1. Introduction
Spinal anesthesia is a safe and effective technique of anesthesia for various surgeries with the benefits of a quick onset of action, affordability, and ease of administration, as well as a low rate of side effects and a brief stay in the post-anesthesia care unit [
1,
2]. On the other hand, these benefits can be outweighed by the short duration of action and a higher chance of delay in motor power recovery associated with some drugs used in spinal anesthesia which can result in delaying ambulation and lengthening hospital stay [
3,
4]. It has been found that numerous medications can be added to intrathecal local anesthetics as adjuvants to boost the effectiveness of spinal anesthesia [
5]. These medications include α2 adrenergic agonists (clonidine and dexmedetomidine), opioids (morphine, sufentanil, and fentanyl), neostigmine, magnesium sulfate, midazolam, and ketamine [
5]. Some of these adjuvant medications such as clonidine and opioids have negative side effects including pruritus, micturition problems, bradycardia, sedation, hypotension, and respiratory depression. Fentanyl, one of the most widely used neuraxial opioids, has a faster onset and lesser duration of action than morphine during intrathecal or epidural administration [
6].
Dexmedetomidine is an adrenoreceptor agonist that selectively targets α2-receptors [
7]. Dexmedetomidine exerts its analgesic effects by downregulating the α2-receptor-mediated nociceptive process in the brain (locus coeruleus) and the spinal cord [
8,
9,
10,
11]. Uncertainty surrounds the method by which dexmedetomidine extends the local anesthetic's sensory and motor block [
12]. Directly preventing the release of pro-nociceptive transmitters such substance P and glutamate may be possible through binding to the α2-receptors [
13]. Dexmedetomidine is superior to clonidine (α2 adrenergic agonists) as dexmedetomidine possesses higher specificity to α2-receptors than clonidine [
14]. The α2/α1 activity of dexmedetomidine is 1620:1 while the α2/α1 activity of clonidine is only 200:1 [
14]. This improvement in specificity to α2-receptors makes dexmedetomidine a more potent and effective sedative and analgesic when compared to clonidine [
15]. Moreover, dexmedetomidine was associated with a significant drop in the rate of adverse drug reactions including cardiovascular ones [
15,
16].
Many uses of dexmedetomidine have been reported including sedation of intubated patients in the Intensive Care Units (ICUs) and during surgical procedures performed under local anesthesia as well [
17]. Furthermore, dexmedetomidine has been also used with local anesthetic in performing different types of nerve block procedures [
18].
Several studies have measured the effect of intrathecal dexmedetomidine on the duration of the spinal sensory block [
6,
19,
20]. Furthermore, it was found that the use of intrathecal dexmedetomidine was associated with a lower risk of experiencing nausea and vomiting, bradycardia, and hypotension during cesarian sections [
21]. There is little evidence of the effect of intrathecal dexmedetomidine on pain scores and the consumption of analgesia postoperatively. One study has demonstrated that intrathecal administration of dexmedetomidine lowers post-operative pain scores and consecutively analgesic consumption [
22]. Furthermore, the reduction of analgesic consumption including opioids provided by intravenous dexmedetomidine administration may result in a reduction of gastrointestinal adverse events and a decrease in the hospital stay duration [
23].
The aim of this study is to provide more knowledge regarding the impact of adding intrathecal dexmedetomidine in spinal anesthesia in improving post-operative pain and reducing the need for post-operative analgesic consumption in comparison with the usually used intrathecal fentanyl.
2. Materials and Methods
Design, Setting, and Participants
This is a retrospective cohort study conducted on patients undergoing spinal anesthesia for different surgical procedures at Princess Basma Teaching Hospital, Irbid, Jordan, a teaching hospital affiliated with Yarmouk University, Irbid, Jordan between 2021 and 2022.
Clinical data collected from electronic medical records included age, sex, height, weight, American Society of Anesthesiologists (ASA) score, type of surgical procedure, duration of the procedure, medication used in anaesthesia, post-operative Visual Analogue Scale (VAS) score, and the used post-operative analgesia.
