Submitted:
11 January 2024
Posted:
12 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
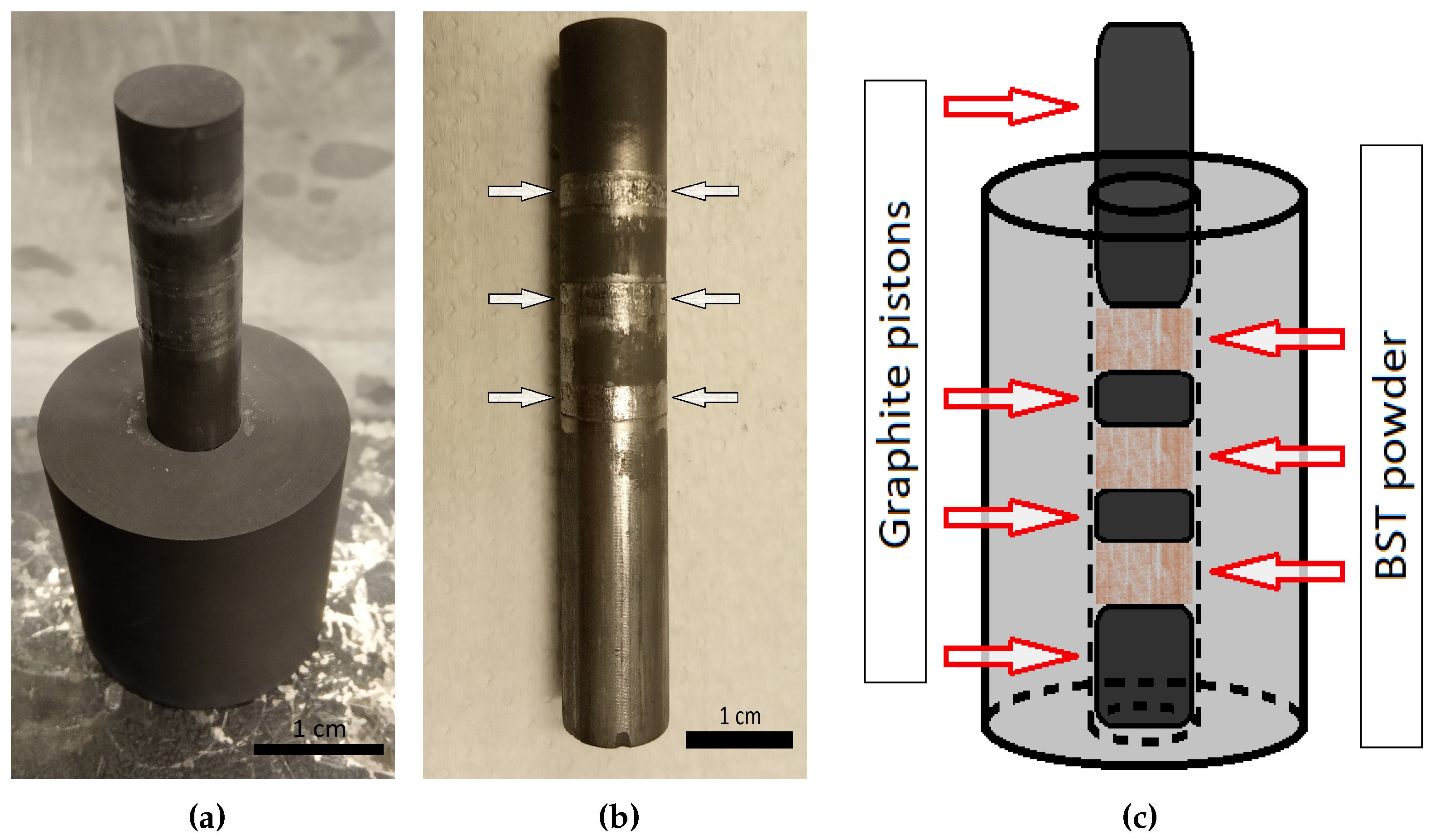
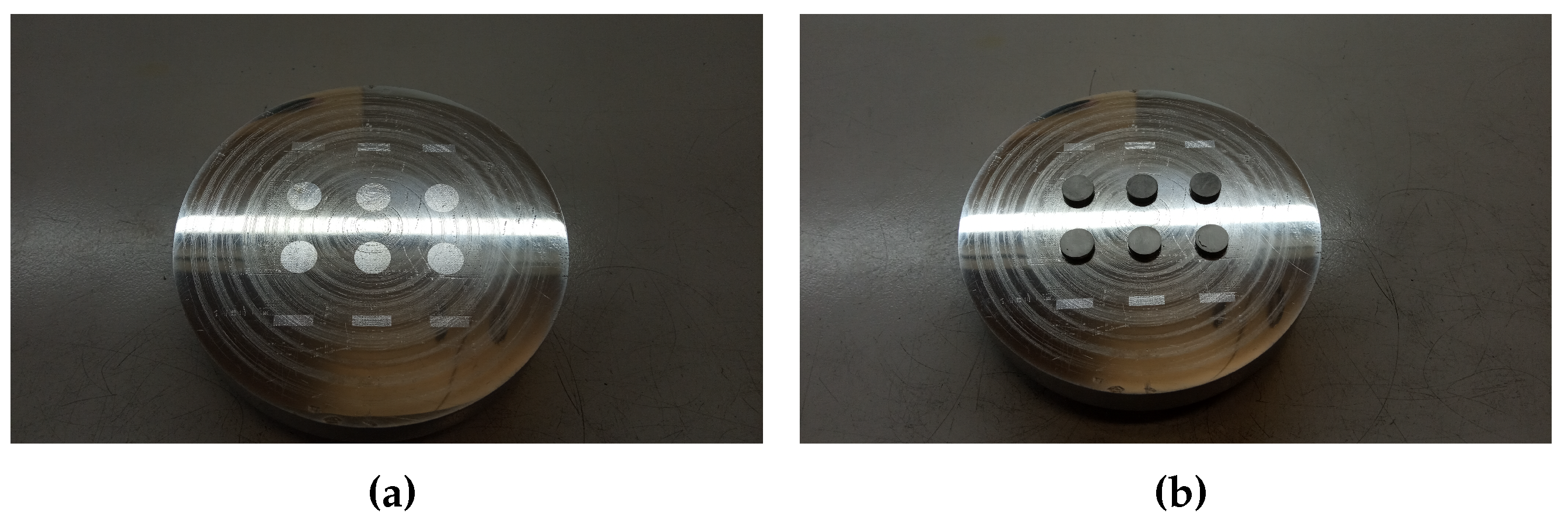
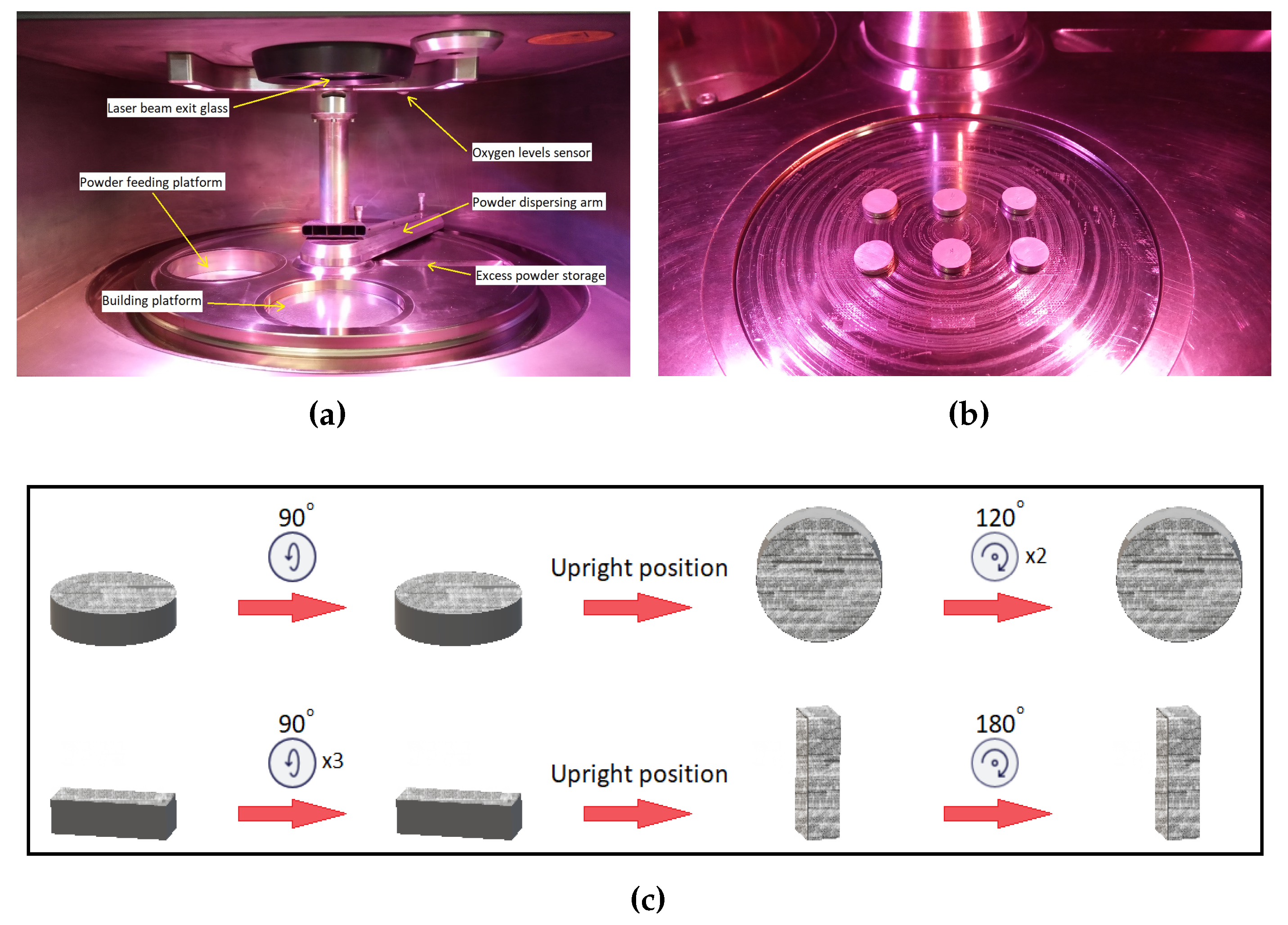
2. Experimental procedures
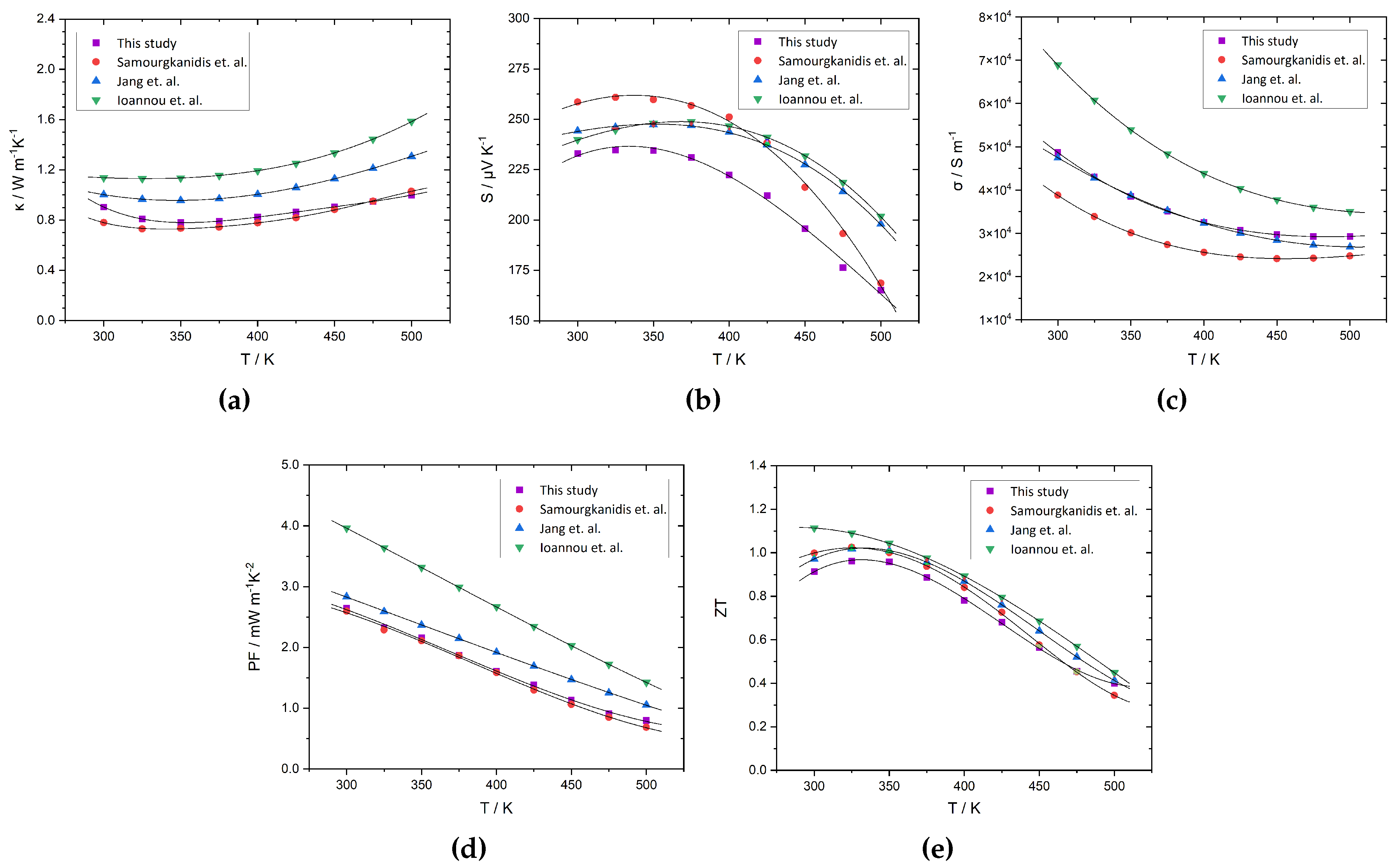
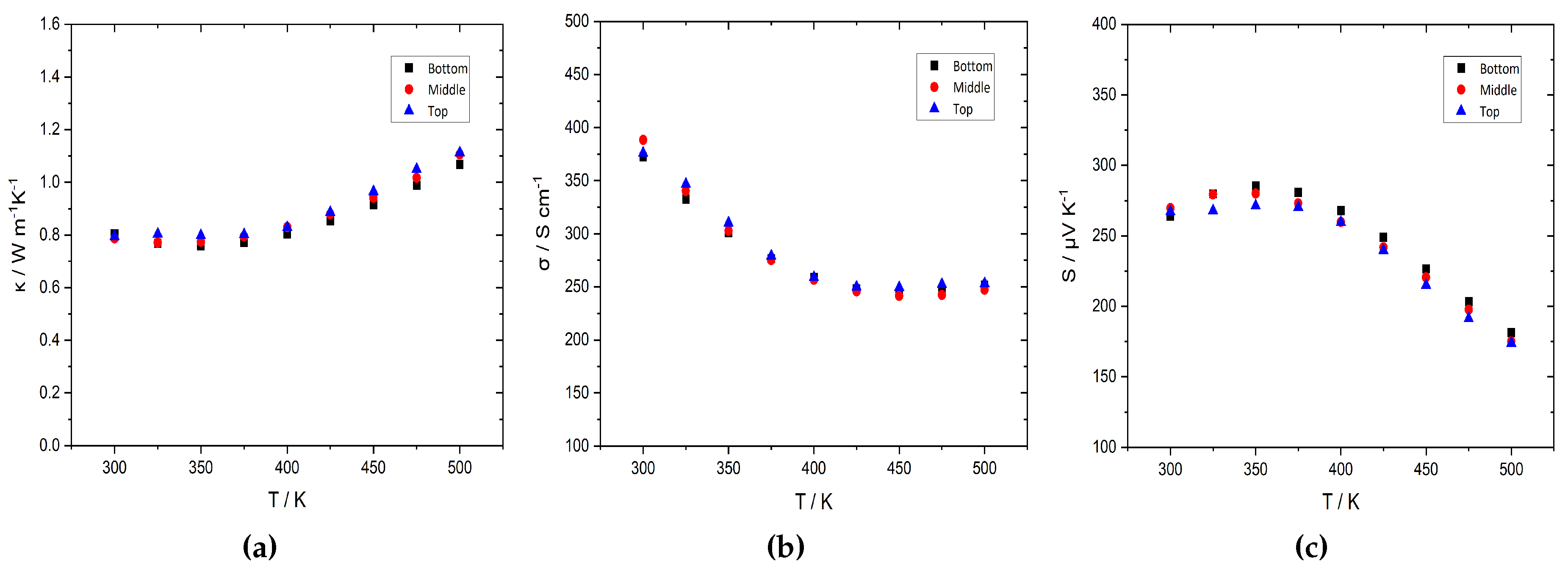
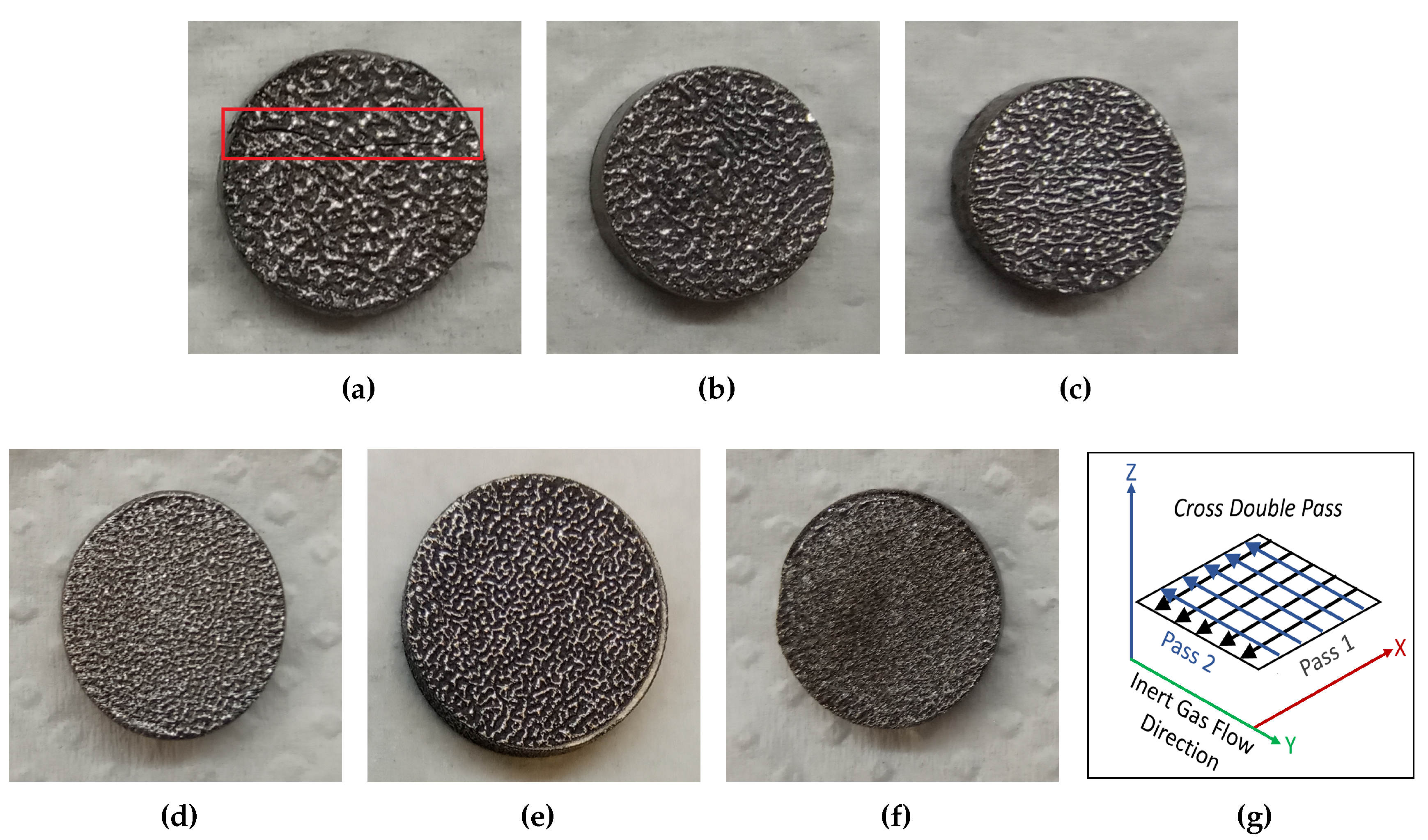
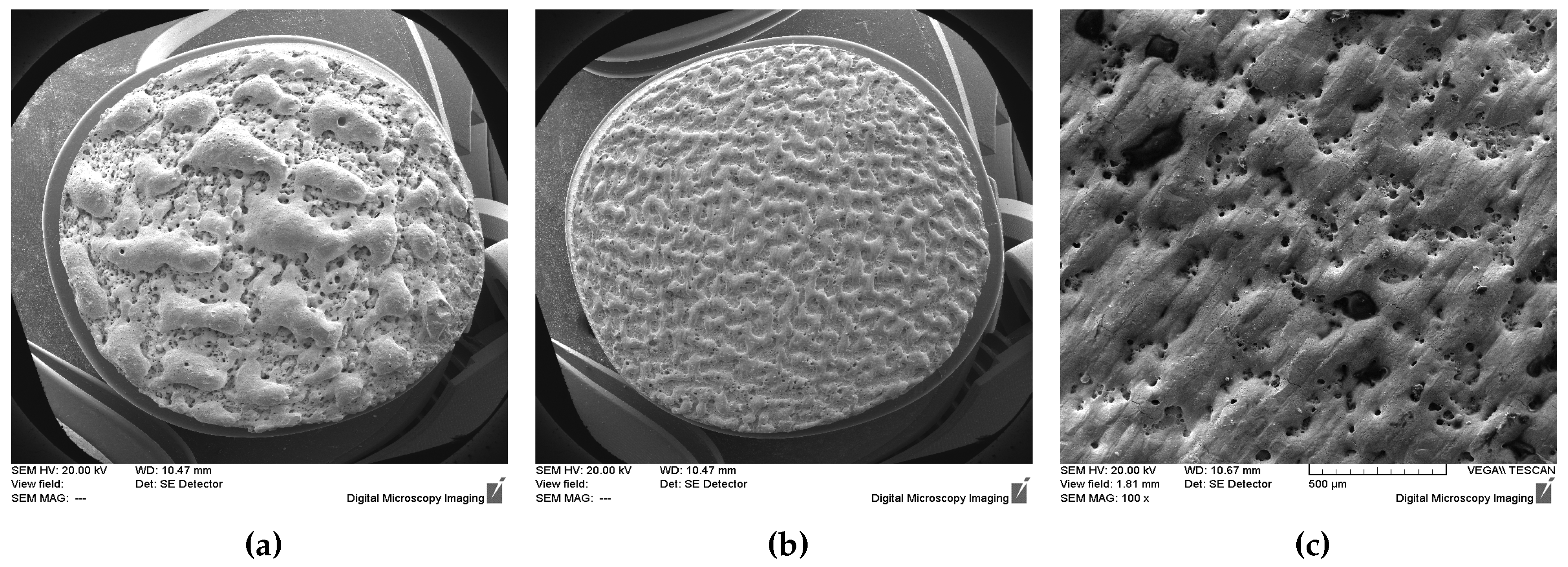
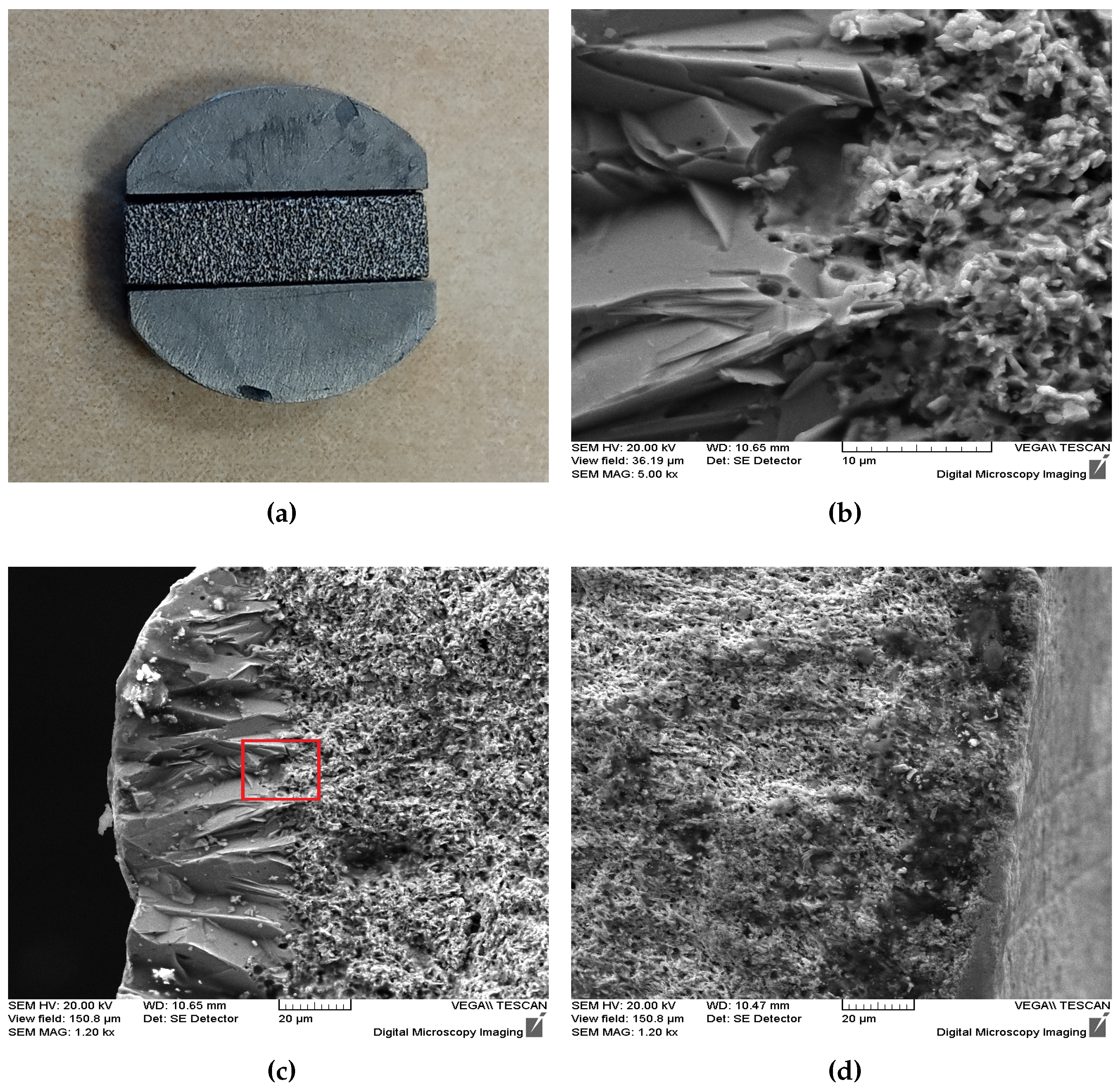
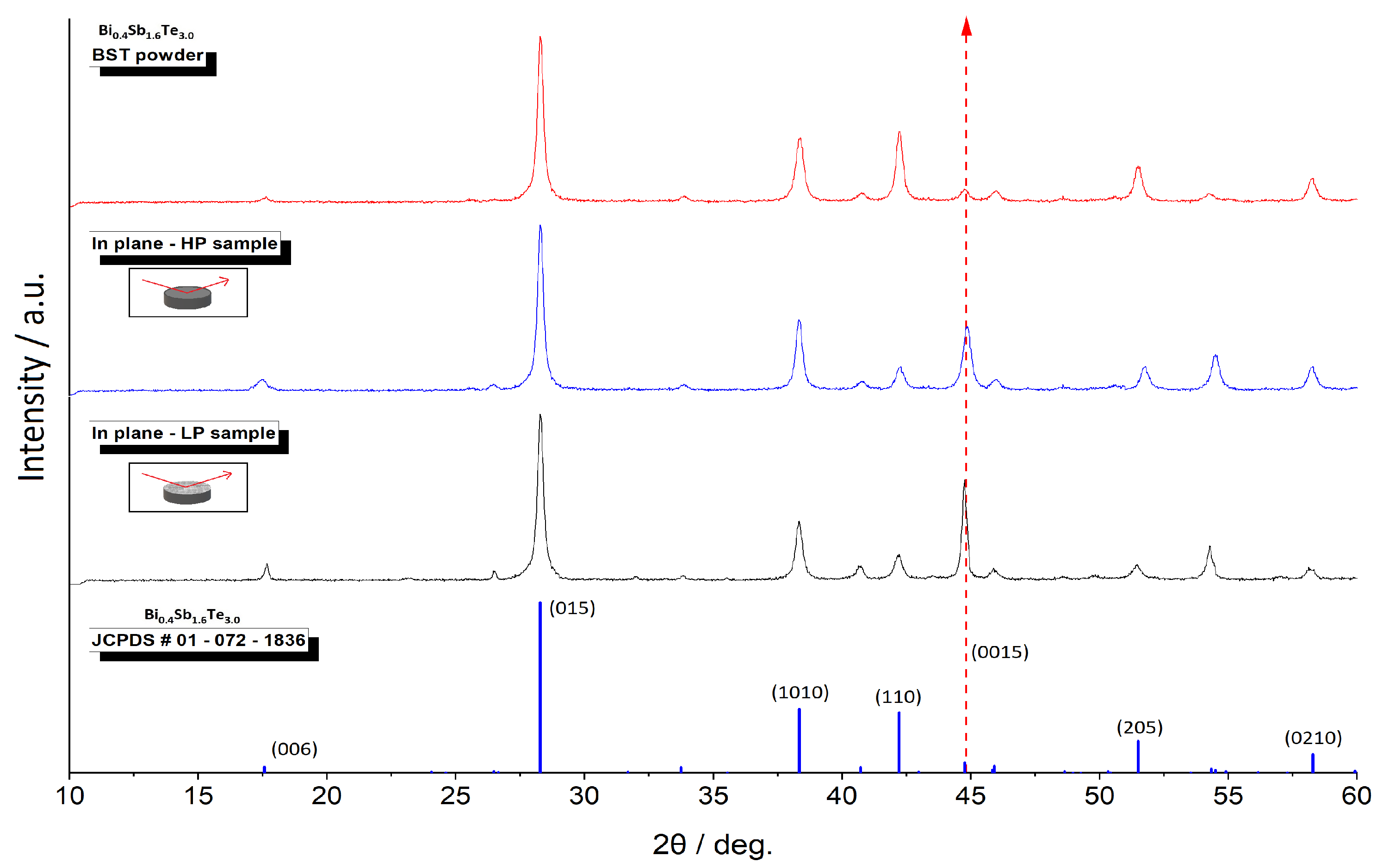
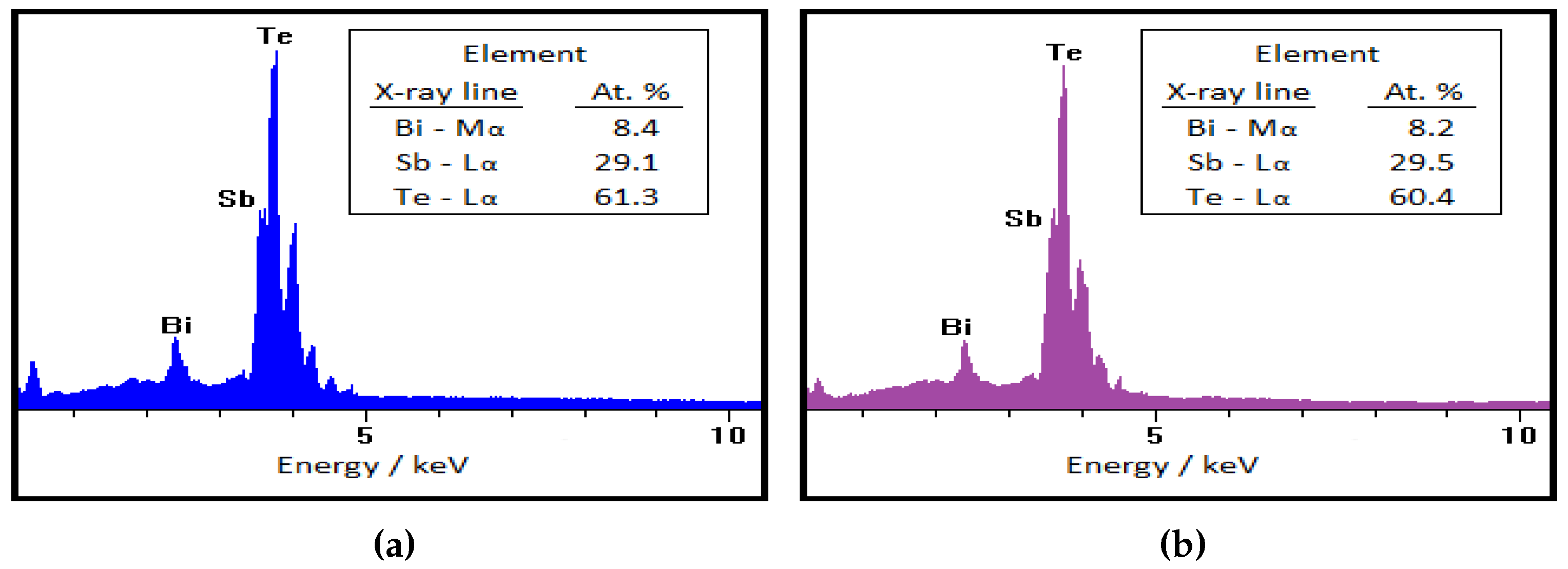
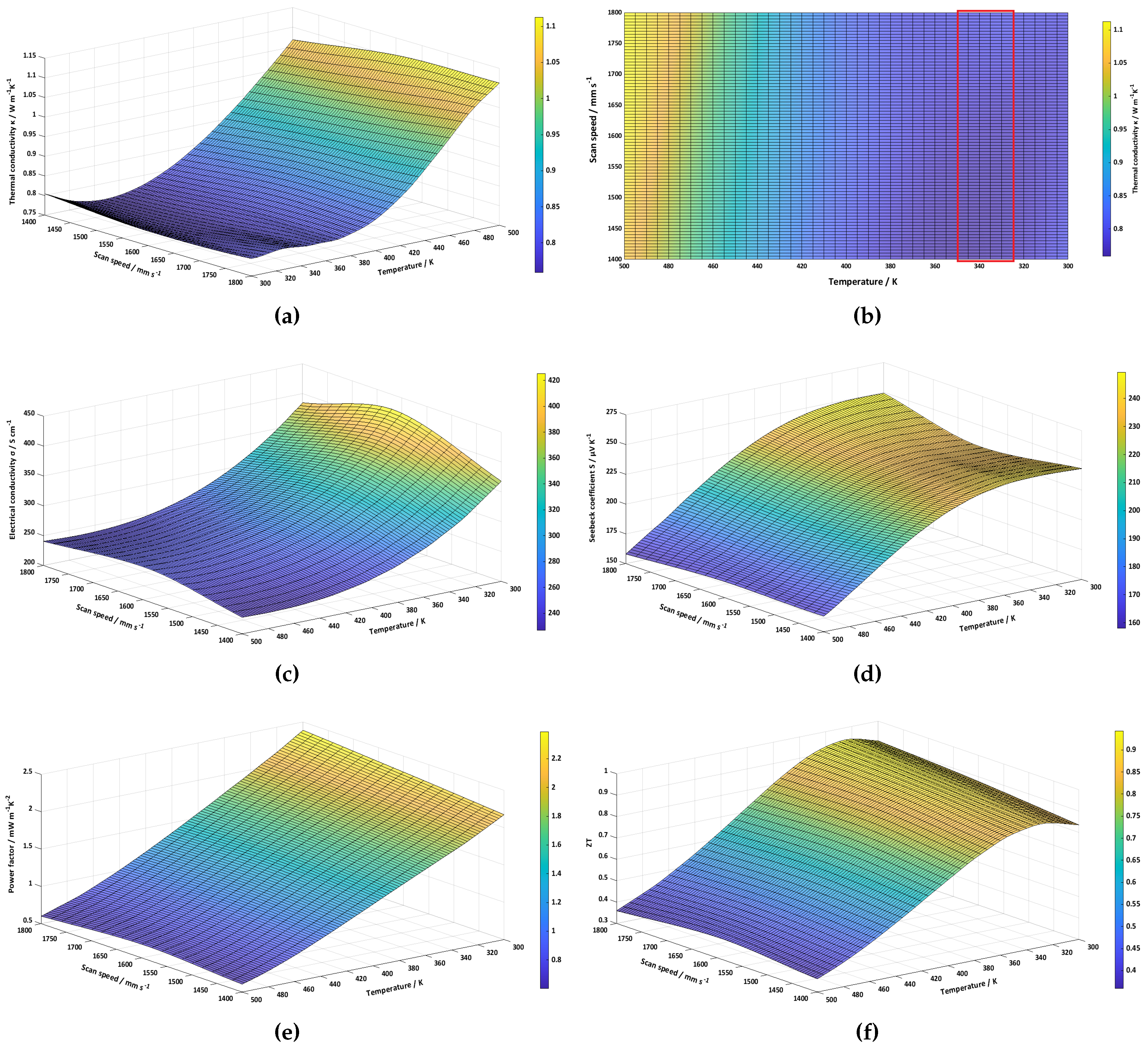
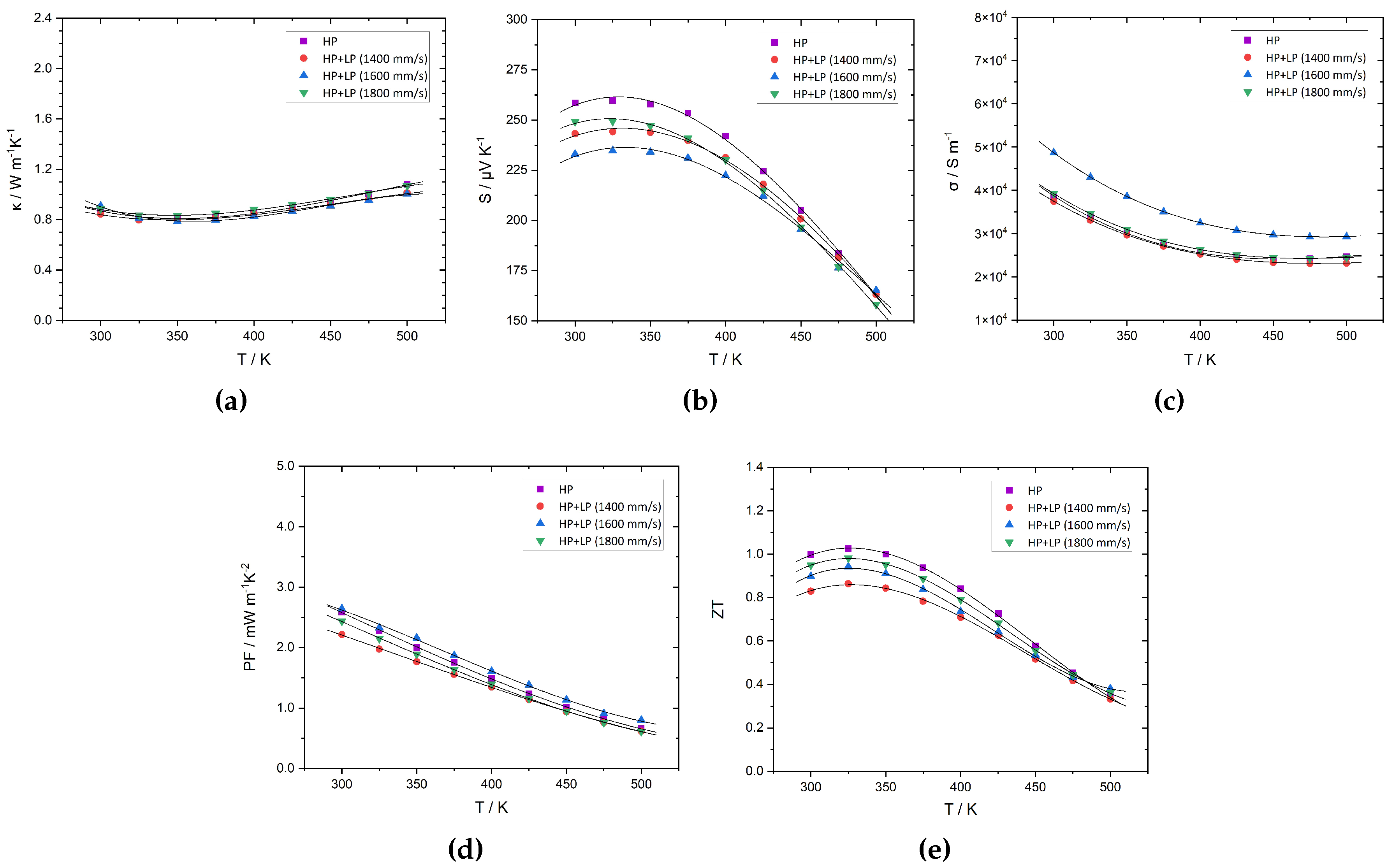
3. Results and discussion
3.1. Laser processing
3.2. Thermoelectric characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Hu, J.; Zhang, S.; Deng, M.; Han, C.G.; Liu, Y. New trends, strategies and opportunities in thermoelectric materials: a perspective. Materials Today Physics 2017, 1, 50–60. [Google Scholar] [CrossRef]
- Wei, J.; Yang, L.; Ma, Z.; Song, P.; Zhang, M.; Ma, J.; Yang, F.; Wang, X. Review of current high-ZT thermoelectric materials. Journal of Materials Science 2020, 55, 12642–12704. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, C.; Snoussi, H.; Zhang, G. Machine learning approaches for thermoelectric materials research. Advanced Functional Materials 2020, 30, 1906041. [Google Scholar] [CrossRef]
- Wahab, J.; Ghazali, M.; Yusoff, W.; Sajuri, Z. Enhancing material performance through laser surface texturing: A review. Transactions of the IMF 2016, 94, 193–198. [Google Scholar] [CrossRef]
