peruviana |
3,19 |
1,89 |
5,92 |
3,66 |
| Urticaceae |
Cecropia membranacea |
1,60 |
5,66 |
3,10 |
3,45 |
| Lauraceae |
Nectandra spp. |
5,32 |
3,77 |
1,25 |
3,45 |
| Subtotal |
|
76,60 |
58,49 |
74,72 |
69,94 |
| Silvopasture high zone (1601-2000 masl) |
| Moraceae |
Ficus spp. |
5,85 |
12,50 |
53,51 |
23,95 |
| Malvaceae |
Heliocarpus americanus |
25,15 |
18,75 |
21,41 |
21,77 |
| Myrtaceae |
Psidium guajava |
38,01 |
9,38 |
6,38 |
17,92 |
| Lauraceae |
Ocotea spp. |
11,11 |
15,63 |
5,07 |
10,60 |
| Fabaceae |
Inga spp. |
9,94 |
9,38 |
5,56 |
8,29 |
| Lauraceae |
Nectandra spp. |
4,68 |
9,38 |
0,99 |
5,01 |
| Rutaceae |
Citrus limon |
1,75 |
6,25 |
0,33 |
2,78 |
| Meliaceae |
Cedrela montana |
0,58 |
3,13 |
2,41 |
2,04 |
| Phyllanthaceae |
Hieronyma oblonga |
0,58 |
3,13 |
1,80 |
1,84 |
| Euphorbiaceae |
Croton lechleri |
0,58 |
3,13 |
1,55 |
1,75 |
| Subtotal |
|
98,25 |
90,63 |
99,00 |
95,96 |
Table 4.
Tree species density, status in Ecuador with their IUCN conservation status, and wood densities for tree species in silvopastoral systems in Low Zone (N=12), Middle Zone (N=8), High Zone (N=6), total 26 plots (2826 m2) along the studied gradient, Napo, SBR.
Table 4.
Tree species density, status in Ecuador with their IUCN conservation status, and wood densities for tree species in silvopastoral systems in Low Zone (N=12), Middle Zone (N=8), High Zone (N=6), total 26 plots (2826 m2) along the studied gradient, Napo, SBR.
Familia
|
Species |
Tree density
(Ind ≥10 cm DAP/ha) |
Status |
category IUCN |
Wood density g/cm3
|
| Low |
Middle |
High |
Annonaceae
|
Annona papilionella |
0 |
1 |
0 |
Native |
LC |
0,48 |
|
Annona sp. |
9 |
1 |
0 |
Native |
NE |
0,47 |
|
Rollinia sp. |
0 |
0 |
1 |
Native |
NE |
0,61 |
Arecaceae
|
Apeiba membranaceae |
1 |
0 |
0 |
Native |
NE |
0,27 |
| Bactris gasipaes |
9 |
0 |
0 |
Native |
NE |
0,43 |
| Iriartea deltoidea |
3 |
1 |
0 |
Native |
LC |
0,27 |
| Wettinia maynensis |
0 |
5 |
0 |
Native |
LC |
0,31 |
Asteraceae
|
Piptocoma discolor |
6 |
26 |
0 |
Native |
LC |
0,47 |
| Vernonanthura patens |
2 |
0 |
0 |
Native |
LC |
0,54 |
Bignoniaceae
|
Crescentia cujete |
1 |
0 |
0 |
Native |
LC |
0,70 |
| Jacaranda copaia |
84 |
3 |
0 |
Native |
LC |
0,60 |
| Boraginaceae |
Cordia alliodora |
0 |
18 |
0 |
Native |
LC |
0,51 |
Burseraceae
|
Dacryodes peruviana |
0 |
6 |
0 |
Native |
LC |
0,61 |
| Protium nodulosum |
0 |
4 |
0 |
Native |
LC |
0,55 |
| Calophyllaceae |
Calophyllum brasiliense |
1 |
1 |
0 |
Native |
LC |
0,47 |
| Combretaceae |
Terminalia oblonga |
1 |
0 |
0 |
Native |
LC |
0,69 |
| Cordiaceae |
Cordia alliodora |
122 |
0 |
0 |
Native |
LC |
0,51 |
Euphorbiaceae
|
Croton lechleri |
0 |
0 |
1 |
Native |
NE |
0,47 |
| Sapium glandulosum |
0 |
1 |
0 |
Native |
LC |
0,44 |
Fabaceae
|
Dussia tessmannii |
2 |
0 |
0 |
Native |
LC |
0,47 |
| Erythrina poeppigiana |
1 |
0 |
0 |
Native |
LC |
0,47 |
| Inga edulis |
2 |
6 |
0 |
Native |
LC |
0,51 |
|
Inga spp. |
0 |
8 |
16 |
Native |
NE |
0,57 |
| Piptadenia pteroclada |
9 |
0 |
0 |
Native |
LC |
0,76 |
Lauraceae
|
Nectandra spp. |
14 |
3 |
3 |
Native |
NE |
0,53 |
|
Ocotea spp. |
12 |
0 |
14 |
Native |
NE |
0,54 |
| Persea americana |
1 |
0 |
0 |
Native |
LC |
0,60 |
| Lecythidaceae |
Grias neuberthii |
8 |
3 |
0 |
Native |
LC |
0,62 |
Malvaceae
|
Ceiba samauma |
2 |
0 |
0 |
Native |
NE |
0,57 |
| Heliocarpus americanus |
0 |
0 |
0 |
Native |
LC |
0,47 |
| Sterculia tessmannii |
2 |
2 |
37 |
Native |
LC |
0,47 |
| Melastomataceae |
Miconia spp. |
7 |
0 |
0 |
Native |
NE |
0,63 |
Meliaceae
|
Cabralea canjerana |
5 |
0 |
0 |
Native |
LC |
0,53 |
| Cedrela montana |
0 |
0 |
1 |
Native |
VU |
0,47 |
| Cedrela odorata |
