Submitted:
11 January 2024
Posted:
12 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
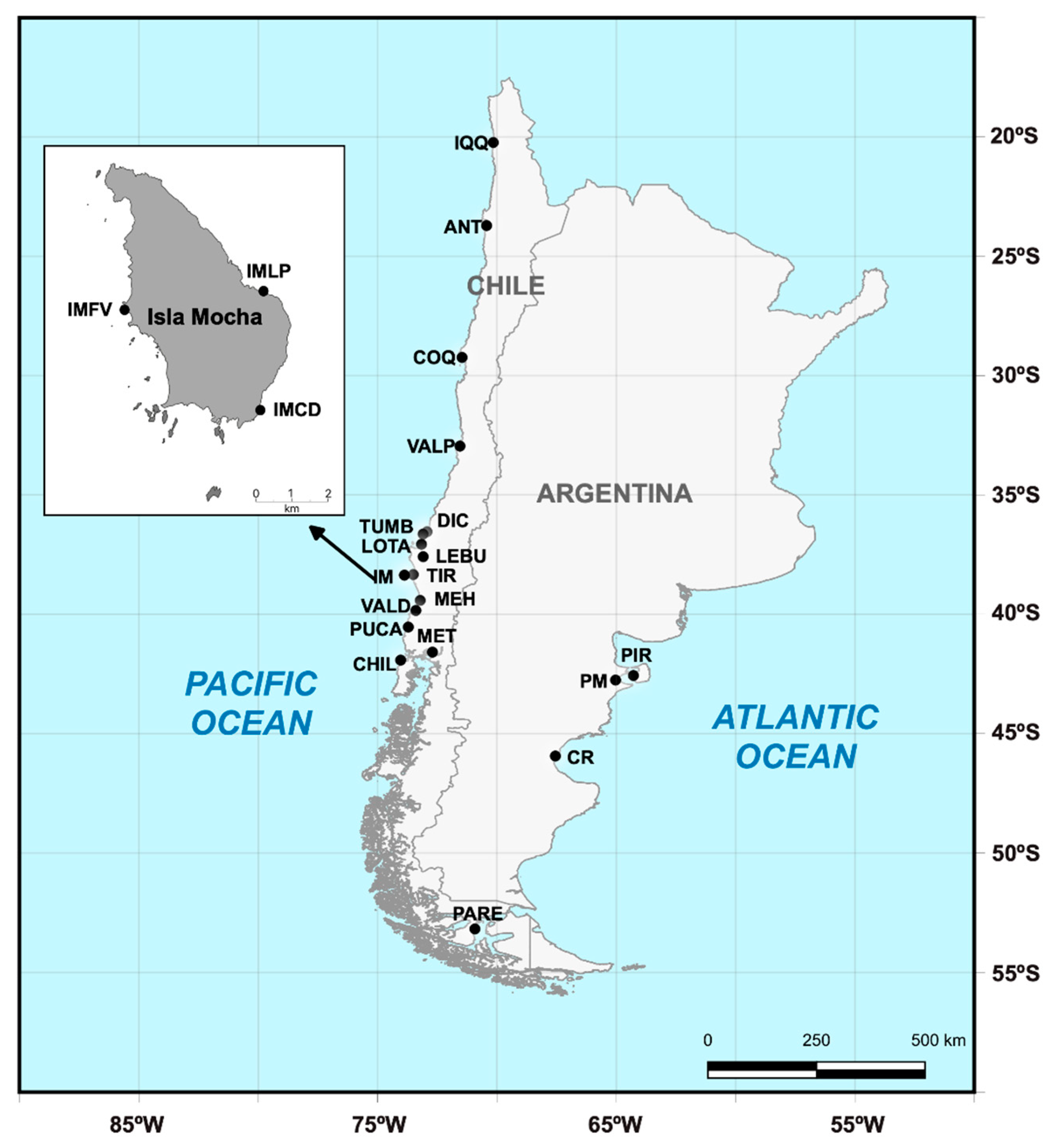
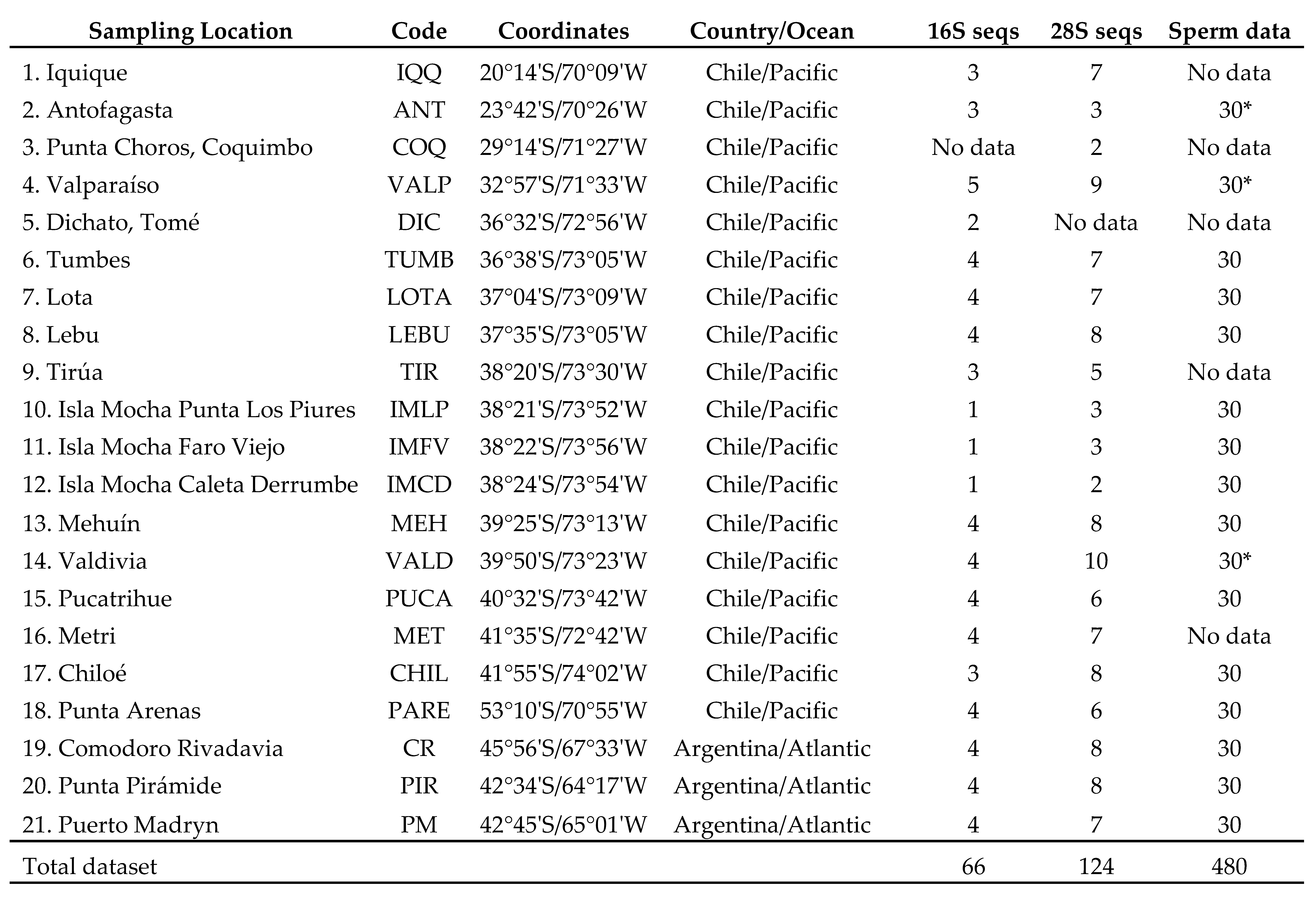
2.1. Sample collection
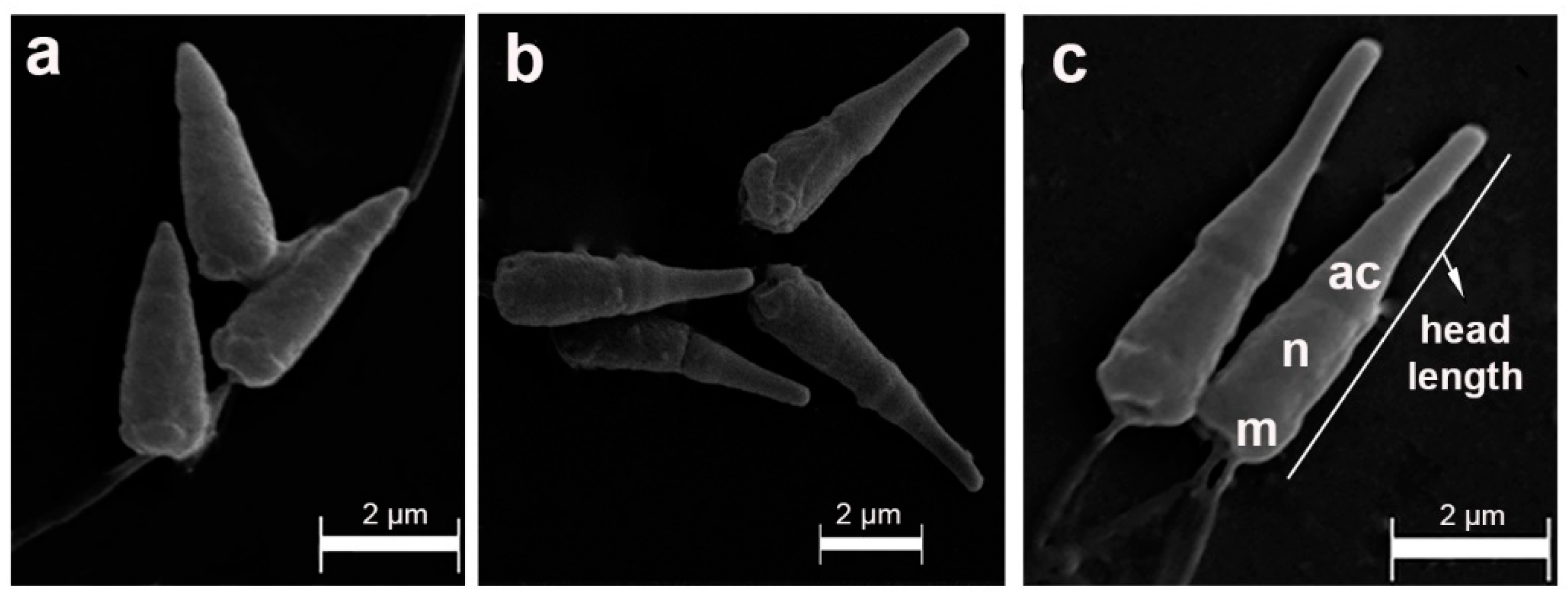
2.2. Sperm morphology analyses
2.3. Scanning electron microscopy (SEM)
2.4. Statistical analyses
2.5. Molecular analyses
2.6. DNA extraction, amplification, and sequencing
2.7. Bayesian reconstructions and genetic structure
2.8. Genetic diversity and demographic history estimation
3. Results
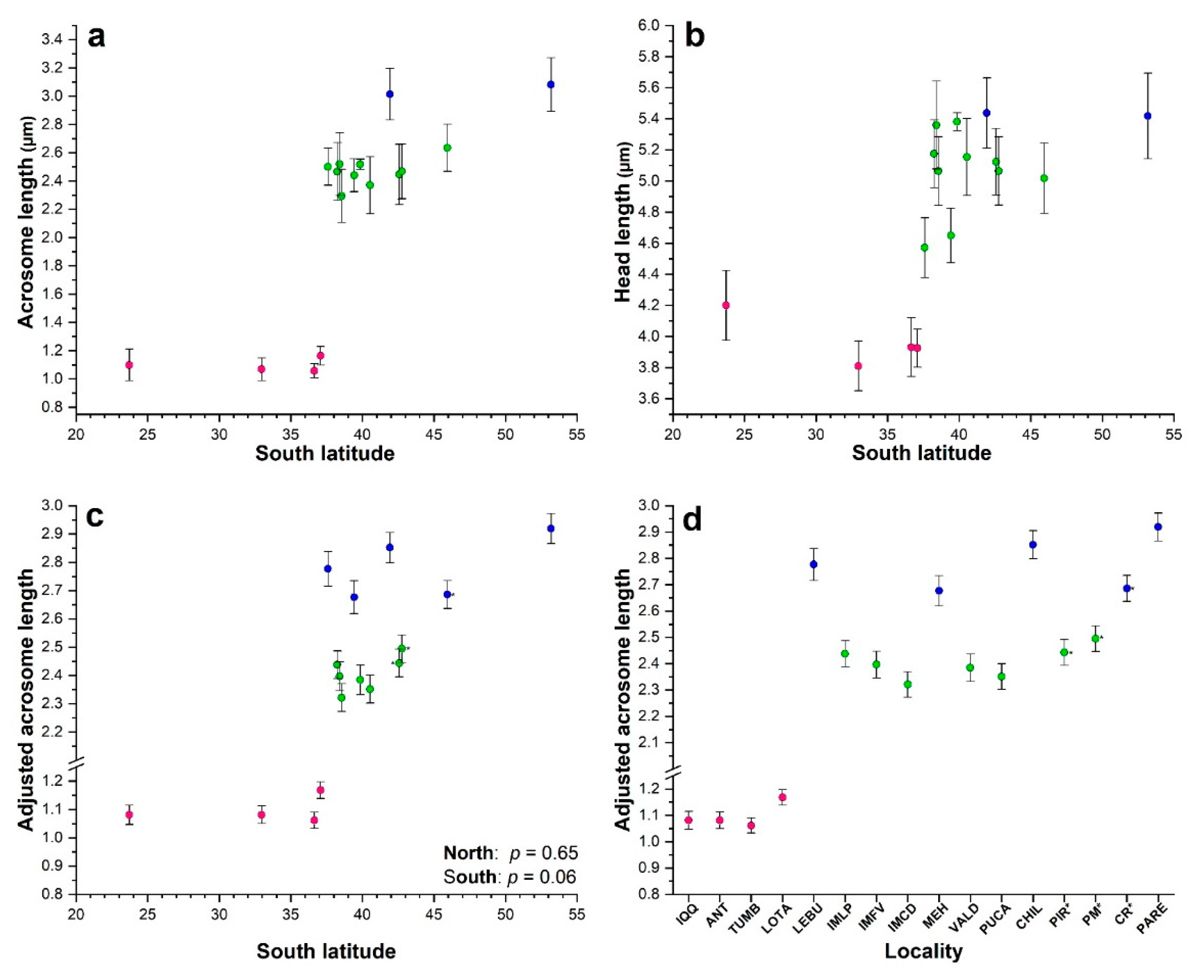
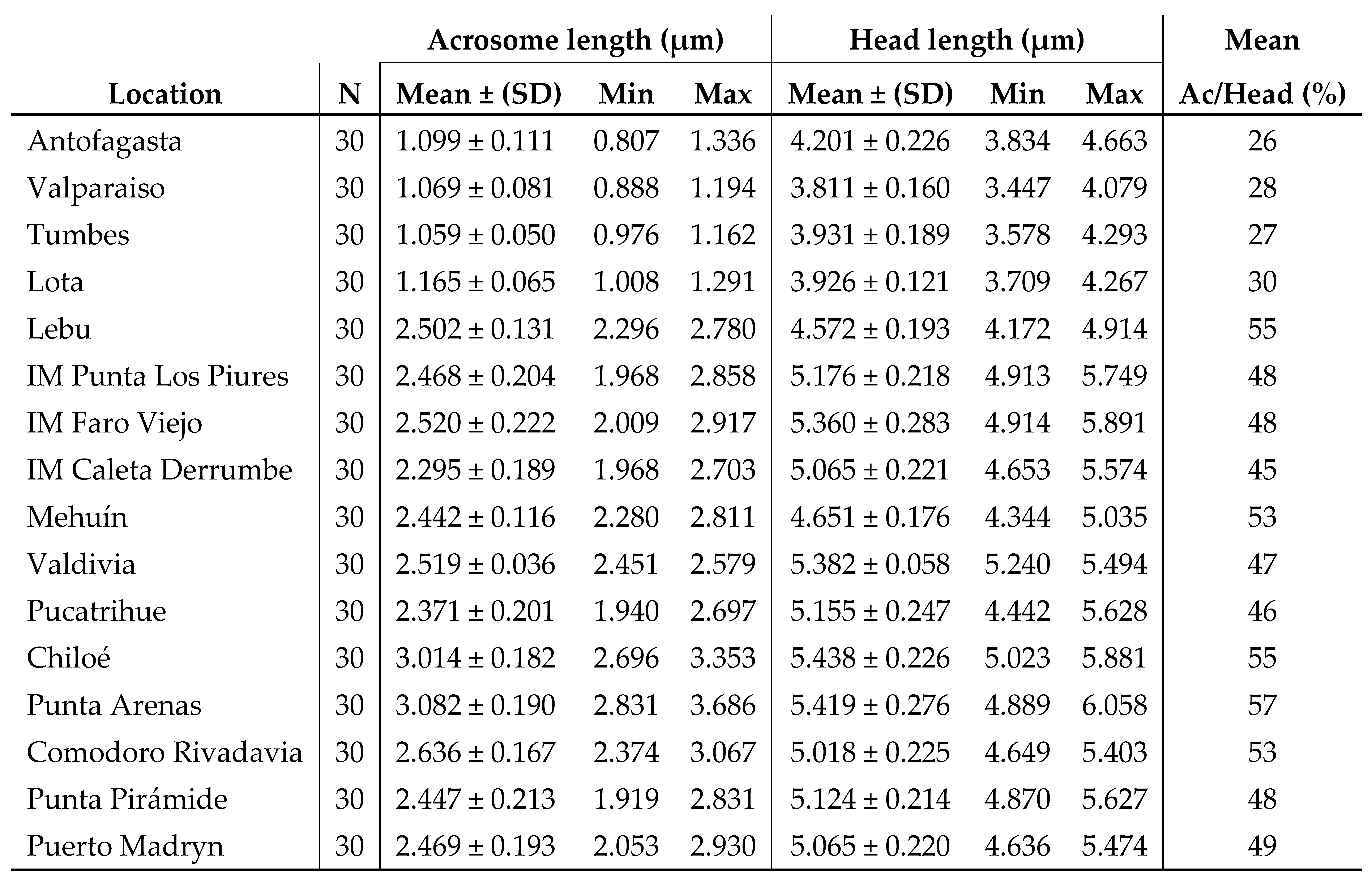
