1. Introduction
Technical and scientific advancements have improved the outcomes of patients undergoing liver transplantation (LT), but long-term survival is still burdened by the complications of chronic immunosuppression [
1]. The utilization of extended criteria donors (ECD), donors after cardiocirculatory death (DCD), and machine perfusion (MP) technology has increased in the evolving scenario of LT [
2,
3,
4]. Additionally, strategies to reduce long-term immunosuppression attrition rates, non-immunosuppressive co-medication, and aging of recipients are widely advocated [
5].
Although several international transplant centers have more than 20 years of clinical experience in LT, only a few studies have analyzed the long-term outcomes of LT recipients [
6,
7,
8,
9,
10]. In Italy, there is a lack of data on the long-term outcomes of adult liver transplant recipients. Recent studies have provided estimates up to 10 years after transplantation [
11]. To address this gap, we conducted a retrospective, comparative study on adult transplant recipients who survived for at least 20 years. Our aim was to identify clinical predictors of outcomes and showcase the long-term health conditions of the survivors.
2. Materials and methods
2.1. study design
This was a retrospective, single-center study at an Italian National Health System (NHS)-based liver transplant center.
2.2. population
The study involved all adult patients (≥18 years old) who underwent LT from deceased donors (DDLT) between January 1996 (when the transplant program began at our institution) and December 31st, 2002. Patients were excluded from the analysis if they received a graft from a donor after cardiocirculatory death (DCD) or if they received combined transplantation.
2.3. data source
For the current study, we used data from the regional transplant authority (CRT, Centro Regionale Trapianti) and the prospectively maintained recipient database of our institution. The CRT database contains information on all donors, candidates on the waiting list, and transplant recipients, and ensures the accuracy, validity, and transparency of the data. The local ethics committee of the University of Pisa (Prot. 0036349/2020) approved all procedures.
2.4. primary outcomes
Our primary outcomes were post-transplant graft failure, patient death, and the incidence of hepatic and extrahepatic complications during the follow-up period. All measures were treated as time-to-event occurrences.
2.5. secondary outcome
Our secondary outcome was to evaluate the prevalence of long-term complications until December 2022.
2.6. statistical analyses
Descriptive statistics were calculated for the donor and recipient demographics and transplant characteristics. Patient characteristics were compared between groups using the appropriate statistical test and survival data were censored at the time of graft failure, death, or latest follow-up (December 2022). We utilized Cox proportional hazards models to assess the independent risks associated with post-transplant graft failure and patient mortality. As complications within the first year after transplantation are more likely to be related to surgical/medical management, we performed sensitivity analyses that censored graft failure and death at 1 year after transplantation. Hazards of graft failure and death were again assessed using Cox proportional hazard models and adjusted as appropriate.
2.7. special considerations
During the study period (1996-2002), the transplant procedure, perioperative management, and immunosuppressive schedules changed according to technological advancements and scientific evidence. The University of Wisconsin (UW) perfusion solution was used until 2001, whereas Celsior® (IGL, Lissieu, France) was utilized thereafter. Bypass, or the classical technique, was standard until 2017. De novo immunosuppression consisted of a triple regimen of microemulsion cyclosporine (CyA) (Neoral®, Novartis, Origgio (VA), Italy) steroids (S), and azathioprine (AZA) until 1999; CyA, S, and mycophenolate mofetil (MMF) until 2001; and quadruple regimen with anti-CD25 (basiliximab, Simulect®, Novartis, Origgio (VA), Italy) since 2002. Use of tacrolimus (TAC) was initiated at our institution in 1999 and has become the standard de novo calcineurin inhibitor since 2009. The use of everolimus (EVR) (Certican®, Novartis, Origgio (VA), Italy) began in 2005 in maintenance schedules and in 2008 in de novo regimens. From 2002 onward, anti-CMV prophylaxis was administered to recipients without acquired immunity (D+/R − and D −/R − combinations).