Based on five classes, the ASA score is a subjective evaluation of a patient's general health used for pre-operative assessment for patients undergoing different surgeries and anesthesia techniques [
24].
Visual Analogue Scale (VAS) is a psychometric measuring tool that consists of a 10 cm line with two end points that stand for 0 (no pain) and 10 (pain as severe as it can be) created to record the characteristics of symptoms severity in patients [
25].
The study population was divided into two groups based on the medications used for their spinal anesthesia. The first group (Fentanyl group) consisted of patients who received an average dose of 10 mg Bupivacaine 5% and 25mcg Fentanyl intrathecally. The second group (Dexmedetomidine group) received an average dose of 10 mg Bupivacaine 5% and 8 mcg Dexmedetomidine intrathecally.
Patients who had allergies to bupivacaine or any anaesthetic medications, hepatic, or renal impairment, and patients with neurological or psychiatric conditions were all excluded.
This study was approved by the Institutional Review Board (IRB) of Yarmouk University, Irbid, Jordan; IRB Reference: IRB/2023/17. The IRB follows the guidelines of Good Clinical Practice (GCP) and the Declaration of Helsinki.
Statistical Analysis
Continuous variables were assessed for normality using histograms, Quantile-Quantile plots in addition to Kolmogorov–Smirnov and the Shapiro–Wilk tests. The mean with standard deviation were calculated for the normally distributed continuous variables. Median with Interquartile range (IQR) was used to describe non-normal continuous variables whereas frequencies and percentages were used to describe categorical variables.
The Man-Whitney test was used to compare means for continuous variables when the data was not normally distributed, and categorical variables were compared between the two groups using chi-square test.
All data analyses were conducted using Stata version 17 software (StataCorp. 2021. Stata: Release 17. Statistical Software. College Station, TX: StataCorp LLC.). The statistical significance was set at a 2-sided P<0.05.
3. Results
Demographics and clinical characteristics
A total of 96 patients were involved in this study. The mean age of the study population was 51.74-year-old, and the median was 55.5-year-old (
Table 1). The number of males (n=48, 51.06%) involved in this study was higher than females (n=46, 48.94%). The mean BMI was 28.96 kg/m2 and the median was 27.05 kg/m2. The number of patients who were overweight (n=52, 55.32%) was higher than those who had normal BMI (n=21, 22.34%) or were obese (n=21, 22.34%). Most of patients involved in this study had ASA score of 2 (n=52, 55.32%), and 1 (n=30, 30.91%). The median duration of surgery among all study participants was 60 minutes. Of the 96 total participants, 63 patients received post-operative analgesia. Finally, the median VAS score for all patients was 3.
The differences between dexmedetomidine and fentanyl groups
As demonstrated in (
Table 1), 47 patients received intrathecal dexmedetomidine (group D) and 49 patients received intrathecal fentanyl (group F). The median age for group (D) (59-year-old) was significantly higher than group (F) (46-year-old) (p=0.0395). The number of males in group (D) (n=30, 66.67%) was higher than in group (F) (n=18, 36.73%). On the other hand, the number of females was higher in group (F) (n=31, 63.27%) when compared to group (D) (n=15, 33.33%). The distribution of gender was statistically significant between the two groups (p=0.004).
The number of patients with normal BMI in group (D) (n=12, 26.67%) was higher than in group (F) (n=9, 18.37%). Moreover, patients with overweight BMI were higher in group (D) (n=29, 59.18%) compared to group (F) (n=23, 51.11%). Likewise, the number of patients who were obese was slightly higher in group (D) (n=11, 22.45%) when compared to group (F) (n=10, 22.22%). The distribution of the BMI category was not statistically significant between the two groups (p=0.607).
The distribution of the ASA score between the two groups was statistically significant (p=0.011). Moreover, the median duration of surgeries was statistically insignificant between groups (F) and (D) (p=0.0943).