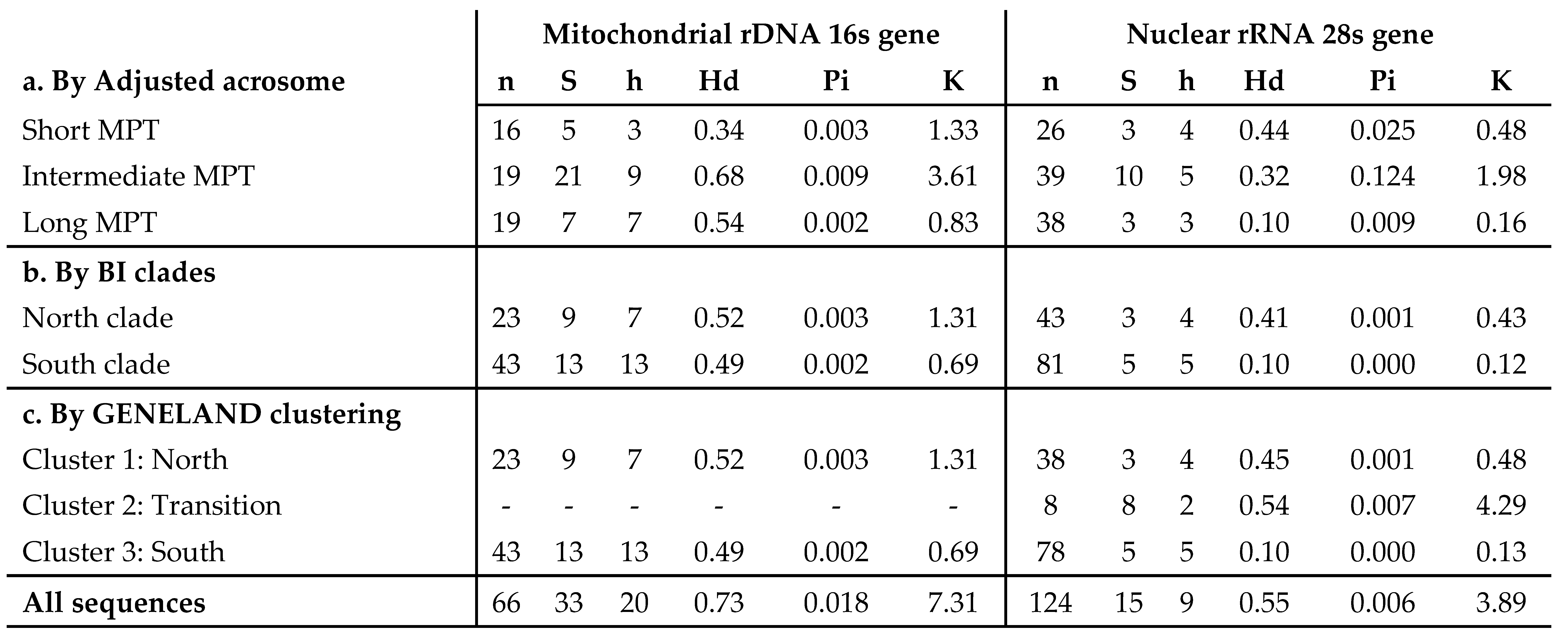
3.1. Sperm morphology analyses
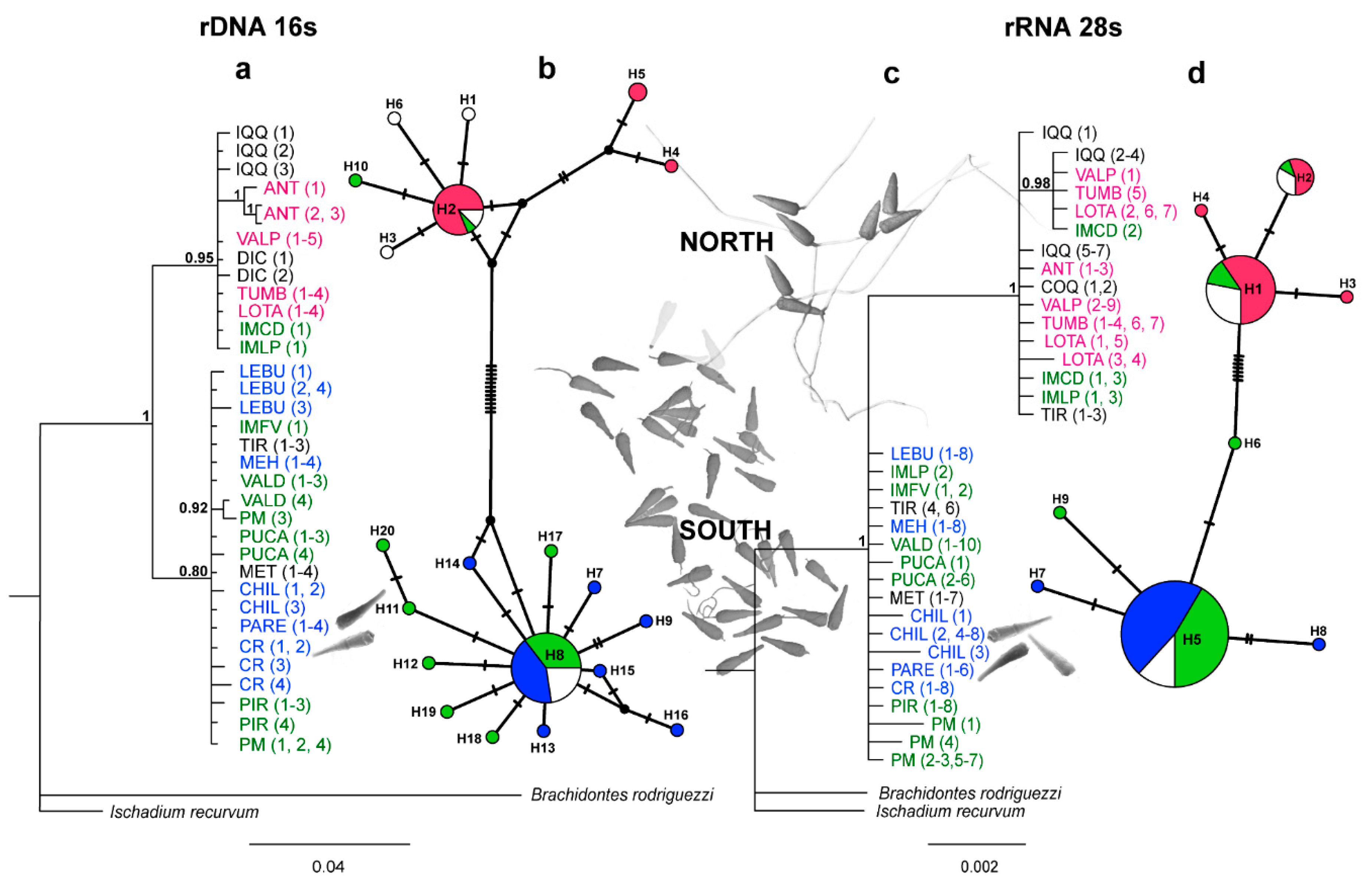
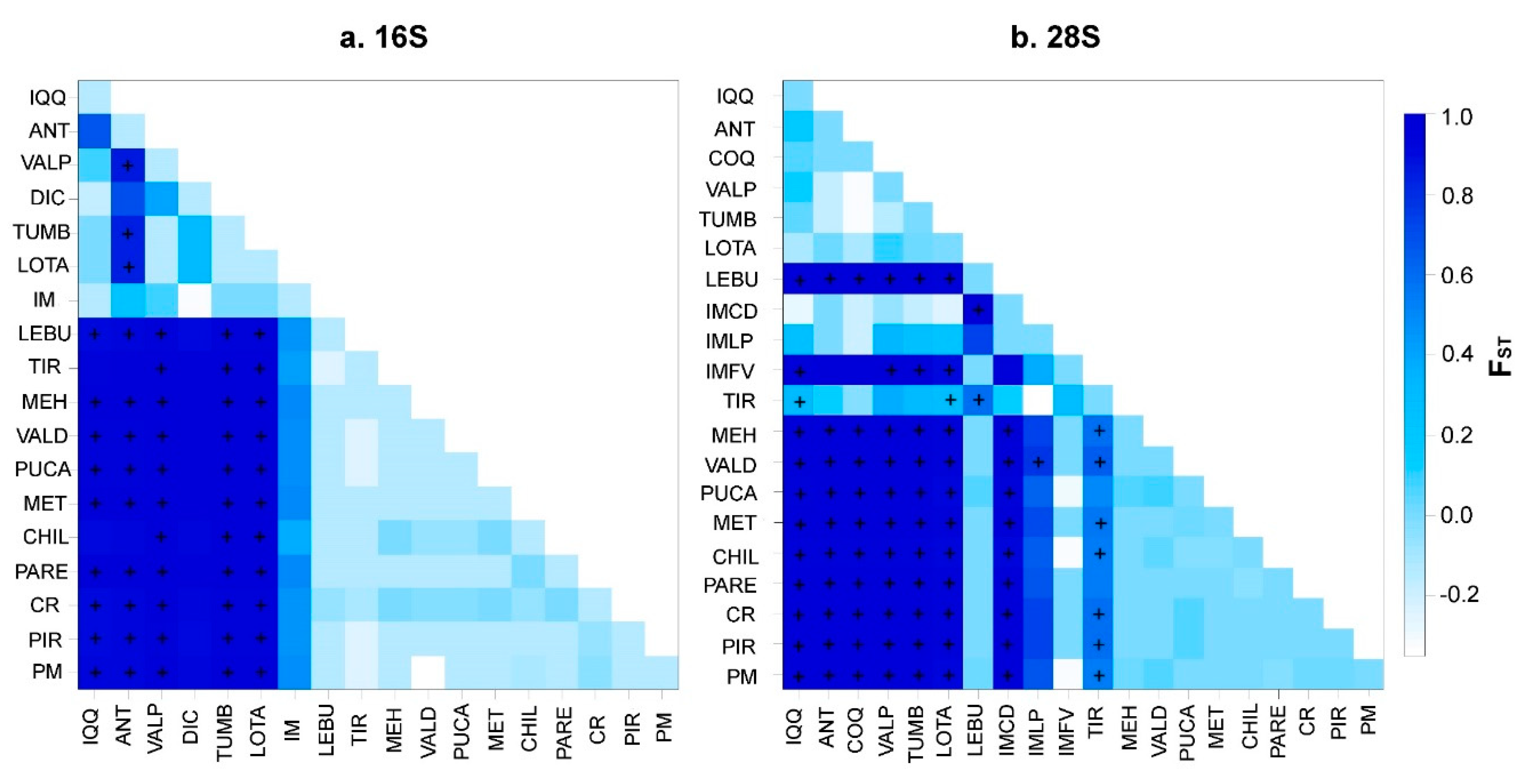
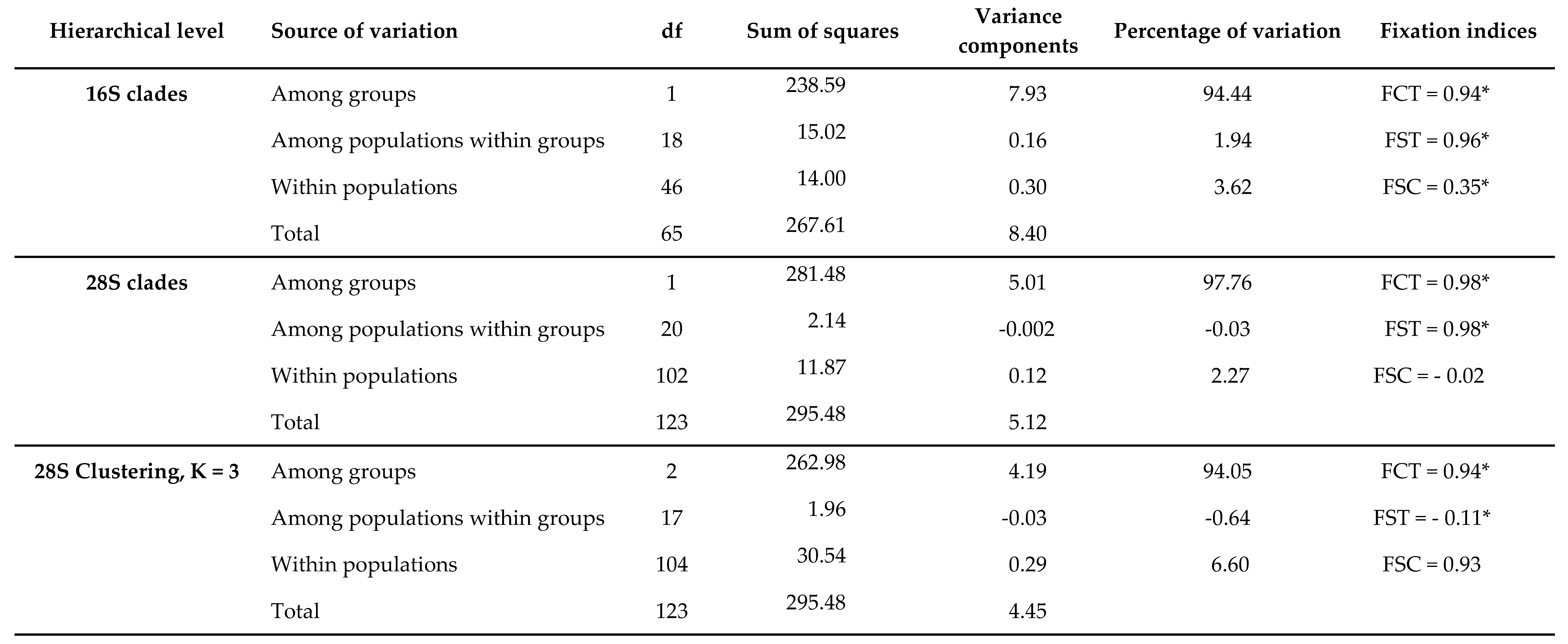
3.2. Molecular analyses
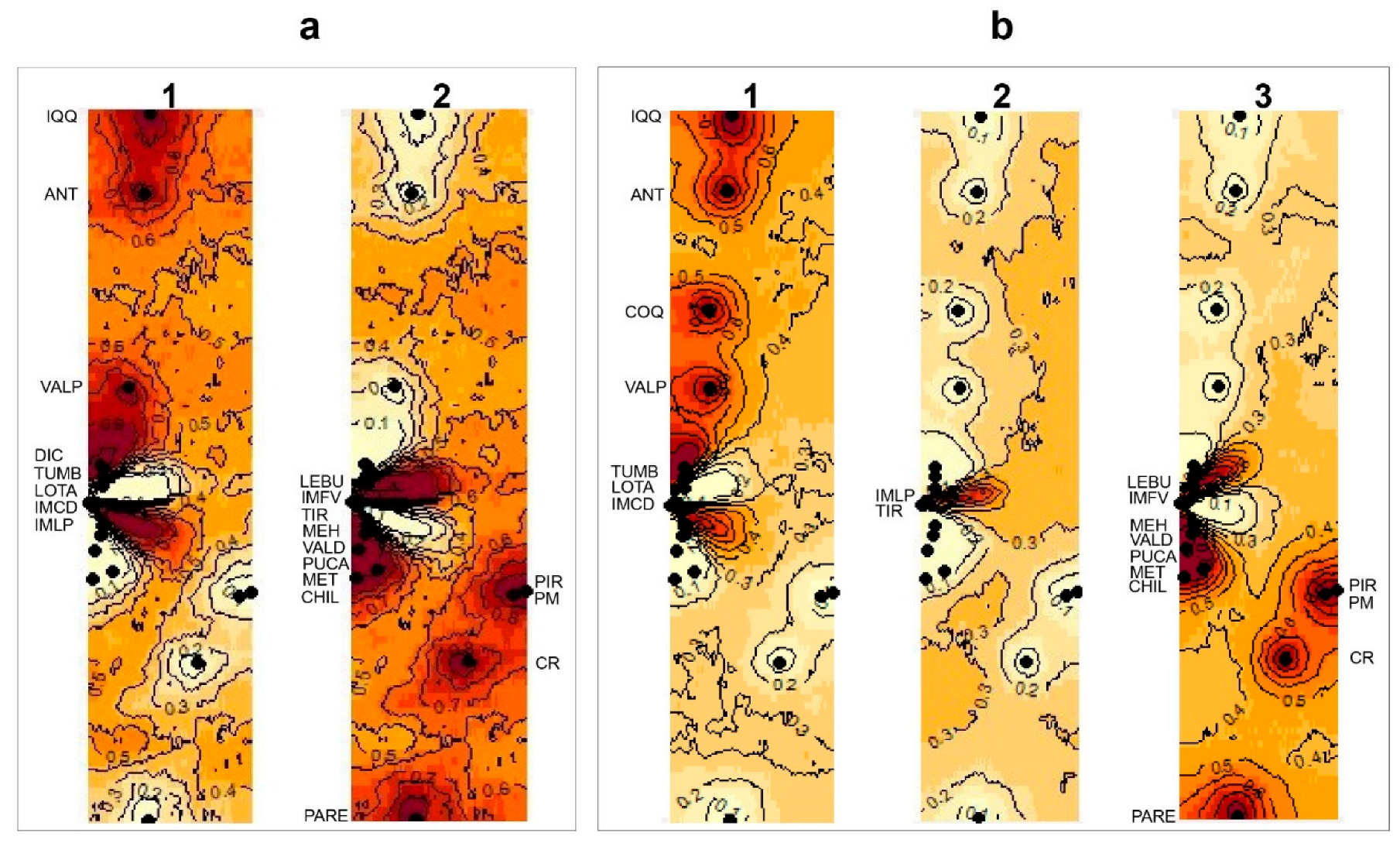
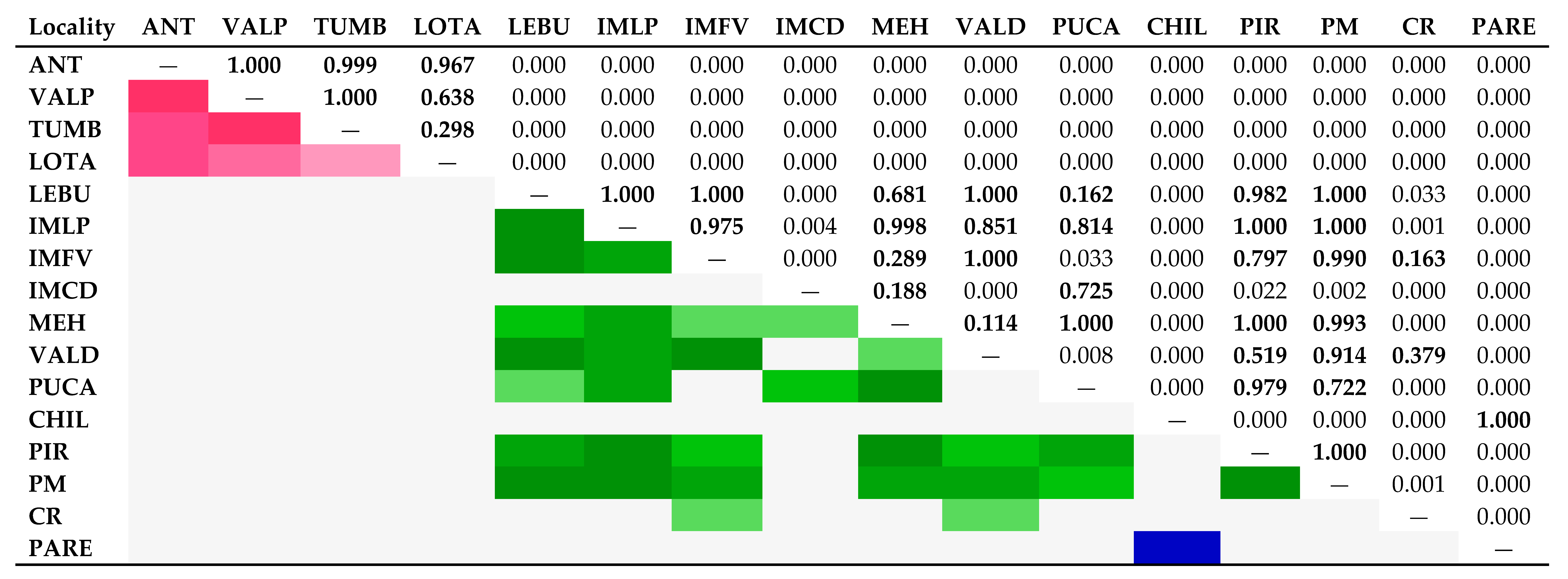
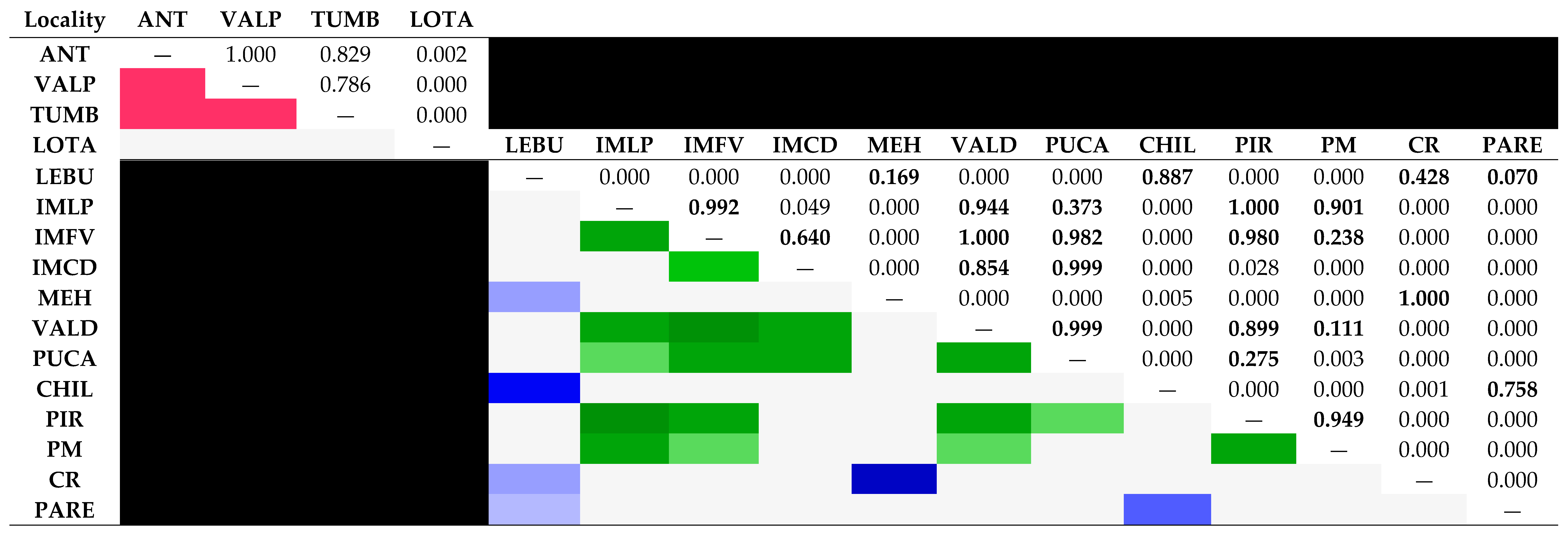
3.3. Bayesian reconstructions and genetic structure and its relationship with acrosomal morphotypes
3.4. Genetic diversity
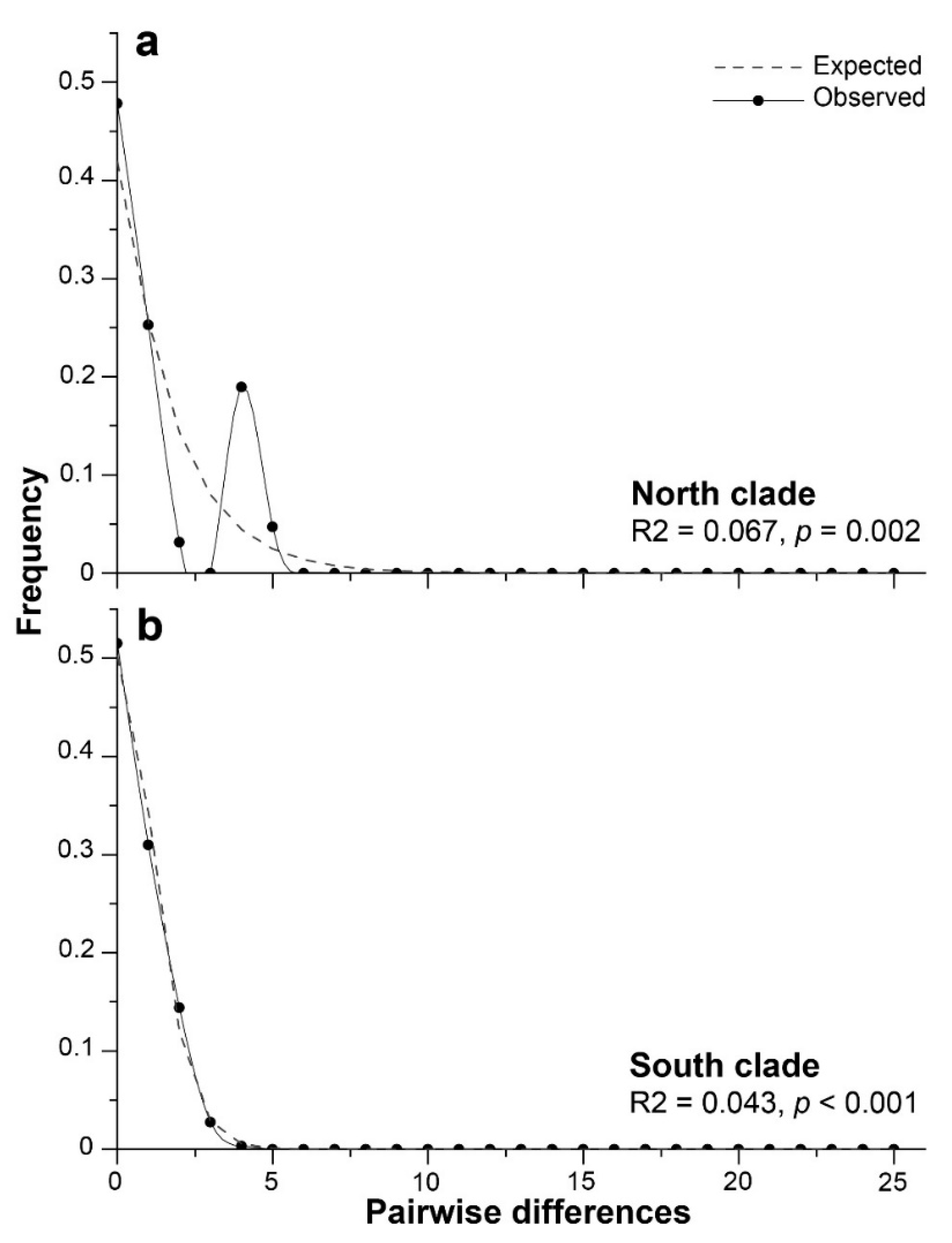
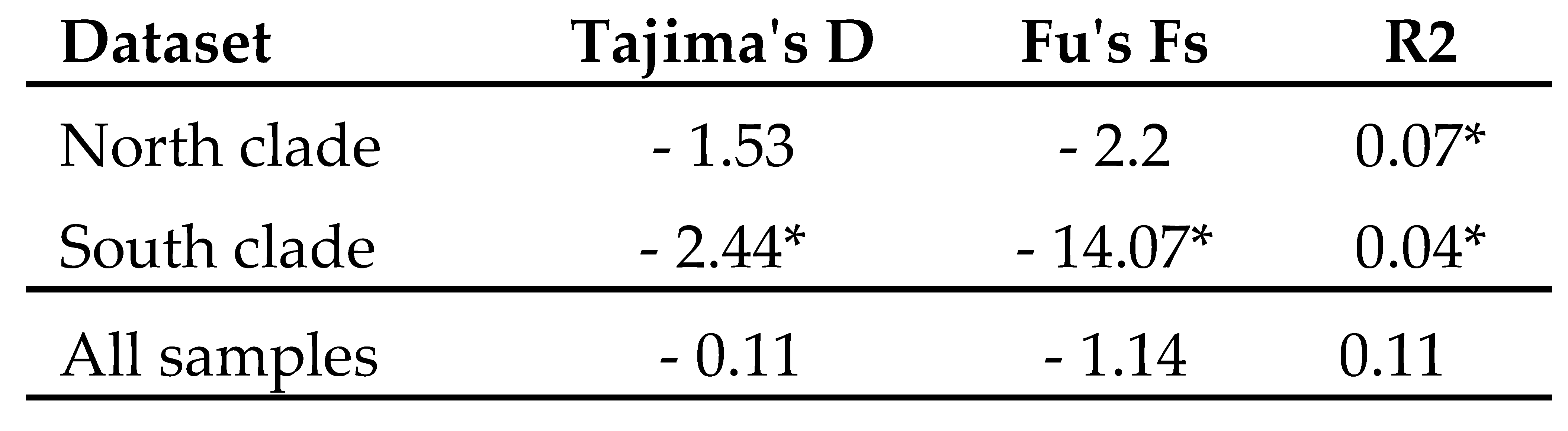
3.5. Demographic history
4. Discussion
4.1. Sperm morphology