2.8. definitions and cut-offs
Cold ischemia time (CIT) was defined as the time from cross-clamping until removal of the organ from the ice for implantation, and warm ischemia time (WIT) was defined as the time of ischemia during graft implantation. EAD was defined according to Olthoff et al. [
12]. MELD scores at transplant were recalculated retrospectively based on available laboratory data. Rejection episodes were graded according to the BANFF scale [
13]. HCV recurrence was diagnosed by liver biopsy in the presence of HCV-RNA. HBV infection recurrence was defined as the reappearance of HBsAg (± HBV DNA) in previously seroconverted patients, irrespective of liver function. Renal function was evaluated as the estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease (MDRD)-4 formula. Renal dysfunction was defined as eGFR <60 mL/min/ 1.73 m
2 or need for dialysis. Arterial hypertension was defined as the need for medication or a blood pressure of 140/90 mmHg at the following two visits. Diabetes mellitus was defined as the need for medication or a fasting plasma glucose level >126 mg/dL at the following two visits. Dyslipidemia was defined as hypercholesterolemia of >220 mg/dL and/or hypertriglyceridemia of >200 mg/dL at the following two visits. Body mass index (BMI) was retrospectively derived from clinical charts for compensated patients during follow-up but not at the time of transplantation, as it was not possible to adjust values for the presence of ascites or edema. Obesity was defined as a BMI ≥30 kg/m2. For the current analysis, we excluded non-melanoma skin malignancies due to their negligible impact on patient survival.
3. Results
3.1. demographics and clinical characteristics
During the study period, 375 procedures were performed on 367 patients (8 re-transplantations). Four patients were excluded from analysis for age at transplantation <18 years and 4 due to combined liver and kidney transplantation. The final cohort included 359 patients. At a median (IQR) follow-up of 144.8 (204) months after transplantation, 123 (34.3%) recipients survived versus 236 (65.7%) who died.
The demographic and clinical characteristics of the study sample are shown in
Table 1. Patients were predominantly male (66.6%) with a median (interquartile range (IQR)) age of 52.5 (13) years. The main indication for transplantation was HCV-related liver disease (54.3%), and hepatocellular carcinoma (HCC) was present in 42.1% of patients. The median (IQR) lab-MELD score at transplant (retrospective) was 13 (7). Diabetes mellitus (DM) was present in 12.2% of patients, CKD in 14.8%, and hypertension in 16.4% (