The effect of dexmedetomidine and fentanyl on post-operative pain scores and use of analgesia
As illustrated in (
Table 1), the median VAS score was significantly higher in patients of group (F) when compared to those in group (D) (4 vs 1, p=0.000). In our study, we compared Visual Analog Scale (VAS) scores across various surgical procedures, categorizing the data into group (F) and group (D) as demonstrated in (
Table 2). The results revealed significant differences in VAS scores for all surgical types—Orthopedic, General, Urology, and Obs/Gyne. In orthopedic surgery, group (D) exhibited a notably lower median VAS score (1.5) compared to group (F) (6.5), with a statistically significant p-value of 0.0004. Similarly, in general surgery, group (D) displayed a significantly lower VAS score (1) compared to group (F) (4), with a p-value of 0.0000. In Urology and Obs/Gyne surgeries, group (D) also demonstrated statistically lower VAS scores, 0 and 2, respectively, compared to group (F), 2.5 and 5, with p-values of 0.0001.
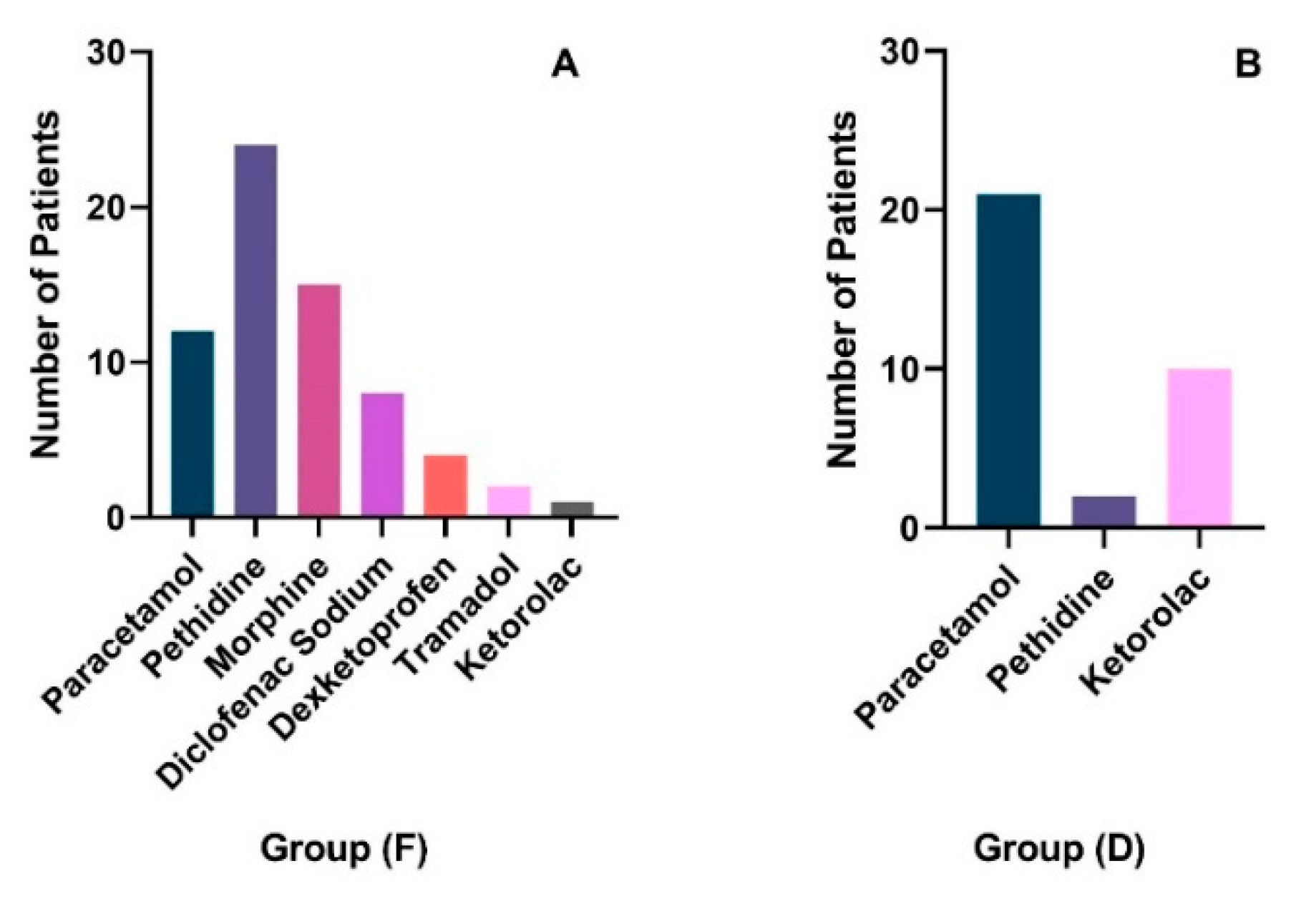
The number of patients who needed post-operative analgesia was significantly higher in group (F) (n=40. 81.63%) than in group (D) (n=23, 51.11%) (p=0.002). Type of analgesia needed post-operatively varied between individuals based on physicians’ judgment as illustrated in (
Figure 1A,B). In group (F), pethidine was the most commonly used analgesic (n=24) followed by morphine (n=15), paracetamol (n=12), Diclofenac Sodium (n=8), Dexketoprofen (n=4), Tramadol (n=2), and ketorolac (n=1). On the other hand, in group (D), Paracetamol was the most commonly used analgesic (n=21, %), followed by Ketorolac (n=10), and pethidine (n=2).
4. Discussion
This study involved 96 patients, with a slight male majority (51.06%). The average age was approximately 51 years, and the median Body Mass Index (BMI) was 28.96 kg/m2. Most participants were overweight (55.32%) and had an ASA score of 2. The average surgery duration was 60 minutes, and 63 patients received post-operative analgesia. The median VAS score for pain was 3.