- Nayak, B.; Gupta, M.; Kolasinski, K. Formation of nano-textured conical microstructures in titanium metal surface by femtosecond laser irradiation. Applied Physics A 2008, 90, 399–402. [Google Scholar] [CrossRef]
- Neves, D.; Diniz, A.E.; Lima, M.S.F. Microstructural analyses and wear behavior of the cemented carbide tools after laser surface treatment and PVD coating. Applied Surface Science 2013, 282, 680–688. [Google Scholar] [CrossRef]
- Razi, S.; Madanipour, K.; Mollabashi, M. Laser surface texturing of 316L stainless steel in air and water: A method for increasing hydrophilicity via direct creation of microstructures. Optics & Laser Technology 2016, 80, 237–246. [Google Scholar]
- Zhou, J.; Shen, H.; Pan, Y.; Ding, X. Experimental study on laser microstructures using long pulse. Optics and Lasers in Engineering 2016, 78, 113–120. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, W. Direct fabrication of cone array microstructure on monocrystalline silicon surface by femtosecond laser texturing. Optical Materials 2017, 72, 508–512. [Google Scholar] [CrossRef]
- Liu, Y.; Su, J.; Tan, C.; Feng, Z.; Zhang, H.; Wu, L.; Chen, B.; Song, X. Effect of laser texturing on mechanical strength and microstructural properties of hot-pressing joining of carbon fiber reinforced plastic to Ti6Al4V. Journal of Manufacturing Processes 2021, 65, 30–41. [Google Scholar] [CrossRef]
- Long, J.; Chu, P.; Li, Y.; Lin, J.; Cao, Z.; Xu, M.; Ren, Q.; Xie, X. Dual-scale porous/grooved microstructures prepared by nanosecond laser surface texturing for high-performance vapor chambers. Journal of Manufacturing Processes 2022, 73, 914–923. [Google Scholar] [CrossRef]