Table 1). All recipients received a graft from a brain-dead donor (DBD) and a split liver was utilized in 6 cases (1.7%) only. The median (IQR) CIT was 536 (78) min. Eight (2.2%) patients were retransplanted due to primary non-function of the liver graft in 6 cases and hepatic artery thrombosis in 2.
Immunosuppression at transplantation was based on CyA in 73.0% of patients, while anti-IL2R monoclonal antibody was used in 37.3%of cases (
Table 1).
3.2. patient and graft survival
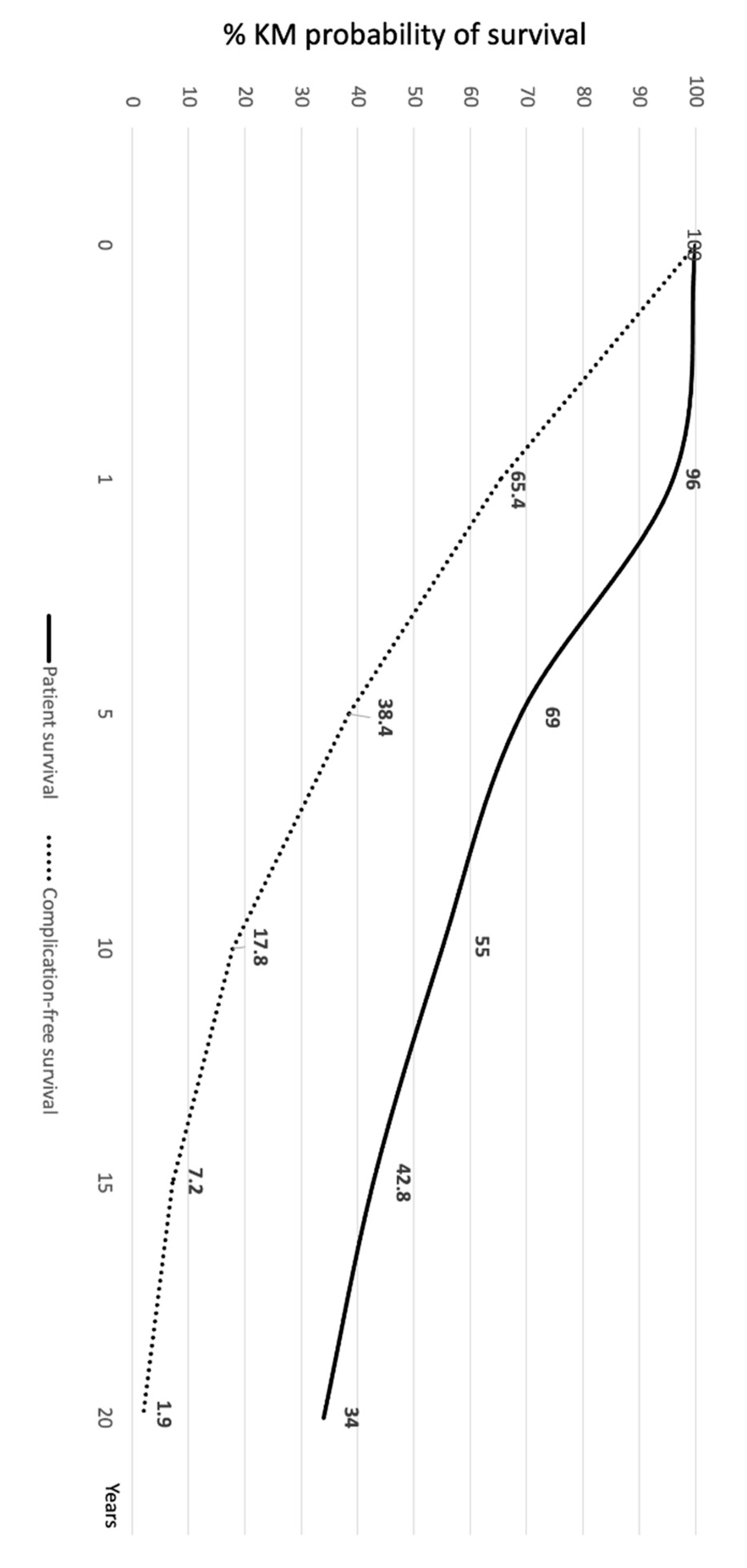
Patient death and re-transplantation were considered graft loss. The actuarial (95% CI) patient survival of the overall study cohort was 96% (94.6%-98.3%), 69% (64.2%-73.6%), 55% (49.8%-59.9%), 42.8% (37.6%-47.8%), and 34% (29.2%-38.9%) at 1, 5, 10, 15, and 20 years, respectively (
Figure 1).
The actuarial (95% CI) graft survival was 93.8% (91.6%-97.2%), 67.6% (62.2%-71.5%), 54.3% (49.6%-59.3%), 42.1% (37.3%-47.4%), and 33.8% (29.0%-38.4%) at 1, 5, 10, 15, and 20 years, respectively.
3.3. causes of death
After a median (IQR) follow-up of 144.8 (204) months, 236 (65.7%) patients had died. The causes of death are presented in
Table 2. Recurrent HCV-related liver dysfunction was the most common cause of death, accounting for 24.6% of all the deaths. This was followed by extrahepatic malignancies (16.9%), infection (14.4%), HCC recurrence (14.4%), and major cardiovascular events (MACE) (10.6%). Half of all deaths occurred within 5.5 years after the transplant.
3.4. rejection
The incidence of treated and biopsy-proven acute rejection (t/BPAR) is shown in
Table 2. Seventy-seven (21.4%) patients presented with at least one episode of t/BPAR, the proportion of patients with more than one episode was 3.9%. Half of all rejection episodes occurred within 6 months after transplantation (data not shown).
3.5. health conditions of long-term survivors
As of December 2022, a cross-sectional analysis of the 123 survivors revealed that the most frequent co-morbidities were hypertension (53.6%), obesity (18.7%), DM (17.1%), hyperlipidemia (14.7%), CKD (14.7%), extrahepatic malignancies (13.8%), and MACE (13.0%) (
Table 3). Most patients (73.9%) experienced more than one complication.
Maintenance immunosuppression consisted of TAC monotherapy in 38.2% of the patients, CyA monotherapy in 29.2%, EVR monotherapy in 13.8%, TAC + EVR in 8.9%, and TAC + MMF (or AZA) + S in 2.4% (Supplementary table 1).