4.2. Linking sperm morphotypes with genetic divergence
4.3. Cryptic and incipient species scenarios: an evolutionary perspective
4.4. Incipient species and postzygotic isolation inside southern lineage of P. purpuratus?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. Geometric morphometrics: ten years of progress following the ‘revolution’. Ital. J. Zool. 2004, 71, 5–16. [CrossRef]
- Tyurin, S.A.; Drozdov, A.L. Ultrastructure of Sperm in Mercenaria stimpsoni and Mactra chinensis (Mollusca: Bivalvia) from the Sea of Japan. Russ. J. Mar. Biol. 2005, 31, 391–395. [CrossRef]
- Healy, J.M.; Buckland-Nicks, J.A.; Jamieson, B.G.M. Spermatozoal ultrastructure of spiny oysters (Spondylidae, Bivalvia) including a comparison with other bivalves. Invertebr. Reprod. Dev. 2001, 40, 27–37. [CrossRef]
- Healy, J.M.; Mikkelsen, P.M.; Bieler, R. Sperm ultrastructure in honeycomb (foam) oysters (Mollusca, Bivalvia, Gryphaeidae, Pycnodontinae): comparison with other Ostreoidea and taxonomic implications. Invertebr. Biol. 2015, 134, 136–150. [CrossRef]
- Garrido, O.; Gallardo, C.S. Ultrastructure of sperms in bivalve molluscs of the Mytilidae family. Invertebr. Reprod. Dev. 1996, 29, 95–102. [CrossRef]