16 |
12 |
1 |
Native |
VU |
0,44 |
Moraceae
|
Brosimum guianense |
2 |
0 |
0 |
Native |
LC |
0,47 |
| Ficus cuatrecasana |
0 |
0 |
1 |
Native |
NE |
0,47 |
| Ficus maxima |
2 |
1 |
0 |
Native |
LC |
0,47 |
|
Ficus spp. |
6 |
0 |
10 |
Native |
NE |
0,42 |
| Myristicaceae |
Virola flexuosa |
16 |
0 |
0 |
Native |
LC |
0,47 |
| Myrtaceae |
Psidium guajava |
48 |
5 |
28 |
Native |
LC |
0,71 |
| Phyllanthaceae |
Hieronyma oblonga |
0 |
0 |
1 |
Native |
LC |
0,47 |
Rutaceae
|
Citrus limon |
1 |
1 |
2 |
No Native |
NE |
0,71 |
| Citrus sinensis |
7 |
0 |
0 |
No Native |
NE |
0,71 |
Sapotaceae
|
Pouteria caimito |
6 |
2 |
1 |
Native |
LC |
0,81 |
|
Pouteria spp. |
0 |
0 |
0 |
Native |
NE |
0,77 |
Urticaceae
|
Cecropia membranacea |
0 |
3 |
0 |
Native |
LC |
0,33 |
|
Cecropia spp. |
1 |
0 |
0 |
Native |
NE |
0,36 |
| Pourouma cecropiifolia |
4 |
1 |
0 |
Native |
LC |
0,36 |
Vochysiaceae
|
Vochysia braceliniae |
28 |
0 |
0 |
Native |
LC |
0,39 |
| Vochysia ferruginea |
6 |
0 |
0 |
Native |
LC |
0,36 |
Table 5.
Use of plants reported in trees, palms and fruit trees in silvopastoral systems in the low zone (N=12), middle zone (N=8), high zone (N=6), total 26 plots (2826 m2) along the studied gradient, Napo, SBR.
Table 5.
Use of plants reported in trees, palms and fruit trees in silvopastoral systems in the low zone (N=12), middle zone (N=8), high zone (N=6), total 26 plots (2826 m2) along the studied gradient, Napo, SBR.
| Scientific name |
Family |
Use |
| Human and livestock feed |
Medicinal use |
Craft use |
Material for construction |
| Annona papilionella |
Annonaceae |
x |
x |
x |
x |
|
Annona sp. |
x |
x |
x |
x |
|
Rollinia sp. |
x |
x |
x |
x |
| Apeiba membranacea |
Arecaceae |
x |
x |
x |
x |
| Bactris gasipaes |
x |
x |
x |
x |
| Iriartea deltoidea |
x |
x |
x |
x |
| Wettinia maynensis |
x |
x |
x |
x |
| Piptocoma discolor |
Asteraceae |
x |
x |
|
x |
| Vernonanthura patens |
|
x |
x |
x |
| Crescentia cujete |
Bignoniaceae |
x |
x |
|
x |
| Jacaranda copaia |
|
x |
|
x |
| Cordia alliodora |
Cordiaceae |
|
x |
|
x |
| Dacryodes peruviana |
Burseraceae |
x |
x |
x |
x |
| Protium nodulosum |
x |
x |
x |
x |
| Calophyllum brasiliense |
Calophyllaceae |
|
x |
|
x |
| Terminalia oblonga |
Combretaceae |
|
|
x |
x |
| Croton lechleri |
Euphorbiaceae |
|
x |
|
x |
| Sapium glandulosum |
x |
x |
|
x |
| Dussia tessmannii |
Fabaceae |
|
|
x |
x |
| Erythrina poeppigiana |
|
|
|
x |
| Inga edulis |
x |
x |
|
x |
|
Inga spp. |
x |
x |
|
x |
| Piptadenia pteroclada |
|
x |
|
x |
|
Nectandra spp. |
Lauraceae |
|
|
|
x |
|
Ocotea spp. |
|
x |
|
x |
| Persea americana |
x |
x |
|
x |
| Grias neuberthii |
Lecythidaceae |
x |
x |
|
x |
| Ceiba samauma |
Malvaceae |
|
|
x |
x |
| Heliocarpus americanus |
|
x |
x |
x |
| Sterculia tessmannii |
|
|
x |
x |
|
Miconia spp. |
Melastomataceae |
|
x |
|
x |
| Cabralea canjerana |
Meliaceae |
|
|
|
x |
| Cedrela montana |
|
|
|
x |
| Cedrela odorata |
|
x |
|
x |
| Brosimum guianense |
Moraceae |
x |
|
|
x |
| Ficus cuatrecasana |
|
|
|
x |
| Ficus maxima |
x |
x |
|
x |
|
Ficus spp. |
x |
x |
|
x |
| Virola flexuosa |
Myristicaceae |
|
|
|
x |
| Psidium guajava |
Myrtaceae |
x |
x |
|
x |
| Hieronyma oblonga |
Phyllanthaceae |
x |
|
|
x |
| Citrus limon |
Rutaceae |
x |
x |
|
x |
| Citrus sinensis |
x |
x |
|
x |
| Pouteria caimito |
Sapotaceae |
x |
x |
|
x |
|
Pouteria spp. |
x |
x |
|
x |
| Cecropia membranacea |
Urticaceae |
x |
x |
|
x |
|
Cecropia spp. |
x |
x |
|
x |
| Pourouma cecropiifolia |
x |
|
|
x |
| Vochysia braceliniae |
Vochysiaceae |
|
x |
|
x |
| Vochysia ferruginea |
|
|
x |
x |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).