The study compared two groups: 47 patients received dexmedetomidine (Group D) and 49 received fentanyl (Group F). Group D had a higher median age (59 years) and a larger proportion of males (66.67%) compared to Group F. The distribution of BMI and ASA scores differed significantly between the groups, but the surgery duration did not.
Group D reported significantly lower VAS scores, indicating less pain, across all types of surgeries (Orthopedic, General, Urology, and Obs/Gyne) compared to Group F. The need for post-operative analgesia was also significantly lower in Group D (51.11%) than in Group F (81.63%). The types of analgesics used varied, with Paracetamol being the most common in Group D, whereas Pethidine was more common in Group F.
In different studies, Dexmedetomidine demonstrated analgesic effects belonging to α2-receptors binding activity when it was initially used in the clinical settings in the Intensive Care Unit (ICU) as a short-term intravenous sedative medication [
26]. Moreover, the application of dexmedetomidine as a systemic analgesic adjuvant has been examined, mostly in the acute perioperative context [
27,
28,
29]. Despite that the half-life elimination time of systemic dexmedetomidine was short (2-3 hours), its analgesic effect was noticed to last for 24 hours [
28,
29]. This extended analgesic-sparing impact was related to dexmedetomidine's sedative, anxiolytic, and thymoanleptic characteristics. Furthermore, it was found that systematic dexmedetomidine extended the duration of action of local anesthesia’s sensory block during peripheral nerve block and spinal anesthesia [
30,
31]. To sum up everything that has been stated so far, it is clearly observed that systematic dexmedetomidine is an effective sedative and analgesic that can be used during various procedures (under general or local anesthesia).