- Yu, X.; He, S.; Cai, R. Frictional characteristics of mechanical seals with a laser-textured seal face. Journal of Materials Processing Technology 2002, 129, 463–466. [Google Scholar] [CrossRef]
- Bai, S.; Peng, X.; Li, Y.; Sheng, S. A hydrodynamic laser surface-textured gas mechanical face seal. Tribology Letters 2010, 38, 187–194. [Google Scholar] [CrossRef]
- Wang, T.; Huang, W.; Liu, X.; Li, Y.; Wang, Y. Experimental study of two-phase mechanical face seals with laser surface texturing. Tribology International 2014, 72, 90–97. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Modin, E.B.; Sayfutdinova, A.R.; Emelyanenko, K.A.; Vasiliev, A.L.; Emelyanenko, A.M. Combination of functional nanoengineering and nanosecond laser texturing for design of superhydrophobic aluminum alloy with exceptional mechanical and chemical properties. ACS nano 2017, 11, 10113–10123. [Google Scholar] [CrossRef]
- Pereira, R.; Moura, C.; Henriques, B.; Chevalier, J.; Silva, F.; Fredel, M. Influence of laser texturing on surface features, mechanical properties and low-temperature degradation behavior of 3Y-TZP. Ceramics International 2020, 46, 3502–3512. [Google Scholar] [CrossRef]
- Etsion, I. Improving tribological performance of mechanical components by laser surface texturing. Tribology letters 2004, 17, 733–737. [Google Scholar] [CrossRef]
- Vilhena, L.; Podgornik, B.; Vižintin, J.; Možina, J. Influence of texturing parameters and contact conditions on tribological behaviour of laser textured surfaces. Meccanica 2011, 46, 567–575. [Google Scholar] [CrossRef]