3.6. complication-free survival
Figure 1 shows the Kaplan-Meier probability (95% CI) of survival without any complication/comorbidity during the follow-up period. At 1, 5, 10, 15, and 20 years this was 65.4% (60.2%-70.3%), 38.4% (33.4%-43.7%), 17.8% (14.1%-22.2%), 7.2% (4.8%-10.5%), and 1.94% (0.85%-4.15%).
3.7. predictors of survival
The univariable analysis showed that the chances of surviving after transplant were higher for recipients of younger grafts (p=0.0009), younger patients (p<0.0001), female patients (p=0.005), those with non-HCV liver disease as the reason for transplantation (p<0.0001), no presence of HCC at transplant (p=0.001), lower laboratory MELD score (p=0.002), use of anti-IL2R as an induction agent (p<0.0001), TAC as the primary immunosuppressant (p<0.0001), and no occurrence of DM one year after transplant (p=0.01). The Cox proportional hazards analysis revealed that several independent factors were associated with the survival probability (
Table 4). These factors associated with successful liver transplantation include younger donor and recipient ages (p=0.001 and 0.004, respectively), female recipient sex (p<0.001), absence of HCV (p<0.01), absence of HCC (p=0.001), and absence of DM at one year (p<0.01).
4. Discussion
To our knowledge, this is the first report of adults surviving at least 20 years after liver transplant in Italy. There is a lack of information on long-term survivors of LT in our country, despite its expanding practice in recent years (11). We conducted this single-center study to address this gap and provide data for comparison with international benchmarks.
Comparing long-term results across international series is challenging due to biases related to recipients, donors, indications for transplantation, eras, and scarcity of publications. Although our one-year patient survival was favorable at 96%, the 5-, 10-, and 20-year survival rates following transplantation were lower (69%, 55%, and 34%, respectively) than those reported in previous studies conducted in North America (6,7) and Germany (8). This may be due to differences in age, HCV status, and the reason for transplantation in our cohort of donors and recipients. In their analyses, Jain et al. [
6] and Duffy et al. [
7] included pediatric recipients (12% and 38.9%, respectively), whereas Schoening et al. [
8] reported that HCV infection accounted for 10% of indications only versus 54.3% in our cohort. Inferior (22%) 20-year survival was reported by Dopazo et al. for a population of recipients with HCV infection in one-third of the cases [
10]. Finally, the reported 10-year survival rate (53.6%) by Lai et al. may be affected by HCV-cohort and era-related bias, recipients' clinical status, and disease severity at transplantation [
11].
Despite variations in survival rates among studies, all long-term reports highlight the burden of comorbidities related to immunosuppression, including cardiovascular, metabolic, renal, and oncological complications. Our study observed that almost all patients who survived 20 years post-transplantation had at least one comorbidity, and 73.9% of them had multiple comorbidities. Similar values were reported by Rubin et al. [
9], with most deaths related to recurrent HCV graft disease, followed by
de novo tumors and cardiovascular events. In their experience, the 1-, 3-, 5-, and 10-year cumulative rates of cardiovascular events and
de novo tumors since baseline were 2%, 5%,10% and 17% and 1%, 3%, 6%, and 13%, respectively [
9]. These values are consistent with the incidence of complications observed in our cohort and the prevalence of complications/comorbidities observed at the latest follow-up in the group of long-term survivors.
After analyzing national and international studies, it has been found that early outcomes of transplant surgeries are more dependent on the graft quality, the surgery itself (measured by CIT and WIT), and the clinical severity of the recipient. On the other hand, long-term results are influenced by demographic characteristics of the patients (such as age and sex), the indication for transplantation (such as HCC), and any complications that may occur in the first few years after the surgery, especially diabetes and chronic kidney dysfunction. According to previous studies [
6], it has been observed that female recipients have a higher probability of long-term survival. This finding should be analyzed in the context of the higher life expectancy rates of females, as well as the differences in the prevalence of liver diseases like hepatitis C between male and female patients. The study by Dopazo et al. [
10] highlighted the predictive role of diabetes one year after transplantation. This may be an end-point surrogate for pre- and clinical studies on novel immunosuppressive agents and therapeutic technologies.