Coombs and colleagues were the first to notice the analgesic characteristics of intrathecal α2-receptors agonists [
32]. Several researchers found that spinal α2-receptors agonists exert analgesic effects by decreasing the secretion of substance P and C-fiber transmitters [
33,
34,
35]. Furthermore, hyperpolarization of post-synaptic neurons of the dorsal horn of the spinal cord may play a role in the effect of these medications [
33,
34,
35]. The analgesic effect of these medications was noticed to be directly correlated with the binding affinity to α2-receptors in the spinal cord [
36]. After that, several studies have investigated the effect of spinal administration of dexmedetomidine (high binding affinity to α2-receptors) in upper abdominal and thoracic surgeries [
22,
37,
38].
This was a retrospective cohort study designed to compare the effect of spinal dexmedetomidine and bupivacaine vs fentanyl and bupivacaine on postoperative pain scores and analgesic need. We have found that despite the significantly higher age in patients who received intrathecal dexmedetomidine, postoperative pain scores and need of analgesia were significantly lower compared to those who received intrathecal fentanyl. This study compared the Visual Analog Scale (VAS) scores across various surgical procedures. The findings of the present study collectively suggest that dexmedetomidine significantly improved post-operative pain management across various surgical specialties, highlighting its effectiveness in reducing patients' pain levels.
In one study investigated the effect of intravenous vs spinal dexmedetomidine in knee arthroscopy, it was found that intrathecal dexmedetomidine significantly prolonged the duration of action of spinal chloro-procaine when the surgery lasted more than 40 minutes compared to intravenous dexmedetomidine [
19].
Recently, a systematic review and meta-analysis was conducted to compare spinal dexmedetomidine and fentanyl as adjuvants to local anesthesia [
6]. From 9 studies, a total of 639 patients were included in this systematic review. This meta-analysis showed that patients who received intrathecal dexmedetomidine had a significantly longer duration of sensory block and motor block. Also, those patients had a significantly longer duration of pain-free periods.
Liu et al conducted a trial to evaluate the effect of spinal dexmedetomidine and morphine on daily morphine consumption in patients with cancer [
39]. All patients entered two phases of the trial (phase M and phase D+M). In phase M, intrathecal morphine was administered, while in phase M+D, intrathecal morphine and dexmedetomidine were administered. A monitoring period of 7-day duration was done for all patients in each phase. Half of the patients were in phase M and the other half were in phase M+D for one week, then patients in both phases were cross-overed. Both phases showed a considerable reduction in pain frequency and intensity compared to baseline. Moreover, the intrathecal morphine and dexmedetomidine phase show a marked reduction in daily consumption of morphine and bolus injections compare to the intrathecal morphine phase.
In a recent randomized clinical trial conducted by Li et al, the analgesic effect of intrathecal dexmedetomidine (5 µg) or sufentanil (5 µg) vs normal saline (0.9%) was compared [
40]. All patients received epidural ropivacaine (0.1%) and sufentanil (0.2 µg/mL). It was found that the analgesia was significantly higher in patients who received spinal dexmedetomidine or sufentanil when compared to those who received normal saline.
This study was strengthened by the fact that it compared intrathecal dexmedetomidine compared to fentanyl (opioid analgesic) instead of normal saline. One of the limitations is that data regarding the side effects of patients in both groups was incomplete or missing so we could compare the effect of dexmedetomidine on the incidence of adverse effects.
5. Conclusions
This study was designed to compare the effect of intrathecal dexmedetomidine vs fentanyl as adjuvant to bupivacaine on post-operative pain scores and analgesic need. Patients who received intrathecal dexmedetomidine had significantly lower the post-operative pain scores and analgesic need compared to those who received intrathecal fentanyl.
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board (IRB) of Yarmouk University, Irbid, Jordan; IRB Reference: IRB/2023/17.