- Qiu, Y.; Khonsari, M. Experimental investigation of tribological performance of laser textured stainless steel rings. Tribology International 2011, 44, 635–644. [Google Scholar] [CrossRef]
- Bathe, R.; Sai Krishna, V.; Nikumb, S.; Padmanabham, G. Laser surface texturing of gray cast iron for improving tribological behavior. Applied Physics A 2014, 117, 117–123. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, F.; Jiang, F.; Liu, G. Study of the effect of surface laser texture on tribological properties of cemented carbide materials. Proceedings of the Institution of Mechanical Engineers, Part B: Journal of Engineering Manufacture 2020, 234, 993–1006. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Y.; Bai, Z.; Liu, J.; Huo, L.; Wang, Q. Evaluation of tribological performance for laser textured surfaces with diverse wettabilities under water/oil lubrication environments. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2022, 645, 128949. [Google Scholar] [CrossRef]
- Soltani-Kordshuli, F.; Miller, C.; Harris, N.; Zou, M. Laser surface texturing of both thin polytetrafluoroethylene coatings and stainless steel substrates for improving tribological properties. Polymer Testing 2023, 117, 107852. [Google Scholar] [CrossRef]
- Dobrzański, L.; Drygała, A.; Gołombek, K.; Panek, P.; Bielańska, E.; Zięba, P. Laser surface treatment of multicrystalline silicon for enhancing optical properties. journal of materials processing technology 2008, 201, 291–296. [Google Scholar] [CrossRef]
- Iyengar, V.V.; Nayak, B.K.; Gupta, M.C. Ultralow reflectance metal surfaces by ultrafast laser texturing. Applied optics 2010, 49, 5983–5988. [Google Scholar] [CrossRef]