Our research has specific constraints that need to be considered, and it is essential to compare and validate the findings on a larger scale. First, its retrospective design does not always allow for the granular information that real-practice clinical studies need to produce clinically transferable data. Additionally, it focused on the initial experience at our center and might inevitably be biased by a learning curve effect in terms of technical refinements, patient selection, and post-transplant therapeutic strategies. Finally, the quality of chronic care provided at our center over a patient’s lifetime horizon changed during the follow-up period and might have affected the individual patient's probability of post-transplant survival.
In conclusion, based on our results, aging with liver grafting is associated with an increased risk of comorbidities and requires a chronic care model to reduce the long-term attrition rate resulting from chronic immunosuppression.
Acknowledgments
The authors would like to express their gratitude to the nursing team of the Coordinamento Trapianti di Fegato, University of Pisa Medical School Hospital, and the Centro Regionale Allocazione Organi e Tessuti, Careggi Florence Hospital.
Conflicts of Interest
PDS has served as advisory board member for Novartis, Astellas, and Chiesi. The other authors have no interest to declare.
References
- Adam, R.; Karam, V.; Cailliez, V.; OGrady, J.G.; Mirza, D.; Cherqui, D.; Klempnauer, J.; Salizzoni, M.; Pratschke, J.; Jamieson, N. 2018 annual report of the European liver transplant registry (ELTR) - 50-year evolution of liver transplantation. Transpl. Int. 2018, 31, 1293–1317. [Google Scholar] [CrossRef] [PubMed]
- De Simone P, Ghinolfi D, Palladino S, Catalano G, Martinelli C, Ducci J, et al. Am J Transplant Sep 25: S1600-6135(23)00702-5. [CrossRef]
- Patrono D, Cussa D, Sciannameo V, Montanari E, et al. Outcome of liver transplantation with grafts from brain-dead donors treated with dual hypothermic oxygenated machine perfusion, with particular reference to elderly donors. Am J Transplant 2022, 22, 1382–1395.
- Grat M, Moeawski M, Zhylko A, Rykowski P, et al. Routine end-ischemic hypothermic oxygenated perfusion in liver transplantation from donors after brain death: a randomized controlled trial. Ann Surg 2023; July 27. [CrossRef]
- De Simone P, Carrai P, Coletti L, Ghinolfi D, Petruccelli S, Filipponi F. Modification of immunosuppressive therapy as risk factor for complications after liver transplantation. Best Pract Res Clin Gastroenterol 2017 Apr;31(2):199-209.
- Jain A, Reyes J, Kashyap R, Dodson SF, Demetris A, Ruppert K, et al. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg 2000; 232(4):490-500.
- Duffy JP, Kao K, Ko CY, Farmer DG, McDiarmid SV, Hong JC, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010;252(4):652-661.
- Schoening WN, Buescher N, Rademacher S, Andreou A, Kuehn S, Neuhaus R, Guckelberger O, Puhl G, Seehofer D, Neuhaus P. Twenty-year longitudinal follow-up after orthotopic liver transplantation: a single-center experience of 313 consecutive cases. Am J Transplant. 2013;13:2384–2394.
- Rubín, A.; Sánchez-Montes, C.; Aguilera, V.; Juan, F.S.; Ferrer, I.; Moya, A., et al. Long-term outcome of 'long-term liver transplant survivors'. Transpl Int 2013, 26, 740–50.
- Dopazo C, Bilbao I, Castells LL, Sapisochin G, Moreiras C, Campos-Varela I, Echeverri J, Caralt M, Lázaro JL, Charco R. Analysis of adult 20-year survivors after liver transplantation. Hepatol Int 2015;9:461–470.
- Lai Q, Mennini G, Ginanni Corradini S, Ferri F, Fonte S, Pugliese F, Merli M, Rossi M. Adult 10-year survivors after liver transplantation: a single-institution experience over 40 years. Updates Surg 2023.
- Olthoff, K.M.; Kulik, L.; Samstein, B.; Kaminski, M.; Abecassis, M.; Emond, J.; Shaked, A.; Christie, J.D. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010, 16, 943–949. [Google Scholar] [CrossRef] [PubMed]
- [No authors listed] Banff schema for grading liver allograft rejection: an international consensus document. Hepatology 1997;25(3):658-663.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).