- Briones, C.; Guiñez, R.; Garrido, O.; Oyarzun, P.A.; Toro, J.E.; Perez, M. Sperm polymorphism and genetic divergence in the mussel Perumytilus purpuratus. Mar. Biol. 2012, 159, 1865–1870. [CrossRef]
- Introini, G.O.; De Magalhaes, C.A.; Aguiar-Jr, O.; Quaresma, A.J.C.; Lino-Neto, J.; Recco-Pimentel, S.M. Spermatozoan morphology of Brachidontes darwinianus and Brachidontes solisianus (Bivalvia, Mytilidae), from the southern Brazilian coast. Invertebr. Reprod. Dev. 2004, 46, 149–158. [CrossRef]
- Oyarzún, P.A.; Toro, J.E.; Garrido, O.; Briones, C.; Guinez, R. Differences in sperm ultrastructure between Mytilus chilensis and Mytilus galloprovincialis (Bivalvia, Mytilidae): could be used as a taxonomic trait? Lat. Am. J. Aquat. Res. 2014, 42, 172–179. [CrossRef]
- Howard, D.J.; Palumbi, S.R.; Birge, L.M.; Manier, M.K. Sperm and speciation. In Sperm biology: an evolutionary perspective, Birkhead, T., Pitnick, S.S., Hosken, D.J., Eds.; Academic Press: Burlington, MA, USA, 2009; pp. 367–403.
- Pitnick, S.; Hosken, D.J.; Birkhead, T.R. Sperm morphological diversity. In Sperm biology: an evolutionary perspective, Birkhead, T., Pitnick, S.S., Hosken, D.J., Eds.; Academic press: Burlington, MA, USA, 2009.