- Sani, E.; Sciti, D.; Silvestroni, L.; Bellucci, A.; Orlando, S.; Trucchi, D.M. Tailoring optical properties of surfaces in wide spectral ranges by multi-scale femtosecond-laser texturing: A case-study for TaB2 ceramics. Optical Materials 2020, 109, 110347. [Google Scholar] [CrossRef]
- Sharma, A.; Marla, D. Understanding the effects of picosecond laser texturing of silicon solar cells on optical and electrical properties. International Journal of Materials Engineering Innovation 2022, 13, 23–32. [Google Scholar] [CrossRef]
- Bouchard, F.; Soldera, M.; Lasagni, A.F. PMMA Optical Diffusers with Hierarchical Surface Structures Imprinted by Hot Embossing of Laser-Textured Stainless Steel. Advanced Optical Materials 2023, 11, 2202091. [Google Scholar] [CrossRef]
- Ryk, G.; Kligerman, Y.; Etsion, I. Experimental investigation of laser surface texturing for reciprocating automotive components. Tribology Transactions 2002, 45, 444–449. [Google Scholar] [CrossRef]
- Tomanik, E.; Profito, F.J.; Zachariadis, D.C. Modelling the hydrodynamic support of cylinder bore and piston rings with laser textured surfaces. Tribology international 2013, 59, 90–96. [Google Scholar] [CrossRef]
- Profito, F.J.; Vlădescu, S.C.; Reddyhoff, T.; Dini, D. Transient experimental and modelling studies of laser-textured micro-grooved surfaces with a focus on piston-ring cylinder liner contacts. Tribology International 2017, 113, 125–136. [Google Scholar] [CrossRef]
- Abdullah, M.; Alghoul, M.; Naser, H.; Asim, N.; Ahmadi, S.; Yatim, B.; Sopian, K. Research and development efforts on texturization to reduce the optical losses at front surface of silicon solar cell. Renewable and Sustainable Energy Reviews 2016, 66, 380–398. [Google Scholar] [CrossRef]