Informed Consent Statement
This study was approved by the Institutional Review Board (IRB) of Yarmouk University, Irbid, Jordan; IRB Reference: IRB/2023/17.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boublik, J.; Gupta, R.; Bhar, S.; Atchabahian, A. Prilocaine spinal anesthesia for ambulatory surgery: A review of the available studies. Anaesthesia, critical care & pain medicine 2016, 35, 417–421. [Google Scholar] [CrossRef]
- Abdallah, F.W.; Brull, R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. British journal of anaesthesia 2013, 110, 915–925. [Google Scholar] [CrossRef]
- Sapate M, Sahu P, Thatte WS, Dubey R. A randomized, double blind, control study of the effects of adding nalbuphine to spinal bupivacaine for lower abdominal surgeries in elderly patients. Anaesthesia, Pain & Intensive Care 2013, 17, 145–148.
- Chung, F.; Mezei, G. Factors contributing to a prolonged stay after ambulatory surgery. Anesthesia and analgesia 1999, 89, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Koyyalamudi, V.; Sen, S.; Patil, S.; Creel, J.B.; Cornett, E.M.; Fox, C.J.; Kaye, A.D. Adjuvant Agents in Regional Anesthesia in the Ambulatory Setting. Current pain and headache reports 2017, 21, 6. [Google Scholar] [CrossRef]
- Sun, S.; Wang, J.; Bao, N.; Chen, Y.; Wang, J. Comparison of dexmedetomidine and fentanyl as local anesthetic adjuvants in spinal anesthesia: a systematic review and meta-analysis of randomized controlled trials. Drug design, development and therapy 2017, 11, 3413–3424. [Google Scholar] [CrossRef] [PubMed]
- Venn, R.M.; Karol, M.D.; Grounds, R.M. Pharmacokinetics of dexmedetomidine infusions for sedation of postoperative patients requiring intensive caret. British journal of anaesthesia 2002, 88, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Kamibayashi, T.; Maze, M.; Weiskopf, Richard B.; Weiskopf, Richard B.; Todd, Michael M. Clinical Uses of α2-Adrenergic Agonists. Anesthesiology 2000, 93, 1345–1349. [CrossRef]
- Owesson, C.A.; Seif, I.; McLaughlin, D.P.; Stamford, J.A. Different alpha(2) adrenoceptor subtypes control noradrenaline release and cell firing in the locus coeruleus of wildtype and monoamine oxidase-A knockout mice. The European journal of neuroscience 2003, 18, 34–42. [Google Scholar] [CrossRef]
- Janumpalli, S.; Butler, L.S.; MacMillan, L.B.; Limbird, L.E.; McNamara, J.O. A point mutation (D79N) of the alpha2A adrenergic receptor abolishes the antiepileptogenic action of endogenous norepinephrine. The Journal of neuroscience: the official journal of the Society for Neuroscience 1998, 18, 2004–2008. [Google Scholar] [CrossRef]
- Stone, L.S.; MacMillan, L.B.; Kitto, K.F.; Limbird, L.E.; Wilcox, G.L. The alpha2a adrenergic receptor subtype mediates spinal analgesia evoked by alpha2 agonists and is necessary for spinal adrenergic-opioid synergy. The Journal of neuroscience: the official journal of the Society for Neuroscience 1997, 17, 7157–7165. [Google Scholar] [CrossRef]
- Grewal, A. Dexmedetomidine: New avenues. Journal of anaesthesiology, clinical pharmacology 2011, 27, 297–302. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, F.; Li, C.; Kong, M.; Liu, H.; Zhang, P.; Zhang, S.; Cao, J.; Zhang, L.; Ma, H. Molecular mechanisms underlying the analgesic property of intrathecal dexmedetomidine and its neurotoxicity evaluation: an in vivo and in vitro experimental study. PloS one 2013, 8, e55556. [Google Scholar] [CrossRef]