- Zagal, C.; Hermosilla, C. Guía de Invertebrados Marinos del Litoral Valdiviano, 1st edn, independent edition ed.; Quebecor World Chile S.A. : Santiago de Chile, 217p., 2001; p. 217p.
- Osorio, C.; Bahamonde, N. Moluscos bivalvos en pesquerías Chilenas. Biol. Pesq., Chile 1968, 3, 69–128.
- Prado, L.; Castilla , J.C. The bioengineer Perumytilus purpuratus (Mollusca: Bivalvia) in central Chile: biodiversity, habitat structural complexity and environmental heterogeneity. J. Mar. Biol. Assoc. U.K. 2006, 86, 417-421. [CrossRef]
- Franzen, A. Ultrastructural studies of spermatozoa in three bivalve species with notes on evolution of elongated sperm nucleus in primitive spermatozoa. Gamete Res. 1983, 7, 199–214. [CrossRef]
- Jamieson, B.G.M.; Rouse, G.W. The spermatozoa of the Polychaeta (Annelida): an ultrastructural review. Biol. Rev. 1989, 64, 93–157. [CrossRef]
- Dгozdov, А.L.; Rеuпov, A.A. Sреrm morрhologу in Mytilid bivalves. Russ. J. Mar. Biol. 1997, 23, 136–142.
- Drozdov, A.L.; Vinnikova, V.V.; Zezina, O.N.; Tyurin, S.A. Morphology of gametes of mollusks, echinoderms, and brachiopods in systematics and phylogeny. Paleontol. J. 2012, 46, 936–944. [CrossRef]
- Gwo, J.C.; Yang, W.T.; Sheu, Y.T.; Cheng, H.Y. Spermatozoan morphology of four species of bivalve (Heterodonta, Veneridae) from Taiwan. Tissue Cell 2002, 34, 39–43. [CrossRef]
- Costa, G.C.; Garda, A.A.; Teixeira, R.D.; Colli, G.R.; Báo, S.N. Comparative analysis of the sperm ultrastructure of three species of Phyllomedusa (Anura, Hylidae). Acta Zool. (Stockholm) 2004, 85, 257–262. . [CrossRef]
- Trovant, B.; Orensanz, J.M.L.; Ruzzante, D.E.; Stotz, W.; Basso, N.G. Scorched mussels (Bivalvia: Mytilidae: Brachidontinae) from the temperate coasts of South America: Phylogenetic relationships, trans-Pacific connections and the footprints of Quaternary glaciations. Mol. Phylogenet. Evol. 2015, 82, 60–74. http://doi.org/10.1016/j.ympev.2014.10.002.
- Guiñez, R.; Pita, A.; Pérez, M.; Briones, C.; Navarrete, S.A.; Toro, J.; Presa, P. Present-day connectivity of historical stocks of the ecosystemengineer Perumytilus purpuratus along 4500 km of the Chilean Coast. Fish. Res. 2016, 7, 322–332. http://doi.org/10.1016/j.fishres.2016.02.013.
- Conover, W.J.; Iman, R.L. Rank transformations as a bridge between parametric and nonparametric statistics. Am. Stat. 1981, 35, 124–133. http://dx.doi.org/10.2307/2683975.
- Packard, G.C.T.J.B. The misuse of ratios, indices, and percentages in ecophysiological research. Physiol. Zool. 1988, 61, 1–9. [CrossRef]
- Vargas, J.; Pérez, M.; Toro, J.; Astorga, M. Presence of two mitochondrial genomes in the mytilid Perumytilus purpuratus: Phylogenetic evidence for doubly uniparental inheritance. Genet. Mol. Biol. 2015, 38, 173–181. [CrossRef]
- Śmietanka, B.; Lubośny, M.; Przyłucka, A.; Gérard, K.; Burzyński, A. Mitogenomics of Perumytilus purpuratus (Bivalvia: Mytilidae) and its implications for doubly uniparental inheritance of mitochondria. PeerJ 2018, 6, e5593. [CrossRef]
- Briones, C.; Presa, P.; Pérez, M.; Pita, A.; Guiñez, R. Genetic connectivity of the ecosystem engineer Perumytilus purpuratus north to the 32°S southeast Pacific ecological discontinuity. Mar. Biol. 2013, 160, 3143–3156. [CrossRef]
- Palumbi, S.R.; Martin, A.; Romano, S.; McMillan, W.O.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR 1991.
- Park, J.K.; Ó Foighil, D. Sphaeriid and corbiculid clams represent separate heterodont bivalve radiations into freshwater environments. Mol. Phylogenet. Evol. 2000, 14, 75–88. [CrossRef]
- Filatov, D.A. Processing and population genetic analysis of multigenic datasets with ProSeq3 software. Bioinformatics 2009, 25, 3189–3190. [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2017, 1–7. [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [CrossRef]
- Lee, T.; Foighil, D.O. Placing the Floridian marine genetic disjunction into a regional evolutionary context using the scorched mussel, Brachidontes exustus, species complex. Evolution 2005, 59, 2139–2158. [CrossRef]
- Trovant, B.; Ruzzante, D.E.; Basso, N.G.; Orensanz, J.M. Distinctness, phylogenetic relations and biogeography of intertidal mussels (Brachidontes, Mytilidae) from the south-western Atlantic. J. Mar. Biol. Assoc. U. K. 2013, 93, 1843–1855. [CrossRef]
- Trovant, B.; Basso, N.G.; Orensanz, J.M.; Lessa, E.P.; Dincao, F.; Ruzzante, D.E. Scorched mussels (Brachidontes spp., Bivalvia: Mytilidae) from the tropical and warm-temperate southwestern Atlantic: the role of the Amazon River in their speciation. Ecol. Evol. 2016, 6, 1778–1798. [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [CrossRef]
- Rambaut, A. FigTree v 1.4.4. A graphical viewer of phylogenetic trees. University of Edinburgh: Institute of Evolutionary Biology. 2018. http://tree.bio.ed.ac.uk/software/figtree/.
- Guillot, G.; Mortier, F.; Estoup, A. Geneland: A computer package for landscape genetics. Mol. Ecol. Notes 2005, 5, 712–715. [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [CrossRef]
- Bandelt, H.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [CrossRef]
- Torroglosa, M.; Giménez, J. Sperm ultrastructure in two species of Brachidontes (Bivalvia, Mytilidae) from the south-western Atlantic Ocean. J. Mar. Biol. Assoc. U. K. 2015, 95, 991–998. [CrossRef]
- Walker, G.K.; Black, M.G.; Edwards, C.A. Comparative morphology of zebra (Dreissena polymorpha) and quagga (Dreissena bugensis) mussel sperm: Light and electron microscopy. Can. J. Zool. 1996, 74, 809–815. [CrossRef]
- Carstensen, D.; Laudien, J.; leese, F.; Arntz, W.; Held, C. Genetic variability, shell and sperm morphology suggest that the surf clams Donax marincovichi and D. obesulus are one species. J. Mollus. Stud. 2009, 75, 381–390. [CrossRef]
- Bieler, R.; Mikkelsen, P.M.; Collins, T.M.; Glover, E.A.; Gonzalez, V.L.; Graf, D.L.; Harper, E.M.; Healy, J.; Kawauchi, G.Y.; Sharma, P.P.; et al. Investigating the Bivalve Tree of Life - an exemplar-based approach combining molecular and novel morphological characters. Invertebr. Syst. 2014, 28, 32–115. [CrossRef]
- Pérez, M.; Guiñez, R.; Llavona, A.; Toro, J.E.; Astorga, M.; Pablo, P. Development of microsatellite markers for the ecosystem bioengineer mussel Perumytilus purpuratus and cross-priming testing in six Mytilinae genera. Mol. Ecol. Resour. 2008, 8, 449–451. [CrossRef]
- Mayr, E. Systematics and the origin of species; Columbia Univ. Press: New York, 1942.
- Mayr, E. Animal species and Evolution; Harvard University Press: Cambridge, Massachusetts, 1963.
- Camus, P. Biogeografía Marina de Chile Continental. Rev. Chil. Hist. Nat. 2001, 74, 587– 617. http://doi.org/10.4067/S0716-078X2001000300008.