- Abbott, M.; Cotter, J. Optical and electrical properties of laser texturing for high-efficiency solar cells. Progress in Photovoltaics: Research and Applications 2006, 14, 225–235. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Laser surface treatment for porous and textured Ca–P bio-ceramic coating on Ti–6Al–4V. Biomedical Materials 2007, 2, 274. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.R.; Nayak, B.K.; Nair, L.S.; Gupta, M.C.; Laurencin, C.T. Improved bio-implant using ultrafast laser induced self-assembled nanotexture in titanium. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2011, 97, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Scharnweber, T.; Pfleging, W.; Besser, H.; Majumdar, J.D. Laser surface textured titanium alloy (Ti–6Al–4V)–Part II–Studies on bio-compatibility. Applied Surface Science 2015, 357, 750–758. [Google Scholar] [CrossRef]
- Pratap, T.; Patra, K. Mechanical micro-texturing of Ti-6Al-4V surfaces for improved wettability and bio-tribological performances. Surface and Coatings Technology 2018, 349, 71–81. [Google Scholar] [CrossRef]
- Kumar, A.; Bano, S.; Govind, B.; Bhardwaj, A.; Bhatt, K.; Misra, D. A review on fundamentals, design and optimization to high ZT of thermoelectric materials for application to thermoelectric technology. Journal of Electronic Materials 2021, 50, 6037–6059. [Google Scholar] [CrossRef]
- Ioffe, A.F.; Stil’Bans, L.; Iordanishvili, E.; Stavitskaya, T.; Gelbtuch, A.; Vineyard, G. Semiconductor thermoelements and thermoelectric cooling. Physics Today 1959, 12, 42–42. [Google Scholar] [CrossRef]