- Dyck, J.B.; Maze, M.; Haack, C.; Vuorilehto, L.; Shafer, S.L. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology 1993, 78, 813–820. [Google Scholar] [CrossRef]
- Aantaa, R.; Jalonen, J. Perioperative use of alpha2-adrenoceptor agonists and the cardiac patient. European journal of anaesthesiology 2006, 23, 361–372. [Google Scholar] [CrossRef]
- Naaz, S.; Bandey, J.; Ozair, E.; Asghar, A. Optimal Dose of Intrathecal Dexmedetomidine in Lower Abdominal Surgeries in Average Indian Adult. Journal of clinical and diagnostic research: JCDR 2016, 10, Uc09–13. [Google Scholar] [CrossRef]
- Grewal, A.J.J.o.a. clinical pharmacology. Dexmedetomidine: new avenues 2011, 27, 297. [Google Scholar]
- Yoshitomi, T.; Kohjitani, A.; Maeda, S.; Higuchi, H.; Shimada, M.; Miyawaki, T. Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor. Anesthesia and analgesia 2008, 107, 96–101. [Google Scholar] [CrossRef]
- Breebaart, M.B.; Saerens, L.; Branders, J.; Casaer, S.; Sermeus, L.; Van Houwe, P. Spinal or Intravenous Dexmedetomidine for Spinal Anesthesia with Chloroprocaine in Ambulatory Knee Arthroscopies: A Double-Blind Randomized Trial. Local and regional anesthesia 2021, 14, 153–160. [Google Scholar] [CrossRef]
- Nayagam, H.A.; Singh, N.R.; Singh, H.S. A prospective randomised double blind study of intrathecal fentanyl and dexmedetomidine added to low dose bupivacaine for spinal anesthesia for lower abdominal surgeries. Indian journal of anaesthesia 2014, 58, 430–435. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Zhang, X.-J.; Wang, Y. Effect of intrathecal dexmedetomidine on cesarean section during spinal anesthesia: a meta-analysis of randomized trials. Drug design, development and therapy 2019, 13, 2933–2939. [Google Scholar] [CrossRef]
- Elhakim, M.; Abdelhamid, D.; Abdelfattach, H.; Magdy, H.; Elsayed, A.; Elshafei, M. Effect of epidural dexmedetomidine on intraoperative awareness and post-operative pain after one-lung ventilation. Acta anaesthesiologica Scandinavica 2010, 54, 703–709. [Google Scholar] [CrossRef]
- Morris, C.; Gold, S. Enhanced recovery programmes; coming to a hospital near you! Anaesthesia 2011, 66, 864–868. [Google Scholar] [CrossRef]
- Daabiss, M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth 2011, 55, 111–115. [Google Scholar] [CrossRef]
- Klimek, L.; Bergmann, K.C.; Biedermann, T.; Bousquet, J.; Hellings, P.; Jung, K.; Merk, H.; Olze, H.; Schlenter, W.; Stock, P.; et al. Visual analogue scales (VAS): Measuring instruments for the documentation of symptoms and therapy monitoring in cases of allergic rhinitis in everyday health care: Position Paper of the German Society of Allergology (AeDA) and the German Society of Allergy and Clinical Immunology (DGAKI), ENT Section, in collaboration with the working group on Clinical Immunology, Allergology and Environmental Medicine of the German Society of Otorhinolaryngology, Head and Neck Surgery (DGHNOKHC). Allergo J Int 2017, 26, 16–24. [Google Scholar] [CrossRef]
- Wagner, D.S.; Brummett, C.M. Dexmedetomidine: as safe as safe can be. In Proceedings of the Seminars in Anesthesia, Perioperative Medicine and Pain; 2006; pp. 77–83. [Google Scholar]
- Choi, Y.M.; Choi, E.J.; Ri, H.S.; Park, J.Y.; You, J.A.; Byeon, G.J. The effect of dexmedetomidine and midazolam on combined spinal-epidural anesthesia in patients undergoing total knee arthroplasty. Anesthesia and pain medicine 2020, 15, 111–119. [Google Scholar] [CrossRef]