- Sugden, D.E.; Bentley, M.J.; Fogwill, C.J. Late-glacial glacier events in southernmost South America: a blend of ‘northern’ and ‘southern’ hemispheric climatic signals. Geogr. Ann. Ser. A-phys. Geogr. 2005, 273–288. [CrossRef]
- Ruzzante, D.E.; Walde, S.J.; Gosse, J.C.; Cussac, V.E.; Habit, E.; Zemlak, T.S.; Adams, E.D.M. Climate control on ancestral population dynamics: insight from Patagonian fish phylogeography. Mol. Ecol. 2008, 17, 2234–2244. [CrossRef]
- Ortlieb, L.; Guzman, N.; Candia, M. Late Pleistocene nearshore mollusks from Antofagasta area, Chile: First determinations and paleoceanographic inferences Estud. Oceanol. 1994, 13, 1–57.
- Ortlieb, L.; Diaz, A.; Guzman, N. A warm interglacial episode during oxygen isotope stage 11 in northern Chile. Quat. Sci. Rev. 1996, 15, 857–871. [CrossRef]
- Dawson, M.N.; Louie, K.D.; Barlow, M.; Jacobs, D.K.; Swift, C.C. Comparative phylogeography of sympatric sister species, Clevelandia ios and Eucyclogobius newberryi (Teleostei, Gobiidae), across the California Transition Zone. Mol. Ecol. 2002, 11, 1065–1075. [CrossRef]
- Bos, D.H.; Gopurenko, D.; Williams, R.N.; Dewoody, J.A. Inferring population history and demography using microsatellites, mitochondrial DNA, and major histocompatibility complex (MHC) genes. Evolution 2008, 62, 1458–1468. [CrossRef]
- Schneider, N.; Chikhi, L.; Currat, M.; Radespiel, U. Signals of recent spatial expansions in the grey mouse lemur (Microcebus murinus). BMC Evol. Biol. 2010, 10, 105. [CrossRef]
- Fernández Iriarte, P.J.; González-Wevar, C.A.; Segovia, N.I.; Rosenfeld, S.; Hüne, M.; Fainburg, L.; Nuñez, J.D.; Haye, P.A.; Poulin, E. Quaternary ice sheets and sea level regression drove divergence in a marine gastropod along Eastern and Western coasts of South America. Sci. Rep. 2020, 10, 844. [CrossRef]
- Montecinos, A.; Broitman, B.R.; Faugeron, S.; Haye, P.A.; Tellier, F.; Guillemin, M.L. Species replacement along a linear coastal habitat: phylogeography and speciation in the red alga Mazzaella laminarioides along the south east pacific. BMC Evol. Biol. 2012, 12, 17. [CrossRef]
- Fraser, C.I.; Thiel, M.; Spencer, H.G.; Waters, J.M. Contemporary habitat discontinuity and historic glacial ice drive genetic divergence in Chilean kelp. BMC Evol. Biol 2010, 10: 203. [CrossRef]
- Macaya, E.C.; Zuccarello, G.C. Genetic structure of the giant kelp Macrocystis pyrifera along the southeastern Pacific. Mar. Ecol. Prog. Ser. 2010, 420, 103–112. [CrossRef]
- Astorga, M.P.; Valenzuela, A.; Segovia, N.I.; Poulin, E.; Vargas-Chacoff, L.; Gonzalez-Wevar, C.A. Contrasting patterns of genetic diversity and divergence between landlocked and migratory populations of fish Galaxias maculatus, evaluated through mitochondrial DNA sequencing and nuclear DNA microsatellites. Front. Genet. 2022, 13, 1–13. [CrossRef]
- Xu, J.W.; Perez-Losada, M.; Jara, C.G.; Crandall, K.A. Pleistocene glaciation leaves deep signature on the freshwater crab Aegla alacalufi in Chilean Patagonia. Mol. Ecol. 2009, 18, 904–918. [CrossRef]
- Gonzalez-Wevar, C.A.; Rosenfeld, S.; Segovia, N.I.; Hune, M.; Gerard, K.; Ojeda, J.; Mansilla, A.; Brickle, P.; Diaz, A.; Poulin, E. Genetics, gene flow, and glaciation: The case of the South American limpet Nacella mytilina. Plos One 2016, 11, 24. [CrossRef]
- Bierne, N.; Welch, J.; Loire, E.; Bonhomme, F.; David, P. The coupling hypothesis: why genome scans may fail to map local adaptation genes. Mol. Ecol. 2011, 20, 2044–2072. [CrossRef]
- Ravinet, M.; Faria, R.; Butlin, R.K.; Galindo, J.; Bierne, N.; Rafajlović, M.; Noor, M.A.F.; Mehlig, B.; Westram, A.M. Interpreting the genomic landscape of speciation: a road map for finding barriers to gene flow. J. Evol. Biol. 2017, 30, 1450–1477. [CrossRef]
- Johannesson, K.; Le Moan, A.; Perini, S.; André, C. A Darwinian laboratory of multiple contact zones. Trends Ecol. Evol. 2020, 35, 1021–1036. [CrossRef]
- Anderson, E.; Hubricht, L. Hybridization in Tradescantia. III. The Evidence for Introgressive Hybridization. , 25(6), 396. AJB. 1938, 25, 396–402. [CrossRef]
- Anderson, E. Introgressive hybridization; New York: John Wiley & Sons: 1949.
- Rawson, P.D.; Agrawal, V.; Hilbish, T.J. Hybridization between the blue mussels Mytilus galloprovincialis and M. trossulus along the Pacific coast of North America: evidence for limited introgression. Mar. Biol. 1999, 134, 201-211. [CrossRef]
- Toro, J.E.; Thompson, R.J.; Innes, D.J. Reproductive isolation and reproductive output in two sympatric mussel species (Mytilus edulis, M. trossulus) and their hybrids from Newfoundland. Mar. Biol. 2002, 141, 897–909. [CrossRef]
- Riginos, C.; Cunningham, C.W. Local adaptation and species segregation in two mussel (Mytilus edulis x Mytilus trossulus) hybrid zones. Mol. Ecol. 2005, 14, 381–400. [CrossRef]
- Fraisse, C.; Belkhir, K.; Welch, J.J.; Bierne, N. Local interspecies introgression is the main cause of extreme levels of intraspecific differentiation in mussels. Mol. Ecol. 2016, 25, 269–286. [CrossRef]
- Oyarzún, P.A.; Toro, J.E.; Cañete, J.I.; Gardner, J.P.A. Bioinvasion threatens the genetic integrity of native diversity and a natural hybrid zone: smooth-shelled blue mussels (Mytilus spp.) in the Strait of Magellan. Biol. J. Linn. Soc. 2016, 117, 574–585. [CrossRef]
- Arnold, M.L.; Kentner, E.K.; Johnston, J.A.; Cornman, S.; Bouck, A.C. Natural hybridisation and fitness. Taxon 2001, 50, 93–104. [CrossRef]
- Bierne, N.; David, P.; Boudry, P.; Bonhomme, F. Assortative fertilization and selection at larval stage in the mussels Mytilus edulis and M. galloprovincialis. Evolution 2002, 56, 292–298. [CrossRef]
- Toro, J.E.; Thompson, R.J.; Innes, D.J. Fertilization success and early survival in pure and hybrid larvae of Mytilus edulis (Linnaeus, 1758) and M. trossulus (Gould, 1850) from laboratory crosses. Aquac. Res. 2006, 37, 1703–1708. [CrossRef]
- Toro, J.E.; Oyarzun, P.A.; Penaloza, C.; Alcapan, A.; Videla, V.; Tilleria, J.; Astorga, M.; Martinez, V. Production and performance of larvae and spat of pure and hybrid species of Mytilus chilensis and M. galloprovincialis from laboratory crosses. Lat. Am. J. Aquat. Res. 2012, 40, 243–247. [CrossRef]
- Brannock, P.M.; Hilbish, T.J. Hybridization results in high levels of sterility and restricted introgression between invasive and endemic marine blue mussels. Mar. Ecol. Prog. Ser. 2010, 406, 161–171. [CrossRef]
- Guedj, B.; Guillot, G. Estimating the location and shape of hybrid zones. Mol. Ecol. Resour. 2011, 11, 1119–1123. [CrossRef]
- Palumbi, S.R. Marine speciation on a small planet. Trends Ecol. Evol. 1992, 7, 114–118. [CrossRef]
- Vacquier, V.D.; Swanson, W.J.; Hellberg, M.E. What have we learned about sea urchin sperm bindin? Dev. Growth Differ. 1995, 37, 1–10. [CrossRef]
- Gavrilets, S. Rapid evolution of reproductive barriers driven by sexual conflict. Nature 2000, 403, 886–889. [CrossRef]
- Riginos, C.; Wang, D.; Abrams, A.J. Geographic variation and positive selection on M7 lysin, an acrosomal sperm protein in mussels (Mytilus spp.). Mol. Biol. Evol. 2006, 23, 1952–1965. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