- Goldsmid, H.J. Bismuth telluride and its alloys as materials for thermoelectric generation. Materials 2014, 7, 2577–2592. [Google Scholar] [CrossRef]
- Zhao, P.; Yu, F.; Wang, B.; Zhao, H.; Chen, C.; Wang, D.; Ying, P.; Wu, Y.; Li, P.; Zhang, B.; et al. Porous bismuth antimony telluride alloys with excellent thermoelectric and mechanical properties. Journal of Materials Chemistry A 2021, 9, 4990–4999. [Google Scholar] [CrossRef]
- El-Makaty, F.M.; Ahmed, H.K.; Youssef, K.M. The effect of different nanofiller materials on the thermoelectric behavior of bismuth telluride. Materials & Design 2021, 209, 109974. [Google Scholar]
- Saberi, Y.; Sajjadi, S.A. A comprehensive review on the effects of doping process on the thermoelectric properties of Bi2Te3 based alloys. Journal of Alloys and Compounds 2022, 904, 163918. [Google Scholar] [CrossRef]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef]
- Shin, W.H.; Ahn, K.; Jeong, M.; Yoon, J.S.; Song, J.M.; Lee, S.; Seo, W.S.; Lim, Y.S. Enhanced thermoelectric performance of reduced graphene oxide incorporated bismuth-antimony-telluride by lattice thermal conductivity reduction. Journal of Alloys and Compounds 2017, 718, 342–348. [Google Scholar] [CrossRef]
- Jang, K.W.; Kim, H.J.; Jung, W.J.; Kim, I.H. Charge transport and thermoelectric properties of p-type Bi 2-x Sb x Te 3 prepared by mechanical alloying and hot pressing. Korean Journal of Metals and Materials 2018, 56, 66–71. [Google Scholar]