- Al-Zaben, K.R.; Qudaisat, I.Y.; Al-Ghanem, S.M.; Massad, I.M.; Al-Mustafa, M.M.; Al-Oweidi, A.S.; Abu-Halaweh, S.A.; Abu-Ali, H.M.; Saleem, M.M. Intraoperative administration of dexmedetomidine reduces the analgesic requirements for children undergoing hypospadius surgery. European journal of anaesthesiology 2010, 27, 247–252. [Google Scholar] [CrossRef]
- Unlugenc, H.; Gunduz, M.; Guler, T.; Yagmur, O.; Isik, G. The effect of pre-anaesthetic administration of intravenous dexmedetomidine on postoperative pain in patients receiving patient-controlled morphine. European journal of anaesthesiology 2005, 22, 386–391. [Google Scholar] [CrossRef]
- Kaya, F.N.; Yavascaoglu, B.; Turker, G.; Yildirim, A.; Gurbet, A.; Mogol, E.B.; Ozcan, B. Intravenous dexmedetomidine, but not midazolam, prolongs bupivacaine spinal anesthesia. Canadian journal of anaesthesia = Journal canadien d'anesthesie 2010, 57, 39–45. [Google Scholar] [CrossRef]
- Rutkowska, K.; Knapik, P.; Misiolek, H. The effect of dexmedetomidine sedation on brachial plexus block in patients with end-stage renal disease. European journal of anaesthesiology 2009, 26, 851–855. [Google Scholar] [CrossRef]
- Coombs, D.W.; Saunders, R.L.; Lachance, D.; Savage, S.; Ragnarsson, T.S.; Jensen, L.E. Intrathecal morphine tolerance: use of intrathecal clonidine, DADLE, and intraventricular morphine. Anesthesiology 1985, 62, 358–363. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, N.Y.; Lee, H.S.; Kil, H.K. Effects of intrathecal dexmedetomidine on low-dose bupivacaine spinal anesthesia in elderly patients undergoing transurethral prostatectomy. Biological & pharmaceutical bulletin 2013, 36, 959–965. [Google Scholar] [CrossRef]
- Lawhead, R.G.; Blaxall, H.S.; Bylund, D.B. Alpha-2A is the predominant alpha-2 adrenergic receptor subtype in human spinal cord. Anesthesiology 1992, 77, 983–991. [Google Scholar] [CrossRef]
- Smith, C.; Birnbaum, G.; Carter, J.L.; Greenstein, J.; Lublin, F.D. Tizanidine treatment of spasticity caused by multiple sclerosis: results of a double-blind, placebo-controlled trial. US Tizanidine Study Group. Neurology 1994, 44, S34–42. [Google Scholar]
- Asano, T.; Dohi, S.; Ohta, S.; Shimonaka, H.; Iida, H. Antinociception by epidural and systemic alpha(2)-adrenoceptor agonists and their binding affinity in rat spinal cord and brain. Anesthesia and analgesia 2000, 90, 400–407. [Google Scholar] [CrossRef]
- Schnaider, T.B.; Vieira, A.M.; Brandão, A.C.; Lobo, M.V. [Intraoperative analgesic effect of epidural ketamine, clonidine or dexmedetomidine for upper abdominal surgery.]. Revista brasileira de anestesiologia 2005, 55, 525–531. [Google Scholar] [CrossRef]
- Kanazi, G.E.; Aouad, M.T.; Jabbour-Khoury, S.I.; Al Jazzar, M.D.; Alameddine, M.M.; Al-Yaman, R.; Bulbul, M.; Baraka, A.S. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta anaesthesiologica Scandinavica 2006, 50, 222–227. [Google Scholar] [CrossRef]
- Liu, H.J.; Gao, X.Z.; Liu, X.M.; Xia, M.; Li, W.Y.; Jin, Y. Effect of intrathecal dexmedetomidine on spinal morphine analgesia in patients with refractory cancer pain. Journal of palliative medicine 2014, 17, 837–840. [Google Scholar] [CrossRef]
- Li, G.; Wang, H.; Qi, X.; Huang, X.; Li, Y. Intrathecal dexmedetomidine improves epidural labor analgesia effects: a randomized controlled trial. The Journal of international medical research 2021, 49, 300060521999534. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).