- Symeou, E.; Nicolaou, C.; Delimitis, A.; Androulakis, J.; Kyratsi, T.; Giapintzakis, J. High thermoelectric performance of Bi2-xSbxTe3 bulk alloys prepared from non-nanostructured starting powders. Journal of Solid State Chemistry 2019, 270, 388–397. [Google Scholar] [CrossRef]
- Ioannou, I.; Ioannou, P.S.; Kyratsi, T.; Giapintzakis, J. Low-cost preparation of highly-efficient thermoelectric BixSb2-xTe3 nanostructured powders via mechanical alloying. Journal of Solid State Chemistry 2023, 319, 123823. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, W.; Wang, Y.; Zhu, P.; Peng, K.; Deng, Y. Towards high integration and power density: Zigzag-type thin-film thermoelectric generator assisted by rapid pulse laser patterning technique. Applied Energy 2020, 275, 115404. [Google Scholar] [CrossRef]
- Samourgkanidis, G.; Kyratsi, T. Continuous and Intermittent Planetary Ball Milling Effects on the Alloying of a Bismuth Antimony Telluride Powder Mixture. Inorganics 2023, 11, 221. [Google Scholar] [CrossRef]
- Stylianou, R.; Evangelou, A.; Loizou, A.; Kim, D.; Wharton, J.; Koutsokeras, L.; Constantinides, G.; Delimitis, A.; Kyratsi, T. Laser powder bed fusion of 316 L stainless steel with 2 wt% nanosized SiO2 additives: Powder processing and consolidation. Powder Technology 2023, 427, 118714. [Google Scholar] [CrossRef]
- Evangelou, A.; Stylianou, R.; Loizou, A.; Kim, D.; Liang, A.; Reed, P.; Constantinides, G.; Kyratsi, T. Effects of process parameters and scan strategy on the microstructure and density of stainless steel 316L produced via laser powder bed fusion. Journal of Alloys and Metallurgical Systems 2023, 3, 100027. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).